Abstract
Objective
To determine the presence of SIBLING and bone components in Juvenile Dermatomyositis (JDM) pathologic calcifications.
Methods
Calcifications, removed from 4 girls with JDM symptoms for 36.9 ± 48.3 months, were stained for SIBLING proteins: osteopontin OPN (full length), bone sialoprotein (BSP), dentin matrix protein 1 (DMP1), dentin phosphoprotein (DPP), matrix extracellular phosphoglycoprotein (MEPE); bone markers: osteocalcin (OC), core binding factor alpha 1 (Cbfa1), and alkaline phosphatase (ALP) for osteoblasts; tartrate resistant acid phosphatase (TRAP) for osteoclasts, as well as the mineral regulators osteonectin (ON) and matrix Gla protein (MGP). The deposit center, periphery, adjacent connective tissue, and vascular endothelial cells were examined.
Results
Alizarin red stained calcified deposits, which did not localize with collagen, like bone, under polarized light. H+E stain revealed a paucity of connective tissue and absence of bone-like structures. The deposits, connective tissue, and vascular endothelial cells were positive for BSP, DPP, DMP1, and ALP; MEPE was not detected. OC, ON and MGP were present in the deposits and vascular endothelial cells; OPN and Cbfa1 were present in deposits and connective tissue. TRAP positive osteoclasts were localized to the calcification periphery.
Conclusion
The disorganized JDM calcifications differ in structure, composition and protein content from bone, suggesting that they may not form through an osteogenic pathway. Osteoclasts at the deposit surface represent an attempt to initiate its resolution.
Juvenile Dermatomyositis (JDM), the most common pediatric inflammatory myopathy, is a small vessel systemic vasculopathy in which the children present with symmetrical proximal muscle weakness and a characteristic rash (1, 2) . As many as 30% of JDM patients develop the painful complication of pathological soft tissue calcifications (3). These calcifications are associated with chronic inflammation, usually occurring after a long period of untreated symptoms (4).
The pathological soft tissue calcifications found in JDM, although similar in composition to bone, however, are quite distinct. A previous study using Western Blot analysis identified osteopontin (OPN), osteonectin (ON), and bone sialoprotein (BSP) in JDM calcifications (5). These proteins are also found in bone, but based on Fourier Transform Infrared spectroscopy (FTIR), the JDM calcifications exhibit a higher mineral to matrix ratio than bone (5), leading to the speculation that the mechanism of mineral deposition in JDM might differ from that of bone formation. There may be other mediators of mineralization present in both bone and JDM calcifications, since calcifications of soft tissue found in other diseases, such as rheumatic valvular heart disease and scleroderma contain markers of bone formation (6, 7).
OPN and BSP both belong to the Small Integrin-Binding Ligand N-Linked Glycoprotein (SIBLING) protein family, along with fellow members: dentin matrix protein 1 (DMP1), dentin sialophosphoprotein (DSPP), and matrix extracellular phosphoglycoprotein (MEPE). The SIBLING proteins play crucial roles in regulating bone and dentin formation, in addition to serving as cell signals. Their roles are governed by post-translational modifications such as phosphorylation and glycosylation (8). Encoded on human chromosome 4, they contain an Arg-Gly-Asp (RGD) integrin binding domain which mediates cell-cell interactions (9). Other non-SIBLING mineralization mediators such as Matrix Gla protein (MGP) and ON are also of interest as they bind to calcium (10, 11). Previous studies found JDM patients with calcifications have a higher urinary MGP output (12), and identified MGP within the calcifications (13). Recent studies reported increased phosphorylated MGP within JDM muscle from children with calcifications as compared to JDM muscle from children without calcifications and normal controls (14). For the present study, osteoblast specific markers core binding factor alpha 1 (Cbfa1), osteocalcin (OC), alkaline phosphatase (ALP), and tartrate resistant acid phosphatase (TRAP) activity were also used to identify osteoblasts and osteoclasts respectively. The proposed functions and tissue localization of these markers are summarized in Table 1.
Table 1.
Bone related protein antigens: Localization and Function
| Antigen | Tissue Localization | Proposed Function |
|---|---|---|
| BSP (LFMb-25) | bone, dentin, salivary glands, sweat glands, and kidney | Mineral nucleator |
| OPN full length (LFMb-14) | bone, dentin, salivary glands, sweat glands, and kidney inflammatory cells and activated T-cells | Osteoclast recruitment, mineralization inhibitor |
| DMP1 (LFMb-31) | bone, dentin, salivary glands, sweat glands, and kidney | Mineral nucleator, mineralization regulator, regulator of osteocalcin |
| DSPP (LFMb-21) (DPP domain CSRGDASYNSDE SKDNG) | bone, dentin, salivary glands, sweat glands, and kidney | Mineralization regulator, recruiter of inflammatory cells |
| MEPE (LFMb-33) | bone, dentin, salivary glands, sweat glands, and kidney | Mineralization regulator |
| ALP | Cell surface of osteoblasts | Marker of bone remodeling |
| Cbfa1 | Osteoblast precursor cells | Transcription factor for osteoblast lineage |
| OC | Bone, osteoblasts | Mineralization regulator; hormone |
| ON | bone | Regulate vascular homeostasis, tissue repair, collagen fibrillogenesis, mineralization regulator |
| MGP | Arteries, cartilage | Mineralization inhibitor |
Patients and Methods
Patient Population
Four girls with probable JDM, diagnosed by the attending physician (LMP) based on clinical criteria of Bohan and Peter (15), were enrolled in the study after obtaining age-appropriate informed consent, (IRB # 2001–11530). Their demographic and clinical data are summarized in Table 2.
