Summary
Megakaryocytes are rare polyploid bone marrow cells whose function is to produce blood platelets. Since the purification and cloning of the major megakaryocyte cytokine, thrombopoietin, in 1994, considerable progress has been made in understanding the biology of megakaryocyte development. Remarkably, these advances have revealed a number of key features of megakaryocytes that are shared with hematopoietic stem cells (HSCs), such as common surface receptors, lineage-specific transcription factors, and specialized signaling pathways. Why there should be such a close connection between these two cell types remains unclear. In this Prospects article, we summarize the data supporting these shared features and speculate on possible teleological bases. In particular, we focus on common links involving developmental hierarchy, endothelial cells, and bone marrow niche interactions. This discussion highlights new data showing close ontologic relationship between HSCs and specialized “hemogenic” endothelial cells during development, and functional overlap between megakaryocytes/platelets and endothelial cells. Overall, these findings may be of relevance in the development of techniques for HSC ex vivo culture and/or possible generation of HSCs via somatic cell reprogramming.
The hematopoietic system provides a constant supply of blood cells of diverse function. This is a highly active process, with nearly a trillion new blood cells produced every day in an adult human. The ability to sustain this activity throughout the lifetime of an individual is dependent on hematopoietic stem cells (HSCs). These rare, specialized cells are characterized by self-renewal capacity, high proliferative potential, and the ability to differentiate into at least ten distinct blood cell types. Since the identification of HSCs by Till and McCullough in 1961, considerable knowledge has been gained about their biology. In particular, key genes have been identified that are required for their ontogeny, maintenance, and function.
Platelets are anucleate blood elements that play essential roles in hemostasis. Resting platelets circulate as small discoid structures. Upon activation, they undergo dramatic changes that include flattening, spreading, membrane surface alterations, and release of vasoactive and thrombogenic substances. In addition to acting as the “first line of defense” at the time of vascular injury, they serve to repair microscopic vascular damage that occurs on a daily basis. Platelets are also thought to play roles in wound healing and angiogenesis through the delivery of stored growth factors, such as PDGF, bFGF, VEGF, platelet factor-4, TGF-β, thrombospondin-1 to sites of injury.
Platelets are derived directly from megakaryocytes (Mks), which are rare, large, polyploid cells that reside predominantly within the bone marrow. During terminal stages of maturation, megakaryocytes produce long cytoplasmic extensions called proplatelets, which then release platelets from their tips. Because of the extreme rarity of megakaryocytes, accounting for <0.05% of nucleated bone marrow cells, an understanding of the molecular biology of megakaryocyte development has lagged behind many of the other blood lineages. However, the purification and cloning of the major cytokine for megakaryopoiesis, thrombopoietin, in 1994, has enabled efficient culturing of these cells and more detailed analysis of their biology. As more has been learned about the key factors involved in megakaryocyte development, and as studies of HSCs have progressed, one of the surprises has been that these two cell types share a remarkable number of key cell-specific factors. Why this should be remains unknown. In this Prospect article, we first review the common features of megakaryocytes and HSCs, and in the second part, offer possible explanations and future research directions.
Shared Features of Megakaryocytes and Hematopoietic Stem Cells
Cell Surface Receptors
Thrombopoietin (TPO) Receptor (c-mpl)
One of the first noted similarities between Mks and HSCs was the finding that both express the thrombopoietin (TPO) receptor (c-mpl) on their cell surface. TPO receptor expression is functionally important in HSCs activity, as bone marrow transplant studies show that HSCs from c-mpl−/− mice have a strong disadvantage in long-term repopulating activity compared to those from wild type mice, even when injected at ten times greater number [Kimura et al., 1998]. Likewise, knock out of TPO itself reduces HSC function [Murone et al., 1998]. In humans, germline inactivating mutations of the TPO receptor gene cause severe congenital amegakaryocytic thrombocytopenia (CAMT). Children with this disorder are at high risk of developing complete bone marrow failure, typically within the first decade of life [van den Oudenrijn et al., 2000]. This finding is consistent with a role for TPO signaling in human HSC maintenance.
CXCR4
The receptor CXCR4 is a seven membrane spanning G-coupled receptor that binds the chemokine SDF-1. It plays an important role in HSC bone marrow retention [Mohle et al., 1998]. In fact, CXCR4 antagonists have recently been developed for use as mobilization agents in the collection of peripheral blood HSCs from stem cell transplant donors. CXCR4 is also expressed on Mks, and plays a critical role in homing of Mks to the vascular sinusoidal space, where terminal Mk maturation and proplatelet formation occurs [Wang et al., 1998].
CD150
Morrison and colleagues recently identified the SLAM receptor molecule CD150 as a surface marker that enriches for cells with HSC activity when used in conjunction with previously characterized markers [Kiel et al., 2005]. CD150 is also expressed abundantly on committed megakaryocyte progenitors (MkPs) [Pronk et al., 2007] and on mature primary murine fetal liver Mks (H. Huang and A. Cantor, unpublished observation). In fact, protocols incorporating CD150 for the immunophenotypic isolation of murine HSCs and MkPs are now widely used.
CD41 (GPIIb)
The integrins CD41 (GPIIb) and CD61 (GPIIIa) form a heterodimeric complex, which is present at high copy number on the surface of platelets. The GPIIb/IIIa complex has been extensively studied with respect to its function as a receptor for fibrinogen during platelet aggregation. CD41 expression was initially thought to be unique to the megakaryocyte lineage. However, it is now clear that CD41 is also expressed at lower levels on early multipotential hematopoietic progenitors cells, and, in fact, is one of the earliest markers of hematopoietic development [Mikkola et al., 2003a]. Some studies suggest that it is also contained on populations of cells with HSC activity [Debili et al., 2001].
