Abstract
In addition to its classical role in mineral homeostasis, the vitamin D receptor has been implicated in diverse physiologic and pathophysiologic processes including immunoregulation and cancer. Interestingly, the vitamin D receptor has been evolutionarily and functionally linked to a select group of nuclear receptors based on a common organism-wide tissue expression profile. These members of the nuclear receptor superfamily, which include the bile acid receptor, xenobiotic receptors, and several orphan nuclear receptors, comprise a transcriptional regulatory network that functions in nutrient uptake, xenobiotic metabolism, and mucosal protection. The major homeostatic functions of the enteric nuclear receptor network are the topic of this review.
Keywords: bile acids, gastrointestinal physiology and disease, metabolism, nuclear receptor, transcriptional regulation
INTRODUCTION
As the body’s eye to the nutrient world, the gut plays a vital role in both nutrient absorption and the elimination of unwanted dietary components. It is also the first line of defense against the world of bacteria that lives within the intestine. The gastrointestinal tissues express a distinct group of nuclear receptors that include the vitamin D receptor (VDR), bile acid receptor (FXR), and the xenobiotic receptors (PXR and CAR). By acting as sensors of absorbed lipids, this group of transcription factors allows the enterocyte to activate appropriate transcriptional programs in response to nutrients, toxic dietary components, and microbial metabolites.
We begin this review with pertinent background on the nuclear receptor superfamily and the basis for the classification of an enteric nuclear receptor network. We then explore the role of VDR and its relatives as components of the nuclear receptor network that coordinates nutrient uptake, elimination of toxic dietary components, and protection against bacterial invasion. We conclude with recent discoveries highlighting the ever-expanding role of this group of nuclear receptors and speculations regarding additional roles of these receptors in gastrointestinal physiology and disease.
THE NUCLEAR RECEPTOR SUPERFAMILY
The nuclear receptor superfamily comprises 48 ligand-activated transcription factors that have been grouped on the basis of shared structural features.1 A DNA binding domain (DBD) allows nuclear receptors to bind conserved elements in the regulatory regions of specific groups of genes, while the adjacent ligand binding domain (LBD) functions to activate or inactivate transcription when bound by a small diffusible molecule referred to as its ligand. This unique property has rendered nuclear receptors particularly useful as intracellular sensors for endocrine hormones and dietary lipids. Nuclear receptors typically bind DNA either as homodimers or heterodimers, although some also bind as monomers. The retinoid X receptors (RXRs) are obligate heterodimer partners, meaning that all nuclear receptor heterodimers must include one of the RXRs.
The nuclear receptor superfamily can be subdivided into four groups based on the type of ligand bound and the DNA binding mechanism.2 The first of these are the endocrine receptors that bind DNA as homodimers. These include the estrogen, androgen, progesterone, glucocorticoid, and mineralocorticoid receptors, which all bind with nanomolar affinity to systemically circulating steroid hormones synthesized by endocrine tissues. The transcriptional programs activated by these receptors provide negative feedback to the endocrine system to turn off ligand synthesis. The second group of receptors form heterodimers with RXR and are activated by a wide range of dietary lipids including fatty acids (bind PPARs), bile acids (bind FXR), xenobiotics (bind PXR, CAR), and a variety of cholesterol metabolites referred to as oxysterols (bind LXRs). Such ligands exist at relatively high concentrations, particularly within enterohepatic tissues and portal circulation, where they bind their cognate receptors with micromolar affinity. In contrast to the endocrine receptors, the metabolic receptors activate transcriptional programs to turn on catabolic pathways for dietary lipids. The third group comprises retinoic acid (vitamin A), 1,25-dihydroxyvitamin D3, and thyroid hormone receptors. These also function as RXR heterodimers, yet their ligands are synthesized in the body from precursors that are derived from the diet; or in the case of vitamin D, sunlight. Like other endocrine receptors, this group binds these ligands with high affinity. It is now known that the vitamin D receptor (VDR) also binds bile acids and this satisfies the criteria of both the second and third groups.3,4 The final group includes receptors with no known ligands, which are referred to as orphan nuclear receptors.
An additional layer of complexity is afforded to receptors that function as RXR heterodimers. RXR itself is activated by the endogenous vitamin A derivative, 9-cis retinoic acid. In theory, therefore, heterodimers can be activated by two ligands, that of RXR and that of its partner. In reality, RXR heterodimers exhibit three modes of activation: permissive, conditional, and non-permissive. 5 Permissive heterodimers can be activated by either receptor’s ligand and are exemplified by the dietary lipid-sensing receptors (e.g., LXRs), while conditional heterodimers can only be activated by RXR agonist when the partner ligand is also present (e.g., retinoic acid receptors). Non-permissive heterodimers are endocrine receptors in which the RXR agonist has no effect on the activity of the heterodimer (e.g., thyroid hormone receptor). RXR/VDR heterodimers can function as both non-permissive and permissive types, since the VDR can bind both an endocrine hormone and bile acid.
THE ENTERIC NUCLEAR RECEPTOR CLADE
Sequence-based clustering of the nuclear receptor superfamily has provided valuable information regarding the evolutionary relatedness of receptors and has led to a better understanding of the function of individual receptors. One way to provide more information regarding this large family of transcription factors is to use anatomically based expression profiling to cluster the nuclear receptor superfamily on the basis of shared expression patterns. The discovery of bile acids as the endogenous ligands for FXR is a prime example of how expression analysis was used to characterize receptor function. The tell-tale expression pattern of FXR in tissues involved in bile acid synthesis and circulation was part of the rationale to pursue the bile acid receptor hypothesis.3 The advantages of understanding the global expression pattern of all nuclear receptors are that 1) new targets or functions can be identified on the basis of common expression; 2) predictions can be made regarding coordinated transcriptional programs within a given tissue involving multiple receptors with known functions; 3) physiologic processes regulated by distinct receptors can be linked; 4) information can be gathered regarding the coordination of physiological processes involving multiple organs and levels of regulation; and finally 5) predictions can be made regarding the systems that regulate the expression of nuclear receptors themselves.
