Abstract
The incidence of fungal infections has increased significantly over the past decades. Very often these infections are associated with biofilm formation on implanted biomaterials and/or host surfaces. This has important clinical implications since fungal biofilms display properties that are dramatically different from planktonic (free-living) populations, including increased resistance to antifungal agents. Here we describe a rapid and highly reproducible 96 well microtiter-based method for the formation of fungal biofilms which is easily adaptable for antifungal susceptibility testing. This model is based on the ability of metabolically active sessile cells to reduce a tetrazolium salt (XTT) to water-soluble orange formazan compounds, the intensity of which can then be determined using a microtiter-plate reader. The entire procedure takes approximately two days to complete. This technique simplifies biofilm formation and quantification, making it more reliable and comparable among different laboratories, a necessary step towards the standardization of antifungal susceptibility testing of biofilms.
INTRODUCTION
Fungal infections caused by yeasts and moulds represent an escalating problem in health care as advances in modern medicine prolong the lives of severely ill patients, including HIV-infected, cancer, transplant, surgical and ICU patients, but also newborn infants. Use of broad spectrum antibiotics, neutropenia, parenteral nutrition, indwelling catheters, immunosupression and disruption of mucosal barriers due to surgery, chemotherapy and radiotherapy represent the most important predisposing factors for these infections. Candida spp., Aspergillus spp. and Cryptococcus neoformans are among the most common etiologic agents of fungal infections 1, 2. Fungi being eukaryotic cells and more complex than bacteria cause infections that are often difficult to diagnose and treat, and carry unacceptably high mortality rates 3.
Most microbiology investigations have traditionally used free living (planktonic) cells in pure-culture resulting in the common perception that microorganisms are independent unicellular life forms. However, in their natural ecosystems, most microbes exist as attached communities of cells within an organized biofilm and rarely as planktonic organisms 4. Thus, a biofilm is defined as a surface-associated and highly structured community of microorganisms that are enclosed within a protective extracellular matrix. Microbial biofilms can form in nature but also inside a host, and in recent years there has been an increased appreciation of the role that microbial biofilms play in human medicine: it is now estimated that about 65% of all human infections have a biofilm etiology 5.
Many different groups of investigators have demonstrated that fungal biofilms show increased levels of resistance against various classes of antifungal drugs, most notably azoles and polyenes 6. This resistance is likely multifactorial and, among other mechanisms, may be due to i) high cellular density within the biofilm; ii) the protective effect of the biofilm exopolymeric material; iii) differential expression of genes linked to resistance, including those encoding efflux pumps; and iv) presence of a subpopulation of “persister” cells. However, newer antifungal agents, such as the echinocandins and liposomal formulations of amphotericin B, display increased efficacy against fungal biofilms 7, 8. Of note, the commonly used Clinical and Laboratory Standards Institute (CLSI) broth microdilution techniques for antifungal susceptibility testing are based on the use of planktonic populations and will not enable prediction of the drugs’ efficacy against fungal biofilms 9. The increased levels of resistance typically associated with biofilms underscores the importance of developing standardized assays to test biofilm antifungal susceptibilities and to thereby systematically determine the effectiveness of different antifungal agents and regimens against fungal biofilms.
