Abstract
Human respiratory syncytial virus (hRSV) is a pneumovirus that causes significant respiratory disease in premature and full-term infants. It was our hypothesis that a common strain of RSV, strain A2, would infect, cause pulmonary pathology, and alter respiratory epithelial innate immune responses in neonatal lambs similarly to RSV infection in human neonates. Newborn lambs between 2 and 3 days of age were inoculated intrabronchially with RSV strain A2. The lambs were sacrificed at days 3, 6, and 14 days postinoculation. Pulmonary lesions in the 6-day postinoculation group were typical of RSV infection including bronchiolitis with neutrophils and mild peribronchiolar interstitial pneumonia. RSV mRNA and antigen were detected by qPCR and immunohistochemistry, respectively with peak mRNA levels and antigen at day 6. Expression of surfactant proteins A and D, sheep beta-defensin-1 and thyroid transcription factor-1 mRNA were also assessed by real-time qPCR. There was a significant increase in surfactant A and D mRNA expression in RSV-infected animals at day 6 postinoculation. There were no significant changes in sheep beta-defensin-1 and thyroid transcription factor-1 mRNA expression. This study shows that neonatal lambs can be infected with RSV strain A2 and the pulmonary pathology mimics that of RSV infection in human infants thereby making the neonatal lamb a useful animal model to study disease pathogenesis and therapeutics. RSV infection induces increased expression of surfactant proteins A and D in lambs, which may also be an important feature of infection in newborn infants.
Keywords: defensin, innate immunity, pulmonary, respiratory syncytial virus, surfactant protein, thyroid transcription factor
Respiratory syncytial virus (RSV) is a pneumovirus that causes severe respiratory disease in infants and recurrent upper airway tract infections in older children and adults. Approximately 75,000–125,000 hospitalizations in the United States are attributed to RSV-induced bronchiolitis or pneumonia (Shay et al. 1999). Populations at increased risk for severe disease or death include premature infants, elderly adults and individuals with respiratory, cardiac or immune compromise (Welliver 2003; Murata & Falsey 2007). There are many factors both host-related and environmental that appear to be involved in the increased risk for premature infants (Welliver 2003).
The innate immune system initiates the first line of defence against pathogens through phagocytosis and secretion of inflammatory and antimicrobial mediators. Epithelial and phagocytic cells produce and secrete antimicrobial peptides and surfactant proteins that complement and synergize with inflammatory and chemotactic mediators (Bals & Hiemstra 2004). Epithelial cells are especially integral as they are infected by the virus that activates receptors such as retinoic acid-induced gene 1 (RIG-1), which induces epithelial cell activation.
Lesions associated with human RSV infection include bronchiolitis, interstitial pneumonia and consolidation resulting in coughing and wheezing (Gilca et al. 2006). Histologically, patients with RSV have bronchioles that are obstructed by mucus, fibrin and sloughed epithelial cells and neutrophils (Johnson et al. 2007). Previously, our laboratory has shown that preterm lambs infected with bovine respiratory syncytial virus (bRSV) develop clinical responses (coughing, temperature, increased respiratory rates) and lesions that parallel those of human disease. We have also demonstrated a correlation between lamb age and disease susceptibility with more severe disease demonstrated in younger, preterm lambs, which is similar to increased severity of RSV infection seen in human preterm infants (Welliver 2003; Meyerholz et al. 2004).
The pulmonary development and cellular composition of the neonatal lamb lung are similar to that of human infants. Alveologenesis begins prenatally in both humans and lambs, in contrast to the postnatal alveolar development in mice and rodents (Langston et al. 1984; Scheuermann et al. 1988; Flecknoe et al. 2003). In addition, epithelial cells of the airways, distal bronchioles and alveoli in lambs are similar to those of humans; in comparison, mouse lung has a large population of Clara cells in bronchiolar airways (Pack et al. 1981; Mariassy & Plopper 1983). Sheep have been used to model a variety of human pulmonary diseases including asthma, pulmonary hypertension, cystic fibrosis, chronic obstructive pulmonary disease, congenital diaphragmatic hernia and numerous others (Davey et al. 2005; Abraham 2008; Scheerlinck et al. 2008).
