Abstract
Objective
Worldwide, cerebral vasospasm after subarachnoid hemorrhage (SAH) has an estimated morbidity and mortality of 1.2 million annually. While it has long been suspected that reactive oxygen species play a major role in the etiology of cerebral vasospasm after SAH, promising results in animal works were not borne out in human clinical trials, despite intensive research effort. The purpose of this study is to investigate the role of glutathione peroxidase in the SAH cerebrospinal fluid milieu.
Methods
We utilized commercially available kits, for the quantitation of glutathione peroxidase 1 (glutathione peroxidase) activity and oxygen radical capacity and sodium dodecyl sulfate polyacrylamide gel electrophoresis with Western blotting with specific antibodies to human glutathione peroxidase to determine the enzyme content of the cerebrospinal fluid samples. Human cerebrospinal fluid was obtained in an Institutional Review Board-exempt manner for this study in the following groups: control (no SAH), CSFC (SAH but no vasospasm on angiography) and CSFV (SAH with clinical and angiographic vasospasm).
Results
We found that glutathione peroxidase activity is significantly higher in CSFV compared with CSFC, and this is reflected in a higher total oxidative capacity in CSFV. Despite similar levels of glutathione peroxidase protein, CSFV had significantly greater activity than CSFC.
Discussion
These results further elucidate previous research from this laboratory, showing increased oxidative stress in CSFV compared with CSFC. In conclusion, there appears to be increased glutathione peroxidase activity in CSFV, despite there being increased levels of oxidative stress markers, suggesting overwhelming oxidative stress may play a role in cerebral vasospasm after SAH.
Keywords: Hemorrhagic stroke, subarachnoid hemorrhage, cerebral vasospasm, oxidative stress, peroxidase
INTRODUCTION
Worldwide, subarachnoid hemorrhage (SAH) and its sequel, cerebral vasospasm (CV), kill or seriously debilitate an estimated 1.2 million people annually. While the initial hemorrhagic event is in itself highly lethal (fourth most common intracranial cause of death)1, its sequelae, specifically cerebral vasospasm, afflicts up to 50% of those surviving the initial event in the following 3–10 days, leading to significantly increased mortality and morbidity, through delayed neurological deficit and secondary ischemia1.
It has long been postulated that reactive oxygen species such as peroxide, superoxide and peroxynitrite are likely involved in the etiology of cerebral vasospasm after SAH2, either by induction of the spasm itself or by prevention of relaxation3. The reactive oxygen species in cerebrospinal fluid from SAH patients are thought to come from three sources: (1) oxyhemoglobin, (2) immune cells and (3) aberrant activity of nitric oxide synthase and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase4,5. These reactive oxygen species are thought to elicit smooth muscle contraction by peroxidation of membrane lipids both in the vascular smooth muscle and the endothelial cells6.
Intrinsic antioxidant responses in the vascular and endothelial tissue include superoxide dismutase, catalase and the selenoprotein, glutathione peroxidase (GSH-Px)2. Based on this, there have been several attempts to use antioxidants in trials to prevent/reverse cerebral vasospasm after SAH. These include, notably, tirilazad mesylate and ebselen1 among others. Despite promising animal trials, antioxidant therapies have proven largely ineffective in the prevention or reversal of human cerebral vasospasm after SAH1 due at least in part to a lack of understanding of antioxidant events during this pathology.
In this study, we aimed to improve the understanding of antioxidant response, particularly glutathione peroxidase, in cerebral vasospasm after SAH by examining the cerebrospinal fluid (CSF) from SAH patients with (CSFV) and without (CSFC) vasospasm.
MATERIALS AND METHODS
Human cerebrospinal fluid
Cerebrospinal fluid was collected from patients admitted to the neurointensive care unit who had confirmed SAH and had an intraventricular drain in place. Control (non-hemorrhagic) cerebrospinal fluid was collected from patients undergoing therapeutic spinal tap. All human tissue collection was performed in accordance with local Institutional Review Board. Patient demographics are shown in Table 1.