Traditionally, most models for the formation of microbial biofilms, including those formed by fungal species, are cumbersome, requiring expert handling, longer processing times and the use of specialized equipment not generally available in a regular microbiology laboratory. In the case of fungal biofilms, models used by different groups of investigators include the use of catheter disks, sheets and tubing from a variety of materials normally placed inside some type of sterile receptacle, glass and plastic slides, a perfused biofilm fermentor, microfermentors, cylindrical cellulose filters, acrylic strips and discs, germanium substratum, tissue culture flasks, syringes, modified Robbins devices, the Calgary biofilm device, the CDC reactor, etc., also including both biofilms formed under static and flow-through conditions 10–21. Perhaps with the exception of the Calgary biofilm device, most of these models are complex, technically demanding and generally not amenable to high throughput screening since relatively few equivalent biofilms can be produced at the same time 6. Here, we describe a rapid and robust 96 well microtiter plate model for the formation of fungal biofilms. This technique involves formation of multiple equivalent fungal biofilms on the bottom of wells of microtiter plates, coupled with a colorimetric method that measures the metabolic activities of cells within the biofilm based on the reduction of 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT). Upon processing by metabolically active cells, the XTT yields a water-soluble formazan colored product that can be measured spectrophotometrically in a microtiter plate reader (Fig. 1). We (and others) have previously shown that the XTT-reduction assay shows excellent correlation between cellular density and metabolic activity, thus providing a semiquantitative measurement of biofilm formation 22–26. This colorimetric assay is non-invasive and non-destructive, requiring minimal post-processing of samples as compared to other alternative methods (such as viable cell counts) and has the additional advantage that, in contrast to other methods such as crystal violet staining and dry mass measurements, it correlates with cell viability which is particularly useful for measuring the effects of drugs on biofilm cells. However, some caveats and limitations about the use of this reagent have been noted 27. For example, results using the XTT-colorimetric assay to compare biofilm-forming ability by different isolates need to be interpreted with caution since it has been reported that different fungal species and even strains from the same species show marked differences in their ability to metabolize the XTT substrate 27. In addition, alterations in the metabolic states during the different phases of biofilm formation may lead to fluctuations in the ability of cells within the biofilms to metabolize this dye 23, 27. Some more recent articles indicate that the use of other vital stains (SYTO9, propidium iodide), fluorogenic assays or bioluminescence may represent alternatives and have some practical advantages over the use of XTT 23, 24, 28. This 96 well microtiter plate model for biofilm formation was originally developed for C. albicans and other Candida spp. 25 but more recently has successfully been adapted for the formation of biofilms by other biofilm-forming fungal species of clinical interest, such as C. neoformans and A. fumigatus 29, 30. Overall this microtiter plate-based model of biofilm formation offers a simple, flexible, relatively inexpensive, accurate and reproducible alternative for biofilm formation that is compatible with the widely available 96 well microplate platform. It can be used to examine multiple parameters and factors influencing biofilm formation, to estimate the biofilm-forming ability of multiple fungal isolates and mutant strains, and more importantly for the purpose of these studies, it can be easily adapted for antifungal susceptibility testing of fungal biofilms following the procedures described below 25, 31, 32.
Figure 1.
A flow chart diagram summarizing the different steps of the procedure.
Experimental design
Fungal isolates
C. albicans SC5314, A. fumigatus AF293 (NCPF 7367, CBS 101355) and C. neoformans H99 (serotype A) are all type strains that were used initially to optimize parameters of biofilm formation. Importantly, these are also the strains that were used for the corresponding genome sequencing projects of each fungal species, and also represent parental strains used for the construction of a variety of mutants. These methods have been used to assess the biofilm-forming ability and antifungal susceptibility profiles of different clinical isolates of the three species and mutant strains, as well as representative isolates of other Candida spp. Stock cultures of these strains are typically prepared on Sabouraud dextrose agar plates or slopes and stored at 4 °C. For long term storage, glycerol stocks and − 70°C are recommended.
Optimization of conditions
These procedures have already been optimized for each of the fungal species described here. Hence, we recommend that readers follow our suggested protocol, at least initially. On the basis of results obtained from their first experiments, readers can then try to optimize certain parameters as they apply to their own experimental design or particularities of their model (i.e. testing of clinical isolates, testing of different fungal species, testing biofilm-forming ability by mutant strains, testing different antifungal agents, etc.).
MATERIALS
REAGENTS
Sabouraud-dextrose agar (Becton Dickinson, cat. no. 211584), to prepare plates or slants for maintenance and short term storage of fungalisolates.
YPD: Yeast peptone dextrose (1% w/v yeast extract, 2% w/v peptone, 2% w/v dextrose, 1.5% agar) (US Biological, cat. no. Y2076) liquid medium for propagation of fungal cultures.
RPMI-1640 without sodium bicarbonate supplemented with L-glutamine (Cellgro, cat. no. 50–020-PB) and buffered with 165 mM morpholinepropanesulfonic acid (Fisher, cat. no. BP308) to pH 7. From now on this medium will be referred to simply as RPMI 1640.
Dulbecco’ Modified Eagle’s Medium (DMEM, Cellgro, cat. no. 10-013-CV)
Phosphate buffered saline, PBS (10 mM phosphate buffer, 2.7 mM potassium chloride, 137 mM sodium chloride, pH 7.4) (Sigma, cat. no. P4417)
Tween-20 (Bio-Rad, cat no. 170-6531)
Dimethyl sulfoxide (DMSO, Sigma, cat. no. D8418)
XTT sodium salt (Sigma, cat. no. X4251)
Ringer’s lactate (Hospira, cat. no. NDC0409-7953-09)
Menadione (Sigma, cat. no. M5625) !CAUTION Menadione (2-Methyl-1,4-naphthoquinone, also known as vitamin K3) acts as an electron acceptor and can interfere with theelectron transport chain in humans and as such is potentially toxic at high enough concentrations. It is extremely hazardous in case of skin contact, inhalation or ingestion. Always wear lab coat, gloves and mask when handling the powder.
