Abstract
Objective
This study evaluates the performance of highly reactive novel monomethacrylates characterized by various secondary moieties as reactive diluent alternatives to TEGDMA in BisGMA filled dental resins. We hypothesize that these monomers improve material properties and kinetics over TEGDMA because of their unique polymerization behavior.
Methods
The cure rates and final double bond conversion of the resins were measured using real-time FTIR spectroscopy. The glass transition temperature and storage modulus of the formed polymers were measured using dynamic mechanical analysis. Flexural modulus and flexural strength values were obtained using a three-point bending flexural test carried out with a MTS® 858 Mini Bionix system.
Results
Polymerization kinetics and polymer mechanical properties were evaluated for the novel resin composites. It was observed that upon the use of novel monomethacrylates as reactive diluents, polymerization kinetics increased by up to 3-fold accompanied by increases in the extent of cure from 5% to 13% as compared to the BisGMA/TEGDMA control. Polymer composites formed from resins of BisGMA/novel monomethacrylates exhibited comparable Tg values to the control, along with 27–37% reductions in the glass transition half widths indicating the formation of more homogeneous polymeric networks. The BisGMA/monomethacrylate formulations exhibited equivalent flexural modulus and flexural strength values relative to BisGMA/TEGDMA.
Significance
Formulations containing novel monovinyl methacrylates exhibit dramatically increased curing rates while also exhibiting superior or at least comparable composite polymer mechanical properties. Thus, these types of materials are attractive for use as reactive diluent alternatives to TEGDMA in dental formulations.
Introduction
A variety of methacrylate resins have been examined previously for use in dental formulations [1–3]. Due to its high mechanical strength, low volatility, and relatively low polymerization shrinkage, (2,2-bis[p-(3-methacryloxy-2-hydroxypropoxy)phenyl]propane (BisGMA) is the primary component in a majority of commercial dental resins [2]. However, BisGMA is also characterized by a very high viscosity and low polymerization conversion. Hence, 20–50 wt% of less viscous dimethacrylates such as triethylene glycol dimethacrylate (TEGDMA) have been added to improve handling of the dental formulations, as well as to achieve higher degrees of conversion [2,4].
The addition of TEGDMA is also associated with an increased volumetric shrinkage due to the higher double bond concentration of TEGDMA and increased overall double bond conversion [5]. BisGMA/TEGDMA resins also exhibit a high shrinkage stress which contributes to premature failure or reduced overall performance of the restoration [6,7]. Hence, many alternative reactive diluents to TEGDMA have been explored as a means to mitigate shrinkage stress [8,9]. Monomethacrylates and acrylates such as hydroxypropyl methacrylate and isobornyl acrylate have been explored and shown to be characterized by reduced volumetric shrinkage and increased double bond conversions [10]. However, the reaction rate is significantly lower for these traditional functional compounds compared with that available from dimethacrylates.
Recently a series of mono-vinyl (meth)acrylic monomers characterized by unique secondary moieties and greatly enhanced polymerization kinetics has been developed. The secondary moieties include, carbamates, carbonates, cyclic carbonates, cyclic acetals, hydroxyl/carboxy, oxazolidones, morpholine, and aromatic rings [11–20]. The cure rates of these mono-vinyl acrylates and methacrylates rival or exceed those of multifunctional acrylates while also achieving much higher extents of cure, hence reducing the amount of extractable material [13]. The more rapid curing kinetics of these monomers facilitate the use of lower light intensities and/or reduced initiator concentrations. Reduced initiator concentrations effectively minimize certain drawbacks associated with initiator usage such as discoloration, optical opacity, and photodegradation of the cured product. Also, since there is significant light attenuation during polymerization, a more reactive resin enables more complete polymerization at the bottom of thick composite systems irradiated only from the upper surface.
Previous work by Lu et al. has explored the performance of mono-(meth)acrylates characterized by carbonate, carbamate and oxazolidone secondary moieties, as reactive diluents in unfilled dental polymers [21]. It was observed that in addition to enhanced polymerization kinetics, mixtures of these (meth)acrylates with BisGMA also exhibited reduced volumetric shrinkage as well as comparable glass transition temperatures with a narrower Tg half-width. This current study focuses on evaluating the impact of monomethacrylate monomers on the polymerization and polymer properties of filled, composite systems as relative to dental restorative systems. The experimental procedures were designed to eliminate other considerations, including differences associated with the initiation rate. As such, an ultraviolet initiating system was chosen that utilizes a cleavage initiator. The initiation rate for cleavage initiating systems is far less affected by monomer composition than the traditional camphorquinone/amine systems in dental materials. This selection, therefore, enabled us to focus only on the differences in kinetics associated with the monomers, while maintaining an initiation rate that was approximately the same across the various samples, including the controls. Further, the previous work with these systems also utilized an ultraviolet initiation system light and thus this approach maintained consistency. All results of polymerization kinetics and mechanical properties are compared to the control BisGMA/TEGDMA system at identical curing conditions.