In this study, we hypothesized that a common strain of human RSV, strain A2, would infect neonatal lambs and cause pathology similar to human neonates. In addition to characterizing the pulmonary pathology we examined the duration of infection to determine the time of peak viral lesions and time at which clearance was obtained. We hypothesized that the virus would alter expression of respiratory epithelial innate immune genes known to have anti-RSV activity including surfactant proteins A (SP-A) and D (SP-D) and sheep beta-defensin-1 (SBD-1). Expression of thyroid transcription factor-1 (TTF-1), a key SP-A transcription factor, was also measured.
Materials and methods
Experimental design
Animal use and experimental procedures were approved by Iowa State University’s Animal Care and Use Committee. Neonatal lambs (2–3 days of age) were randomly assigned to two groups, a control group (n = 10) or RSV inoculated group (n = 18). Lambs were anaesthetized with an intramuscular injection of xylazine (0.1 mg/kg), placed in right lateral recumbency and inoculated with human respiratory syncytial virus (hRSV), strain A2 (5 ml of 2 × 107 pfu/ml RSV) via fibreoptic bronchoscope in the right terminal bronchus followed by a sterile saline flush (10 ml). Control animals were inoculated with cell growth media (5 ml) without the virus followed by a sterile saline flush (10 ml). RSV (A2 strain) was a gift from Barney S. Graham (National Institutes of Health; NIH, Bethesda, MD, USA) and was grown in HEp-2 cells (American Type Culture Collection; ATCC, Manassas, VA, USA). Lambs were given daily antibiotics (ceftiofur, 2.2 mg/kg, intramuscular) to prevent bacterial complications. Lambs were monitored for clinical signs of respiratory disease (coughing and wheezing) and daily temperatures were measured. Animals were euthanized by sodium pentobarbital on days 3 (control n = 3, RSV n = 6), 6 (control n = 4, RSV n = 6) and 14 postinoculation (control n = 3, RSV n = 6).
Tissue
The thorax was opened and the lungs were examined for gross lesions. The lungs were then removed for tissue collection. In all animals, tissue samples were taken at the same location of the right cranial, middle and caudal lobes and left cranial lung lobe. Samples were placed in cassettes and then in 10% neutral-buffered formalin for histological and immunohistochemical analysis. Additional samples were taken from these sites and snap-frozen on dry ice for real-time quantitative PCR (Meyerholz et al. 2004).
One-step real-time qPCR
Total RNA was isolated from whole lung tissue (affected areas as determined grossly or by immunohistochemistry for RSV antigen) via Trizol according to manufacturer’s guidelines (Invitrogen, Carlsbad, CA, USA). RNA samples were assessed by spectrophotometry and DNase treated (Ambion, Austin, TX, USA, TURBO DNase). Real-time quantitative PCR (qPCR) was carried out as a fluorogenic one-step process in a GeneAmp 5700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using previously described methods. Primer and probe sequences used in our laboratory have been previously described (Hu et al. 2003; Kawashima et al. 2006) (Table 1). All samples were run in duplicate and each target gene amplification was converted to a relative quantity and normalized to the geometric mean of two housekeeping genes, hRibo18S and ovRPS15 (Gallup & Ackermann 2006).
Table 1.
Primers and probe sequences for ovine gene expression assessed by real-time qPCR
| hRSV | Fwd: | 5′-GCTCTTAGCAAAGTCAAGTTGAATGA |
| Rev: | 5′-TGCTCCGTTGGATGGTGTATT | |
| Probe: | 5′-6FAM-ACACTCAACAAAGATCAACTTCTGTCATCCAGC-TAMRA | |
| SP-A | Fwd: | 5′-TGACCCTTATGCTCCTCTGGAT |
| Rev: | 5′-GGGCTTCCAAGACAAACTTCCT | |
| Probe: | 5′-6FAM-TGGCTTCTGGCCTCGAGTGCG-TAMRA | |
| SP-D | Fwd: | 5′-ACGTTCTGCAGCTGAGAAT |
| Rev: | 5′-TCGGTCATGCTCAGGAAAGC | |
| Probe: | 5′-6FAM-TTGACTCAGCTGGCCACAGCCCAGAACA-TAMRA | |
| SBD-1 | Fwd: | 5′-CCATAGGAATAAAGGCGTCTGTGT |
| Rev: | 5′-CGCGACAGGTGCCAATCT | |
| Probe: | 5′-6FAM-CCGAGCAGGTGCCCTAGACACATGA-TAMRA | |
| TTF-1 | Fwd: | 5′-TCCCAGGCGCAGGTGTAT |
| Rev: | 5′-CGGACAGGTACTTCTGCTGCTT | |
| Probe: | 5′-6FAM-AGCTGGAGCGACGCT-MGBNFQ | |
| hRibo18S (18S rRNA) | Fwd: | 5′-CGGCTACCACATCCAAGGAA |
| Rev: | 5′-GCTGGAATTACCGCGGCT | |
| Probe: | 5′-VIC-TGCTGGCACCAGACTTGCCCTC-TAMRA | |
| ovRiboProteinS15 | Fwd: | 5′-CGAGATGGTGGGCAGCAT |
| Rev: | 5′-GCTTGATTTCCACCTGGTTGA | |
| Probe: | 5′-VIC-CCGGCGTCTACAACGGCAAGACC-TAMRA |
6FAM or VIC, 5′ Fluorescent reporter dye; TAMRA, Fluorescent quencher dye; MGBNFQ, Minor groove binding non-fluorescent quencher.