Acetone (Sigma, cat. no. 34850)
Antifungal drugs: Typically fluconazole (Pfizer, Inc.), amphotericin B (Bristol-Myers Squibb) and caspofungin (Merck & Co., Inc.) as representatives of the three main classes of antifungal agents most frequently used in the clinics: azoles, polyenes and echinocandins respectively.
EQUIPMENT
Autoclave (Sanyo)
Disposable plastic vacuum filter system, 500 ml (Corning, cat no. 430769)
Petri dishes (Fisher, cat no. 08-757-12)
15 ml conical centrifuge tubes (Corning, cat no. 430790)
50 ml conical centrifuge tubes (Corning, cat no. 430828)
Erlenmeyer flasks (Corning)
Biological safety cabinet (Nuaire)
Vortex mixer (Fisher)
Orbital shaker (New Brunswick)
Centrifuge (Eppendorf)
Hemacytometer (Hausser Scientific)
Microscope (Fisher)
96 well microtiter plates: Polystyrene, flat-bottomed, tissue culture treated (Corning, cat. no. 3595).
Parafilm
Multichannel pipette and tips (Eppendorf)
Incubator (Fisher)
Inverted Microscope (optional) (Fisher)
Microtiter plate reader (Bio-Rad)
REAGENTS SET UP
If prepared from powder forms, after preparing the corresponding solutions in water following manufacturer’s instructions, microbiological media need to be autoclaved (YPD) or filter-sterilized (RPMI 1640, DMEM).
The XTT is prepared as a saturated solution at 0.5 g/L in sterile Ringer’s lactate. Ringer’s lactate can be substituted for PBS or any other physiological buffer, as these have been shown to give similar reproducible results. The XTT solution is light sensitive, so it should be covered with aluminium foil during preparation. The solution needs to be filter-sterilized using a 0.22 μm-pore size filter; since it is a saturated solution, the filtration step will leave yellow residues on the filter, but this does not constitute a problem. Once prepared and filter-sterilized, aliquote into 10 ml working volumes, and store at −70 °C. We recommend wrapping the tubes containing this XTT solution in aluminum foil to prevent light penetration. We do not recommend storage of the XTT solution, even the frozen aliquots, for prolonged periods (longer than one year) since the activity of the reagent may decrease over time. Once the XTT is effectively metabolized by live fungal cells, this originally clear solution is transformed into an orange color. We have observed batch to batch variations between XTT solutions and even aliquots, but analysis and interpretation of results should not be problematic if proper intra-experimental controls are used. However, this variability makes it imperative to exercise caution when trying to compare absorbance readings from experiments performed on different days.
Prepare a 10 mM stock solution of menadione in 100% acetone. Aliquot into smaller volumes (about 50 μl) and store at −70°C.
Antifungal agents are selected based on the testing required. The drugs are initially solubilized following manufacturer’s instructions (most typically in DMSO, but some antifungals are also soluble in water). If needed, concentrated stock solutions (i.e. 1 mg/ml) of the antifungals can be aliquoted into smaller volumes and stored at −70 °C until required.
PROCEDURE
Growing the fungal cultures and preparation of inocula Timing ~ 12–16 h (overnight)
1] For propagation of fungal cells, for Candida spp. and C. neoformans, flasks containing YPD liquid medium (typically 20 ml of medium in a 150 ml flask) are inoculated with a loopful of cells from the stock cultures and incubated overnight in an orbital shaker (150 – 180 rpm) at 30 °C. In the case of A. fumigatus, conidia are harvested from 3 day old cultures on Sabouraud dextrose agar plates by flooding the surface of the plates with 5 ml of PBS containing 0.025% (v/v) Tween-20 and rocking gently. The conidial suspension is then recovered and dispensed into a 15 ml sterile tube. This conidial suspension can be retained for use for up to two weeks when stored at 4 °C.