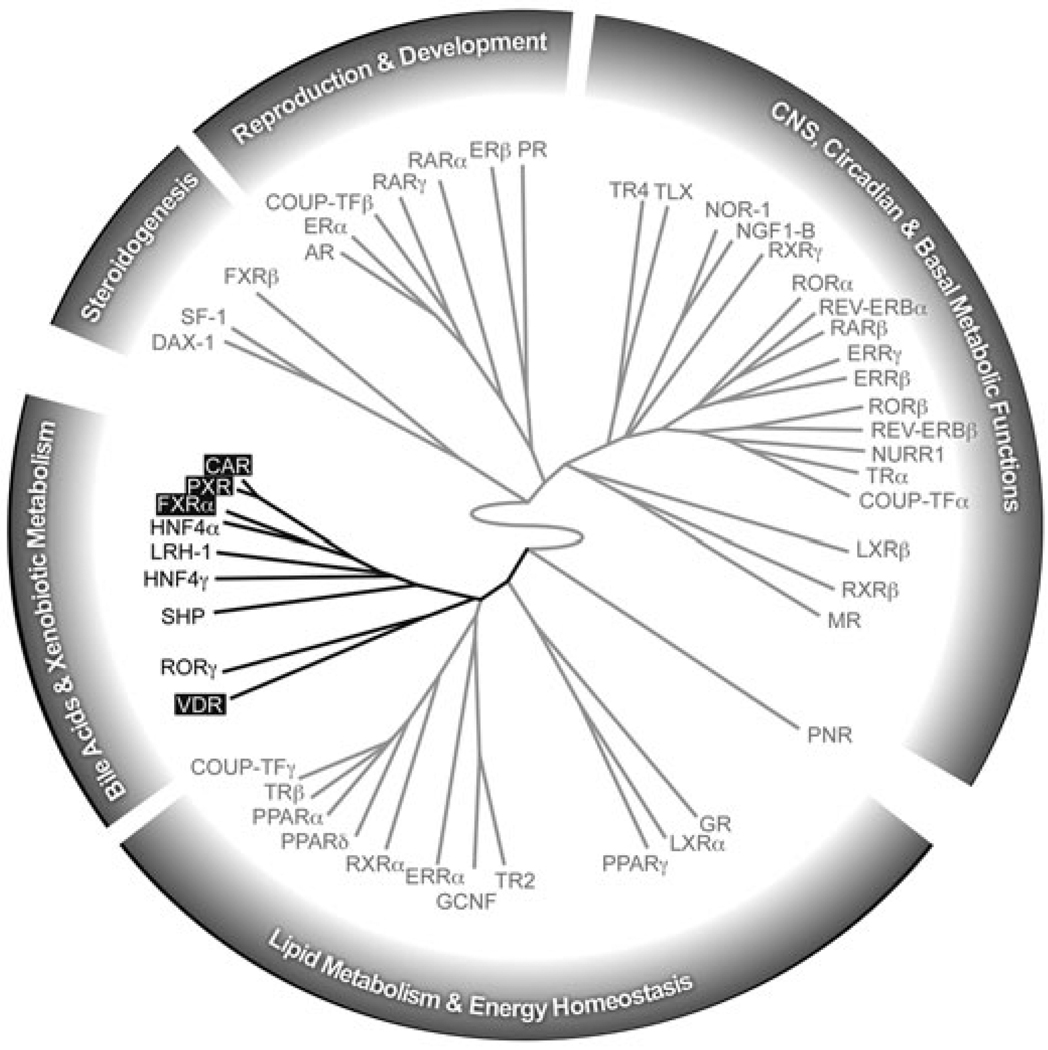
A recent study used quantitative real-time PCR to determine the relative expression level of the entire nuclear receptor superfamily in 39 distinct tissues.6 A systems biology approach was taken to cluster the nuclear receptors based on the similarity of these expression profiles. Broadly, the superfamily divides into two major divisions: one whose members regulate reproduction, development, and growth; the other whose members regulate nutrient uptake, metabolism, and excretion. VDR falls into a clade of nuclear receptors that function largely in bile acid and xenobiotic metabolism. The members of this group are all expressed in the gastrointestinal tract (Figure 1) and, like VDR, most are involved in nutrient uptake and/or the elimination of toxic dietary components. Within this group VDR, FXR, PXR, and CAR all function as RXR heterodimers and are activated by dietary lipids. Although the other members of this group, LRH-1, HNF4α, HNF4γ, SHP, and RORγ are not ligand activated, some of them are known to mediate or moderate the activity of the ligand activated receptors. In order to emphasize the common role they play in intestinal physiology, the following sections will focus mainly on the functions of the four ligand-activated members of the enteric nuclear receptor clade: the vitamin D receptor (VDR), the bile acid receptor (FXR), and the xenobiotic receptors (PXR and CAR).
Figure 1. Dendrogram of enteric nuclear receptors defining a distinct clade within the nuclear receptor superfamily.