Table 2.
Demographic, Clinical, and Genetic Data for 4 Children with JDM with Removed Calcinosis
| Sample ID | Sex | Age at JDM onset (years) | Duration of untreated disease (months) | Age at Calcification removal (years) | Location | DAS Musclea | DAS skinb | TNFα-308 | DQA1 *0501 | ERLc/mm |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 2 | 24 | 7.8 | left patellar tendon | 0 | 3 | GA | + | 3.6 |
| 2 | F | 2.1 | 3.4 | 12.2 | Elbow connective tissue | 1 | 0 | GA | - | 4.6 |
| 3 | F | 6.2 | 108.3 | 18.5 | Elbow connective tissue | 1 | 7 | GG | + | 3.7 |
| 4 | F | 1.1 | 12 | 18.2 | Biceps muscle | 9 | 7 | GA | + | 3.0 |
| Mean ± SD | 2.9 ± 2.3 | 36.9 ± 48.3 | 14.2 ± 5.2 | 2.8 ± 4.2 | 4.3 ± 3.4 | 3.7 ± 0.33 |
Disease Activity Score (DAS) for muscle strength and function; range from 0 (normal) to 9 (maximal impairment)
Disease Activity Score (DAS) for skin involvement severity and intensity from 0 (normal) to 11 (severe)
Nailfold Capillary End Row Loop (ERL); decreased ERL/mm in chronic JDM with calcifications (normal range > 7 ERL/mm)
Clinical Definitions
The Disease Activity Score (DAS), a validated scoring of disease activity in skin and muscle (16), was determined by the same physician (LMP) during the clinical visit prior to the removal of the calcification. This score is a clinical estimate of disease activity that rates the active involvement of both skin and muscle for a total score of 20 points (16). The duration of untreated disease was defined as the interval of time between the first symptom (disease onset) and the date of diagnostic evaluation (4).
Genetic Testing
The primers and probes were obtained from Invitrogen (Madison, WI). Peripheral blood mononuclear cells (PBMCs) were isolated from anti-coagulated whole blood and stored at −80°C until DNA isolation was performed using a Puregene DNA isolation kit (Gentra Systems, Inc. Minneapolis, MN). The TNFα-308 polymorphism consists of a single base pair substitution of an A for the more common G; and PCR was used to amplify a 107 bp fragment of that gene containing a region that incorporated a NcoI restriction site as previously described (17). Digestion with NcoI confirmed genotype as GG, GA or AA. Testing for DQA1*0501 was performed as described elsewhere (18).
Sample Collection
Four girls requested surgical removal of painful calcifications. The specimens were fixed overnight in 10% formalin (pH 7.0). Due to the difficulty of sectioning calcified tissue, patient sample #4, was extensively de-calcified (10% hydrochloric acid). All tissues were paraffin embedded and sectioned at 4 µm (Histology Department, Children’s Memorial Hospital, Chicago).
Immunohistochemistry
Standard techniques were followed to deparaffinize, rehydrate, and retrieve antigen with citrate buffer (pH 6.0) for 20 minutes at 95°C (19). The sections were incubated with the following primary antibodies at the specified dilutions: Cbfa1 1:25, OC 1:10 (R&D Systems, Minneapolis, MN), ON 1:10 (Developmental Studies Hybrdioma Bank Iowa), ALP 1:50 (Sigma-Aldrich, St. Louis, MO, USA), BSP 1:100, DMP1 1:50, DPP 1:400, MEPE 1:300, OPN full length 1:200 (Larry W. Fisher, PhD, National Institute of Dental and Craniofacial Research, National Institutes of Health), and MGP 1:50 (Proteintech Group, Chicago, IL). The primary antibody was detected by standard methodology (20). Substitution of the primary antibody with mouse IgG control, rabbit IgG control (cat. #08–6599, #08–6199 Zymed, San Francisco, CA), and rat IgG control (cat. #R2b00, Invitrogen) served as negative controls. Human osteosarcoma (MG-63) cell lines (ATCC, Manassas, VA) and paraffin embedded human iliac crest sections served as marker positive controls.
Histochemistry
The sections were stained with Hematoxylin and Eosin (21), Alizarin red (22), and Tartrate-resistant acid phosphatase (TRAP) (23) by standard methodology.
Image Acquisition
Serial sections of each patient sample were examined, and areas of calcified tissue (confirmed by Alizarin red staining) were photographed under the same conditions. Images of stained sections were acquired using Openlab computer software 4.04 (Improvision Inc., Lexington, MA) and a Leica DMR-HC microscope (Leica Microsystems GmbH, Wetzlar, Germany) coupled to a Photometric Cool Snap chargecoupled device camera and edited using Adobe Photoshop CS2 software.
Data Analysis
Images of serial sections stained for each marker were examined by two independent observers. Positive staining was defined as areas of dark brown comparable to positive control slides. The presence or absence of each marker within the center or periphery (outer edge) of calcium deposits, surrounding connective tissue, and endothelial cells was recorded as the number of positive samples over the total number of samples tested. For each marker, a tissue location was defined as "positive" staining if at least 2 out of 3 calcified samples stained showed localized antigen-specific antibody.
Results
Hematoxylin and Eosin & Alizarin red staining
Calcium deposits were confirmed by Alizarin red staining. No cellular or bone-like trabecular structures were observed within the deposits (Figure 1).