!CAUTION Most fungalorganisms to be tested here have the potential to cause infections in humans, particularly immunosuppressed individuals. Thus, adequate biosafety practices need to be followed. For C. neoformans and Aspergillus spp., all manipulations should be performed within a biological safety cabinet (in the case of Aspergillus spp. this is also important to prevent contamination of other laboratory surfaces). Work with Candida spp. can be performed on an open bench but proper microbiological handling techniques to prevent contamination and universal precautions must be followed.
2] Harvest cells from the overnight grown liquid cultures (for Candida spp. and C. neoformans) or from the conidial suspension (for A. fumigatus) by centrifugation (approximately 3,000g for 5 min at 4 °C), remove supernatant and wash twice in sterile PBS (by resuspending the pellet in approximately 20 ml of ice-cold buffer, vortexing vigorously, followed by centrifugation as above).
▵ CRITICAL STEP Because fungal cells tend to settle and/or aggregate, the cell suspensions must be vortexed vigorously after washings and before pipetting for the different manipulations used in this and successive steps.
3] Resuspend the final pellet of cells in approximately 20 ml of the appropriate medium that has been prewarmed to 37 °C: RPMI-1640 for Candida and Aspergillus and DMEM for Cryptococcus.
4] From the resulting cell suspension prepare 1:100 and/or 1:1,000 fold dilutions in the same medium and count using a hemocytometer and a bright field microscope with a 40 × objective.
5] After counting, calculate the volumes needed to prepare a suspension of cells at a final density of 1.0 × 106 cells/ml in RPMI 1640 for Candida, 1.0 × 105 cells/ml in RPMI 1640 for Aspergillus, or 1.0 × 107 cells/ml in DMEM for Cryptococcus. The total volume needed will depend on the aggregate number of wells (or plates) that need to be seeded for biofilm formation for each isolate under investigation.
▴ CRITICAL STEP The fungal cell density of the initial inoculum is very important for correct biofilm development, as quorum sensing mechanisms play an important role in biofilm formation. Cell densities that are too high or too low will likely result in poor biofilms. The cell densities for the inocula suggested here have been empirically optimized for experiments using 96 well microtiter plates for each different fungal species.
Biofilm formation Timing ~ 24 h
6] Open as many 96 well microtiter plates as needed according to the experimental design (these plates are sterile and come individually packaged). We recommend performing a minimum of 2 – 4 replicates (entire rows of the microtiter plate(-s)) for each combination of fungal isolate/antifungal agent to be tested.
7] From the standardized inoculum prepared in step 5, pipette 100 μl (for Candida) or 200 μl (for Aspergillus and C. neoformans) into selected wells of the microtiter plate(-s). Typically, wells in column 12 on each plate should remain unseeded, as these 8 wells will act as negative background controls during subsequent analyses. Repeat as necessary for each different fungal isolate to be tested.
CRITICAL STEP: It is important that the seeding of the wells occurs promptly after preparation of the inocula.
CRITICAL STEP: If multiple rows in the same plate, the entire plate or if multiple plates are to be seeded with the same fungal isolate, the use of a multichannel pipette is strongly recommended for this and successive steps.
8] When all the selected wells have been seeded, cover the entire microtiter plate with its original lid, seal with parafilm, place inside an incubator and incubate statically for 24 h at 37 °C. For Candida spp., Aspergillus spp. and C. neoformans after 24–48 h incubation biofilms should already display the complex three dimensional architecture characteristic of multicellular communities, and the resulting biofilms can be used for antifungal susceptibility testing. However, after seeding the plates the time of incubation for biofilm formation may be varied depending on the specific objectives of the study: for example, if the main objective of the study is examination of initial adherence the incubation time can be reduced to 4 h, other investigators may want to study “fully mature” biofilms after 72–96 h incubation.
!CAUTION Note that A. fumigatus biofilms with greater than 48 h growth may begin to sporulate, which could create post processing problems and increase the possibilities for unwanted laboratory contamination.
9] After biofilm formation, aspirate the medium carefully as not to touch and disrupt the biofilm. The best way to do this is, using a multi-channel pipette, angle the pipette tips towards the corners of the wells, thereby minimizing contact with the biofilm.
10] Using a multichannel pipette wash plates three times in sterile PBS (200 – 300 μl per well) to remove planktonic and/or non-adherent cells that remain in the wells.
11] After each wash the microtiter plates should be drained in an inverted position by blotting with paper towels to remove any residual PBS. Biofilms are now ready to be processed for antifungal susceptibility testing assays.