The dendrogram was created using hierarchical, unsupervised clustering of the 49 mouse nuclear receptor tissue expression profiles. Branch length denotes relatedness in organism-wide tissue expression profiles. The enteric nuclear receptor clade is shown in black. All members of this group are expressed in the intestine. Receptors in black boxes denote ligand-activated RXR heterodimers discussed in the text. Note that in mice there are two FXR genes (FXRα and FXRβ). FXRβ is a pseudogene in humans and in mice its ligand and function are unknown. For simiplicity, the text refers to FXRα as FXR. Figure adapted from Bookout et al. (2006).6
ABSORBING THE GOOD
Prolonged vitamin D deficiency causes rickets in children and osteomalacia in adults. In 1988, Hughes et al. discovered that mutations in the DNA binding domain of the vitamin D receptor were the cause of an autosomal recessive disease now known as hereditary vitamin D-resistant rickets (HVDRR). This was the first report of a mutation in a nuclear receptor gene in human disease.7 HVDRR is characterized by resistance of target organs to the action of vitamin D. Patients typically present during infancy with bone pain, hypotonia, and generalized muscle weak-ness. Severe cases are marked by seizures or convulsions. Laboratory abnormalities include hypocalcemia, hypophosphatemia, hyperparathyroidism, and elevated serum 1,25-dihydroxyvitamin D3.8 The primary defect stems from the inability to absorb sufficient calcium from the diet. While VDR functions in the kidney and bone to regulate mineral homeostasis, probably the most important site of VDR action is in the intestine. Here, the transcriptional regulation of ion channels and transport proteins by VDR is essential for the uptake of calcium. This is exemplified by the VDR knock-out mouse, which at the time of weaning develops hypocalcemia, secondary hyperparathyroidism, and subsequently osteomalacia.9 Interestingly, the rachitic bone phenotype can be prevented, provided serum calcium and phosphate levels are normalized with a high calcium/phosphorus and high lactose diet.10 This suggests that insufficient calcium absorption is the primary cause of decreased bone mineralization resulting from the loss of VDR activity.
In the intestine, VDR directly regulates calcium transport and binding proteins. Calcium transport can potentially be regulated at three distinct steps at the level of the enterocyte; namely, influx across the apical membrane, intracellular transport/trafficking, and basolateral efflux.11 The major ion channels responsible for transcellular calcium transport are the apical transporters ECaC1 (TRPV5) and ECaC2 (TRPV6), and the basolateral ATP-dependent transporter PMCA1 (ATP2B1).11 ECaC1 and ECaC2 are regulated by VDR both in the intestine and kidney.12,13 In mice, ECaC2 is the predominant calcium transporter in the intestine and is also regulated by vitamin D.14 Although there is a report of PMCA transcriptional regulation by 1,25-dihydroxyvitamin D3,15 evidence is still lacking for a direct regulation of PMCA1 by VDR. The calbindins belong to a family of high-affinity cytosolic calcium binding proteins. They are thought to facilitate calcium transport from the apical to the basolateral membrane and to act as a buffer for intracellular calcium, which may otherwise be toxic to the cell.16 Calbindin D9K is the predominant vitamin D-responsive calcium protein in the intestine. Both its basal expression and induction by vitamin D is dependent on VDR.9
While calcium is largely absorbed in the proximal intestine, bile acids are taken up by the distal small intestine. Here, the reabsorption of bile acids synthesized and secreted by the liver is a highly efficient process that is regulated by FXR. As has been proposed for intestinal calcium absorption, the regulation of bile acid transport by the enterocyte may occur at three levels. Transport of conjugated bile acids across the brush border membrane is facilitated by the ileal bile acid transporter, IBAT (SLC10A2), also known as the apical sodium-dependent bile acid transporter or ASBT. At the basolateral membrane, bile acid efflux occurs via the heterodimeric organic solute transporter OSTalpha/beta.17 Finally, the ileal bile acid binding protein (I-BABP, also known as Fabp6) binds to bile acids in the cytosol.18While its function is not entirely known, it may facilitate intracellular bile acid transport and, by binding bile acids, reduce their cytotoxic effects in a manner analogous to the calbindins with calcium. Each of these proteins is regulated at the transcriptional level by FXR.19,20 Thus, there are striking similarities in the function of VDR and FXR target genes in the intestine, although differences exist in how these nuclear receptors regulate nutrient absorption.
NEUTRALIZING THE BAD
While only a subset of the enteric nuclear receptors regulate nutrient absorption, they all play a role in eliminating toxic substances, drugs, or xenobiotics absorbed by the gut. Living up to their designation as xenobiotic sensors, PXR and CAR are the principle modulators of detoxifying pathways involving phase I and II enzymes and phase III transporters. While the liver is the major site of bile acid and xenobiotic metabolism, it is now recognized that the intestine expresses many of the same enzymes and contributes to the metabolism of drugs/xenobiotics entering the body by the oral route. Major insight into the metabolism of bile acids in the intestine came with the discovery that VDR is a receptor for the toxic bile acid, lithocholic acid (LCA).3 Since VDR can activate bile acid metabolizing enzymes, this discovery provided a mechanism for the direct detoxification of bile acids within the gut. The following paragraphs describe the major target genes regulated by multiple nuclear receptors in the intestine. A more complete description of PXR/CAR target genes involved in drug metabolism can be found in a number of plenary reviews on the topic.21–23
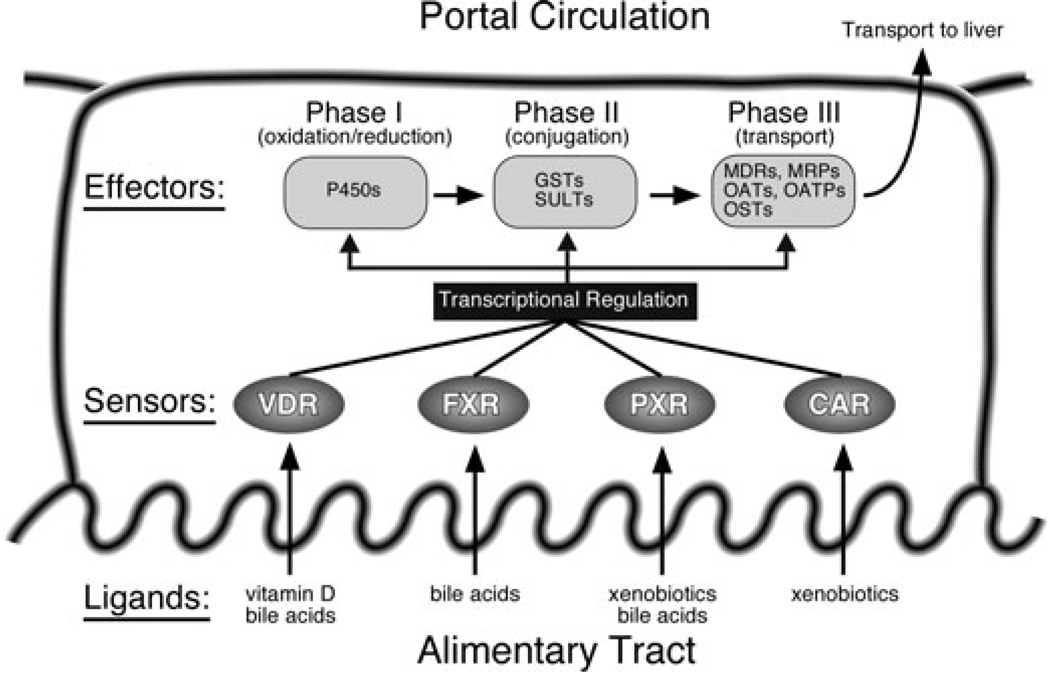