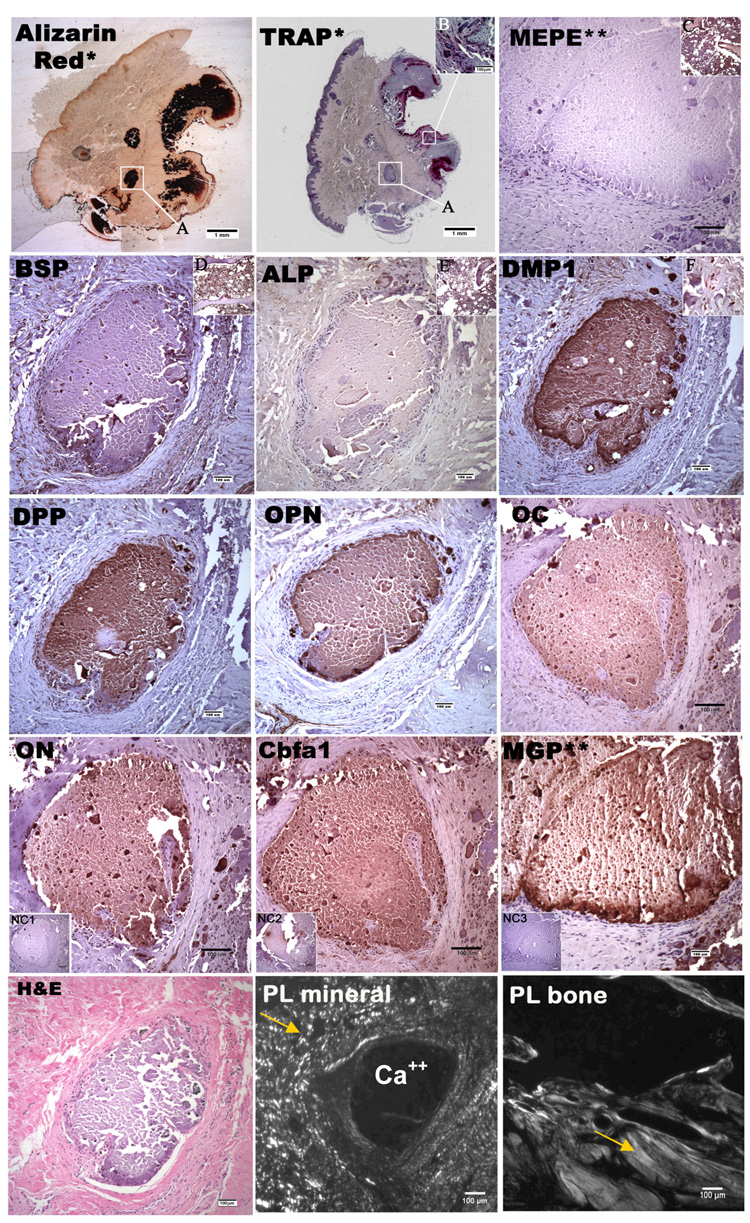
Figure 1.
Alizarin red stain, TRAP stain, H&E stain and immunohistological stain for DPP, OPN, MEPE, BSP, DMP1, ALP, Cbfa1, ON, OC, MGP in mineral deposits and adjacent connective tissue taken at 10x magnification. Brown indicates positive stain. *picture taken at 1x magnification, **picture taken at different site, NC1 is negative control for DPP, OPN, MEPE, BSP, DMP1, ALP, ON, OC,; NC2 is the negative control for Cbfa1; NC3 is the negative control for MGP (A) magnified region in the other panels, (B) indicated region of TRAP positive osteoclasts at 10x magnification (C) human bone as positive control for MEPE, (D) positive control for BSP, (E) positive control for ALP, (F) representative 63x magnified region of surrounding cells demonstrating DMP1 reactivity within the cytoplasm; (PL Mineral) represents JDM mineral deposits under polarized light; (PL bone) represent normal bone taken under polarized light. Arrows indicate collagen.
Immunohistochemical staining
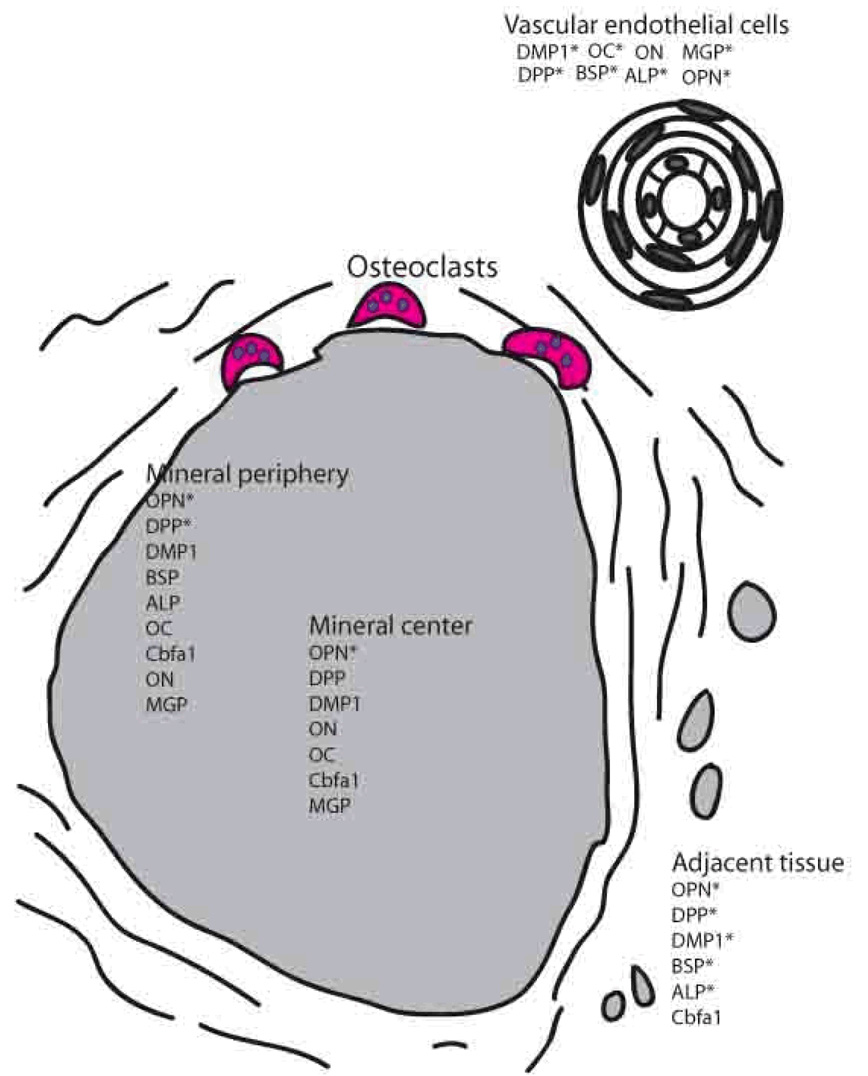
Typical results for the immunostaining of JDM calcification samples are shown in Figure 1 & 2, while a summary of the results from patient samples for OPN (full length), BSP, DMP1, DPP, MEPE, ALP, OC, Cbfa1, ON, and MGP are presented in Table 3 and as a schematic representation in Figure 3. The extensively decalcified sample was not included in the final tallies in Table 3, and the presence of the markers in this sample was noted by an asterisk in Figure 3.