Other Receptors
Several other cell surface markers have also been reported to be shared between Mks and HSCs, including c-kit, CD34, CD105 (Endoglin), CD31 (PECAM-1), JAM-A, Tie-2, and KDR (VEGF receptor 2).
Transcription Factors
Runx-1
Runx-1 (formerly known as AML-1, Cbf-α2, and PEBPA2B) is a hematopoietic-expressed member of the runt family of transcription factors. These factors share a highly conserved 128 amino acid domain that was first identified in the drosophila runt gene. This region mediates sequence-specific DNA binding, as well as protein-protein interactions with the cofactor molecule Cbf-β. Runx1−/− mice die between embryonic day 12.5 to 13.5 (e12.5–13.5) from central nervous system hemorrhage and failure of all definitive hematopoiesis [Wang et al., 1996]. The latter is due to impaired emergence of the first definitive HSCs from aorto-gonadal-mesonephros (AGM) region during embryogenesis [North et al., 1999]. In adult mice, Runx-1 haploinsufficiency causes a decrease in the number of HSCs (long-term repopulating cells) [Sun and Downing, 2004]. Conditional knock out studies using the Mx1-Cre system, which allows inducible deletion of targeted alleles within the bone marrow, also show a requirement for Runx1 during megakaryocyte differentiation in adult animals [Growney et al., 2005; Ichikawa et al., 2004]. Runx-1 deficient megakaryocytes have hypolobulated nuclei, underdeveloped cytoplasm, low DNA ploidy and enhanced replating activity in semisolid media culture assays. Haploinsufficiency of Cbf-β also perturbs megakaryopoiesis in mice [Talebian et al., 2007]. In humans, heterozygous Runx-1 germline mutations cause Familial Platelet Disorder with Propensity to Develop AML (FPD/AML), a rare autosomal dominant disorder characterized by thrombocytopenia, platelet dysfunction, and markedly elevated risk for myelodysplastic syndrome (MDS) and leukemia (median incidence ~35%) [Song et al., 1999].
GATA-2
GATA family transcription factors play essential roles during development and in the maintenance of certain adult tissues. There are six known family members in mammals, three of which (GATA-1, -2, and -3) are expressed in hematopoietic tissues. In general, GATA-1 and GATA-2 play reciprocal roles during hematopoiesis, with GATA-2 required for early multipotent stages, and GATA-1 required for terminal maturation of certain lineages. Complete loss of GATA-2 in mice causes a marked defect in HSCs and multipotential progenitor cell expansion resulting in early embryonic lethality [Tsai et al., 1994]. Haploinsufficiency of GATA-2 in adult mice results in abnormal HSC homeostasis [Rodrigues et al., 2005]. GATA factors also play crucial roles in megakaryopoiesis. GATA-1 is required for terminal megakaryocyte maturation and is mutated in X-linked macrothrombocytopenia and acute megakaryoblastic leukemia in children with Down syndrome [Cantor, 2005]. GATA-2 plays an overlapping functional role with GATA-1 during early stages of megakaryopoiesis, both of which require interaction with the cofactor Friend of GATA-1 (FOG-1) [Chang et al., 2002].
Evi-1
The ecotropic viral integration site-1 (Evi-1) is an oncogenic transcription factor in murine and human myeloid leukemia. Evi-1−/− embryos have markedly reduced numbers of phenotypic HSCs [Goyama et al., 2008]. Functional studies using the Mx1-Cre conditional knock out system show defective HSC self-renewing activity and repopulating capacity. Interestingly, the mice also develop marked thrombocytopenia, but no significant alteration in total white blood cell counts or hemoglobin levels [Goyama et al., 2008]. The mice also show selective delayed recovery of platelets following treatment with the cytotoxic agent 5-fluorouracil (5-FU). Collectively, these findings suggest lineage-specific functional roles for Evi-1 in both HSC and megakaryocyte development/maintenance. Some of the hematopoietic effects of Evi-1 may be mediated through GATA-2 [Yuasa et al., 2005] and/or Runx-1 [Senyuk et al., 2007].
Ets Family Transcription Factors
Ets family transcription factors shared a conserved DNA binding motif, and often integrate cell signaling events with transcriptional activity. At least 28 different Ets family members have been identified and play diverse developmental roles. Several members have been shown to be involved in HSC ontogeny/maintenance and megakaryopoiesis. TEL/ETV6 is frequently rearranged in human leukemias of myeloid or lymphoid origins. TEL−/− mice are embryonic lethal due to a yolk sac angiogenic defect. Conditional knock out studies using the Mx1-Cre system in adult mice show a dramatic loss of HSCs and lineage-selective impairment of megakaryocyte maturation [Hock et al., 2004].
Fli-1 was first identified as a gene product activated by Friend viral complex insertion in murine erythroleukemia cells [Ben-David et al., 1991]. Knock-out of the Fli-1 gene in mice results in dysmegakaryopoiesis and impaired vascular integrity, leading to death at day e11.5–12.5 from hemorrhage [Hart et al., 2000; Spyropoulos et al., 2000]. Fetal livers from the knockout animals have a marked decrease in total colony forming cells. In humans, heterozygous loss of the Fli-1 gene causes a congenital macrothrombocytopenia associated with Jacobsen or Paris-Trousseau syndrome [Hart et al., 2000; Raslova et al., 2004].
SCL/TAL1
SCL/TAL1 is a basic helix-loop-helix transcription factor that binds E-Box elements as a heterodimer with E-protein transcription factors. SCL−/− mice die during early embryogenesis with a “bloodless” phenotype [Porcher et al., 1996]. Loss of SCL during adult hematopoiesis results in impaired megakaryopoiesis and erythropoiesis, but normal myelopoiesis and lymphopoiesis [Mikkola et al., 2003b].