Numerous studies have focused on the transcriptional targets and drug metabolizing roles of nuclear receptors such as PXR, CAR, and FXR, particularly with respect to drug interactions and first-pass metabolism; however, this system was not designed with our current pharmacopeia in mind, rather it likely evolved to protect the body from ingested toxins, toxins produced by pathogenic bacteria, or even endogenous compounds converted to toxins by commensal bacteria. In the first phase of drug/xenobiotic metabolism, a substrate is typically modified through the addition of a hydroxyl group by a cytochrome P450 (CYP). The reactions carried out by this family of monooxygenases include oxidation, reduction, and hydrolysis. In phase II, the chemically activated moiety is further modified by conjugating enzymes that add a larger, more hydrophilic group such as glucuronic acid, glutathione, sulfate, glycine, taurine, or acetate. Phase III involves transporting the modified and typically more water-soluble compound out of the cell. All components of this system are under transcriptional regulation by the four ligand-activated members of the enteric nuclear receptor clade: CAR, PXR, FXR, and VDR (Figure 2). Together, these four nuclear receptors function as the sensors of the intestinal detoxifying system and thus have the important role of activating the effectors (metabolizing enzymes and transporters) upon increased or chronic exposure of the enterocyte to dietary toxins. Two additional members of the enteric group, the orphan receptors HNF4α and LRH-1, have sometimes been referred to as competence factors since they potentiate the activity of the ligand-activated receptors at certain promoters such as CYP7A1 and CYP3A4.24,25
Figure 2. Regulation of the intestinal detoxification system by the enteric nuclear receptor clade.
As a group, the vitamin D receptor (VDR), the bile acid receptor (FXR), and the xenobiotic receptors (PXR and CAR) function as sensors in the gut to protect the body from excess exposure to toxic dietary lipids. All three phases of xenobiotic and bile acid metabolism, including oxidation/reduction, conjugation, and transport, are regulated at the transcriptional level by this group of receptors within the intestine. The robustness of the intestinal detoxification system is a consequence of the ligand binding characteristics of these receptors. While each receptor binds a unique set of ligands with high affinity, most also bind common ligands with lower affinity (e.g., bile acids). The result is a system adapted to a wide range of insults that is able to limit damage should one of its members become compromised. The redundancy in ligand binding reflects the important evolutionary conservation of this subgroup of receptors.
The two most abundantly expressed phase I (oxidation) enzymes in the intestine are CYP3A4 and CYP2B6 (the murine homologs are Cyp3a11 and Cyp2b10, respectively). The former is a prototypical PXR target gene while the latter is a prototypical CAR target gene. However, both genes can be activated by these two receptors.26–29 CYP3A4 is also activated by VDR and possibly even FXR.3,30 The significance of CYP3A4 regulation by VDR in the colon is discussed in a subsequent section.
Phase II (conjugating) enzymes regulated by nuclear receptors in the intestine include sulfotransferases (SULTs) and glutathione S-transferases (GSTs). SULT2A1 is a known PXR target gene in the liver, where it functions in the inactivation of steroids such as dehydroepiandrosterone and the excretion of bile acids such as lithocholate. Recent reports have identified SULT2A1 also as a VDR and an FXR target gene in the intestine. 31,32 Since VDR and FXR are activated by toxic secondary bile acids and sulfonation of bile acids is exclusively carried out by SULT2A1, this system provides a rapid mechanism to detoxify bile acids and protect the intestine even before the passively absorbed secondary bile acids reach the liver. A number of GSTs are upregulated by PXR and CAR ligands in the intestine. 28 In general, this class of enzymes that catalyze the conjugation of glutathione to various chemical substrates, including xenobiotic substrates and carcinogens, are thought to reduce oxidative stress and protect from cancer.33
The enterocyte expresses many members of the two major classes of membrane transporters, the ATP binding cassette (ABC) transporters and the solute carrier (SLC) transporters. Members of the ABC transporter superfamily use energy in the form of ATP to actively transport a wide range of substrates across plasma membranes. They include the multidrug resistance transporters (MDRs) and multidrug resistance-associated proteins (MRPs). Transporters of the SLC superfamily, on the other hand, use electrochemical gradients to move substrates by coupled or passive transport. SLCs include transporters of polar substrates such as glucose (GLUTs) and amino acids; organic cation and anion transporters (OCTs, OATs, and OATPs); ion channels (Na+, K+, Cl−, Ca++); and many more. MDR1 (ABCB1, also known as P-glycoprotein) is an efflux pump for hydrophobic compounds, and MRP2 (ABCC2) transports organic anions such as sulfated or glucuronidated products of phase II enzymes. Both transporters are regulated by PXR, while MRP2 is also regulated by CAR.27,28 Since these pumps are located in the apical membrane of the enterocyte, they directly return toxic compounds to the gut lumen.34 MRP3 (ABCC3), on the other hand, is a basolateral transporter of a wide variety of organic anions including bile acids. MRP3 is regulated by PXR and CAR in the liver and, specifically, by VDR in the colon.28,35 Another basolateral organic anion transporter, OATP2 (SLCO1A4), has recently been shown to be upregulated by PXR in the intestine of humanized PXR mice.27
As a final point, not only do PXR, CAR, FXR, and VDR regulate many of the same targets, there is also overlap in the chemical ligands by which they are activated. Notably, molecules of the steroid class, such as bile acids, activate all four receptors, albeit with different potencies. This redundancy in the action of the enteric nuclear receptor clade reflects the important evolutionary conservation of this subfamily of receptors and speaks to the importance of a functional detoxification system in the gut.