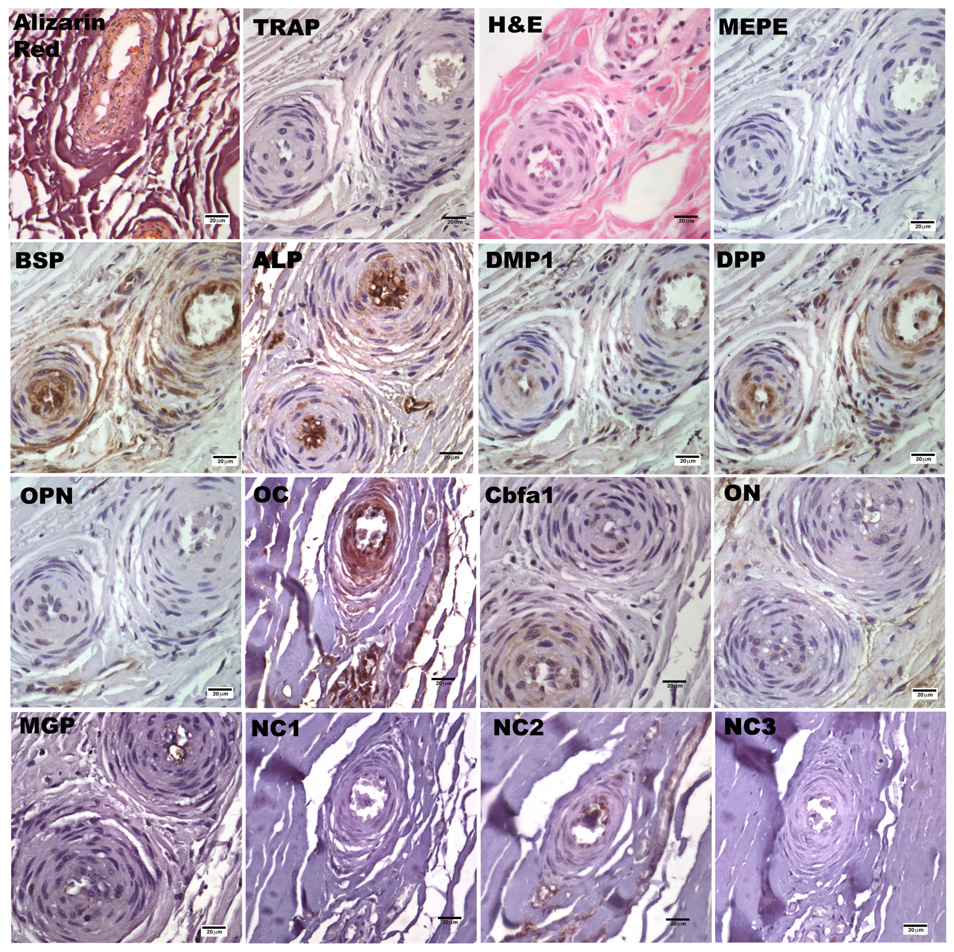
Figure 2.
Alizarin red stain, TRAP stain, H&E stain and immunohistological stain for DPP, OPN, MEPE, BSP, DMP1, ALP, Cbfa1, ON, OC, MGP in vascular endothelial cells at 40x magnification. Brown indicates positive stain. NC1 is the negative control for DPP, OPN, MEPE, BSP, DMP1, ALP, ON, OC; NC2 is the negative control for Cbfa1; NC3 is the negative control for MGP
Table 3.