Histological examination
The lung histological lesion score was determined by the percentage of parenchymal consolidation of each section of lung and immunohistochemical antigen staining for RSV. The scoring scale for the alveolar consolidation was: 0, no consolidation; 1, <30%; 2, 30–60%; 3, >60%. The scoring scale for the RSV antigen staining was: 0, no staining; 1, 1–5 positive cells; 2, 6–10 positive cells; 3, >10 positive cells. All evaluations were made based on the average of 10, 20X fields per section and four sections per lung per animal. Group averages were calculated for the RSV antigen score and the alveolar consolidation score.
Immunohistochemistry
Immunohistochemistry for RSV antigen was performed on paraffin-embedded tissue as described previously (Meyerholz et al. 2004). Briefly, sections were cut at 5 μm thickness onto positively charged slides. Following routine deparaffinization, sections were treated with Pronase E (Protease Type XIV from Streptomyces griseus, Sigma, St Louis, MO, USA) for 12 min at 37°C. Non-specific binding was blocked by incubation in 20% normal swine serum. The sections were incubated with polyclonal goat anti-RSV antibody (BioDesign/Meridian, Saco, ME, USA) overnight at a concentration of 1:50 with 5% normal swine serum. The slides were rinsed and then incubated with biotinylated rabbit anti-goat secondary antibody (KPL, Inc, Gaithersburg, MD, USA) at a concentration of 1:300 in 5% normal sheep serum. A peroxidase block with 3% peroxide was followed by incubation with peroxidase-conjugated streptavidin (BioGenex, San Ramon, CA, USA) for 45 min. After two PBS washes, the colour was developed with Nova Red. The slides were then counterstained with Harris’ haematoxylin, dehydrated and cover-slipped.
Statistical analysis
Data are expressed as mean values ± SEM. For the temperature data, the group mean values on each day were analysed for significance (RSV-infected vs. control) using unpaired Student’s t-tests. The histological lesion score data was analysed using the Wilcoxon–Mann–Whitney test. The qPCR data was log-transformed to stabilize variances across mean values and bring data to a normal distribution. A two-way anova (unbalanced design) was used to test for significant effects of treatments (control vs. RSV-infected), groups (3, 6, and 14 days postinoculation time-points), and their interaction. A two-sample t-test was used to compare target genes expression at postinoculation time-points. The Pearson product–moment correlation coefficient was used to evaluate relationships between hRSV levels and expression of various target genes.
Results
Clinical and postmortem findings
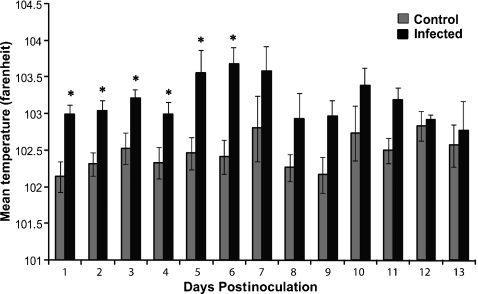
RSV-infected lambs had significantly higher temperatures than control animals at days 1–6 postinoculation (Figure 1, P< 0.05). Clinically, three of the six lambs in the 6-day postinoculation group developed a slight-to-moderate cough on days 4 and 5 postinoculation. Two of the six lambs in the 14-day postinoculation group developed a moderate cough on day 5 postinoculation with resolution of the cough at day 10. At postmortem examination, gross lesions were characterized by multifocal to locally extensively reddened areas of pulmonary consolidation, which varied in severity from moderate to severe. In the 6-day postinoculation group, five of the six infected animals had gross lesions. No gross lesions were present in the 3- and 14-day postinoculation group.