PROTECTING FROM THE UGLY
The enteric tract is host to a large microbial community. The complex commensal and symbiotic relationship has extensively shaped the evolution of both man and microorganism. In addition to the mucosal barrier formed by tight junctions between epithelial cells; mucus, gastric acid, pancreatic secretions, biliary secretions, antimicrobial peptides, and secretory IgA represent the major mucosal defenses. On the other hand, bacteria that colonize the enteric niche have evolved unique protective mechanisms such as modified surface molecules and a microbiome capable of modifying antimicrobial chemicals secreted by the host. The regulation of host immune defenses in the intestine is still poorly understood and the effect of host gene expression on the composition of gut microbiota is just beginning to be explored.36
It has long been known that bile acids are toxic to bacteria. The microbicidal activity of these host-synthesized steroids has been attributed to their intrinsic detergent properties. In the pathologic condition of cholestasis, the luminal concentration of bile acids is significantly decreased and bacterial overgrowth ensues. Recent work has shown that the antimicrobial activity of bile acids could be mediated, in part, by their activation of FXR in the intestine.37 This discovery was made from a genome-wide survey of FXR targets in the intestine that yielded a group of genes involved in innate mucosal immunity. Upon induction of cholestasis in mice by bile duct ligation, bacterial overgrowth, mucosal translocation, and lymph node invasion occurred. Amazingly, bacterial overgrowth and mucosal invasion was completely prevented by the administration of a synthetic FXR agonist. Furthermore, many of the FXR target genes involved in mucosal immunity originally identified by microarray analysis were upregulated in agonist-treated mice. Importantly, the synthetic agonist had no effect on bacterial growth or target gene expression in FXR-null mice that had undergone the same procedure. This study clearly demonstrated that activation of FXR is sufficient to prevent bacterial overgrowth in the gut caused by cholestasis, and it identifies bile acids as a double-edged sword in the armamentarium of intestinal defenses. To what extent FXR plays a role in normal mucosal immunity and whether the importance of the signaling function of bile acids exceeds that of their direct detergent properties in protecting from bacterial overgrowth remains to be determined.
VDR is expressed in cells of the adaptive immune system and vitamin D has been shown to prevent or reduce autoimmune disease in various experimental models.38 Vitamin D inhibits the activation of T cells, and promotes tolerance in dendritic cells.39,40 VDR has also been implicated in toll-like-receptor (TLR) signaling and regulation of the innate immune response in macrophages. 41 In the latter instance, themechanism was shown to involve induction of the cathelicidin gene, which encodes an antimicrobial peptide with potent antimycobacterial activity. Antimicrobial peptides are not only made by circulating cells of the innate immune system, they are also synthesized by epithelial cells including the intestinal epithelium.42 Interestingly, the product of the cathelicidin gene LL-37, is also made by cells of the colonic epithelium. In a study in rabbits, induction of LL-37 in the colon by butyrate promoted the elimination of shigella and improved the outcome of disease.43 Whether VDR induces antimicrobial peptides or other antimicrobial factors in the intestine has not yet been determined.
Collectively referred to as inflammatory bowel disease (IBD), ulcerative colitis and Crohns’ disease are chronic inflammatory disorders of the intestine. While the etiology is unclear, the pathogenesis of IBD is thought to involve abnormal immune responses to intestinal microbes. A number of studies in animals and humans have indicated that loss of VDR or PXR can contribute to the pathogenesis of IBD.44–47
In a landmark study, Ley et al.48 used comparative metagenomic analysis to identify quantitative differences in the gut microbiota of mice deficient for the leptin gene. Subsequently, it was shown that colonization of gnotobiotic mice with the microbiota of obese mice was sufficient to increase the body fat mass of the recipient, strongly suggesting that the gut microbiome can be a significant factor in the pathogenesis of obesity. Importantly, these studies demonstrate that the host genome can shape the gut microbiota, which in turn affects host physiology and disease. As intestinal sensors of bacterial metabolites and regulators of transcriptional programs, the enteric nuclear receptors may play a role in shaping the intestinal ecosystem. Applying comparative metagenomic and metaproteomic analysis to targeted genetic models of the enteric nuclear receptors may help elucidate new roles for these already busy sentinels of the gut.