Materials and Methods
Materials
2,2-Bis[4-(2-hydroxy-3-methacryloxyprop-1-oxy)phenyl]propane (BisGMA) and triethylene glycol dimethacrylate (TEGDMA) were donated by Esstech, Inc. (Essington, PA, USA). The initiator used was 0.1 wt% 2,2-dimethoxy-2-phenylacetophenone (DMPA/Irgacure 651 Ciba-Geigy, Hawthorne, NY). The reactive diluents 2-(methacryloyloxy)ethyl morpholine-4-carboxylate (morpholinecarbonyl methacrylate) and 2-(phenoxycarbonyloxy)ethyl methacrylate (phenyl carbonate methacrylate) were synthesized by the reaction of morpholine carbonyl chloride or phenyl chloroformate with hydroxyethyl methacrylate, respectively. The detailed synthesis and purification methods of these monomers are available elsewhere [15]. Barium glass filler (4 μm average diameter) treated with methacroyloxypropyltriethoxysilane was donated by Confi-Dental Products (Louisville, CO). Chemical structures of monomers are given in Figure 1. All resins contain 70 wt% BisGMA, 30 wt% of the reactive diluent TEGDMA, morpholinecarbonyl methacrylate, or phenyl carbonate methacrylate, and 0.1 wt% DMPA. Composites were prepared in 5 gram batches and contain 70 wt% barium glass filler. Composites were mixed with a Flacktek SpeedMixer (Landrum, SC). All samples were protected from ultraviolet light exposure.
Figure 1.
Chemical structures of monomers.
Fourier Transform Infrared Spectroscopy
Fourier Transform Infrared Spectroscopy (FTIR) experiments were utilized for kinetic analysis and conducted in the mid-infrared (4000 – 600 cm−1) using a Nicolet 750 Magna FTIR spectrometer (Madison, WI) with a KBr beam splitter and an MCT/A detector. All samples were laminated between NaCl windows and a horizontal transmission accessory (HTA) was utilized to redirect the IR beam vertically, which allows the samples to remain in a horizontal configuration during analysis [15]. Series scans were recorded at a rate of approximately 2 scans per second until the reaction was complete, as indicated by the reactive group absorption peak no longer decreasing. Methacrylate functional group conversion was monitored utilizing the methacrylate absorption peak at 1630 cm−1 (C=C stretching vibration) or 810 cm−1 (C=C twisting vibration) in real time. Samples were irradiated for 5 min with 5.0 mW/cm2 UV light utilizing an EFOS Ultracure with a 320–500 nm filter. Irradiation intensity was measured at the sample surface level with an International Light, Inc. Model IL1400A radiometer (Newburyport, MA). For each system, experiments were performed in triplicate.
Methods
Samples were irradiated utilizing an EFOS Ultracure with a 320–500 nm bandpass filter. Irradiation intensity was measured at the sample surface level with an International Light, Inc. Model IL1400A radiometer (Newburyport, MA).
Dynamic mechanical analysis
Samples for dynamic mechanical analysis (DMA) were irradiated for 10 min at 5.0 mW/cm2. Dynamic mechanic analysis in extension mode (TA Instruments, Q800 DMA, CT) was utilized for measurement of the material properties. Loss tangent and storage modulus were determined as a function of temperature, applying a sinusoidal stress at a frequency of 1 Hz. The temperature of the sample was increased from −10 to 250 °C at a rate of 3 °C/min. The glass transition temperature (Tg) was taken to be the maximum of the loss tangent versus temperature curve. Glass transition and modulus data are from the second heating. For each system, experiments were performed in triplicate.