SCL/Runx1/GATA-2/Ets Factor Transcriptional Network
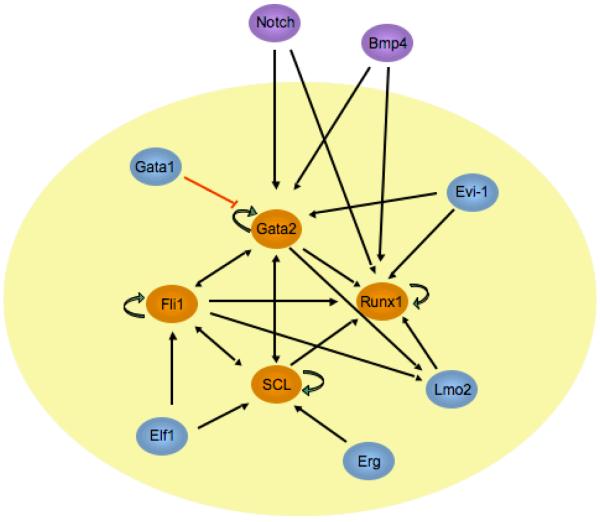
Recent work from several groups indicates that the transcription factors discussed above act in a cross- and auto- regulatory transcriptional network (Fig. 1). de Bruijn and colleagues identified a key enhancer element of the Runx-1 gene that is controlled by GATA-2/Lmo2/Ldb-1 /SCL/Ets factors during HSC ontogeny [Nottingham et al., 2007]. Gottgens and colleagues found that key enhancers of the GATA-2, Fli-1, SCL, and Lmo2 genes are occupied by all four of these factors in vivo, and that these factors autoregulate themselves through a recursively wired gene regulatory subcircuit [Gottgens et al., 2002; Landry et al., 2009; Pimanda et al., 2007].
Fig. 1.
Transcription factor network involved in HSC ontogeny. Diagram indicating key transcription factors and their cross- and auto-regulatory circuits. Core factors are indicated in orange, and associated factors are shown in blue. Possible involvement of signaling through Notch and BMP4 pathways are shown in purple. Most of these same factors are specifically involved in megakaryocyte development.
In addition to these cross-regulatory loops, many of these factors physically interact with one another. GATA-1 forms a pentameric complex with SCL/E47/LMo2/Ldb1, and directly interacts with Fli-1 and Runx-1 [Eisbacher et al., 2003; Elagib et al., 2003; Wadman et al., 1997]. GATA-2 also interacts with Runx-1 (H. Huang and A. Cantor, unpublished observation). We recently demonstrated that Runx-1 and Fli-1 physically interact directly themselves, resulting in enhanced transcriptional activity at megakaryocyte-specific genes (H. Huang, A. Cantor, submitted). The combination of direct physical interaction and extensive cross/auto-regulatory relationships between small groups of key transcription factors is an emerging theme of gene regulatory networks [Kim et al., 2008]. Gottgens and colleagues have proposed that GATA-2/SCL/LMo2/Ets (and Runx-1) factors comprise a gene regulatory “kernel” involved in the specification of HSCs. This same “kernel” appears to be active in megakaryocyte development.
HOX-related Genes
In addition to the network factors described above, several other transcription factors have been shown to play key roles in both megakaryocyte and HSC development; notably, several homeobox genes and related proteins. Meis1 and Pbx1 are important cofactors for HOX gene function. Meis1 is expressed at high levels in the AGM mesenchyme, aortic endothelium, intra-aortic clusters, and HSCs [Azcoitia et al., 2005; Hisa et al., 2004]. Meis1-deficient embryos die between e11.5 to 14.5 of gestation from internal hemorrhage, liver hypoplasia and anemia [Azcoitia et al., 2005; Hisa et al., 2004]. The AGM region and fetal liver have markedly reduced colony forming potential. In addition, there is a complete agenesis of the megakaryocyte lineages and patterning defects of the vasculature.
Pbx1 is expressed in hematopoietic progenitors during murine embryonic development. Its absence results in severe anemia and embryonic lethality at e15 to 16 [DiMartino et al., 2001]. Pbx1−/− embryos display impaired HSC function and reduced numbers of common myeloid progenitors and megakaryocyte- and erythrocyte-committed progenitors.
HOXA9 is expressed in CD34+ bone marrow cells and is inactivated as cells leave the CD34+ compartment. Loss of HOXA9 in mice leads to a defect in committed progenitors of the myeloid/erythroid and pre-B cell lineages [Lawrence et al., 1997]. Competitive repopulation assays show that the major defect in Hoxa9−/− animals is a dramatic impairment of their HSCs to repopulate lethally irradiated recipients after bone marrow transplantation, suggesting a key role in early HSCs function [Argiropoulos and Humphries, 2007]. There are no reports about whether HOXA9 is expressed in Mks. However, Kaushansky and colleagues showed that TPO induces HOXA9 nuclear transport in immature hematopoietic cells [Kirito et al., 2004].
Rbm15 (OTT)
Rbm15 (also called OTT for “one: twenty-two”) was first identified as a fusion partner of MKL1 (also call MAL) in acute megakaryocytic leukemias harboring the recurrent chromosomal translocation t(1,22)(p13;q13). This translocation produces a fusion molecule that includes essentially all of the coding regions of both molecules. Rbm15 and MKL1, individually, have subsequently been shown to play important roles during normal megakaryopoiesis. Rbm15 conditional knock out mice display enhanced megakaryopoiesis in both spleen and bone marrow, compared to wild type mice (~5 and 2-fold, respectively)[Raffel et al., 2007]. Interestingly, these mice also have about a 50% increase in the size of the bone marrow Lin−Kit+Sca-1+ (LKS) population of cells, which is highly enriched in HSCs.