Positive samples over number examined for each antigen/marker
| Vascular Endothelial Cells | Calcification Center | Calcification Periphery | Adjacent Tissue | |
|---|---|---|---|---|
| BSP | 3/3 | 1/3 | 2/3 | 3/3 |
| OPN | 1/3 | 3/3 | 3/3 | 2/3 |
| DMP1 | 3/3 | 3/3 | 3/3 | 2/3 |
| DPP | 2/3 | 3/3 | 2/3 | 3/3 |
| MEPE | 0/3 | 0/3 | 0/3 | 0/3 |
| ALP | 2/3 | 1/3 | 3/3 | 2/3 |
| Cbfa1 | 1/3 | 2/3 | 2/3 | 2/3 |
| OC | 3/3 | 3/3 | 3/3 | 0/3 |
| ON | 2/3 | 3/3 | 3/3 | 1/3 |
| MGP | 2/3 | 3/3 | 3/3 | 0/3 |
| Alizarin red | 0/3 | 3/3 | 3/3 | 0/3 |
| TRAP | 0/3 | 0/3 | 3/3 | 1/3 |
Figure 3.
Schematic representation of the composition of a JDM calcification. * indicates presence of marker in both calcified and decalcified sample
Calcium deposits within three samples stained dark red with Alizarin red. In contrast, sample #4, which was extensively decalcified prior to sectioning, did not exhibit the dark red staining typical of Alizarin red. TRAP staining revealed the presence of osteoclasts (stained dark pink) around the periphery of mineral deposits in all three calcified samples, but not in the decalcified one. Figure 1, inset F is a representative picture of all the stains taken from the DMP1 stain which demonstrates that the pattern of reactivity within the cells surrounding the calcifications and vascular endothelial cells (Figure 2) is primarily cytoplasmic in nature. After inspecting H&E sections of JDM calcification samples under polarized light (Figure 1), we identified the total absence of embedded collagen fiber within mineral deposits, as compared with sections of normal trabecular bone, despite its presence in other parts of the tissue sample.
OPN, full length form, is present within the center and periphery of mineral deposits in three JDM calcification samples, as well as the decalcified sample. DMP1, MGP, OC and ON were identified both in the center and the periphery of mineral deposits in the three calcified samples, while these molecules were not present within the decalcified sample deposits. DPP was observed within the center of mineral deposits in three calcified samples and in the periphery of two calcified samples as well as in the decalcified sample. BSP was observed in the center of one calcification sample and in the periphery of two calcification samples, but not in the decalcified sample. ALP positive cells were found on the periphery of mineral deposits in the three calcified samples, but only in the center of mineral deposits in one of the samples. Cbfa1 was present in the center and periphery of mineral deposits in two of the samples. The site of the mineral deposits in the decalcified sample did not exhibit ALP, or Cbfa1 in the center or periphery.
With respect to staining of vascular endothelial cells, BSP, DMP1, and OC were present in all three calcified samples, as well as in the decalcified sample. DPP, MGP, ON and ALP were expressed in two calcified samples and each of these markers, with the exception of ON, were present in the decalcified sample. OPN and Cbfa1 were observed in only one calcified sample.
In the adjacent connective tissue, BSP and DPP were observed in three calcified samples as well as the decalcified sample. OPN, DMP1, and ALP were expressed in two calcified samples, as well as the decalcified sample. Cbfa1 also was present in two calcified samples, but not in the decalcified sample. ON was also detected in one calcified sample, but not in the decalcified sample. Neither MGP nor OC were observed in the connective tissue of any of the samples.
The presence of MEPE was not observed in any tissues analyzed.
Mouse isotype controls (for DPP, OPN, MEPE, BSP, DMP1, ALP, ON, OC) and rabbit isotype controls (for MGP) were negative in all tissues, while rat isotype control (for Cbfa1) showed slight positive staining within mineral deposits.
Discussion
This is the first immunohistochemical study of JDM calcifications assessing the mineralized tissue for the presence of SIBLING proteins OPN, BSP, DMP1, DPP, non- SIBLING mineralization regulators ON and MGP, osteoblast specific markers OC, ALP, and Cbfa1, and osteoclast specific TRAP.
SIBLING proteins initially identified in bone and dentin (8) were then demonstrated in specific cancers in which calcified tissue occurred, such as breast and thyroid carcinomas (24). Additionally, osteoblast-like phenotypes have been observed within calcified tissue in cases of rheumatic valvular heart disease and scleroderma (6, 7), which first led us to investigate the presence and location of bone forming and reabsorbing cells within JDM calcifications.
OPN has been proven to be an important mineralization inhibitor and has aspartic acid rich calcium binding domains (25) as well as extensive phosphorylation which may account for its accumulation and localization at calcium deposits. OPN has also been shown to play a role in recruitment and adhesion of osteoclasts (26), which could be associated with the positive TRAP staining for osteoclasts around the periphery of mineral deposits. Furthermore, macrophages, present in the inflammatory infiltrate in JDM muscle (27), produce OPN, which may opsonize the calcifications, thus serving as an attractant for other macrophages and phagocytic cells interacting via the RGD binding domain (28).
Macrophages migrating into the injury site could also differentiate into osteoclasts, under the influence of the abundant chemokines at the inflammation sites. Previous studies have shown that biopsies from JDM patients with chronic muscle weakness exhibit elevated IL-1 expression, and JDM patients positive for the TNF-α 308 A allele exhibit higher TNF-α expression in muscle compared to those patients without this allele (29, 30). Both IL-1 and TNF-α have been proven to be potent initiators of osteoclast formation (31). In the present study, osteoclasts were identified by TRAP staining at the outer edge of calcium deposits. The larger deposits seemed to attract more osteoclasts than smaller deposits. Nevertheless, the persistence of calcifications despite the presence of osteoclasts indicates that the mineral reabsorbing activity of these cells appears to be neither sufficient nor effective in dissolving the deposited mineral.