Flexural modulus testing
Flexural strength studies were performed with monomer samples of dimensions, 25 mm×2 mm×2 mm photopolymerized in a glass/Teflon mold with 5.0 mW/cm2 of UV irradiation for 10 min on each side. A three-point bending flexural test was carried out with a MTS® 858 Mini Bionix system (MTS Systems Corporation, Eden Prairie, MN, USA) using a span width of 20 mm and a crosshead speed of 1.0 mm/min. The flexural strength and modulus were obtained from the following equations:
where σ is the flexural strength and Ef is the flexural modulus, F is the peak load in Newtons, l is the span length in mm, b is the width of the specimen in mm, h is the thickness of the specimen in mm, and d is the deflection of the specimen in mm, at load F1 in N during the straight line portion of the trace [22]. For each system, at least four repeat experiments were performed.
Statistical analysis
The experimental results were analyzed using one-way analysis of variance (ANOVA) based on triplicate specimens for FTIR and DMA and quadruplicate speciments for flexural modulus testing. Multiple pairwise comparisons were further conducted using Tukey’s test with a significance level of 0.05.
Results
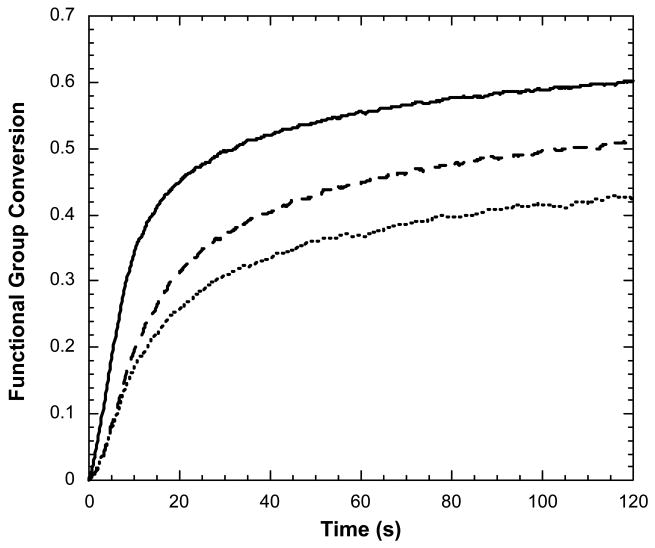
The photopolymerization kinetics of morpholinecarbonyl methacrylate and phenyl carbonate methacrylate as compared to TEGDMA, when utilized as reactive diluents to BisGMA, were evaluated with 70 wt% barium glass fillers. The polymerization kinetics are shown in Figure 2. It was observed that the BisGMA/morpholinecarbonyl methacrylate and BisGMA/phenyl carbonate methacrylate formulations exhibited enhanced polymerization rates and final conversions as compared to the BisGMA/TEGDMA formulations (Table 1). However, only the BisGMA/morpholinecarbonyl methacrylate system exhibits statistically different polymerization rate and conversion values. DMA analysis results are given in Table 2. The BisGMA/TEGDMA control system and BisGMA/morpholinecarbonyl methacrylate systems did not provide statistically different Tg values. The Tg of the BisGMA/phenyl carbonate methacrylate systems was lower than the other two systems. The storage modulus values of both experimental monomethacrylate-containing composites were significantly greater than that of the control. Due to thermal post-curing effects when performing DMA analysis [21], all DMA results are reported for the third heating cycle to assure that these are stable materials undergoing no additional post-cure. The final conversions range from 91–95 %. Flexural strength and modulus results are given in Table 3. The BisGMA/phenyl carbonate methacrylate system yielded equivalent flexural modulus and decreased flexural strength relative to the BisGMA/TEGDMA control. The BisGMA/morpholinecarbonyl methacrylate system exhibited increased flexural modulus and equivalent flexural strength relative to the control.
Figure 2.
Acrylate functional group conversion as a function of time for filled resins of (—) 70/30 BisGMA/morpholinecarbonyl methacrylate, (---) 70/30 BisGMA/phenyl carbonate methacrylate, and (…) 70/30 BISGMA/TEGDMA. Polymerization conditions: 70 wt% 4 micron barium glass as filler, initiator concentration = 0.1 wt% DMPA, light intensity = 5.0 mW/cm2.
Table 1.
The mean (s.d) polymerization kinetics and the final double bond conversions for triplicate samples of the formulations of BisGMA with reactive diluents. All systems contain 70 wt% 4 micron barium glass as filler, 0.1 wt% DMPA, and are irradiated at 5.0 mW/cm2 at ambient temperature. The rates mean values calculated from 5% to 25 % conversion. Significantly different groups (P < 0.05) within each column are identified by letter.
| System (70/30 wt% monomer formulations ) | Average polymerization Rate (s−1) | Final double bond conversion (%) |
|---|---|---|
| BisGMA/TEGDMA | 0.013 ± 0.004 a | 50 ± 3a |
| BisGMA/Phenyl Carbonate Methacrylate | 0.020 ± 0.002 a | 55 ± 1 a |
| BisGMA/Morpholinecarbonyl Methacrylate | 0.040 ± 0.005 b | 63 ± 3 b |
Table 2.