Figure 1.
Average body temperatures by control and RSV-infected lambs. RSV-infected lambs had significant increases in body temperature (*P < 0.05). Values are expressed as mean values ± SD.
Histology and immunohistochemistry
Histologically, RSV-induced changes were characterized by mild-to-moderate suppurative bronchiolitis with sloughed epithelial cells, cellular debris and neutrophils in the lumen of the medium to small airways at days 3 and 6. Also present was a mild-to-moderate peribronchiolar mononuclear interstitial pneumonia containing lymphocytes and plasma cells and locally extensive areas of alveolar consolidation (Figure 2a,b). In the 3-day postinoculation group, all six lambs inoculated with RSV had histological changes consistent with RSV infection. In the 6-day postinoculation group, five of the six RSV-inoculated lambs had histological changes associated with RSV virus. In the 14-day postinoculation group, three of the six RSV-inoculated lambs had minimal changes characterized by multifocal small areas of alveolar consolidation.
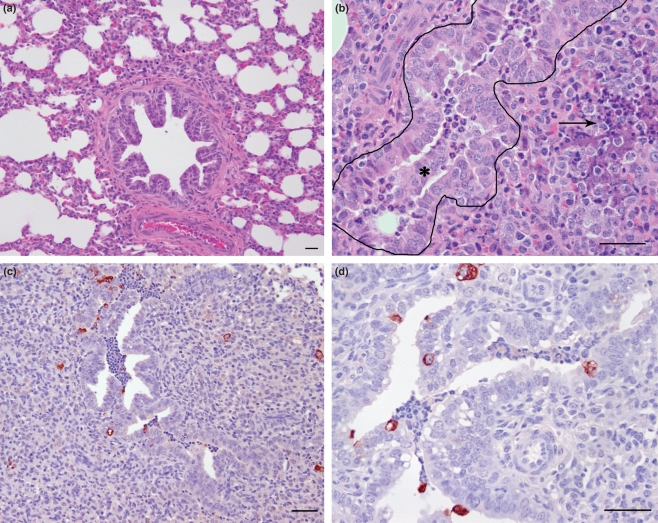
Figure 2.
(a) Lung from a control lamb not infected with RSV that contains a bronchiole surrounded by non-collapsed alveoli with a central lumen. (b) Lung from a lamb 6 days postinoculation with RSV. Within the lumen of the bronchiole (outlined) are sloughed epithelial cells mixed with degenerate neutrophils (*). The alveoli surrounding the bronchiole are collapsed with accumulation of degenerate neutrophils and areas of necrosis (arrow). (c) and (d) Immunohistochemistry for RSV antigen on lung tissue from a lamb 6 days postinoculation with RSV, in which there is immunoreactivity within the bronchi, epithelial cells lining the bronchi, and the syncytial cells in areas of alveolar consolidation. Bars = 25 μm.
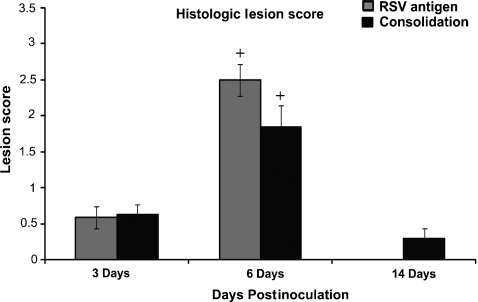
Immunoreactivity for RSV antigen was present within the slonghed epithelial cells in the airway, bronchiolar epithelium and the syncytial cells in consolidated areas in the 3- and 6-day postinoculation groups (Figure 2c,d). Immunoreactivity to RSV antigen was not present in the 14-day postinoculation group. A histological score was calculated for RSV immunoreactivity and alveolar consolidation for each group (Figure 3). Animals in the 6-day RSV postinoculation group had significantly higher scores for RSV immunoreactivity and alveolar consolidation as compared to the 3-day RSV postinoculation group (P < 0.05). There was a significant decrease in both lesion scores in the 14-day RSV postinoculation group (P < 0.05).
Figure 3.