Signaling Pathways
Notch Signaling
Studies of drosophila germ cells have established a critical role for Notch signaling pathways in maintaining stem cell pluripotency. Although somewhat controversial, Notch signaling has also been shown to play roles in HSC development, and this appears to require the transcription factor Runx-1 [Burns et al., 2005; Maillard et al., 2008]. Recently, Gilliland and his colleagues showed that Notch signaling also positively regulates megakaryopoiesis both in vitro and in vivo [Mercher et al., 2008]. Activated Notch signaling was shown to markedly increase specification of megakaryocytes from HSCs in a co-culture system, and this effect could be abrogated by inhibition of Notch signaling either with gamma-secretase inhibitors or by expression of the dominant-negative Mastermind-like 1. Notch signaling has also been implicated in modulating GATA-2 expression [Robert-Moreno et al., 2005]. Thus, Notch may play an important role in stimulating the hematopoietic stem cell and megakaryocyte “gene regulatory kernel” (Fig. 2).
Fig. 2.
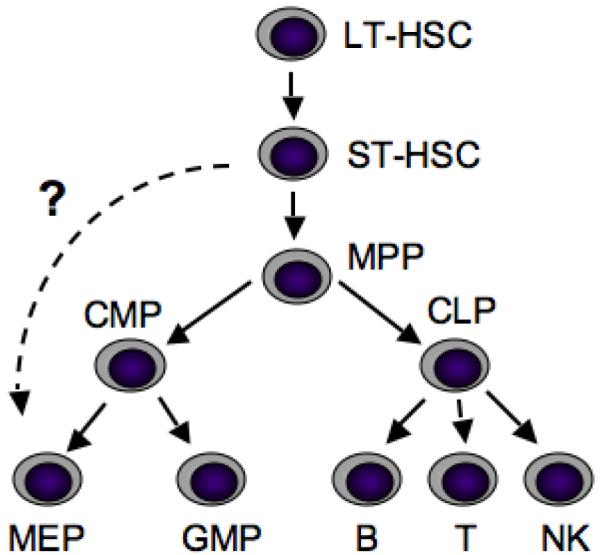
Hierarchal relationship among hematopoietic cell populations. Schematic diagram depicting the classical pathways of hematopoietic development. A potential direct relationship between ST-HSCs and MEPs is indicated by a dashed line. LT-HSCs, long-term repopulating hematopoietic stem cells; ST-HSCs, short-term repopulating hematopoietic cells; MPP, multipotent progenitor cells; CMP, common myeloid progenitor; CLP, common lymphoid progenitor; MEP, megakaryocyte-erythroid progenitor; GMP, granulocyte-macrophage progenitor; B, B-cell; T, T-cell; NK, natural killer cell. Adapted from [Adolfsson et al., 2005].
Role of Prostaglandins
Prostaglandins are lipid metabolites that play key roles in vascular function and platelet activity. Recently, a chemical screening study in zebrafish showed that agents that enhance prostaglandin E2 synthesis markedly enhance HSC expansion, and those that block prostaglandin synthesis decrease stem cell numbers [North et al., 2007]. Follow-up studies in mice show that PGE2 caused amplification of multipotent progenitors and increased the frequency of long-term repopulating HSCs present in murine bone marrow. Prostaglandins also play important roles in platelet function and are synthesized in megakaryocytic cell lines. In fact, aspirin's mode of action in inhibiting platelet function is via irreversible inactivation of the key prostaglandin metabolic enzyme cyclooxygenase.
Collectively, the findings highlighted above point towards a broad array of cell-specific factors that are shared between megakaryocytes and HSCs. These are summarized in Table 1.
Table 1.
Common features of murine megakaryocytes, HSCs, endothelial cells and hemangioblasts. N, not reported. LT-HSC, long term repopulating hematopoietic stem cells; ST-HSCs, short term repopulating hematopoietic cells.
| Megakaryocyte | HSC | Endothelial cell | Hemangioblast | |
|---|---|---|---|---|
|
| ||||
| Receptors | ||||
| c-mpl | + | + | + | + |
| CXCR4 | + | + | + | + |
| CD 150 | + | + (LT-HSC) | N | N |
| CD41 | + | + | − | − |
| CD34 | + | + (ST-HSC) | + | + |
| CD 105 | + | + | + | + |
| ACE/CD143 | N | + | + | + |
| VE-Cadherin | N | + | + | + |
| CD31/PECAM-1 | + | + | + | + |
| Tie-2 | + | + | + | + |
| KDR | + | + | + | + |
| c-kit | + | + | + | + |
| Transcription Factors | ||||
| RUNX-1 | + | + | + | + |
| FLI-1 | + | + | + | + |
| GATA2 | + | + | + | + |
| SCL/TAL1 | + | + | + | + |
| TEL/ETV6 | + | + | + | N |
| EVI-1 | + | + | N | N |
| HOXA9 | N | + | + | N |
| MEIS1 | + | + | + | N |
| PBX1 | + | + | + | N |
| Other markers | ||||
| vWF | + | − | + | N |
| P-Selectin | + | − | + | N |
| Signaling | ||||
| Prostaglandin | + | + | + | N |
| Notch | + | +/− | + | N |
| Residence | ||||
| BM vascular sinusoid | + | + | + | N |
Possible Origins of HSC and Megakaryocytes Commonalities
Why do HSCs and megakaryocytes share such a large set of cell-specific factors? Possible explanations include: (1) close hierarchical developmental relationship; (2) common functional requirements; and/or (3) shared microenvironmental interactions. In the following section, we consider these possibilities in greater detail.
