Abstract
Lysophosphatidylcholine (LPC) levels are elevated in sera in patients with atherosclerosisand in atherosclerotic tissue. Previous studies have shown that reactive chlorinating species attack plasmalogens in human coronary artery endothelial cells (HCAEC), forming LPC and LPC-chlorohydrin (LPC-ClOH). The results herein demonstrate for the first time that LPC-ClOH is elevated over 60-fold in human atherosclerotic lesions. In cultured HCAEC, LPC-ClOH led to a statistically significant increase in P-selectin cell-surface expression. These data show that LPC-ClOH is elevated in atherosclerotic tissue and may have unique pro-atherogenic properties compared to LPC.
Keywords: Phospholipid analysis; Analytical Techniques, Atherosclerosis; Physiology, Coronary artery disease; Physiology, Inflammations; Physiology, Plasmalogens; Specific Lipids; Myeloperoxidase; reactive chlorinating species (RCS); plasmalogen; P-selectin; chlorohydrins; lysophosphatidylcholine
Introduction
Activated monocytes and a subpopulation of macrophages release myeloperoxidase (MPO), which is an enzyme that catalyzes the production of reactive chlorinating species (RCS) such as hypochlorous acid (HOCl) (1). Levels of MPO are elevated in atherosclerotic plaques (2), as well as MPO-derived oxidation products such as 3-chlorotyrosine (3) and 2-chlorohexadecanal (4). Plasmenylcholine is a subclass of glycerophospholipid enriched in the cells of the cardiovascular system, and is thought to play an important role in normal heart function, as well as serve as an endogenous lipoprotein antioxidant (5). Recently, our lab and others have shown that RCS attack of plasmenylcholine in liposomes and in human coronary artery endothelial cells (HCAEC) yields LPC (6, 7) and LPC-chlorohydrin (LPC-ClOH) (7–9). Additionally, we have shown that HOCl preferentially targets LPC in comparison to PC (8). Since LPC is elevated in atherosclerotic lesions and is preferentially targeted by RCS in vitro, the present study was designed to determine that LPC-ClOH levels increase in atherosclerotic lesions. We show here, for the first time, that LPC-ClOH is elevated in atherosclerotic lesions, and that LPC-ClOH induces P-selectin cell-surface expression on HCAEC.
Experimental Procedures
Plasmenylcholine (PC) 1-O-hexadec-1’-enyl-2-heptadec-10’-enoyl-GPC (16:0–17:1 PC), 1-O-hexadec-1’-enyl-2-octadec-9’-enoyl-GPC (16:0-18:1 PC), and 1-O-hexadec-1’-enyl-2-nonadec-10’-enoyl-GPC (16:0–19:1 PC) were synthesized by an anhydrous reaction utilizing 1-O-hexadec-1’-enyl-GPC and heptadec-10’-enoyl chloride, octadec-9’-enoyl chloride, and nonadec-10’-enoyl chloride, respectively, as precursors and dimethylaminopyridine as a catalyst. 16:0–17:1 and 16:0–19:1 plasmenylcholine were treated with 10-fold molar excess HOCl (10, 11), which simultaneously cleaved the vinyl ether bond and formed 17:0 and 19:0 LPC–ClOH, respectively. The desired reaction product, LPC-ClOH, was purified by HPLC, quantified by Bartlett (12), extracted in chloroform and stored at −20 °C under N2.
Human tissue
Atherosclerotic and normal aortic tissue was harvested from human postmortem autopsy specimens, rinsed, and submerged in PBS supplemented with 100 µM diethylenetriaminepentaacetic acid and 100 µM butylhydroxytoluene, frozen in liquid N2, and stored at −80 °C until analysis. Frozen tissue was weighed and then crushed in a liquid nitrogen-chilled mortar and pestle. Lipids and internal standards 17:0 and 19:0 LPC-ClOH were added to the crushed tissue in methanol/chloroform/saline (2.5:1.5:1 v/v/v). After 5 min, chloroform was added resulting in a 1:1:0.8 methanol/chloroform/saline ratio (13). The extracted organic phase was stored in chloroform at −20 °C under N2 until analysis.