Histological lesion score based on RSV antigen staining and alveolar consolidation of RSV-infected animals at 3, 6, and 14 days postinoculation. RSV antigen staining and alveolar consolidation scores were highest at day 6 postinoculation. Values are expressed as mean values ± SD. +P< 0.05 vs. other time points.
Epithelial innate immune gene expression
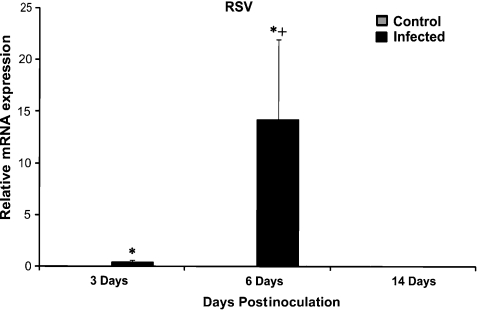
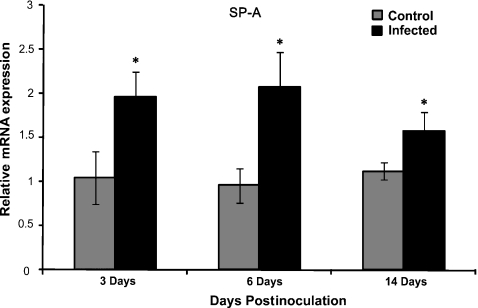
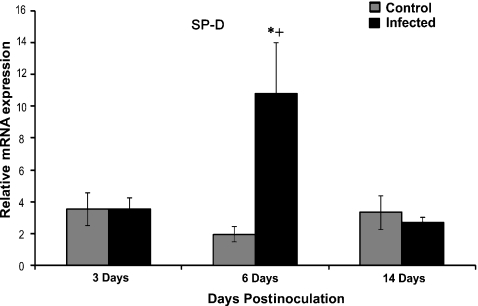
Expression of RSV, SP-A, SP-D, SBD-1 and TTF-1 were measured by qPCR. The analysis showed significant increase in RSV mRNA from day 3 to day 6 postinoculation (P < 0.01) and a significant decrease from day 6 to 14 postinoculation (P < 0.01) (Figure 4). The analysis showed statistically significant differences in SP-A and SP-D levels between infected and control groups. There was a significant (P < 0.05) increase in expression of SP-A between RSV-infected and control animals in all three groups. However, there was no significant difference in expression of SP-A in the RSV-infected lambs between different postinoculation time-points (Figure 5). There was a significant increase in expression of SP-D between 3-day and 6-day postinoculation animals and a significant decrease between 6-day and 14-day postinoculated animals (Figure 6, P < 0.03). There was a significant difference between control and infected animals in the 6-day postinoculation group (P < 0.03). In addition, there was a correlation between RSV levels and SP-D expression in infected animals. There were no significant differences in SBD-1 and TTF-1 expression between the infected and non-infected groups.
Figure 4.
Comparison of mRNA levels of RSV between groups. There was a significant increase in the expression of viral mRNA between RSV-infected vs. control animals at 3 and 6 days postinoculation (*P < 0.01). There was a significant increase in expression of RSV between RSV-infected animals at 6 days postinoculation vs. other time points (+P < 0.01). Control animals lack RSV mRNA. Values are expressed as mean values ± SD.
Figure 5.
Comparison of mRNA levels of SP-A between groups. There was a significant increase in expression of SP-A between RSV-infected vs. control animals (*P< 0.05) at all postinoculation time points. There was no significant difference in expression of SP-A in the RSV-infected lambs between different postinoculation time-points. Values are expressed as mean values ± SD.
Figure 6.
Comparison of mRNA levels of SP-D between groups. There was a significant increase in expression of SP-D between RSV-infected vs. control animals at the 6-day postinoculation time point (*P < 0.03). There was a significant increase in expression of SP-D between RSV-infected animals at 6 days postinoculation compared to other time points (+P < 0.03). There was a correlation between RSV levels and SP-D expression in infected animals (r = 0.60, P < 0.008). Values are expressed as mean values ± SD.