Table 1.
Patient demographics
| Patient | Gender | Age (years) | Vasospasm* | Patient | Gender | Age (years) | Vasospasm* |
|---|---|---|---|---|---|---|---|
| Control 1 | M | 48 | n/a | CSFV 1 | M | 42 | Yes |
| Control 2 | F | 53 | n/a | CSFV 2 | M | 45 | Yes |
| Control 3 | M | 57 | n/a | CSFV 3 | M | 50 | Yes |
| CSFC 1 | F | 52 | No | CSFV 4 | F | 46 | Yes |
| CSFC 2 | F | 43 | No | CSFV 5 | M | 46 | Yes |
| CSFC 3 | M | 52 | No | Control (3) | 66% M | Mean 52.67 | n/a |
| CSFC 4 | M | 32 | No | CSFC (5) | 40% M | Mean 50.2 | No |
| CSFC 5 | F | 72 | No | CSFV (5) | 80% M | Mean 45.8 | Yes |
n/a, Non-SAH patient; yes, asymptomatic or symptomatic vasospasm on angiography.
Vasospasm determined by angiography.
Lipid oxidation and malondialdehyde quantification
Commercially available colorimetric assay kits (Calbiochem, San Diego, CA, USA) were used to measure total oxidized lipids (Cat no. 437638) and malondialdehyde (Cat no. 437634). Cerebrospinal fluid was extracted and assayed in triplicate, as previously reported7.
Western blot analysis of GPx protein
Samples were assayed for protein using the bicinchoninic acid (BCA) protein assay (Pierce, Rickford, IL, USA). Each well of a sodium dodecyl sulfate-polyacrylamide gel was loaded with 25 μg of total protein and separated using gel electrophoresis. Pure glutathione peroxidase protein (10 ng) was also loaded in a separate well as a positive control. Proteins were transferred onto nitrocellulose membrane and probed for glutathione peroxidase using sheep polyclonal anti-human glutathione peroxidase (AbCam, Cambridge, MA, USA). Bands were visualized using the electrochemiluminescence (ECL) Western blotting development system (Pierce). Image analysis software (ImageJ, NIH) was used to assess band intensity and provide a semi-quantitative analysis.
Total antioxidant capacity assay (ORAC)
The Oxygen Radical Absorbance Capacity assay kit (ORAC) was purchased from Cayman Chemical Company (Ann Arbor, MI, USA; Cat no. 709-001). The kit contains a substrate [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)] which reacts stoichiometrically with oxygen radicals to form a colored compound. A tocopherol analogue (Trolox) is added in various doses for comparison, and the capacity of the cerebrospinal fluid samples to absorb oxygen radicals is expressed as molar Trolox equivalents8.
Glutathione peroxidase activity assay
A commercially available kit (OxiTek Glutathione Peroxidase Activity Kit; Zeptometrix Corp., Franklin, MA, USA; Cat no. 0805002) was used for this assay. Cumene hydroperoxide is provided as a substrate for glutathione peroxidase. The reaction mixture contains glutathione reductase and NADPH, allowing quantitative assessment of sample glutathione peroxidase levels by the coupled recycling reaction catalyzed by glutathione reductase and the concomitant conversion of NADPH (which absorbs at 340 nm) to NADP+, which does not absorb. The kinetic reaction was recorded, and the activity of glutathione peroxidase was calculated.
Determination of interfering factors
The assay we used for glutathione peroxidase activity utilizes a glutathione peroxidase-specific reaction coupling system; however, copper and iron moieties have been shown to act as non-enzymatic ‘pseudoperoxidases’8, and therefore we measured copper and iron in the samples. A colorimetric assay was used to measure total copper concentration in the cerebrospinal fluid samples, as previously described9. Briefly, a series of copper standards were prepared, ranging from 0 to 2.5 μg copper/ml water. These standards as well as the samples were utilized in the following procedure.