Electrospray ionization – tandem mass spectrometry
Lipid extracts were reconstituted in methanol containing 10 µM NaOH and analyzed by ESI-MS in the direct infusion mode at a flow rate of 1–3 µL/min using Thermo Electron TSQ Quantum Ultra instrumentation. The choline glycerophospholipids yield intense [M-Na]+ adduct ions by electrospray ionization in the positive ion mode. Tandem mass spectrometry was performed on selected ions (collision energies were ~ 32–38 eV). To detect LPC and LPC–ClOH molecular species, total ion current (TIC) and neutral loss scanning of 95 a.m.u. (NL 95) was employed. Spectra were averaged at 3 to 5 minutes and processed utilizing Xcalibur software (Thermo), and molecular species were quantified by comparing the ion intensity of individual molecular species to that of the internal standards. The differences in 13C isotope effects (Z1) between the endogenous 16:0 and 18:0 LPC-ClOH and the internal standards 17:0 and 19:0 LPC-ClOH were corrected (14).
P-selectin surface expression
HCAEC, grown to confluence in 24-well plates, were incubated with indicated lipids in Hanks’ buffer for 5 min at 37 °C in 95 % O2/5 % CO2 and P-selectin surface expression was determined as previously described (4).
Data analysis
Data were normalized to the respective control mean values and are expressed as means +/− SEM. Statistical analyses of data were performed by t-test and P ≤ 0.05 was considered statistically significant.