Discussion
Neonatal lambs infected with human respiratory syncytial virus (hRSV), strain A2 develop lesions consistent with those seen in human infant RSV infection (Johnson et al. 2007; Welliver et al. 2007). Histologically, bronchiolitis was present characterized by infiltration of neutrophils and macrophages into the bronchiolar lumen admixed with degenerate epithelial cells and cellular debris. In addition, there were multifocal to locally extensive areas of alveolar consolidation with numerous syncytial cells and infiltrates of lymphocytes and plasma cells at days 6 and 14. Pulmonary lesions are also similar to those that occur experimentally in lambs with bRSV and in natural spontaneous lesions of bRSV-infected cattle (Lehmkuhl & Cutlip 1979). Pulmonary pathology was most severe at 6 days postinoculation at which time animals exhibited cough and high temperatures. By 14 days postinoculation, lambs had almost complete resolution given the lack of gross lesions, histological changes and immunoreactivity for RSV antigen at this time point. The infection and disease progression correlates to a previous study of experimental RSV infection in lambs in which infected lambs showed clinical signs associated with RSV infection; however in that study, tissue collection was carried out 4 weeks following inoculation, therefore no histological changes were present (Lapin et al. 1993).
The perinatal lamb has several key features consistent with human infant RSV infection and pulmonary development that make it a very good animal model. As shown here, lambs develop histological lesions similar to human infection. Robust RSV pathology is lacking in many rodent models of human RSV infection thereby making the ovine model more attractive (Kong et al. 2005). These lesions represent a moderate human infection such that resolution is possible, which commonly occurs in infants. The severity of pneumonia in this model can likely be enhanced by increasing the viral density and volume of viral inoculum.
Associated with gross lesions were significant increases in expression of surfactant proteins A and D (Figures 5 and 6). The increase in SP-A expression is similar to studies with lambs inoculated with bRSV and indicates a cellular response to the virus (Kawashima et al. 2006). Both SP-A and SP-D have anti-RSV activity, which include viral opsonization and activation of macrophages, which are thought to play a critical role in RSV clearance (Hickling et al. 2004; Sano & Kuroki 2005). In severe human RSV infection, SP-A protein levels are decreased (Kerr & Paton 1999). Our previous work has shown that paramyxoviral infection in sheep increases SP-A gene expression but does not significantly increase SP-A protein levels (Grubor et al. 2004). Human individuals with deleterious polymorphisms in the SP-A gene show increased severity of RSV infection – indicating the importance of SP-A in viral clearance and demonstrating the importance of this protein clinically (Lahti et al. 2002). Moreover, SP-D enhances RSV macrophage uptake and plays a role in modulating the immune response. Mice deficient in SP-D exhibit impaired RSV clearance and an increased neutrophilic response and human SP-D polymorphisms are associated with either increased severity of RSV or protection against severe infection (Lahti et al. 2002; LeVine et al. 2004; Pastva et al. 2007).
Beta defensin expression in the lung is developmentally regulated in both humans and sheep (Starner et al. 2003; Meyerholz et al. 2006). Beta defensins are produced by pulmonary epithelial cells and leucocytes and have antimicrobial properties (Schutte & McCray 2002; Hickling et al. 2004). In this study, SBD-1 expression was not significantly altered by RSV infection. In humans, HBD-1 is constitutively produced in the lung and is not inducible by pro-inflammatory mediators (Starner et al. 2005). This may be similar in the ovine and explain the lack of up-regulation of SBD-1, however, in previous studies, SBD-1 was up-regulated with parainfluenza virus (PIV-3) indicating that under certain conditions this gene may be inducible (Grubor et al. 2004).
Thyroid transcription factor-1 is a nuclear transcription factor that is most prevalent in the type II epithelial cells in the alveolus in the perinatal lung. TTF-1 binds to regulatory promoter elements of surfactant protein A in addition to other surfactant proteins (DeFelice et al. 2003). TTF-1 mRNA levels did not increase during RSV infection despite the increases in SP-A mRNA levels. This may indicate that other regulatory factors are involved or that the duration in which there is up-regulation of the transcription factor is at an earlier time-point prior to our sample collection (e.g. day 3). The regulation of surfactant protein expression is complex involving multiprotein signaling complexes. TTF-1 interacts with many other transcription factors and co-factors such as FOXa2, GATA-6, AP-1, C/EBPα, NFATc3 and retinoic acid receptors (Dave et al. 2004; Besnard et al. 2007). It is also possible that SP-A up-regulation could be downstream to RIG-1 activation.
We have previously shown that preterm lambs infected with bRSV have more severe RSV lesions than full-term lambs (Meyerholz et al. 2004). We expect that infection of preterm lambs with this human strain of RSV would similarly have increased lesion severity similar to human premature infant disease.