The mean (s.d) glass transition temperatures, Tg half width, and storage modulus at room temperature for triplicate samples of filled resins of BisGMA/reactive diluents. All samples contain 70 wt% 4 micron barium glass filler and were cured at 5.0 mW/cm2, for 10 min at 25 °C. Significantly different groups (P < 0.05) within each column are identified by letter.
| System (70/30 wt% monomer formulations) | Tg (°C) | Half peak width (°C) | Storage Modulus at 25 °C (GPa) | Conversion* (%) |
|---|---|---|---|---|
| BisGMA/TEGDMA | 144 ± 8 a | 100 ± 6 a | 6.9 ± 0.7 a | 91 ± 6 a |
| BisGMA/Phenyl carbonate Methacrylate | 116 ± 1 b | 65 ± 8 b | 8.8 ± 0.1 b | 95 ± 5 a |
| BisGMA/Morpholine-carbonyl methacrylate | 140 ± 3 a | 56 ± 5 b | 8.7 ± 0.1 b | 92 ± 8 a |
Conversion was measured after three heating cycles in the DMA.
Table 3.
Mean (s.d) flexural modulus and flexural strength of quadruplicate samples of the filled resins of BISGMA/reactive diluents. All samples contain 70 wt% 4 micron barium glass filler and were cured at 5.0 mW/cm2 for 10 min on each side at 25 °C. Significantly different groups (P < 0.05) within each column are identified by letter.
| System (70/30 monomer formulations) with 70 wt% filler | Flexural Modulus (GPa) | Flexural Strength (MPa) |
|---|---|---|
| BisGMA/TEGDMA | 7.2 ± 0.2 a | 133.1 ± 10.5 a |
| BisGMA/Phenyl Carbonate Methacrylate | 7.3 ± 0.5 a | 88.9 ± 11.9 b |
| BisGMA/Morpholinecarbonyl Methacrylate | 9.4 ± 0.3 b | 123.5 ± 10.7 a |
Discussion
As can be inferred from Figure 2 and Table 1, composites with the monovinyl methacrylates exhibited enhanced polymerization kinetics as compared to the BisGMA/TEGDMA formulations, with up to a 3-fold rate enhancement in the mean polymerization rates. The conversion levels are less than the analogous unfilled materials [21], which is not uncommon when comparing resin versus composite systems. In agreement with the previous unfilled resin study, the BisGMA/morpholinecarbonyl methacrylate composite provided the highest reaction rate. Increased curing efficiency is very desirable because a reduced amount of initiator may be utilized to achieve a particular cure rate, thereby improving the long term performance and color stability of the dental composites [1,23]. While only thin film specimens were evaluated in the kinetic studies here, the high reactivity at low light intensities also suggests higher achievable depths of cure in these composite materials. The greatly enhanced reactivity as well as the high mobility of these novel monomers is expected to minimize potential for significant amounts of residual unreacted monomethacrylate monomer in these copolymers with BisGMA, but this point remains to be verified.
The DMA data in Table 2 demonstrate that the BisGMA/morpholinecarbonyl methacrylate system exhibits a Tg that is equivalent to BisGMA/TEGDMA at equivalent conversions. Additionally, both the BisGMA/phenyl carbonate methacrylate and BisGMA/morpholinecarbonyl methacrylate systems provide improved storage moduli compared to that of the BisGMA/TEGDMA system. Flexural modulus data in Table 3 also demonstrates the utility of the BisGMA/novel monomer systems in regards to mechanical properties. Specifically, the BisGMA/morpholinecarbonyl methacrylate system exhibits improved flexural modulus and equivalent flexural strength as compared to BisGMA/TEGDMA. The uniformly favorable mechanical strength properties achieved with the BisGMA/morpholinecarbonyl methacrylate composite material are likely associated with the hydrogen bonding donor/acceptor capabilities possible based on the urethane/cyclic ether structures of the morpholinecarbonyl methacrylate [24].