Results
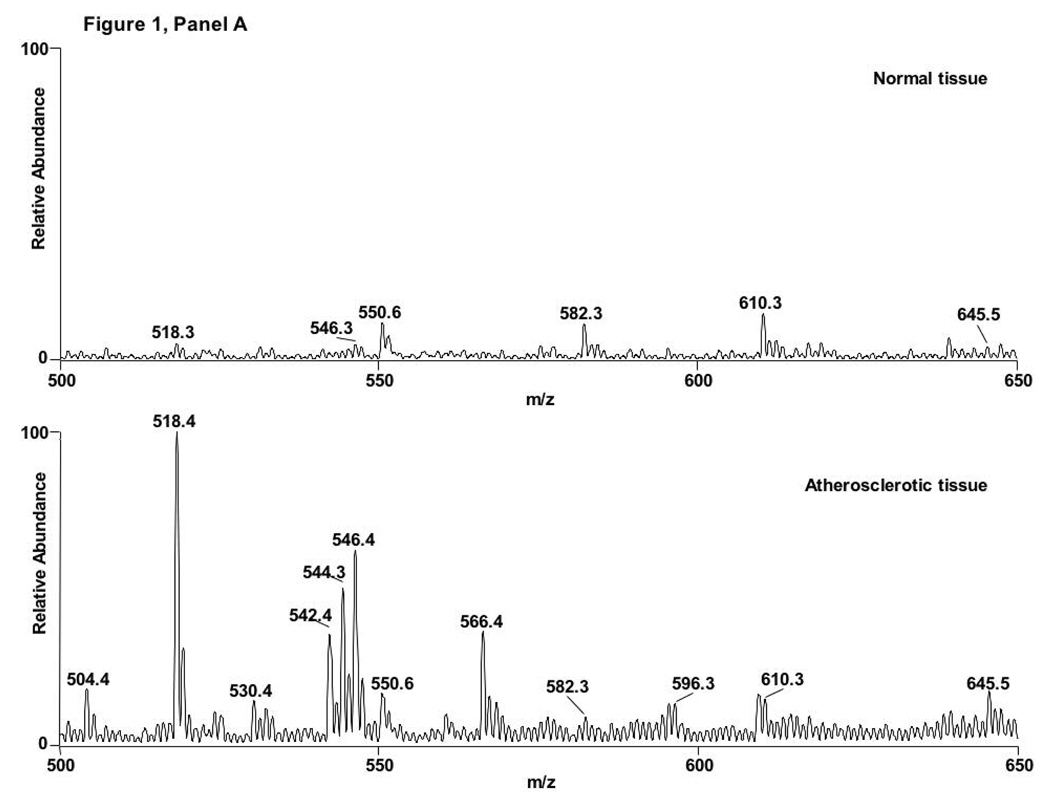
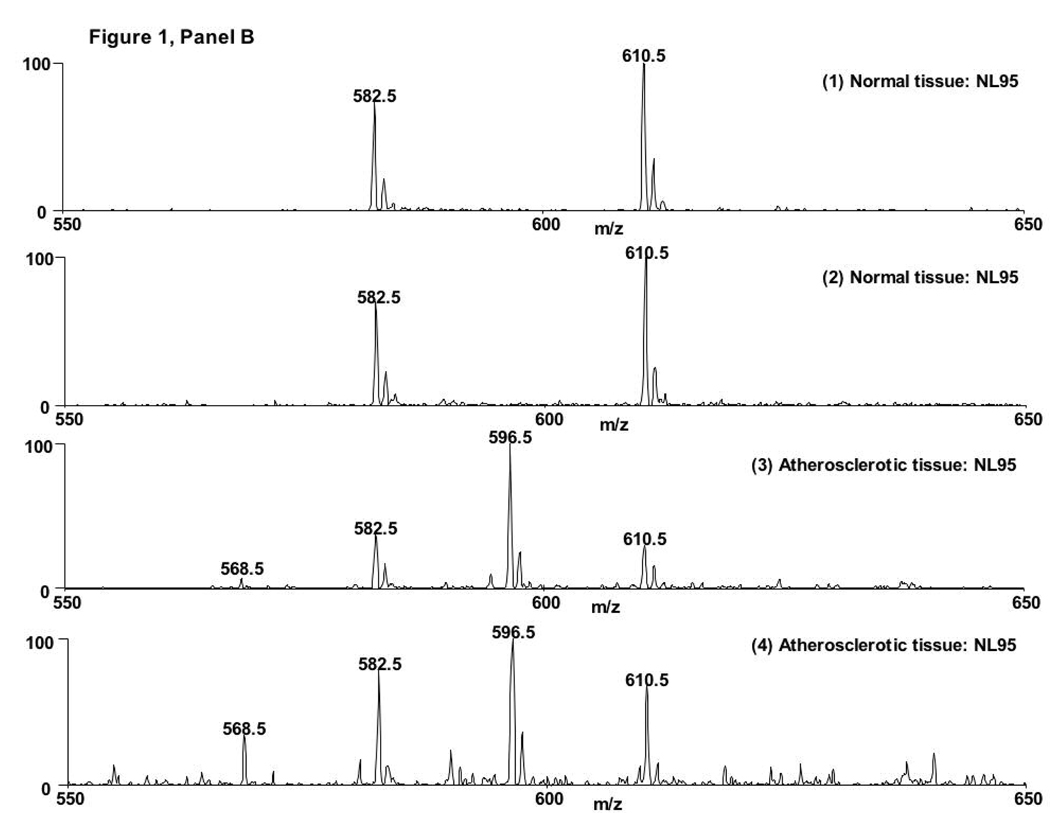
Lipid extracts from both atherosclerotic and normal human aorta samples were analyzed by ESI-MS to assess LPC-ClOH accumulation in atherosclerotic lesions. Initial inspection of the spectra from normal and atherosclerotic tissue in the positive ion mode show noticeable differences, qualitatively revealing an increase in LPC molecular species, which include m/z 518, 542, 544, 546, and 566, corresponding to 16:0, 18:2, 18:1, 18:0, and 20:4 LPC, respectively (Fig 1A). Neutral loss scanning of 95 amu, which corresponds to the combined neutral loss of the trimethylamine and HCl (8), was utilized to detect chlorinated choline glycerophospholipid species. Figure 1B (traces 1 and 2) show molecular ions with NL 95 scanning of molecular ions from normal tissue extracted in the presence of synthetic internal standards 17:0 and 19:0 LPC-ClOH at m/z 582 and 610, respectively. Figure 1B (traces 3 and 4) show molecular ions with NL 95 scanning from atherosclerotic tissue extracted in the presence of synthetic internal standards (e.g., m/z 582 and 610) and demonstrate the appearance of 18:0 LPC-ClOH at m/z 596. Atherosclerotic tissue also contains 16:0 LPC-ClOH (m/z 568).
Figure 1. 18:0 LPC-ClOH is detected in atherosclerotic tissue.