DMP1, BSP, and DPP all showed similar patterns of staining within endothelial cells and adjacent connective tissue in both the calcified and decalcified samples; however those proteins were only detected in the mineral deposits of the calcified samples but not in the decalcified sample. The presence of BSP suggests that it may play a role in osteoclast differentiation (32), despite in vitro studies that demonstrated that BSP expression fosters osteoblast differentiation, leading to increased BSP expression and mineralization (33). DMP1, a transcriptional signal occurring early during differentiation of osteoblasts, initiates mineralization during the final steps of osteoblast differentiation, and is a regulator of the osteoblast gene, osteocalcin (8, 34, 35). DMP1 binds calcium, and is a nucleator of hydroxyapatite, the mineral which is present in high concentrations in JDM calcification (36). DMP1’s centralized location within mineral deposits is consistent with this function. DPP is an effective stimulatory molecule during the dissolution of the dentin matrix, by attracting inflammatory neutrophils and stimulating the release of cytokines such as IL-1β and TNF-α by macrophages (37, 38). The presence of DPP within calcifications, soft tissue, and vascular endothelial cells may serve as an effective attractant for macrophages in an effort to disperse the calcifications, consequently recruiting additional lymphocytes and exacerbating inflammation.
Although MEPE is a component of bone, its role in mineralization is debatable. MEPE inhibits mineralization in some studies (39), while in others it has been shown to promote bone regeneration (40). The absence of MEPE in all of the JDM calcifications further suggests that the mechanism of calcification in JDM differs from that of bone.
ON, one of the most highly expressed non-collagenous proteins in bone, binds calcium, and has been identified in tissues undergoing repair or remodeling, as well in malignant tumors (10, 41). ON also regulates vascular homeostasis by interacting with platelet-derived growth factor PDGF, fibroblast growth factor FGF-2, and vascular endothelial growth factor VEGF (10). Expression of ON within the mineral deposits may indicate a tissue repair mechanism that is insufficient to maintain homeostasis, thus, contributing to deposition of mineral deposits.
One of the few osteoblast-specific proteins, OC is secreted by osteoblasts into the circulation and is regulated notably via the transcription factor Cbfa1 (42), which we identified in the JDM deposits. Additionally, OC deficient mice exhibit increased bone formation, as well as higher bone mass with improved function and defective remodeling (43, 44).
ALP activity detected within vascular endothelial cells, adjacent tissue, and on the periphery of calcifications suggests an osteoblast-like mechanism of mineralization, and the presence of OC, Cbfa1, and ALP within JDM calcifications suggests that osteoblast-like cells may be present, however the structure of the mineral deposits does not exhibit the morphology of physiologic bone, often seen in rheumatic valvular heart disease (7).
In the past, investigators identified MGP as one of the proteins present in JDM calcifications (13), and recent studies have shown phosphorylated MGP to be present within diseased JDM muscle from patients with calcifications as compared with controls (14). However, immunohistological examination to localize MGP within JDM calcification has not been reported. The present study documented that MGP was involved in both the mineral deposits and endothelial cells in all the calcified samples, but it was not detected in the decalcified sample. This observation suggests that MGP was bound to the precipitated mineral, and may be involved at the initiation stage of the calcification process. MGP may possess a function similar to some of the large calcium binding complex proteins, such as fetuin A and OPN, which along with MGP, have been reported to form calciprotein particles (CPP) with calcium phosphate in the circulation to prevent the deposition of minerals (45). Fetuin appears to be increased in the young child (46) and may contribute to the increased frequency of calcifications in this group of children with prolonged untreated inflammation central to JDM.
JDM is characterized as a small vessel systemic vasculopathy. We previously have shown that a long duration of untreated disease was accompanied by a marked decrease in nailfold capillary end row loops, which did not revert to normal with disease control in many cases (47). For this reason we examined the vasculature for the presence of SIBLING and bone markers. From the H&E stain in Figure 2, we observed that the vascular endothelial cells are activated and co-localize with the presence of the observed markers within the samples. To our knowledge there have not been any studies investigating the expression of these proteins in the vasculature of idiopathic calcinosis. However, the presence of BSP and OPN has been identified within the vasculature associated with prostate and breast tumors where calcified tissue occurs. Both BSP and OPN have been proven to be pro-angiogenic factors and interact via the RGD binding domain with the αvβ3 receptor on activated vascular endothelial cells in tumor vasculature (24). Endothelial cells have also been reported to express ON (48) and MGP (49). We have confirmed the previous reports and further identified the expression of DMP1, DPP, ALP and OC in endothelial cells from mineralized tissue. DMP1 and DPP may exhibit functions that are similar to those of BSP and OPN, since they also contain the RGD binding domain. The presence of Cbfa1 in the surrounding tissue indicates osteoblast pro-genitor cells, while observation of the expression of OC and ALP in vascular endothelial cells further identifies osteoblast-like cells in the surrounding tissues and vasculature. These observations reveal the interplay between immune regulation, angiogenesis, and calcification, but further studies are needed to determine the mechanism by which these markers operate in calcifications that occur as a consequence of the chronic inflammation present in JDM.
Previous studies from our lab demonstrated an increased number of apoptotic cells within inflamed JDM muscle as compared with control age-matched pediatric muscle tissue (20). Studies of the JDM calcification by electron microscopy have documented damaged mitochondria (Zhao, unpublished observations) in the surrounding soft tissue, which may release internal calcium stores, thus providing the nidus for mineral deposition. Examination of the deposits with polarized light clearly show that deposition of mineral is not spatially associated with collagen, as is present in normal trabecular bone. After identifying the SIBLING and bone formation associated proteins within JDM calcifications, we are now able to further explore the possible mechanisms by which these proteins operate in JDM calcifications.