One milliliter of copper standard or sample was mixed with 1 ml of 2 mol acetic acid, 5 ml of 0.01 mol/l WO42- solution, 0.3 ml of 0.01 mol/l PO43- and 1 ml of 0.02% polyvinyl alcohol solution. Five milliliter of 3,3′,5,5′-tetramethylbenzidine was added to each tube with 11.7 ml water, bringing the total solution volume to 25 ml. The samples and standards were boiled for 13 minutes and 30 seconds at a uniform temperature of 121°C in an aluminum dry heat block then allowed to cool to room temperature. The absorbance of each sample was measured at 660 nm.
Iron moieties were measured according the colorimetric protocol published by Bleijenberg et al.10. Briefly, 4,7-diphenyl-1,10-phenantroline disulfonate [bathophenantroline disulfate (BPS)] was used as a ferrous iron chelator. Ferrous and ferric iron standard curves were constructed for comparison with samples. BPS-ferrous complex absorbance was recorded at 535 nm. Total ferrous + ferric iron content was determined by incubation with ascorbate, which renders the BPS-ferric complex colored and the plate was reread at 535 nm.
In order to account for the amount of blood in the cerebrospinal fluid, iron and copper measurements are expressed as a ratio with hemoglobin concentration. Hemoglobin concentration was determined by the Drabkin’s method, whereby the sample is incubated with Drabkin’s reagent, a cyanide derivative, thus converting all the hemoglobin moieties in the sample into cyanomethemoglobin. The concentration of cyanomethemoglobin in the sample can be calculated from the known molar extinction coefficient and molecular weight.
All chemicals were obtained from Sigma Chemical Company (St. Louis, MO, USA). Spectrophotometric measurements were made on a SpectraMax M5 (Molecular Devices, Sunnyvale, CA, USA). Significance of results was determined when p<0.05, calculated by using ANOVA.
RESULTS
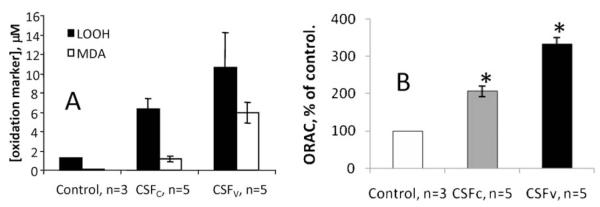
In Figure 1A, we show that both total peroxidized lipids and malondialdehyde specifically are not only increased in human SAH cerebrospinal fluid compared with non-SAH cerebrospinal fluid but that there is significantly more lipid peroxidation in CSFV than CSFC.
Figure 1.
(A) Both total peroxidized lipids (LOOH) and malondialdehyde are increased significantly (p<0.05, ANOVA) in SAH cerebrospinal fluid above control (non-SAH cerebrospinal fluid). In addition, CSFV has significantly more of both these lipid oxidation markers than CSFC. Errors are SD. (B) ORAC of cerebrospinal fluid from non-SAH (control, n=3), SAH, non-vasospastic (CSFC, n=5) and SAH, vasospastic (CSFV, n=5). *Significant difference from control, p<0.01 (ANOVA). Errors are SD
Figure 1B illustrates that the oxygen radical absorbance capacity of the cerebrospinal fluid is increased in vasospastic cerebrospinal fluid, and yet the lipid oxidation is significantly higher. The ORAC of the cerebrospinal fluid samples (Figure 1B) shows that patients without vasospasm appear to mount a lesser antioxidant response than SAH patients with vasospasm. This suggests that the antioxidant systems are working harder in vasospastic patients but that they are still overwhelmed or that some aspect of heightened antioxidant response in the cerebrospinal fluid contributes to vasospasm.
Figure 2 clearly shows the significantly higher activity of glutathione peroxidase in CSFV compared with CSFC, despite similar protein levels (Figure 3). Elimination of confounding factors (Table 2) was necessary to account for blood concentration in the cerebrospinal fluid samples and for pseudoperoxidase activity. Figure 2 shows that there is significantly (p<0.01) lower glutathione peroxidase activity in CSFC than CSFV.