! TROUBLESHOOTING
CRITICAL STEP: The washing procedures are critical, with the main emphasis on preserving biofilm integrity. Other methodologies can potentially be used, such as gently flicking the plates to discard the liquid contents, squirting buffer from a bottle, using an automated microtiter plate washer, etc. Typically the fungal biofilms are strongly attached to the bottom of the wells of microtiter plates and these washing procedures should not disrupt the pre-formed biofilms. In any case, at the end of the washing procedures, any well with clearly disrupted biofilm layer at the bottom (normally this is visible by the naked eye) should be excluded from further subsequent calculations. This is one of the main reasons why we recommend performing sufficient replicates for each isolate/antifungal drug combination to be tested.
▴ CRITICAL The ability to form multiple equivalent biofilms in a reproducible manner is a requirement for the success of these techniques. Thus, we recommend that when these protocols are followed for the very the first time, and before proceeding to antifungal susceptibility testing, investigators corroborate that they have indeed been able to form multiple equivalent biofilms in the wells of the microtiter plate(-s). At this point after biofilm formation, the XTT/menadione reagent can be added and the resulting color read using a microtiter plate reader (see below, follow steps 18–22) (see also “anticipated results”). The same is true if assessment of biofilm-forming ability of fungal isolates (and not antifungal susceptibility testing) is the main objective of a particular set of experiments.
Preparation of antifungal agents and antifungal challenge of pre-formed biofilms Timing ~ 24 h
12] From the stock solution of each antifungal a final “high” working concentration is prepared in RPMI 1640 medium. Typical high concentrations are 1,024 μg/ml for fluconazole, and 16 μg/ml for both amphotericin B and caspofungin. Other concentrations may be used for different agents.
▴ CRITICAL If testing experimental and/or new agents with unknown activity against biofilms we recommend to start with high concentrations of the drug, normally up to 100–1,000 times higher than one would use against planktonic populations.
13] Using a multichannel pipette, add200 μl of the high working concentration of antifungal to the corresponding wells on column 1 of each microtiter plate containing fungal biofilms, being careful not to touch or otherwise disrupt the biofilms.
14] Add 100 μl of RPMI 1640 to each well in columns 2 to 10. Add 100 μl of RPMI 1640 to wells in column 11, these will act as the positive control (biofilm not exposed to antifungal). Wells in column 12 remain empty as negative controls.
15] Remove 100 μl of antifungal agent from the wells of column 1 and add to the adjacent wells in column 2 (already containing 100 μl of medium). The contents are then mixed by gently pipetting up and down to perform a serial doubling dilution, and the pipette tips removed.
16] Remove 100 μl of antifungal agent from the wells of column 2 and add to the adjacent wells in column 3 (already containing 100 μl of medium). Mix gently and repeat moving right until the wells of column 10, after which the final 100 μl volume from the wells of column 10 after mixing is discarded. You have just created serial doubling dilutions of your agent(−s) of interest, from column 1 (most concentrated) to column 10 (least concentrated), with unchallenged biofilms in column 11 serving as positive controls.
17] Cover the plates with their lids, seal with parafilm and incubate for 24 h at 37 °C. Other incubation times can be used depending on the experimental design or particularities of the antifungal agent(−s) being tested.
Post-processing and measurement of metabolic activity after antifungal treatment Timing ~ 2–3 h
18] Thaw as many tubes containing 10 ml of the XTT solution as required for the experimental design (one per plate). To each tube, add 1 μl of the stock solution of menadione to achieve a final menadione concentration of 1 μM. For uniformity, if multiple plates are processed at the same time, we recommend pooling all the resulting XTT/menadione tubes into a single solution in a clean sterile container.
19] Using a multichannel pipette add 100 μl of the XTT/menadione solution to each well containing a pre-washed biofilm as well as to negative control wells (for the measurement of background XTT-colorimetric levels).
20] Cover the plates in aluminium foil and incubate in the dark for 2 – 3 h at 37 °C.
21] Uncover the plates. At this point, visual inspection of the plates will typically demonstrate a gradient of orange color. Using a multichannel pipette remove 75 – 80 μl of the resulting colored supernatant from each well and transfer into the wells of a new microtiter plate.
22] Read the plate(-s) in a microtiter plate reader at 490 nm.