Close Hierarchal Relationship
The ability to purify subpopulations of cells based on cell surface marker expression, and then test their lineage potential in vitro and in vivo has provided powerful means to understand the hierarchal relationships between different hematopoietic lineages. The earliest application of this technology led to a model in which long-term repopulating HSCs give rise to short-term repopulating HSCs, and followed by multipotential progenitor cells (MPPs) (Fig. 2). These MPPs make the first lineage commitment choice by differentiating into either common lymphoid (CLPs) or common myeloid progenitors (CMPs). CMPs then give rise to bipotent megakaryocyte-erythroid progenitors (MEPs), and granulocyte-macrophage progenitors (GMPs). CLPs differentiate into T, B and NK lineages. A relatively recent provocative study suggested that MEPs may also arise directly from ST-HSCs (Lin−Sca-1+c-kit+CD34+Flt3− cells) in mice [Adolfsson et al., 2005]. However, these new findings have been challenged in a subsequent study that took into account differences in the kinetics of distinct progenitor populations and used more sensitive methods [Forsberg et al., 2006]. This area remains controversial, but raises the possibility that megakaryocytes and erythroid cells are closely related to HSCs developmentally.
The Endothelial Cell Link
The developmental origins of hematopoietic and endothelial lineages are closely linked. Some of the earliest evidence for this comes from studies of in vitro differentiated mouse embryonic stem (ES) cells, which showed that a blast-colony forming cell (BL-CFC) could be isolated that gives rise to colonies with both endothelial and hematopoietic components [Choi et al., 1998]. The common precursor cell was termed a “hemangioblast”. Subsequent studies have provided in vivo evidence for the presence of hemangioblasts.
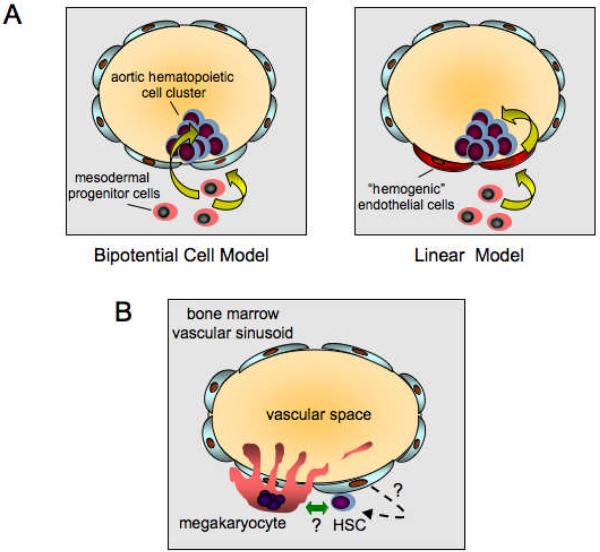
During embryogenesis, HSCs first arise from the ventral wall of the dorsal aorta during a brief developmental window. They then seed the fetal liver, which is the main hematopoietic organ during embryonic development. Emergence of HSCs coincides with the appearance of intra-aortic clusters of hematopoietic cells that are closely associated with specialized endothelial cells lining the ventral aspect of the dorsal aorta. Two models have been proposed for the origins of these cells (Fig. 3A). In the first model, bipotent mesodermal precursors cells in the subaortic mesenchyme migrate toward the lumen of the aorta and independently produce endothelial cells that line the vessel, and HSCs that transmigrate around the endothelial cells to contribute to the hematopoietic aortic cell clusters. This would be akin to the bipotential hemangioblast concept. In the second model, the mesodermal precursor cells give rise exclusively to specialized “hemogenic endothelial cells”, and these cells then give rise to HSCs (a so-called “linear” model). Three recent papers, including one involving single cell continuous imaging analysis, provide strong evidence for the latter [Chen et al., 2009; Eilken et al., 2009; Lancrin et al., 2009].
Fig 3.
Relationship between HSCs, endothelial cells, and megakaryocytes. (A) Models of HSC emergence from the aorto-gonadal-mesonephros region during embryogenesis. Left panel, “Bipotential” model. In this model, bipotential mesodermal precursor cells give rise independently to endothelial cells lining the ventral aspect of the dorsal aorta and cells that migrate around the endothelial cells to form clusters of cells containing HSCs in the lumen of the aorta. Right panel, “Linear” model. In this model, mesodermal precursor cells give rise to specialized “hemogenic” endothelial cells lining the anterior aspect of the dorsal aorta. These cells then give rise directly to the HSC containing clusters in the dorsal aorta lumen. (B) Co-localization of megakaryocytes and HSCs at bone marrow vascular sinusoids. Diagram depicting vascular sinusoids in adult bone marrow. Mature megakaryocytes are shown in apposition to the bone marrow side of a single cell layer of endothelial cells lining the sinusoid. During megakaryocyte maturation, long cytoplasmic proplatelet extensions form and protrude into the vascular space through fenestrations in the endothelial cell layer. Fragments then are shed into the circulation where they are further processed into platelets. Hypothetical interactions between Mks and HSCs at the vascular sinusoidal niche are indicated by the green bi-directional arrow. Hypothetical low-level de novo generation of HSCs from vascular sinusoidal endothelial cells is indicated by the dashed arrow.
The Weissman laboratory recently developed a novel experimental model to study to generation of the hematopoietic bone marrow niche [Chan et al., 2009]. This involves injection of fetal bone-derived cells under the renal capsule, followed by examination for establishment of a bone marrow cavity capable of supporting HSCs. Cell fractionation studies show that the cell population responsible for establishing the hematopoietic microenvironment expresses the endothelial marker CD105 (Endoglin), and that chondrocyte neovascularization is required for establishing the niche. In combination with the previously noted findings, it seems clear that hematopoietic origins have a close ancestral relationship with endothelial cells, at least during embryonic development.
There is considerable functional overlap and interplay between megakaryocytes and endothelial cells. The ultimate function of platelets is to repair disrupted endothelium and “plug” up minute holes. This occurs via adhesion to exposed subendothelium structures, activation, aggregation, cell flattening, and activation of angiogenesis. Both platelets and endothelial cells utilize prostaglandin signaling pathways, and modulate hemostasis and thrombosis. Both megakaryocytes and endothelial cells synthesize and secrete von Willebrand factor (vWF), which is involved in linking platelets to exposed basement membrane, and P-selectin, which acts as a key adhesion molecule during hemostasis. Activated platelets also secrete a large number of vasoactive and angiogenic modulatory factors.