This study shows that the pulmonary pathology of RSV in neonatal lambs is similar to a moderate human infant RSV infection. This is the first time that the pulmonary epithelial innate immune response has been characterized in the neonatal lamb model of human RSV. In this study, RSV infection up-regulated surfactant proteins A and D expression but did not alter the expression of sheep beta-defensin-1 or thyroid transcription factor. Future work in our laboratory using this model will elucidate other key roles of epithelial cells in RSV infection and allow a useful model for therapeutic trials.
Acknowledgments
This work was funded, in part, by J.G. Salsbury Endowment, NIH NIAID grant RO1 AI062787 (to MA), RO1 AI063520 (to SMV).
References
- Abraham WM. Modeling of asthma, COPD and cystic fibrosis in sheep. Pulm. Pharmacol. Ther. 2008;21:743–754. doi: 10.1016/j.pupt.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- Besnard V, Xu Y, Whitsett JA. Sterol response element binding protein and thyroid transcription factor-1 (Nkx2.1) regulate Abca3 gene expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L1395–L1405. doi: 10.1152/ajplung.00275.2007. [DOI] [PubMed] [Google Scholar]
- Dave V, Childs T, Whitsett JA. Nuclear factor of activated T cells regulates transcription of the surfactant protein D gene (Sftpd) via direct interaction with thyroid transcription factor-1 in lung epithelial cells. J. Biol. Chem. 2004;279:34578–34588. doi: 10.1074/jbc.M404296200. [DOI] [PubMed] [Google Scholar]
- Davey MG, Biard JM, Robinson L, et al. Surfactant protein expression is increased in the ipsilateral but not contralateral lungs of fetal sheep with left-sided diaphragmatic hernia. Pediatr. Pulmonol. 2005;39:359–367. doi: 10.1002/ppul.20175. [DOI] [PubMed] [Google Scholar]
- DeFelice M, Silberschmidt D, DiLauro R, et al. TTF-1 phosphorylation is required for peripheral lung morphogenesis, perinatal survival, and tissue-specific gene expression. J. Biol. Chem. 2003;278:35574–35583. doi: 10.1074/jbc.M304885200. [DOI] [PubMed] [Google Scholar]
- Flecknoe SJ, Wallace MJ, Cock ML, Harding R, Hooper SB. Changes in alveolar epithelial cell proportions during fetal and postnatal development in sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L664–L670. doi: 10.1152/ajplung.00306.2002. [DOI] [PubMed] [Google Scholar]
- Gallup JM, Ackermann MR. Addressing fluorogenic real-time qPCR inhibition using the novel custom Excel file system ‘FocusField2-6GallupqPCRSet-upTool-001’ to attain consistently high fidelity qPCR reactions. Biol. Proced. Online. 2006;8:87–152. doi: 10.1251/bpo122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilca R, De Serres G, Tremblay M, et al. Distribution and clinical impact of human respiratory syncytial virus genotypes in hospitalized children over 2 winter seasons. J. Infect. Dis. 2006;193:54–58. doi: 10.1086/498526. [DOI] [PubMed] [Google Scholar]
- Grubor B, Gallup JM, Meyerholz DK, et al. Enhanced surfactant protein and defensin mRNA levels and reduced viral replication during parainfluenza virus type 3 pneumonia in neonatal lambs. Clin. Diagn. Lab. Immunol. 2004;11:599–607. doi: 10.1128/CDLI.11.3.599-607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickling TP, Clark H, Malhotra R, Sim RB. Collectins and their role in lung immunity. J. Leukoc. Biol. 2004;75:27–33. doi: 10.1189/jlb.0703304. [DOI] [PubMed] [Google Scholar]
- Hu A, Colella M, Tam JS, Rappaport R, Cheng SM. Simultaneous detection, subgrouping, and quantitation of respiratory syncytial virus A and B by real-time PCR. J. Clin. Microbiol. 2003;41:149–154. doi: 10.1128/JCM.41.1.149-154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Meyerholz DK, Gallup JM, et al. Differential expression of ovine innate immune genes by preterm and neonatal lung epithelia infected with respiratory syncytial virus. Viral Immunol. 2006;19:316–323. doi: 10.1089/vim.2006.19.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MH, Paton JY. Surfactant protein levels in severe respiratory syncytial virus infection. Am. J. Respir. Crit. Care Med. 1999;159:1115–1118. doi: 10.1164/ajrccm.159.4.9709065. [DOI] [PubMed] [Google Scholar]