23] From the resulting colorimetric readings and after subtracting the corresponding values for negative controls (from wells in column 12 containing XTT only) calculate the sessile minimum inhibitory concentrations (SMICs) for each fungal isolate: SMIC50 and SMIC80 are the antifungal concentrations at which a 50% or 80% decrease in absorbance are detected in comparison to the control biofilms formed by the same fungal isolate in the absence of antifungal drug (in this case values for column 11). SMIC results are normally displayed in tabular form. Alternatively, results can be presented as a graph plotting colorimetric readings or percent inhibition (preferred) versus antifungal concentration (see “anticipated results”).
TIMING
Steps 1–5, growing the fungal cultures and preparation of inocula ~ 12–16 h (up to 3 days for Aspergillus spp.)
Steps 5–11, biofilm formation ~ 24 – 48 h
Steps 12–17, preparation of antifungal agents and antifungal challenge of pre-formed biofilms ~ 24 h
Steps 18–23, post-processing and measurement of metabolic activity after antifungal treatment ~ 2–3 h
TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
TABLE 1.
Troubleshooting table
| Problem | Possible reason | Solution |
|---|---|---|
| Lack of biofilm formation (at the end of Step 11) | Problems with counting the seeding inocula | Make sure the dilutions and calculations are made appropriately |
| Some fungal isolates do not form biofilm | These isolates should be considered “non-biofilm” formers | |
| Establish “cut-off” values to identify non-biofilm forming isolates | ||
| Consider using some other surfaces/materials for biofilm formation | ||
| Biofilms visible by the naked eye but no orange color develops (at the end of step 21) | Old XTT solution | Prepare a fresh solution of XTT |
| Menadione not added | Always remember to add menadione to the XTT before adding to the wells | |
| Microbial contamination (at the end of step 11) | Not maintaining sterility | This is a rare occurrence. |
| Poor handling | Always maintain proper microbiological handling techniques | |
| Storage of reagents | Minimize re-opening of reagents. Discard any reagent with signs of contamination | |
| Biofilms peel-off from bottom of wells (all washing steps, but particularly after step 11) | Aggressive washing | Be gentle during washings (follow recommendations) |
| Most often this will be visible by the naked eye. Do not use these wells for final calculation | ||
| Make sure enough replicates are performed to minimize problems | ||
| Biofilms too old (mature) | Disaggregation should be considered normal in the biofilm life cycle as biofilms reach maturity. Use younger biofilms |
ANTICIPATED RESULTS
As emphasized before, it is important that multiple equivalent biofilms are formed in individual wells of the microtiter plates. Figure 2 shows XTT-colorimetric readings (OD490 values) for each of 10 biofilms of C. albicans strain SC5314 formed in each of the 8 different rows of the same 96 well microtiter plate (a total of 80 replicates). Results for the different rows were compared by one-way analysis of variance and using the Bartlett’s test for homogeneity of variances and the Bonferroni’s multiple comparison post-test. No statistically significant differences were noted when comparing all pairs of rows to each other (P > 0.05).
Figure 2.
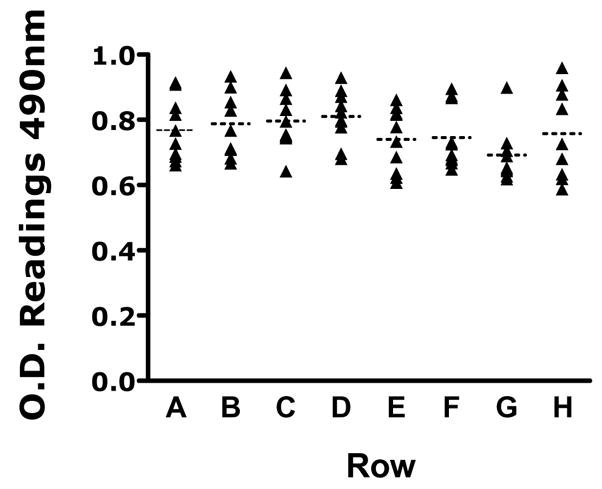
Colorimetric readings (OD490 values) from XTT-reduction assays of biofilms formed by C. albicans strain SC5314 in wells of microtiter plates. Values are for 10 independent biofilms formed in each of 8 different rows of the same 96 well microtiter plate.