In further support of this idea, many of the commonly expressed “lineage-specific” factors of megakaryocytes and HSCs are also present in endothelial cells and/or “hemangioblasts”, many of which have been shown to be functionally important (Table I). These include the surface receptors c-mpl, CXCR4, CD34, CD105, CD31 (PECAM), JAM-A, Tie-2, KDR, and c-kit; and the transcription factors Runx-1, Fli-1, GATA-2, SCL/TAL1, TEL/ETV6, HOXA9, Meis1, and Pbx. In fact, TPO alone supports BL-CFC formation and gives rise to secondary hematopoietic colonies and endothelial cells [Perlingeiro et al., 2003]. Thus, there appears to be close functional and compositional overlap between megakaryocytes, HSCs and endothelial cells.
Common Bone Marrow Localization
HSCs are thought to reside in specialized niches within the bone marrow. Classically, the HSC niche has been defined as a site in close proximity to the endosteum (the “osteoblastic” niche). However, recent work suggests that HSCs also reside in proximity to vascular sinusoids within the bone marrow (the “vascular niche”) [Kiel et al., 2005]. In this study, about 60% of CD150+CD48−CD41−Lin− HSCs were localized in bone marrow sinusoidal endothelium, and 14% were associated with endosteum [Kiel et al., 2005]. In addition, HSCs maintenance is supported by bone marrow endothelial sinusoids, whereas N-cadherin -mediated homophilic adhesion with osteoblasts is dispensable [Kiel et al., 2009]. This area remains somewhat controversial, and it is possible that there is overlap in osteoblastic and vascular niches as some of the vascular sinusoids may be relatively close to the endosteum. Interestingly, mature Mks also reside predominantly at bone marrow sinusoids, juxtaposed to vascular endothelial cells (Fig. 3B). This has most recently been documented in the context of living mice using in vivo imaging techniques [Junt et al., 2007]. This study also demonstrated the extension of long proplatelet processes from the megakaryocytes through fenestrations in the endothelial cell layer and into the vascular space, where proplatelet fragments (and presumably platelets) were shed into the circulation. The homing of Mks to the vascular sinusoid is mediated in large part by the chemoattractive effects of SDF-1 on the CXCR4 receptor, a system shared with HSCs [Avecilla et al., 2004]. Thus, the functional and gene expression similarities of megakaryocytes and HSCs may reflect a common requirement for homing to and interaction with the specialized vascular sinusoidal niche.
Conclusions and Future Prospects
From the work described above, it is clear that megakaryocytes and HSCs share a remarkable number of specialized factors, and this overlap extends to endothelial cells and hemangioblasts. In addition, close physical association of these cell types occurs in the adult bone marrow. It is not known at this time whether vascular sinusoidal megakaryocytes make direct cell-cell contacts with HSCs. However, it is possible that megakaryocytes themselves may contribute to the vascular HSC niche. If so, they could perhaps be utilized for ex vivo HSC culture or expansion.
The recent demonstration of HSC derivation directly from “hemogenic” endothelium during embryogenesis raises the provocative idea that low-level de novo generation of HSCs might occur from vascular sinusoidal endothelial cells at bone marrow sinusoids in adult mice. Monvoisin et al. generated mice expressing tamoxifen-inducible Cre under the control of endothelial-specific vascular endothelial cadherin (VE-Cadherin) gene promoter [Monvoisin et al., 2006]. When these mice were interbred with reporter mice containing the β-galactosidase gene knocked into the ubiquitously expressed ROSA26 locus and preceded by a loxP-flanked stopper cassette, a small but detectable number of hematopoietic bone marrow cells (0.3 −/+ 0.1 %) became labeled after Cre was activated by tamoxifen injection in adult mice. Although there are a number of alternative explanations for this finding, one intriguing possibility is that HSCs may be capable of being generated de novo from vascular sinusoidal endothelial cells at very low levels in adult animals. If present, it is possible that such a system could be more robust following injury and regeneration.
Cellular reprogramming from somatic cells has become a reality with the remarkable demonstration that a small set of key transcription factors can generate pluripotent stem cells when over expressed in adult murine and human fibroblasts. Given the significant overlap in key transcription factors expressed in Mks and HSCs, cultured Mks may represent an efficient source of somatic cells for reprogramming into HSCs.
The discovery of shared key factors between Mks and HSCs suggests that any new findings in Mk biology may have parallels in HSC biology, and vice versa. Thus, one field may help to inform the other. On the other hand, Mks and HSCs do have unique properties. Therefore, comparison of their differentially expressed factors may represent a somewhat simplified system to elucidate the molecular basis of their specialized activities, such as stem cell self-renewal/pluripotency and megakaryocyte endomitosis. While all of the ideas discussed above are highly speculative, they are testable and may have implications for regenerative medicine, stem cell transplantation, and some bone marrow failure disorders.
Acknowledgements
A.B.C. is supported by a grant from the NIH (R01 HL082952). We would like to acknowledge the investigators who have contributed important information to this field, but whose work we were unable to cite due to space limitations. We would also like to thank Zhe Li for useful discussion.