It is also observed that the BisGMA/novel monomer systems exhibited much narrower glass transition half peak widths as compared to BisGMA/TEGDMA. Though the BisGMA/TEGDMA and BisGMA/morpholinecarbonyl systems exhibit comparable Tgs, the glass transition half peak width is much greater for the BisGMA/TEGDMA system (100 ± 6 °C) than for the BisGMA/morpholinecarbonyl system (56 ± 5 °C). It has been shown that the broadness of the glass transition peak is directly associated with the extent of the heterogeneity of the polymer system [25]. Thus, the formulations of BisGMA with the novel methacrylates result in less heterogeneous systems. Due to heterogeneity within the material, a dental composite begins to soften well before the defined Tg. Therefore, even though the BisGMA/morpholinecarbonyl system exhibits a similar Tg to BisGMA/TEGDMA, the narrower Tg width/reduced heterogeneity indicates that this experimental system will exhibit less softening at temperatures below the defined Tg.
Flexural modulus measurements are commonly used in the evaluation of dental material formulations [22,26]. The formulations of BisGMA with morpholinecarbonyl methacrylate and phenyl carbonate were characterized by higher or comparable values of flexural modulus compared with the control formulation containing TEGDMA while also being characterized by comparable to slightly lower values of flexural strength (Table 3). However, these flexural strength values are much higher than the required standard specified in the international standard of dentistry-resin-based filling materials, (that the flexural strength should be higher than the value of [(flexural modulus×0.0025)+40] MPa, and, in any case, not less than 50 MPa) [22].
Conclusions
Highly reactive novel monomethacrylates phenyl carbonate methacrylate and morpholinecarbonyl methacrylate were evaluated in composite systems as reactive diluents to BisGMA. The BisGMA/phenyl carbonate methacrylate and BisGMA/morpholinecarbonyl methacrylate composite systems were compared to a control BisGMA/TEGDMA system for polymerization kinetics, glass transition temperature, and flexural strength and modulus. Utilizing phenyl carbonate methacrylate did not result in significant improvements in polymerization kinetics or mechanical properties. However, utilizing morpholinecarbonyl methacrylate as a reactive diluent resulted in dramatic increases in polymerization rate and extent of overall double bond conversion. Additionally, the morpholinecarbonyl methacrylate system exhibited equivalent glass transition temperature and flexural strength along with improved storage modulus and flexural modulus. Hence, highly reactive monomethacrylates such as morpholinecarbonyl methacrylate, show great promise for being utilized as reactive diluents in dental resins.
Acknowledgments
The authors acknowledge the National Science Foundation Industry University Cooperative Research Center for Fundamentals and Applications of Photopolymerizations, NIH DE grant #10959, and an NIH Ruth Kirschstein 5F32DE015906 Fellowship for Neil Cramer for funding this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferracane JL. Current trends in dental composites. Critical Reviews in Oral Biology and Medicine. 1995;6:302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 2.Peutzfeldt A. Resin composites in dentistry: The resin systems. European Journal of Oral Sciences. 1997;105:97–116. doi: 10.1111/j.1600-0722.1997.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 3.Khatri CA, Stansbury JW, Schultheisz CR, Antonucci JM. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dental Materials. 2003;19:584–588. doi: 10.1016/s0109-5641(02)00108-2. [DOI] [PubMed] [Google Scholar]
- 4.Peutzfeldt A, Asmussen E. Effect of propanal and diacetyl on quantity of remaining double bonds of chemically cured BisGMA/TEGDMA resins. European Journal of Oral Sciences. 1996;104:309–312. doi: 10.1111/j.1600-0722.1996.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 5.Linden LA, Jakubiak J. Contraction (shrinkage) in polymerization - Part II. Dental resin composites. Polimery. 2001;46:590–595. [Google Scholar]
- 6.Burke FJT, Cheung SW. Restoration longevity and analysis of reasons for the placement and replacement of restorations provided by vocational dental practitioners and their trainers in the United Kingdom. Mjor, I. A., Wilson, N. H.F. Quintessence International. 1999;30:234–242. [PubMed] [Google Scholar]