In conclusion, we have presented evidence for the presence of osteoclasts and osteoblast-markers, in the absence of observable osteoblasts. Osteoclasts at the surface of the JDM deposit may be present in an attempt to resolve the calcification. Mineral-binding proteins may attach to the deposits after they form, but the presence of osteoblast specific markers (Cbfa1, OC, ALP) indicate that these cells may also be involved. Since the JDM calcifications differ in structure, composition and protein content from bone, we speculate they may not be formed via a classical osteogenic pathway.
Acknowledgement
We are grateful for the comments given by Dr. Adele L. Boskey, the technical assistance from the core center funded by NIH AR046121 (ALB) and the antibodies provided by Dr. Larry Fisher.
Supported by: R01 AR48289 (NIAMS) and The Cure JM Center of Excellence in Juvenile Myositis Research (to LMP)
References
- 1.Pachman LM, Lipton R, Ramsey-Goldman R, Shamiyeh E, Abbott K, Mendez EP, et al. History of infection before the onset of juvenile dermatomyositis: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Research Registry. Arthritis Rheum. 2005;53(2):166–172. doi: 10.1002/art.21068. [DOI] [PubMed] [Google Scholar]
- 2.Banker BQ, Victor M. Dermatomyositis (systemic angiopathy) of childhood. Medicine (Baltimore) 1966;45(4):261–289. doi: 10.1097/00005792-196607000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Rider LG. Pediatr Rheum On Line Jour. 2003. Calcinosis in Juvenile Dermatomyositis: Pathogenesis and Current Therapies. [Google Scholar]
- 4.Pachman LM, Abbott K, Sinacore JM, Amoruso L, Dyer A, Lipton R, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr. 2006;148(2):247–253. doi: 10.1016/j.jpeds.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Pachman LM, Veis A, Stock S, Abbott K, Vicari F, Patel P, et al. Composition of calcifications in children with juvenile dermatomyositis: association with chronic cutaneous inflammation. Arthritis Rheum. 2006;54(10):3345–3350. doi: 10.1002/art.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies CA, Jeziorska M, Freemont AJ, Herrick AL. Expression of osteonectin and matrix Gla protein in scleroderma patients with and without calcinosis. Rheumatology (Oxford) 2006;45(11):1349–1355. doi: 10.1093/rheumatology/kei277. [DOI] [PubMed] [Google Scholar]
- 7.Rajamannan NM, Nealis TB, Subramaniam M, Pandya S, Stock SR, Ignatiev CI, et al. Calcified rheumatic valve neoangiogenesis is associated with vascular endothelial growth factor expression and osteoblast-like bone formation. Circulation. 2005;111(24):3296–3301. doi: 10.1161/CIRCULATIONAHA.104.473165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15(3):126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 9.Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44 Suppl 1:33–40. [PubMed] [Google Scholar]
- 10.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19(8):816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 11.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386(6620):78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 12.Lian JB, Pachman LM, Gundberg CM, Partridge RE, Maryjowski MC. Gamma-carboxyglutamate excretion and calcinosis in juvenile dermatomyositis. Arthritis Rheum. 1982;25(9):1094–1100. doi: 10.1002/art.1780250910. [DOI] [PubMed] [Google Scholar]
- 13.Lian JB, Skinner M, Glimcher MJ, Gallop P. The presence of gamma-carboxyglutamic acid in the proteins associated with ectopic calcification. Biochem Biophys Res Commun. 1976;73(2):349–355. doi: 10.1016/0006-291x(76)90714-2. [DOI] [PubMed] [Google Scholar]
- 14.van Summeren MJ, Spliet WG, van Royen-Kerkhof A, Vermeer C, Lilien M, Kuis W, et al. Calcinosis in juvenile dermatomyositis: a possible role for the vitamin K-dependent protein matrix Gla protein. Rheumatology (Oxford) 2008 doi: 10.1093/rheumatology/kem360. [DOI] [PubMed] [Google Scholar]
- 15.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 16.Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum. 2003;49(1):7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 17.Bouma G, Oudkerk Pool M, Scharenberg JG, Kolkman JJ, von Blomberg BM, Scheper RJ, et al. Differences in the intrinsic capacity of peripheral blood mononuclear cells to produce tumor necrosis factor alpha and beta in patients with inflammatory bowel disease and healthy controls. Scand J Gastroenterol. 1995;30(11):1095–1100. doi: 10.3109/00365529509101613. [DOI] [PubMed] [Google Scholar]
- 18.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;39(5):225–235. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 19.Taylor C, Shan-Rong Shi. In: Morphology Methods: Cell and Molecular Biology Techniques. Lloyd RV, editor. Totowa: Humana Press; 2001. pp. 23pp. 239–265. [Google Scholar]
- 20.Zhao Y, Fedczyna TO, McVicker V, Caliendo J, Li H, Pachman LM. Apoptosis in the skeletal muscle of untreated children with juvenile dermatomyositis: Impact of duration of untreated disease. Clin Immunol. 2007 doi: 10.1016/j.clim.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd RV. In: Morphology Methods: Cell and Molecular Biology Techniques. Lloyd RV, editor. Totowa: Humana Press; 2001. p. 23. [Google Scholar]
- 22.Kawaguchi J. Generation of Osteoblasts and Chondrocytes From Embryonic Stem Cells. In: Turksen K, editor. Methods in Molecular Biology. Second ed. Totowa: Humana Press, Inc; 2006. [DOI] [PubMed] [Google Scholar]