REGULATING PROLIFERATION, DIFFERENTIATION, AND CANCER
The fact that nuclear receptor expression and activity is often altered in cancer has prompted an interest in defining the role of nuclear receptors in cancer progression. In addition, the amenability of nuclear receptors to pharmacological modulation makes them prime candidates for molecularly targeted therapies. In some cases, nuclear receptor agonists and antagonists are already part of the standard chemotherapeutic regimen. Examples include all-trans-retinoic-acid for the treatment of acute promyelocytic leukemia; anti-androgens and anti-estrogens for the treatment of hormone-responsive prostate and breast cancers; and glucocorticoids for acute lymphoblastic leukemia (ALL).While some of the classic endocrine nuclear hormone receptors have direct effects on differentiation and proliferation, the situation is not as clear for the enteric nuclear receptors. On the one hand, the bile acid and xenobiotic metabolizing nuclear receptors could directly affect the transcription of cell cycle regulators; alternatively, their effects may be indirect and a consequence of their ability to eliminate potentially genotoxic or tumor-promoting agents. In the case of VDR there is evidence to suggest that both mechanisms play a role in protection from colon cancer.49,50
The expression of VDR is most abundant in the large intestine, even though this organ does not contribute to the classical functions of VDR, such as vitamin D metabolism and mineral homeostasis. Mice lacking VDR have enhanced rates of proliferation and increased oxidative stress in the distal large intestine.51 In addition, there is a large body of epidemiological data indicating an inverse relationship between serum vitamin D levels and the occurrence of colon cancer.49 Vitamin D decreases proliferation and increases differentiation in human colon cancer cells, and a variety of mechanisms have been proposed to explain this activity, including the downregulation of cyclins and the upregulation of cell cycle inhibitors such as p21 and p27.52 While vitamin D is thought to reduce the risk of colon cancer, secondary bile acids such as deoxycholic acid (DCA) and lithocholic acid (LCA) are believed to increase the risk of developing colon cancer. Bile acids have been shown to function as tumor promoters in rodent models of colon cancer.53 Diets with a high content of animal fat are believed to increase risk by elevating the levels of fecal bile acids. A unifying hypothesis to explain the effect of VDR and bile acids on tumor promotion was proposed when it was discovered that VDR is activated by the toxic secondary bile acid, LCA.3 This hypothesis is based on a model of LCA detoxification by the VDR target genes CYP3A11 and SULT2A1 in the colon. The CYP3A11 enzyme catalyzes the hydroxylation of LCA, while SULT2A1 sulfates LCA, both of which promote LCA elimination in the feces.31,54,55 On a normal diet, LCA levels are low and systemically circulating vitamin D can prime the system to ensure a basal level of catabolic activity. On a high-fat diet and/or in a vitamin D-deficient state, the rate of LCA metabolism is inadequate, such that it builds up to toxic levels that can damage the colonic epithelium and cause cancer.50 It will be interesting to see whether other members of the enteric nuclear receptor group affect the progression to colorectal cancer. Like VDR, they could have direct effects on proliferation/differentiation or indirect effects, such as the detoxification of carcinogens or reduction of oxidative stress.
THE ENTERIC NUCLEAR RECEPTOR CLADE OUTSIDE THE GASTROINTESTINAL TRACT
While we have focused on targets directly regulated in the intestine by prominent members of the enteric nuclear receptor clade, the regulation of other organs by these same nuclear receptors via secreted factors is an emerging paradigm in nuclear receptor biology. Recent discoveries have focused on transcriptional regulation of a subfamily of fibroblast growth factors (FGFs), including FGF19 (known as FGF15 in the mouse), FGF21, and FGF23. Most FGFs signal in an autocrine or paracrine fashion; however, members of the FGF19 subfamily are released into circulation and function as endocrine hormones.56
FGF23 is a phosphaturic hormone released from mineralized tissues whose major target organ is the kidney. 57 In the proximal tubule of the nephron, the primary site of 1,25-dihydroxyvitamin D3 synthesis, FGF23 binds to its receptor and downregulates the major apical sodium-phosphate cotransporter, NPT2 (SLC34A1), and reduces the active form of vitamin D by downregulating 25-hydroxyvitaminD3-1α-hydroxylase and upregulating 25-hydroxyvitamin D-24-hydroxylase.58Vitamin D increases circulating levels of FGF23, and activates transcription of its gene in bone.59,60 Although VDR is important for transcriptional regulation of FGF23, it is not yet clear whether this involves a direct regulation of the FGF23 promoter.61,62 Thus, VDR controls the secretion of FGF23, which functions in an elegant bone-kidney endocrine feedback axis to regulate phosphate reabsorption and vitamin D synthesis in response to phosphate stores and vitamin D activity. A direction of future research will be to determine if FGF23 has vitamin D-dependent effects in other tissues of the body.
FXR, on the other hand, controls the synthesis of FGF19, which signals in an intestine-liver and intestine-gallbladder axis to regulate bile acid synthesis and secretion. Even before its secretion from the intestine was known, FGF19 was found to repress bile acid synthesis in cultured primary hepatocytes.63 CYP7A1 encodes the microsomal enzyme that catalyzes the first and rate-limiting step in the conversion of cholesterol to bile acids; accordingly, its promoter is regulated in a complex manner by signaling pathways that may originate directly in the nucleus or at the plasma membrane. Although the exact mechanism is not known, repression of CYP7A1 in the liver by FGF19 requires the membrane-localized FGF receptor 4 (FGFR4), an active c-Jun N-terminal kinase (JNK) pathway, and the repressive nuclear receptor SHP.63,64 Interestingly, in experimentally induced cholestasis, where bile acids accumulate in the liver and are absent in the intestine, the hepatic expression of CYP7A1 is high. Thus, the mechanism of CYP7A1 feedback repression by bile acids does not merely involve bile acid signaling in the liver. This conundrum was solved in mice by the discovery that FXR induces the expression of FGF15 (the mouse ortholog of human FGF19) in the ileum, and that FGF15, which is secreted by enterocytes, functions in an endocrine manner to repress CYP7A1 in the liver.64 There is now evidence that FGF19 functions in a comparable manner in humans.65 Thus, activation of FXR by bile acids in the ileum regulates bile acid synthesis in the liver via the endocrine hormone FGF15/19. In a recent report, FGF15/19 has also been shown to stimulate gallbladder filling by causing the relaxation of gallbladder smooth muscle.66 The mechanism is believed to involve an increase in cAMP and antagonism of the gallbladder contracting hormone cholecystokinin (CCK). FGF15/19 is not expressed in the gallbladder; however, it is induced to high levels by FXR in the ileum, suggesting that gallbladder relaxation is regulated in an endocrine fashion by FGF15/19 analogous to the CCK-stimulated gallbladder contraction. The regulation of the secreted FGFs 19 and 23 led to the discovery that the third member of this subfamily, FGF21, is a fasting hormone regulated by the fatty acid receptor PPARα.67 These discoveries have drawn attention to the importance of enteric nuclear receptor signaling not only within the intestine but also in the coordination of complex multi-organ physiologic processes.