- 7.Davidson CL, Feilzer AJ. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. Journal of Dentistry. 1997;25:435–440. doi: 10.1016/s0300-5712(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 8.Stansbury JW, Antonucci JM. Evaluation of methylene lactone monomers in dental resins. Dental Materials. 1992;8:270–273. doi: 10.1016/0109-5641(92)90098-w. [DOI] [PubMed] [Google Scholar]
- 9.Nie J, Lovell LG, Bowman CN. Synthesis and characterization of N-isopropyl, N-methacryloxyethyl methacrylamide as a possible dental resin. Biomaterials. 2001;22:535–540. doi: 10.1016/s0142-9612(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 10.Labella R, Davy KWN, Lambrechts P, Van Meerbeek B, Vanherle G. Monomethacrylate co-monomers for dental resins. European Journal of Oral Sciences. 1998;106:816–824. doi: 10.1046/j.0909-8836.1998.eos106308.x. [DOI] [PubMed] [Google Scholar]
- 11.Decker C, Moussa K. A new class of highly reactive acrylic-monomers .1. Light-induced polymerization. Macromolekulare Chemie, Rapid Communications. 1990;11:159–167. [Google Scholar]
- 12.Decker C, Moussa K. Photopolymerization of multifunctional polymers. 5. Polyurethane-acrylate resins. European Polymer Journal. 1991;27:881–889. [Google Scholar]
- 13.Decker C, Moussa K. A new class of highly reactive acrylic-monomers .2. Light-induced copolymerization with difunctional oligomers. Die Macromolekulare Chemie. 1991;192:507–522. [Google Scholar]
- 14.Decker C, Moussa K. Photopolymerization of multifunctional monomers .4 Acrylates with carbamate or oxazolidone structure. European Polymer Journal. 1991;27:403–411. [Google Scholar]
- 15.Berchtold KA, Nie J, Stansbury JW, Hacioglu B, Beckel ER, Bowman CN. Novel monovinyl methacrylic monomers containing secondary functionality for ultrarapid polymerization: Steady-state evaluation. Macromolecules. 2004;37:3165–3179. [Google Scholar]
- 16.Kilambi H, Stansbury JW, Bowman CN. Deconvoluting the impact of intermolecular and intramolecular interactions on the polymerization kinetics of ultrarapid mono(meth)acrylates. Macromolecules. 2007;40:47–54. [Google Scholar]
- 17.Kilambi H, Beckel ER, Berchtold KA, Stansbury JW, Bowman CN. Influence of molecular dipole on monoacrylate monomer reactivity. Polymer. 2005;46:4735–4742. [Google Scholar]
- 18.Kilambi H, Konopka D, Stansbury JW, Bowman CN. Factors affecting the sensitivity to acid inhibition in novel acrylates characterized by secondary functionalities. Journal of Polymer Science Part A, Polymer Chemistry. 2007;45:1287–1295. [Google Scholar]
- 19.Jansen JFGA, Dias AA, Dorschu M, Coussens B. Fast monomers: Factors affecting the inherent reactivity of acrylate monomers in photoinitiated acrylate polymerization. Macromolecules. 2003;36:3861–3873. [Google Scholar]
- 20.Jansen J, Dias AA, Dorschu M, Coussens B. Effect of preorganization due to hydrogen bonding on the rate of photoinitiated acrylate polymerization. Photoinitiated Polymerization. 2003;847:127–139. [Google Scholar]
- 21.Lu H, Stansbury JW, Nie J, Berchtold KA, Bowman CN. Development of highly reactive mono-(meth)acrylates as reactive diluents for dimethacrylate-based dental resin systems. Biomaterials. 2005;26:1329–1336. doi: 10.1016/j.biomaterials.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 22.ISO/DIS4049. International organization for standardization, Dentistry-Resinbased Filling materials. 1987:1–13. [Google Scholar]
- 23.Asmussen E. Factors affecting the color stability of restorative resins. Acta Odontologica Scandinavica. 1983;41:11–18. doi: 10.3109/00016358309162298. [DOI] [PubMed] [Google Scholar]
- 24.Lemon MT, Jones MS, Stansbury JW. Hydrogen bonding interactions in methacrylate monomers and polymers. Journal of Biomedical Materials Research: Part A. 2007;83A:734–746. doi: 10.1002/jbm.a.31448. [DOI] [PubMed] [Google Scholar]
- 25.Lu H, Lovell LG, Bowman CN. Exploiting the heterogeneity of cross-linked photopolymers to create High-T-g polymers from polymerizations performed at ambient conditions. Macromolecules. 2001;34:8021–8025. [Google Scholar]
- 26.Lovell LG, Newman SM, Donaldson MM, Bowman CN. The effect of light intensity on double bond conversion and flexural strength of a model, unfilled dental resin. Dental Materials. 2003;19:458–465. doi: 10.1016/s0109-5641(02)00090-8. [DOI] [PubMed] [Google Scholar]