Figure 2.
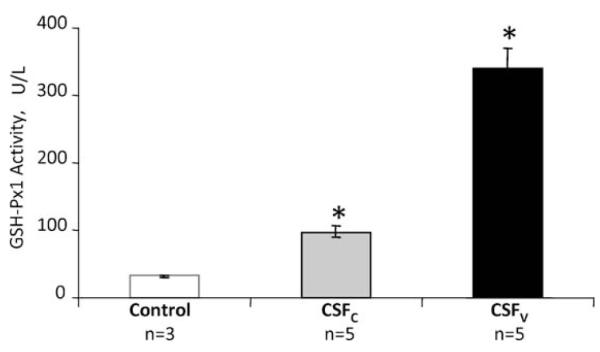
Activity of glutathione peroxidase. SAH cerebrospinal fluid exhibited significantly higher activity than non-SAH cerebrospinal fluid. In addition, CSFV had significantly higher activity than CSFC, over 300% more. Errors are SD, *p<0.05 compared with control (ANOVA)
Figure 3.
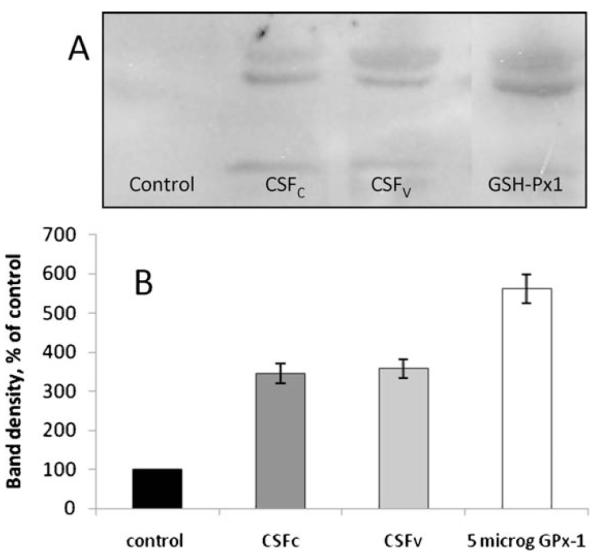
(A) Representative lanes showing the bands resulting from cerebrospinal fluid samples and from a 5-mg GPx-protein standard. Glutathione peroxidase gives a double band, and the intensity of these two bands combined was used to produce the data shown in (B). (B) Relative concentration of glutathione peroxidase protein is calculated from the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)-Western blot using ImageJ software. Errors are SD, n=6. There is no significant difference between SAH cerebrospinal fluid samples
Table 2.
Sources of interference
| Sample | Total protein (g/dl) | Total hemoglobin (g/dl) | Iron (μM) | Copper (μg/g hemoglobin) |
|---|---|---|---|---|
| Control CSF (n=3) | 0.056 ± 0.01* | nd | 1.43 ± 0.02 | nd |
| CSFC (n=5) | 4.303 ± 1.77 | 4.108 ± 1.69 | 20.72 ± 4.98 | 38.50 ± 3.6 |
| CSFV (n=5) | 4.155 ± 1.02 | 3.974 ± 0.98 | 17.99 ± 4.29 | 40.78 ± 5.3 |
Errors are SD.
Although the kit is specific for the enzyme glutathione peroxidase, it is known that some iron and copper moieties can act as pseudoperoxidases. In order to determine the possible contribution of these moieties to the activity we measured in Figure 2, we performed iron and copper determinations in the cerebrospinal fluid samples. Table 2 summarizes these data.
Although here was significantly more copper and iron in the SAH cerebrospinal fluid compared with control, as might be expected, there was no significant difference in any parameter between CSFC and CSFV (p>0.05). Thus, the differences we saw in peroxidase activity are either due to increased glutathione peroxidase protein level or increased activation. We performed SDS-PAGE and Western blotting with a specific anti-human glutathione peroxidase antibody probe in order to assess differences in glutathione peroxidase protein levels. Figure 3 shows the results of this investigation.