- Kong X, Hellermann GR, Patton G, et al. An immunocompromised BALB/c mouse model for respiratory syncytial virus infection. Virol J. 2005;2:3. doi: 10.1186/1743-422X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti M, Lofgren J, Marttila R, et al. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatr. Res. 2002;51:696–699. doi: 10.1203/00006450-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Langston C, Kida K, Reed M, Thurlbeck WM. Human lung growth in late gestation and in the neonate. Am. Rev. Respir. Dis. 1984;129:607–613. [PubMed] [Google Scholar]
- Lapin CD, Hiatt PW, Langston C, Mason E, Piedra PT. A lamb model for human respiratory syncytial virus infection. Pediatr. Pulmonol. 1993;15:151–156. doi: 10.1002/ppul.1950150305. [DOI] [PubMed] [Google Scholar]
- Lehmkuhl HD, Cutlip RC. Experimentally induced respiratory syncytial viral infection in lambs. Am. J. Vet. Res. 1979;40:512–544. [PubMed] [Google Scholar]
- LeVine AM, Elliott J, Whitsett JA, et al. Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 2004;31:193–199. doi: 10.1165/rcmb.2003-0107OC. [DOI] [PubMed] [Google Scholar]
- Mariassy AT, Plopper CG. Tracheobronchial epithelium of the sheep: I. Quantitative light-microscopic study of epithelial cell abundance, and distribution. Anat. Rec. 1983;205:263–275. doi: 10.1002/ar.1092050304. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Grubor B, Fach SJ, et al. Reduced clearance of respiratory syncytial virus infection in a preterm lamb model. Microbes Infect. 2004;6:1312–1319. doi: 10.1016/j.micinf.2004.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Kawashima K, Gallup JM, Grubor B, Ackermann MR. Expression of select immune genes (surfactant proteins A and D, sheep beta defensin 1, and toll-like receptor 4) by respiratory epithelia is developmentally regulated in the preterm neonatal lamb. Dev. Comp. Immunol. 2006;30:1060–1069. doi: 10.1016/j.dci.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Falsey AR. Respiratory syncytial virus infection in adults. Antivir. Ther. 2007;12:659–670. [PubMed] [Google Scholar]
- Pack RJ, Al-Ugaily LH, Morris G. The cells of the tracheobronchial epithelium of the mouse: a quantitative light and electron microscope study. J. Anat. 1981;132:71–84. [PMC free article] [PubMed] [Google Scholar]
- Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc. Am. Thorac. Soc. 2007;4:252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Kuroki Y. The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Mol. Immunol. 2005;42:279–287. doi: 10.1016/j.molimm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Scheerlinck JP, Snibson KJ, Bowles VM, Sutton P. Biomedical applications of sheep models: from asthma to vaccines. Trends Biotechnol. 2008;26:259–266. doi: 10.1016/j.tibtech.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Scheuermann DW, Van Meir F, Adriaensen D, Timmermans JP, De Groodt-Lasseel MH. Development of alveolar septa and formation of alveolar pores during the early postnatal period in the rat lung. Acta Anat. (Basel) 1988;131:249–261. doi: 10.1159/000146524. [DOI] [PubMed] [Google Scholar]
- Schutte BC, McCray PB., Jr [beta]-defensins in lung host defense. Annu. Rev. Physiol. 2002;64:709–748. doi: 10.1146/annurev.physiol.64.081501.134340. [DOI] [PubMed] [Google Scholar]
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- Starner TD, Barker CK, Jia HP, Kang Y, McCray PB., Jr CCL20 is an inducible product of human airway epithelia with innate immune properties. Am. J. Respir. Cell Mol. Biol. 2003;29:627–633. doi: 10.1165/rcmb.2002-0272OC. [DOI] [PubMed] [Google Scholar]
- Starner TD, Agerberth B, Gudmundsson GH, McCray PB., Jr Expression and activity of beta-defensins and LL-37 in the developing human lung. J. Immunol. 2005;174:1608–1615. doi: 10.4049/jimmunol.174.3.1608. [DOI] [PubMed] [Google Scholar]
- Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J. Pediatr. 2003;143:S112–S117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- Welliver TP, Garofalo RP, Hosakote Y, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]