References
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Argiropoulos B, Humphries RK. Hox genes in hematopoiesis and leukemogenesis. Oncogene. 2007;26:6766–76. doi: 10.1038/sj.onc.1210760. [DOI] [PubMed] [Google Scholar]
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, de Sauvage F, Rafii S. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- Azcoitia V, Aracil M, Martinez AC, Torres M. The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev Biol. 2005;280:307–20. doi: 10.1016/j.ydbio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Ben-David Y, Giddens EB, Letwin K, Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991;5:908–18. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–42. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor AB. GATA transcription factors in hematologic disease. Int J Hematol. 2005;81:378–84. doi: 10.1532/ijh97.04180. [DOI] [PubMed] [Google Scholar]
- Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–4. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AN, Cantor AB, Fujiwara Y, Lodish MB, Droho S, Crispino JD, Orkin SH. GATA-factor dependence of the multitype zinc-finger protein FOG-1 for its essential role in megakaryopoiesis. Proc Natl Acad Sci U S A. 2002;99:9237–42. doi: 10.1073/pnas.142302099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–91. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–32. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Debili N, Robin C, Schiavon V, Letestu R, Pflumio F, Mitjavila-Garcia MT, Coulombel L, Vainchenker W. Different expression of CD41 on human lymphoid and myeloid progenitors from adults and neonates. Blood. 2001;97:2023–30. doi: 10.1182/blood.v97.7.2023. [DOI] [PubMed] [Google Scholar]
- DiMartino JF, Selleri L, Traver D, Firpo MT, Rhee J, Warnke R, O'Gorman S, Weissman IL, Cleary ML. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood. 2001;98:618–26. doi: 10.1182/blood.v98.3.618. [DOI] [PubMed] [Google Scholar]
- Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Eisbacher M, Holmes ML, Newton A, Hogg PJ, Khachigian LM, Crossley M, Chong BH. Protein-protein interaction between Fli-1 and GATA-1 mediates synergistic expression of megakaryocyte-specific genes through cooperative DNA binding. Mol Cell Biol. 2003;23:3427–41. doi: 10.1128/MCB.23.10.3427-3441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elagib KE, Racke FK, Mogass M, Khetawat R, Delehanty LL, Goldfarb AN. RUNX1 and GATA-1 coexpression and cooperation in megakaryocytic differentiation. Blood. 2003;101:4333–41. doi: 10.1182/blood-2002-09-2708. [DOI] [PubMed] [Google Scholar]
- Forsberg EC, Serwold T, Kogan S, Weissman IL, Passegue E. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126:415–26. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Gottgens B, Nastos A, Kinston S, Piltz S, Delabesse EC, Stanley M, Sanchez MJ, Ciau-Uitz A, Patient R, Green AR. Establishing the transcriptional programme for blood: the SCL stem cell enhancer is regulated by a multiprotein complex containing Ets and GATA factors. Embo J. 2002;21:3039–50. doi: 10.1093/emboj/cdf286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyama S, Yamamoto G, Shimabe M, Sato T, Ichikawa M, Ogawa S, Chiba S, Kurokawa M. Evi-1 is a critical regulator for hematopoietic stem cells and transformed leukemic cells. Cell Stem Cell. 2008;3:207–20. doi: 10.1016/j.stem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Growney JD, Shigematsu H, Li Z, Lee BH, Adelsperger J, Rowan R, Curley DP, Kutok JL, Akashi K, Williams IR, Speck NA, Gilliland DG. Loss of Runx1 perturbs adult hematopoiesis and is associated with a myeloproliferative phenotype. Blood. 2005;106:494–504. doi: 10.1182/blood-2004-08-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A, Melet F, Grossfeld P, Chien K, Jones C, Tunnacliffe A, Favier R, Bernstein A. Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity. 2000;13:167–77. doi: 10.1016/s1074-7613(00)00017-0. [DOI] [PubMed] [Google Scholar]
- Hisa T, Spence SE, Rachel RA, Fujita M, Nakamura T, Ward JM, Devor-Henneman DE, Saiki Y, Kutsuna H, Tessarollo L, Jenkins NA, Copeland NG. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. Embo J. 2004;23:450–9. doi: 10.1038/sj.emboj.7600038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock H, Meade E, Medeiros S, Schindler JW, Valk PJ, Fujiwara Y, Orkin SH. Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev. 2004;18:2336–41. doi: 10.1101/gad.1239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa M, Asai T, Saito T, Yamamoto G, Seo S, Yamazaki I, Yamagata T, Mitani K, Chiba S, Hirai H, Ogawa S, Kurokawa M. AML-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nat Med. 2004;10:299–304. doi: 10.1038/nm997. [DOI] [PubMed] [Google Scholar]
- Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner DD, Graf T, Italiano JE, Jr., Shivdasani RA, von Andrian UH. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–70. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Acar M, Radice GL, Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell. 2009;4:170–9. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–61. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci U S A. 1998;95:1195–200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirito K, Fox N, Kaushansky K. Thrombopoietin induces HOXA9 nuclear transport in immature hematopoietic cells: potential mechanism by which the hormone favorably affects hematopoietic stem cells. Mol Cell Biol. 2004;24:6751–62. doi: 10.1128/MCB.24.15.6751-6762.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–5. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry JR, Bonadies N, Kinston S, Knezevic K, Wilson NK, Oram SH, Janes M, Piltz S, Hammett M, Carter J, Hamilton T, Donaldson IJ, Lacaud G, Frampton J, Follows G, Kouskoff V, Gottgens B. Expression of the leukaemia oncogene Lmo2 is controlled by an array of tissue specific elements dispersed over 100kb and bound by Tal1/Lmo2, Ets and Gata factors. Blood. 2009 doi: 10.1182/blood-2008-11-187757. [DOI] [PubMed] [Google Scholar]
- Lawrence HJ, Helgason CD, Sauvageau G, Fong S, Izon DJ, Humphries RK, Largman C. Mice bearing a targeted interruption of the homeobox gene HOXA9 have defects in myeloid, erythroid, and lymphoid hematopoiesis. Blood. 1997;89:1922–30. [PubMed] [Google Scholar]
- Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, Pross SE, Aster JC, Bhandoola A, Radtke F, Pear WS. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2:356–66. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercher T, Cornejo MG, Sears C, Kindler T, Moore SA, Maillard I, Pear WS, Aster JC, Gilliland DG. Notch signaling specifies megakaryocyte development from hematopoietic stem cells. Cell Stem Cell. 2008;3:314–26. doi: 10.1016/j.stem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola HK, Fujiwara Y, Schlaeger TM, Traver D, Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003a;101:508–16. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- Mikkola HK, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, Orkin SH. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature. 2003b doi: 10.1038/nature01345. [DOI] [PubMed] [Google Scholar]
- Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–30. [PubMed] [Google Scholar]
- Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML. VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev Dyn. 2006;235:3413–22. doi: 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- Murone M, Carpenter DA, de Sauvage FJ. Hematopoietic deficiencies in c-mpl and TPO knockout mice. Stem Cells. 1998;16:1–6. doi: 10.1002/stem.160001. [DOI] [PubMed] [Google Scholar]
- North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M, Speck NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–75. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, Fitzgerald GA, Daley GQ, Orkin SH, Zon LI. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–11. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottingham WT, Jarratt A, Burgess M, Speck CL, Cheng JF, Prabhakar S, Rubin EM, Li PS, Sloane-Stanley J, Kong ASJ, de Bruijn MF. Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood. 2007;110:4188–97. doi: 10.1182/blood-2007-07-100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlingeiro RC, Kyba M, Bodie S, Daley GQ. A role for thrombopoietin in hemangioblast development. Stem Cells. 2003;21:272–80. doi: 10.1634/stemcells.21-3-272. [DOI] [PubMed] [Google Scholar]
- Pimanda JE, Ottersbach K, Knezevic K, Kinston S, Chan WY, Wilson NK, Landry JR, Wood AD, Kolb-Kokocinski A, Green AR, Tannahill D, Lacaud G, Kouskoff V, Gottgens B. Gata2, Fli1, and Scl form a recursively wired gene-regulatory circuit during early hematopoietic development. Proc Natl Acad Sci U S A. 2007;104:17692–7. doi: 10.1073/pnas.0707045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW, Orkin SH. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996;86:47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- Pronk CJ, Rossi DJ, Mansson R, Attema JL, Norddahl GL, Chan CK, Sigvardsson M, Weissman IL, Bryder D. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–42. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Raffel GD, Mercher T, Shigematsu H, Williams IR, Cullen DE, Akashi K, Bernard OA, Gilliland DG. Ott1(Rbm15) has pleiotropic roles in hematopoietic development. Proc Natl Acad Sci U S A. 2007;104:6001–6. doi: 10.1073/pnas.0609041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raslova H, Komura E, Le Couedic JP, Larbret F, Debili N, Feunteun J, Danos O, Albagli O, Vainchenker W, Favier R. FLI1 monoallelic expression combined with its hemizygous loss underlies Paris-Trousseau/Jacobsen thrombopenia. J Clin Invest. 2004;114:77–84. doi: 10.1172/JCI21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. 2005;132:1117–26. doi: 10.1242/dev.01660. [DOI] [PubMed] [Google Scholar]
- Rodrigues NP, Janzen V, Forkert R, Dombkowski DM, Boyd AS, Orkin SH, Enver T, Vyas P, Scadden DT. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106:477–84. doi: 10.1182/blood-2004-08-2989. [DOI] [PubMed] [Google Scholar]
- Senyuk V, Sinha KK, Li D, Rinaldi CR, Yanamandra S, Nucifora G. Repression of RUNX1 activity by EVI1: a new role of EVI1 in leukemogenesis. Cancer Res. 2007;67:5658–66. doi: 10.1158/0008-5472.CAN-06-3962. [DOI] [PubMed] [Google Scholar]
- Song WJ, Sullivan MG, Legare RD, Hutchings S, Tan X, Kufrin D, Ratajczak J, Resende IC, Haworth C, Hock R, Loh M, Felix C, Roy DC, Busque L, Kurnit D, Willman C, Gewirtz AM, Speck NA, Bushweller JH, Li FP, Gardiner K, Poncz M, Maris JM, Gilliland DG. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23:166–75. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- Spyropoulos DD, Pharr PN, Lavenburg KR, Jackers P, Papas TS, Ogawa M, Watson DK. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20:5643–52. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Downing JR. Haploinsufficiency of AML1 results in a decrease in the number of LTR-HSCs while simultaneously inducing an increase in more mature progenitors. Blood. 2004;104:3565–72. doi: 10.1182/blood-2003-12-4349. [DOI] [PubMed] [Google Scholar]
- Talebian L, Li Z, Guo Y, Gaudet J, Speck ME, Sugiyama D, Kaur P, Pear WS, Maillard I, Speck NA. T-lymphoid, megakaryocyte, and granulocyte development are sensitive to decreases in CBFbeta dosage. Blood. 2007;109:11–21. doi: 10.1182/blood-2006-05-021188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–6. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- van den Oudenrijn S, Bruin M, Folman CC, Peters M, Faulkner LB, de Haas M, von dem Borne AE. Mutations in the thrombopoietin receptor, Mpl, in children with congenital amegakaryocytic thrombocytopenia. Br J Haematol. 2000;110:441–8. doi: 10.1046/j.1365-2141.2000.02175.x. [DOI] [PubMed] [Google Scholar]
- Wadman IA, Osada H, Grutz GG, Agulnick AD, Westphal H, Forster A, Rabbitts TH. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. Embo J. 1997;16:3145–57. doi: 10.1093/emboj/16.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JF, Liu ZY, Groopman JE. The alpha-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood. 1998;92:756–64. [PubMed] [Google Scholar]
- Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci U S A. 1996;93:3444–9. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa H, Oike Y, Iwama A, Nishikata I, Sugiyama D, Perkins A, Mucenski ML, Suda T, Morishita K. Oncogenic transcription factor Evi1 regulates hematopoietic stem cell proliferation through GATA-2 expression. Embo J. 2005;24:1976–87. doi: 10.1038/sj.emboj.7600679. [DOI] [PMC free article] [PubMed] [Google Scholar]