We also measured the concentration of glutathione peroxidase substrate, hydrogen peroxide, in the samples, but the results showed no difference (data not shown).
DISCUSSION
Previous work in this area has focused on analysis of the oxidative damage in the vascular and cerebral tissue following SAH and which, if any, of these molecules may play a role in the etiology of vasospasm, often with a view to using antioxidants as therapy2–6. When undertaking this study, we hoped to further elucidate the sources of oxidative damage and possible endogenous anti-oxidative mechanisms in play in this disease in humans by analysing CSF from patients (as opposed to an animal model; we feel this access to human tissue is a significant advantage, since the etiology of the disease is unknown). Whether the lipid oxidation is a causative or correlative effect of cerebral vasospasm after SAH still remains to be elucidated, but it is supportive of our assertion that the oxidative events occurring in the hemorrhagic cerebrospinal fluid milieu are not simple and may impact whether the patients goes on to develop vasospasm or not. As such, understanding this process could lead not only to insights into the etiology of CV after SAH but also to novel or refined therapeutic strategies. CSFV samples were taken from the patients subsequent to vasospasm onset (or at an equivalent time post-SAH for CSFC samples). This means we are looking at a biochemical snapshot at one time point (an acknowledged weakness of this study), raising the question, which came first, the vasospasm or the ORAC or the reactive oxygen species? These are questions better answered by a larger, prospective study, which we plan to undertake. However, these data show for the first time that not only is there increased evidence of oxidative damage in the CSF of SAH patients with vasospasm compared with SAH patients without vasospasm (shown previously) but that here is a response mounted in the form of increased free radical absorbance capacity, and yet despite this, evidence of oxidative damage is still higher in vasospastic SAH CSF.
From the data in Figure 3 and Table 2, we reasoned that the increased activity of glutathione peroxidase seen in CSFV was not merely due to the presence of more blood in that cerebrospinal fluid population. This might have been a reasonable assumption, since increased blood volume has long been associated with increased risk of cerebral vasospasm in the SAH patient and is in fact used as a predictive diagnostic tool (Fisher Grade). There are reports in the literature of the ‘pseudoperoxidase’ or non-enzymatic activity of copper and iron moieties, such as Cu2+ and Fe3+8. Enzymatic peroxidase activity in our system was confined to glutathione peroxidase, since our method utilizes substrate specificity to rule out catalase and superoxide dismutase interference. This left the glutathione peroxidase protein level. Western blot analysis of the cerebrospinal fluid samples revealed no significant difference between CSFC and CSFV samples, which can be seen in Figure 3. Having also ruled out hydrogen peroxide concentration (data not shown), we postulate that either glutathione itself or selenium may be a key factor in this observation. Work from other laboratories has used exogenous glutathione as a treatment for vasospasm in dogs11, but glutathione levels have not previously been measured in human cerebrospinal fluid.
It has long been known that glutathione peroxidase type 1 (which is found in all mammalian tissues12 is a selenoprotein, that is, it contains selenocysteine residues, without which it cannot perform its function as an antioxidant enzyme12. However, measurement of total cerebrospinal fluid selenium will not yield useful information regarding the activation level of GSH-Px because there are many selenoproteins in the human brain which have been found in SAH cerebrospinal fluid2. The selenium moiety of many of these selenoproteins is not available to be incorporated into glutathione peroxidase, and therefore speciation must be performed to determine the useful selenium content of a sample, with respect to glutathione peroxidase activity. Recent advances in metallomic analysis of biological samples now allow this kind of determination13, and we plan to continue this study using these modalities.