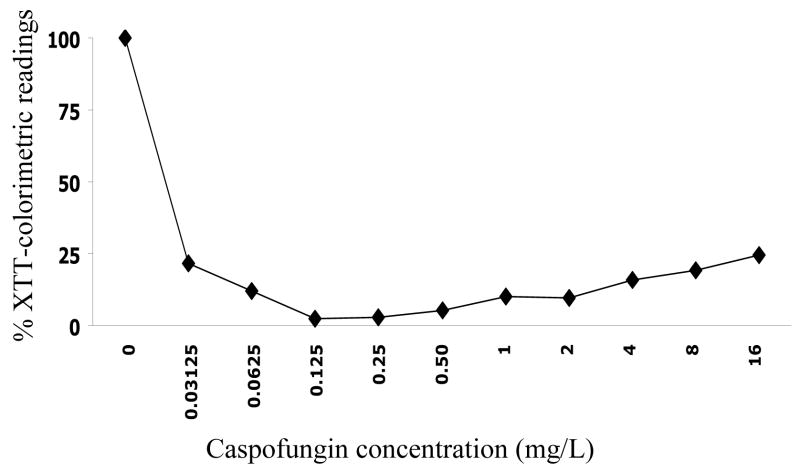
Table 2 shows typical results, expressed as SMIC50s and SMIC80s, of antifungal susceptibility testing for multiple C. albicans isolates against three of the most common antifungal agents used clinically. Note that typically biofilms are intrinsically resistant to fluconazole and most other azole derivatives. Amphotericin B is active against biofilms but normally at concentrations considered to be high, due to the intrinsic toxicity associated with this polyene antibiotic. Caspofungin, a representative of the echinocandin class of new antifungal agents, shows excellent in vitro activity against biofilms formed by most C. albicans isolates. Figure 3 shows the activity of caspofungin at different concentrations against C. albicans biofilms, expressed as percent colorimetric readings for XTT-reduction assays as compared to control wells.
TABLE 2.
Results of antifungal susceptibility testing of biofilms formed by C. albicans type strain SC5314 and several C. albicans clinical isolates against fluconazole, amphotericin B and caspofungin. Values are in mg/L
| Fluconazole | Amphotericin B | Caspofungin | ||||
|---|---|---|---|---|---|---|
| Isolate | SMIC50 | SMIC80 | SMIC50 | SMIC80 | SMIC50 | SMIC80 |
| SC5314 | >1024 | >1024 | 0.5 | 1 | 0.03125 | 0.0625 |
| #1 | >1024 | >1024 | 1 | 2 | 0.0625 | 0.03125 |
| #2 | 1024 | >1024 | 0.5 | 0.5 | 0.125 | 0. 25 |
| #3 | >1024 | >1024 | 0.25 | 0.5 | 0.0625 | 0.0625 |
| #4 | 1024 | >1024 | 0.5 | 1 | 0.03125 | 0.03125 |
| #5 | >1024 | >1024 | 2 | 4 | 0.0625 | 0.125 |
| #6 | >1024 | >1024 | 0.25 | 1 | 0.0625 | 0.0625 |
| #7 | >1024 | >1024 | 0.5 | 1 | 0.0625 | 0.0625 |
Figure 3.
Activity of different caspofungin concentrations against pre-formed biofilms of C. albicans SC5314. Values are expressed as average percent colorimetric readings for XTT-reduction assays as compared to control wells. Note that, as previously described, caspofungin loses efficacy at non-physiological high concentrations. This has been referred to as the “paradoxical” or “Eagle” effect.
Acknowledgments
Work in the laboratory is funded by Grant number 5R21DE017294 from the National Institute of Dental & Craniofacial Research (to J.L.L.-R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCR, the NIAID or the NIH. A.R.T. was supported by the McNair Scholars Program at St. Mary’s University funded by the U.S. Department of Education.