On a final note, many of the enteric nuclear receptors are also expressed in the liver and kidney. In the liver, PXR, CAR, and FXR play a central role in xenobiotic and bile acid metabolism.2,22 As in the intestine, they regulate transcriptional programs to coordinate the three phases of drug/steroid metabolism; namely, oxidation, conjugation, and transport. In this regard, the liver functions as a second line of defense from dietary components by ensuring that toxic lipids do not reach the systemic circulation. Both VDR and FXR are highly expressed in the kidney, an organ that, like the gut, employs active and passive transport to maintain appropriate levels of nutrients and electrolytes. As already noted, VDR regulates calcium and phosphate reabsorption and contributes to the regulation of vitamin D synthesis. However, the function of FXR in the kidney remains elusive. Unlike entero-hepatic tissues, the kidney is not normally exposed to high concentrations of bile acids, suggesting that perhaps additional physiologic ligands for FXR are yet to be identified.
CONCLUSION
As described above, new functions of the enteric nuclear receptor network are being discovered at a record pace. It is beginning to be appreciated that this group of nuclear receptors, particularly VDR and FXR, function in a wide range of physiologic processes to maintain homeostasis within the intestine. New approaches are needed to elucidate as yet unknown functions of the nuclear receptor superfamily in gastrointestinal physiology and disease. Additionally, much remains to be learned about how the receptors are themselves regulated and how they interact with other families of transcription factors. The recently published nuclear receptor expression profile6 and the new functions being discovered for these transcription factors provide the beginning of a quest to elucidate the temporal and spatial regulation of transcriptional programs at the organismal level.
Acknowledgments
Funding. This work was funded by the Howard Hughes Medical Institute, the Robert A. Welch Foundation (# I-1275), and the National Institutes of Health (Atlas Grant #U19DK62434).
Footnotes
Note on nomenclature: Members of the nuclear receptor superfamily have both a trivial name and an official designation (code). The official code reflects a grouping system based on sequence conservation. Throughout the literature, trivial names are almost solely in use, and this manuscript is no exception. Nevertheless, for the sake of clarity we list here the official receptor code of each of the nuclear receptors for which a trivial name appears in the text. PPARα (NR1C1), RXRs (NR2B1, NR2B2, and NR2B3), LXRs (NR1H2 and NR1H3), FXR (NR1H4), VDR (NR1I1), PXR (NR1I2), CAR (NR1I3), LRH-1 (NR5A2), HNF4α (NR2A1), HNF4γ (NR2A2), SHP (NR0B2), and RORα (NR1F3).
Declaration of interest. The authors have no relevant interests to declare.
References
- 1.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 3.Makishima M, Lu TT, Xie W, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 4.Nehring JA, Zierold C, Deluca HF. Lithocholic acid can carry out in vivo functions of vitamin D. Proc Natl Acad Sci USA. 2007;104:10006–10009. doi: 10.1073/pnas.0703512104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shulman AI, Larson C, Mangelsdorf DJ, Ranganathan R. Structural determinants of allosteric ligand activation in RXR heterodimers. Cell. 2004;116:417–429. doi: 10.1016/s0092-8674(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 6.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore DD, Kato S, Xie W, et al. International Union of Pharmacology. LXII. The NR1H and NR1I receptors: constitutive androstane receptor, pregnene X receptor, farnesoid X receptor alpha, farnesoid X receptor beta, liver X receptor alpha, liver X receptor beta, and vitamin D receptor. Pharmacol Rev. 2006;58:742–759. doi: 10.1124/pr.58.4.6. [DOI] [PubMed] [Google Scholar]
- 8.Beer S, Tieder M, Kohelet D, et al. Vitamin D resistant rickets with alopecia: a form of end organ resistance to 1,25 dihydroxy vitamin D. Clin Endocrinol. 1981;14:395–402. doi: 10.1111/j.1365-2265.1981.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 9.Li YC, Pirro AE, Amling M, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amling M, Priemel M, Holzmann T, et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–4987. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 11.Bouillon R, Van Cromphaut S, Carmeliet G. Intestinal calcium absorption: Molecular vitamin D mediated mechanisms. J Cell Biochem. 2003;88:332–339. doi: 10.1002/jcb.10360. [DOI] [PubMed] [Google Scholar]
- 12.van Abel M, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ. Regulation of the epithelial Ca2+ channels in small intestine as studied by quantitative mRNA detection. Am J Physiol Gastrointest Liver Physiol. 2003;285:G78–G85. doi: 10.1152/ajpgi.00036.2003. [DOI] [PubMed] [Google Scholar]
- 13.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okano T, Tsugawa N, Morishita A, Kato S. Regulation of gene expression of epithelial calcium channels in intestine and kidney of mice by 1alpha,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2004;89–90:335–338. doi: 10.1016/j.jsbmb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Pannabecker TL, Chandler JS, Wasserman RH. Vitamin-D-dependent transcriptional regulation of the intestinal plasma membrane calcium pump. Biochem Biophys Res Commun. 1995;213:499–505. doi: 10.1006/bbrc.1995.2159. [DOI] [PubMed] [Google Scholar]
- 16.Christakos S, Liu Y, Dhawan P, Peng X. The Calbindins: Calbindin-D9K and Calbindin-D28K. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. San Diego, CA: Elsevier Academic Press; 2005. pp. 721–735. [Google Scholar]
- 17.Dawson PA, Hubbert M, Haywood J, et al. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong YZ, Everett ET, Schwartz DA, Norris JS, Wilson FA. Molecular cloning, tissue distribution and expression of a 14-kDa bile acid-binding protein from rat ileal cytosol. Proc Natl Acad Sci USA. 1994;91:4741–4745. doi: 10.1073/pnas.91.11.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grober J, Zaghini I, Fujii H, et al. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J Biol Chem. 1999;274:29749–29754. doi: 10.1074/jbc.274.42.29749. [DOI] [PubMed] [Google Scholar]
- 20.Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol. 2006;290:G476–G485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- 21.Handschin C, Meyer UA. Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev. 2003;55:649–673. doi: 10.1124/pr.55.4.2. [DOI] [PubMed] [Google Scholar]