CONCLUSION
As previously reported by various groups, there is evidence for significant oxidative stress in SAH cerebrospinal fluid from vasospastic patients. We found that the total oxygen radical absorbance capacity was actually increased in these samples, where the oxidative stress was highest, indicating either an overwhelming reactive oxygen species production or involvement of the antioxidant pathways in the etiology of vasospasm. This in itself warrants further investigation. Glutathione peroxidase activity, but not concentration, was significantly higher in SAH cerebrospinal fluid from patients with vasospasm, compared with those without. This may indicate alterations in glutathione or selenoprotein content in these cerebrospinal fluid samples. Methods have recently become available to us to measure the specific selenoproteins that act as a cofactor for glutathione peroxidase, and we are actively pursuing this avenue.
ACKNOWLEDGEMENT
This research was supported by an R01 grant from NINDS (Gail J. Pyne-Geithman).
REFERENCES
- 1.Macdonald RL. Cerebral Vasospasm: Advances in Research and Treatment. 1st edn Thieme; New York: 2004. [Google Scholar]
- 2.Macdonald RL, Weir BK, Runzer TD, et al. Malondialdehyde, glutathione peroxidase, and superoxide dismutase in cerebrospinal fluid during cerebral vasospasm in monkeys. Can J Neurol Sci. 1992;19(3):326–332. [PubMed] [Google Scholar]
- 3.Sasaki T, Tanishima T, Asano T, et al. Significance of lipid peroxidation in the genesis of chronic vasospasm following rupture of intracranial aneurysm. Acta Neurochir Suppl (Wien) 1979;28:536–540. [PubMed] [Google Scholar]
- 4.Macdonald RL, Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke. 1991;22(8):971–982. doi: 10.1161/01.str.22.8.971. [DOI] [PubMed] [Google Scholar]
- 5.Takehara Y, Nakahara H, Inai Y, et al. Oxygen-dependent reversible inhibition of mitochondrial respiration by nitric oxide. Cell Struct Funct. 1996;21(4):251–258. doi: 10.1247/csf.21.251. [DOI] [PubMed] [Google Scholar]
- 6.Choi JM, Kim CD, Hong KW. Involvement of NADH/NADPH oxidase-derived superoxide in experimental vasospasm induced by periarterial blood in rat femoral artery. Life Sci. 2001;69(15):1753–1763. doi: 10.1016/s0024-3205(01)01273-5. [DOI] [PubMed] [Google Scholar]
- 7.Pyne-Geithman GJ, Morgan CJ, Wagner K, et al. Bilirubin production and oxidation in CSF of patients with cerebral vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;26(8):1070–1077. doi: 10.1038/sj.jcbfm.9600101. [DOI] [PubMed] [Google Scholar]
- 8.Wang CC, Chu CY, Chu KO, et al. Trolox-equivalent antioxidant capacity assay versus oxygen radical absorbance capacity assay in plasma. Clin Chem. 2004;50(5):952–954. doi: 10.1373/clinchem.2004.031526. [DOI] [PubMed] [Google Scholar]
- 9.Di J, Wu Y, Ma Y. A novel spectrophotometric determination of trace copper based on charge transfer complex. Spectrochim Acta A Mol Biomol Spectrosc. 2005;61(5):937–941. doi: 10.1016/j.saa.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Bleijenberg BG, van Eijk HG, Leijnse B. The determination of nonheme iron and transferrin in cerebrospinal fluid. Clin Chim Acta. 1971;31:277–281. doi: 10.1016/0009-8981(71)90387-1. [DOI] [PubMed] [Google Scholar]
- 11.Hačıyakupoğlu S, Ildan F, Polat S, et al. Effect of GSH on cerebral vasospasm in dogs. Neurosurg Rev. 1994;17(4):283–239. doi: 10.1007/BF00306819. [DOI] [PubMed] [Google Scholar]
- 12.Schweizer U, Bräuer AU, Köhrle J, et al. Selenium and brain function: A poorly recognized liaison. Brain Res Rev. 2004;45:164–178. doi: 10.1016/j.brainresrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 13.B’Hymer C, Caruso JA. Selenium speciation analysis using inductively coupled plasma-mass spectrometry. J Chromatogr A. 2006;1114:1–20. doi: 10.1016/j.chroma.2006.02.063. [DOI] [PubMed] [Google Scholar]