References
- 1.Clark TA, Hajjeh RA. Recent trends in the epidemiology of invasive mycoses. Curr Opin Infect Dis. 2002;15:569–574. doi: 10.1097/00001432-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Dixon DM, McNeil MM, Cohen ML, Gellin BG, La Montagne JR. Fungal infections: a growing threat. Public Health Rep. 1996;111:226–235. [PMC free article] [PubMed] [Google Scholar]
- 3.Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol. 2007;45:321–346. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- 4.Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 6.Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. Candida biofilms: an update. Eukaryot Cell. 2005;4:633–638. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachmann SP, et al. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob Agents Chemother. 2002;46:3591–3596. doi: 10.1128/AAC.46.11.3591-3596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal Susceptibility of Candida Biofilms: Unique Efficacy of Amphotericin B Lipid Formulations and Echinocandins. Antimicrob Agents Chemother. 2002;46:1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeast’s: approved standard. NCCLS Document M27-A. National Committee for Clinical Laboratory Standards; Wayne, PA. 1997.1997. [Google Scholar]
- 10.Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol. 2006;55:999–1008. doi: 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 11.Baillie GS, Douglas LJ. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob Agents Chemother. 1998;42:1900–1905. doi: 10.1128/aac.42.8.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baillie GS, Douglas LJ. Iron-limited biofilms of Candida albicans and their susceptibility to amphotericin B. Antimicrob Agents Chemother. 1998;42:2146–2149. doi: 10.1128/aac.42.8.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Sanchez S, et al. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot Cell. 2004;3:536–545. doi: 10.1128/EC.3.2.536-545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison JJ, Turner RJ, Ceri H. A subpopulation of Candida albicans and Candida tropicalis biofilm cells are highly tolerant to chelating agents. FEMS Microbiol Lett. 2007;272:172–181. doi: 10.1111/j.1574-6968.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 15.Nobile CJ, Mitchell APC. albicans transcription factor Bcr1p: a regulator of cell-surface genes and biofilm formation. Current Biol. 2005;15:1150–1155. doi: 10.1016/j.cub.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Parahitiyawa NB, et al. Interspecies variation in Candida biofilm formation studied using the Calgary biofilm device. Apmis. 2006;114:298–306. doi: 10.1111/j.1600-0463.2006.apm_394.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramage G, Bachmann S, Patterson TF, Wickes BL, Lopez-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother. 2002;49:973–980. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 18.Ramage G, Tomsett K, Wickes BL, Lopez-Ribot JL, Redding SW. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:53–59. doi: 10.1016/j.tripleo.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Ramage G, Wickes BL, Lopez-Ribot JL. A seed and feed model for the formation of Candida albicans biofilms under flow conditions using an improved modified Robbins device. Rev Iberoam Micol. 2008;25:37–40. doi: 10.1016/s1130-1406(08)70009-3. [DOI] [PubMed] [Google Scholar]
- 20.Suci PA, Geesey GG, Tyler BJ. Integration of Raman microscopy, differential interference contrast microscopy, and attenuated total reflection Fourier transform infrared spectroscopy to investigate chlorhexidine spatial and temporal distribution in Candida albicans biofilms. J Microbiol Methods. 2001;46:193–208. doi: 10.1016/s0167-7012(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 21.Vediyappan G, Chaffin WL. Non-glucan attached proteins of Candida albicans biofilm formed on various surfaces. Mycopathologia. 2006;161:3–10. doi: 10.1007/s11046-005-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawser SP, Norris H, Jessup CJ, Ghannoum MA. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-t etrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998;36:1450–1452. doi: 10.1128/jcm.36.5.1450-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honraet K, Goetghebeur E, Nelis HJ. Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. J Microbiol Methods. 2005;63:287–295. doi: 10.1016/j.mimet.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Jin Y, et al. The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia. 2005;159:353–360. doi: 10.1007/s11046-004-6987-7. [DOI] [PubMed] [Google Scholar]
- 25.Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tellier R, Krajden M, Grigoriew GA, Campbell I. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob Agents Chemother. 1992;36:1619–1625. doi: 10.1128/aac.36.8.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhn DM, Balkis M, Chandra J, Mukherjee PK, Ghannoum MA. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J Clin Microbiol. 2003;41:506–508. doi: 10.1128/JCM.41.1.506-508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Y, Samaranayake LP, Samaranayake Y, Yip HK. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol. 2004;49:789–798. doi: 10.1016/j.archoralbio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Martinez LR, Casadevall A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 2006;50:1021–1033. doi: 10.1128/AAC.50.3.1021-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mowat E, Butcher J, Lang S, Williams C, Ramage G. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J Med Microbiol. 2007;56:1205–1212. doi: 10.1099/jmm.0.47247-0. [DOI] [PubMed] [Google Scholar]
- 31.Bachmann SP, et al. Antifungal Combinations against Candida albicans Biofilms In Vitro. Antimicrob Agents Chemother. 2003;47:3657–3659. doi: 10.1128/AAC.47.11.3657-3659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramage G, VandeWalle K, Bachmann SP, Wickes BL, Lopez-Ribot JL. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob Agents Chemother. 2002;46:3634–3636. doi: 10.1128/AAC.46.11.3634-3636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]