- 22.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 23.Urquhart BL, Tirona RG, Kim RB. Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J Clin Pharmacol. 2007;47:566–578. doi: 10.1177/0091270007299930. [DOI] [PubMed] [Google Scholar]
- 24.Lu TT, Makishima M, Repa JJ, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 25.Tirona RG, Lee W, Leake BF, et al. The orphan nuclear receptor HNF4alpha determines PXR- and CAR-mediated xenobiotic induction of CYP3A4. Nat Med. 2003;9:220–224. doi: 10.1038/nm815. [DOI] [PubMed] [Google Scholar]
- 26.Gnerre C, Blattler S, Kaufmann MR, Looser R, Meyer UA. Regulation of CYP3A4 by the bile acid receptor FXR: evidence for functional binding sites in the CYP3A4 gene. Pharmacogenetics. 2004;14:635–645. doi: 10.1097/00008571-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Ma X, Shah YM, Guo GL, et al. Rifaximin is a gut-specific human pregnane X receptor activator. J Pharmacol Exp Ther. 2007;322:391–398. doi: 10.1124/jpet.107.121913. [DOI] [PubMed] [Google Scholar]
- 28.Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 29.Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to Phenobarbital in activating the human CYP2B6 gene. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 30.Thummel KE, Brimer C, Yasuda K, et al. Transcriptional control of intestinal cytochrome P-4503A by 1alpha,25-dihydroxy vitamin D3. Mol Pharmacol. 2001;60:1399–1406. doi: 10.1124/mol.60.6.1399. [DOI] [PubMed] [Google Scholar]
- 31.Echchgadda I, Song CS, Roy AK, Chatterjee B. Dehydroepiandrosterone sulfotransferase is a target for transcriptional induction by the vitamin D receptor. Mol Pharmacol. 2004;65:720–729. doi: 10.1124/mol.65.3.720. [DOI] [PubMed] [Google Scholar]
- 32.Song CS, Echchgadda I, Baek BS, et al. Dehydroepiandrosterone sulfotransferase gene induction by bile acid activated farnesoid X receptor. J Biol Chem. 2001;276:42549–42556. doi: 10.1074/jbc.M107557200. [DOI] [PubMed] [Google Scholar]
- 33.Grubben MJ, Nagengast FM, Katan MB, Peters WH. The glutathione biotransformation system and colorectal cancer risk in humans. Scand J Gastroenterol Suppl. 2001;234:68–76. doi: 10.1080/003655201753265479. [DOI] [PubMed] [Google Scholar]
- 34.Dietrich CG, Geier A, Oude Elferink RP. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut. 2003;52:1788–1795. doi: 10.1136/gut.52.12.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy TC, Li X, Sinal CJ. Vitamin D receptor-dependent regulation of colon multidrug resistance-associated protein 3 gene expression by bile acids. J Biol Chem. 2005;280:23232–23242. doi: 10.1074/jbc.M411520200. [DOI] [PubMed] [Google Scholar]
- 36.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Inagaki T, Moschetta A, Lee YK, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. Faseb J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 39.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 40.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocytederived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 41.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 42.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 43.Raqib R, Sarker P, Bergman P, et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci USA. 2006;103:9178–9183. doi: 10.1073/pnas.0602888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dring MM, Goulding CA, Trimble VI, et al. The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology. 2006;130:341–348. doi: 10.1053/j.gastro.2005.12.008. quiz 592. [DOI] [PubMed] [Google Scholar]
- 45.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 46.Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 2006;117:310–318. doi: 10.1111/j.1365-2567.2005.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langmann T, Moehle C, Mauerer R, et al. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26–40. doi: 10.1053/j.gastro.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 48.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cross HS. Vitamin D and colon cancer. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. Vol. 2. San Diego, CA: Elsevier Academic Press; 2005. pp. 1709–1725. [Google Scholar]
- 50.Mangelsdorf DJ, Motola DL. Vitamin D receptor as a sensor for toxic bile acids. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. Vol. 1. San Diego, CA: Elsevier Academic Press; 2005. pp. 863–870. [Google Scholar]
- 51.Kallay E, Pietschmann P, Toyokuni S, et al. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429–1435. doi: 10.1093/carcin/22.9.1429. [DOI] [PubMed] [Google Scholar]
- 52.Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A. Vitamin D and cancer. J Steroid Biochem Mol Biol. 2006;102:156–162. doi: 10.1016/j.jsbmb.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Nagengast FM, Grubben MJ, van Munster IP. Role of bile acids in colorectal carcinogenesis. Eur J Cancer. 1995;31A:1067–1070. doi: 10.1016/0959-8049(95)00216-6. [DOI] [PubMed] [Google Scholar]
- 54.Araya Z, Wikvall K. 6alpha-Hydroxylation of taurochenodeoxycholic acid and lithocholic acid by CYP3A4 in human liver microsomes. Biochimica et biophysica Acta. 1999;1438:47–54. doi: 10.1016/s1388-1981(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 55.Xie W, Radominska-Pandya A, Shi Y, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogawa Y, Kurosu H, Yamamoto M, et al. Beta Klotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukagawa M, Nii-Kono T, Kazama JJ. Role of fibroblast growth factor 23 in health and in chronic kidney disease. Curr Opin Nephrol Hypertens. 2005;14:325–329. doi: 10.1097/01.mnh.0000172717.49476.80. [DOI] [PubMed] [Google Scholar]
- 58.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 59.Kolek OI, Hines ER, Jones MD, et al. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 60.Saito H, Maeda A, Ohtomo S, et al. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 61.Barthel TK, Mathern DR, Whitfield GK, et al. 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol. 2007;103:381–388. doi: 10.1016/j.jsbmb.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 62.Masuyama R, Stockmans I, Torrekens S, et al. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest. 2006;116:3150–3159. doi: 10.1172/JCI29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holt JA, Luo G, Billin AN, Bisi J, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Lundasen T, Galman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 66.Choi M, Moschetta A, Bookout AL, et al. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12:1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 67.Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]