- 23.Schett G. Tuerk, Birgit Bone Histomorphometry in Arthritis Models. In: Cope AP, editor. Methods in Molecular Medicine. Totowa: Humana Press Inc.; 2007. pp. 269–283. [DOI] [PubMed] [Google Scholar]
- 24.Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008;8(3):212–226. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scatena M, Liaw L, Giachelli CM, Osteopontin A Multifunctional Molecule Regulating Chronic Inflammation and Vascular Disease. Arterioscler Thromb Vasc Biol. 2007 doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 26.Denhardt DT, Guo X. Osteopontin: a protein with diverse functions. Faseb J. 1993;7(15):1475–1482. [PubMed] [Google Scholar]
- 27.Pachman LM. Myositis. In: Isenberg DA, Miller III, John J, editors. Adolescent Rheumatology. New York: Informa Healthcare; 1999. pp. 127–146. [Google Scholar]
- 28.Weber GF, Zawaideh S, Hikita S, Kumar VA, Cantor H, Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol. 2002;72(4):752–761. [PubMed] [Google Scholar]
- 29.Uzel G, Pachman LM. Cytokines in juvenile dermatomyositis pathophysiology: potential and challenge. Curr Opin Rheumatol. 2003;15(6):691–697. doi: 10.1097/00002281-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Fedczyna TO, Lutz J, Pachman LM. Expression of TNFalpha by muscle fibers in biopsies from children with untreated juvenile dermatomyositis: association with the TNFalpha-308A allele. Clin Immunol. 2001;100(2):236–239. doi: 10.1006/clim.2001.5063. [DOI] [PubMed] [Google Scholar]
- 31.Lee SK, Lorenzo J. Cytokines regulating osteoclast formation and function. Curr Opin Rheumatol. 2006;18(4):411–418. doi: 10.1097/01.bor.0000231911.42666.78. [DOI] [PubMed] [Google Scholar]
- 32.Valverde P, Tu Q, Chen J. BSP and RANKL induce osteoclastogenesis and bone resorption synergistically. J Bone Miner Res. 2005;20(9):1669–1679. doi: 10.1359/JBMR.050511. [DOI] [PubMed] [Google Scholar]
- 33.Gordon JA, Tye CE, Sampaio AV, Underhill TM, Hunter GK, Goldberg HA. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone. 2007;41(3):462–473. doi: 10.1016/j.bone.2007.04.191. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, et al. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem. 2003;278(19):17500–17508. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- 35.Qin C, D'Souza R, Feng JQ. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res. 2007;86(12):1134–1141. doi: 10.1177/154405910708601202. [DOI] [PubMed] [Google Scholar]
- 36.Stock S, Ignatiev K, Lee P, Abbott K, Pachman L. Pathological calcification in juvenile dermatomyositis (JDM): microCT and synchrotron x-ray diffraction reveal hydroxyapatite with varied microstructures. Connect Tissue Res. 2004;45(4–5):248–256. doi: 10.1080/03008200490903066. [DOI] [PubMed] [Google Scholar]
- 37.Silva TA, Lara VS, Silva JS, Garlet GP, Butler WT, Cunha FQ. Dentin sialoprotein and phosphoprotein induce neutrophil recruitment: a mechanism dependent on IL-1beta, TNF-beta, and CXC chemokines. Calcif Tissue Int. 2004;74(6):532–541. doi: 10.1007/s00223-003-0159-5. [DOI] [PubMed] [Google Scholar]
- 38.Silva TA, Lara VS, Silva JS, Oliveira SH, Butler WT, Cunha FQ. Macrophages and mast cells control the neutrophil migration induced by dentin proteins. J Dent Res. 2005;84(1):79–83. doi: 10.1177/154405910508400114. [DOI] [PubMed] [Google Scholar]
- 39.Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, et al. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278(3):1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 40.Six N, Septier D, Chaussain-Miller C, Blacher R, DenBesten P, Goldberg M. Dentonin, a MEPE fragment, initiates pulp-healing response to injury. J Dent Res. 2007;86(8):780–785. doi: 10.1177/154405910708600818. [DOI] [PubMed] [Google Scholar]
- 41.Porter PL, Sage EH, Lane TF, Funk SE, Gown AM. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem. 1995;43(8):791–800. doi: 10.1177/43.8.7622842. [DOI] [PubMed] [Google Scholar]
- 42.Ducy P. Cbfa1: a molecular switch in osteoblast biology. Dev Dyn. 2000;219(4):461–471. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1074>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 43.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382(6590):448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 44.Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23(3):187–196. doi: 10.1016/s8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 45.Jahnen-Dechent W, Schafer C, Ketteler M, McKee MD. Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med. 2007 doi: 10.1007/s00109-007-0294-y. [DOI] [PubMed] [Google Scholar]
- 46.Marhaug G, Shah V, Shroff R, Varsani H, Wedderburn LR, Pilkington CA, et al. Age-dependent inhibition of ectopic calcification: a possible role for fetuin-A and osteopontin in patients with juvenile dermatomyositis with calcinosis. Rheumatology (Oxford) 2008;47(7):1031–1037. doi: 10.1093/rheumatology/ken136. [DOI] [PubMed] [Google Scholar]
- 47.Christen-Zaech S, Seshadri R, Sundberg J, Paller AS, Pachman LM. Persistent association of nailfold capillaroscopy changes and skin involvement over thirty-six months with duration of untreated disease in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;58(2):571–576. doi: 10.1002/art.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85(3):979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 49.Sosnoski DM, Gay CV. Evaluation of bone-derived and marrow-derived vascular endothelial cells by microarray analysis. J Cell Biochem. 2007;102(2):463–472. doi: 10.1002/jcb.21307. [DOI] [PubMed] [Google Scholar]