Lipids from normal and atherosclerotic aorta were analyzed by ESI-MS in positive ion mode with spectra from total ion current shown in (A) and neutral loss scanning of 95 amu (B). The spectra of molecular ions from normal tissue (B, spectra 1 and 2) show the synthetic internal standards 17:0 and 19:0 LPC-ClOH at m/z 582 and 610, respectively. The spectra of molecular ions atherosclerotic tissue contain 16:0 LPC-ClOH at m/z 568, 18:0 LPC-ClOH at m/z 596, and the synthetic internal standards (B, spectra 3 and 4).
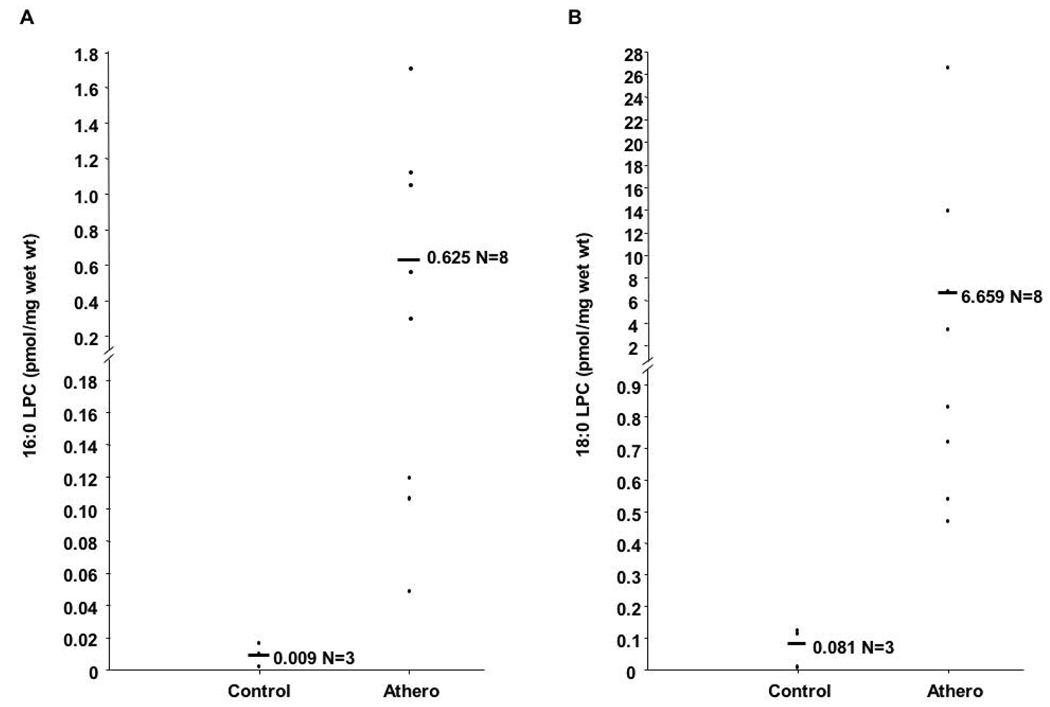
Utilizing neutral loss scanning of 95 amu, the relative abundance of 18:0 and 16:0 LPC-ClOH were compared to the relative intensities of the internal standards for quantification. Atherosclerotic tissue contains approximately 67-fold more 16:0 LPC-ClOH and 82-fold more 18:0 LPC-ClOH than normal tissue (Fig 2). However, 18:0 LPC-ClOH is about 10.7-fold more abundant than 16:0 LPC - ClOH. This likely reflects the abundance of 18:1 LPC compared to 16:1 LPC in atherosclerotic tissue (see Figure 1A, spectra 2). Other unsaturated LPC molecular species, such as 18:2 and 20:4 LPC, are elevated in atherosclerotic tissue compared to control tissue (Figure 1A), however chlorohydrin molecular species of LPC were not observed as derivatives of those LPCs. It should be noted that others have shown that chlorohydrins of phospholipids containing polyunsaturated fatty acids are unstable and are not readily detectable (15).
Figure 2. 16:0 and 18:0 LPC-ClOH molecular species are elevated in atherosclerotic tissue.
Lipids from normal and atherosclerotic aorta tissue were extracted and analyzed by direct infusion ESI-MS using neutral loss scanning of 95 amu in positive ion mode. Quantification of 16:0 and 18:0 LPC-ClOH was performed by comparison to the relative intensities of the internal standards 17:0 and 19:0 LPC-ClOH, respectively, as described in “Materials and Methods.”
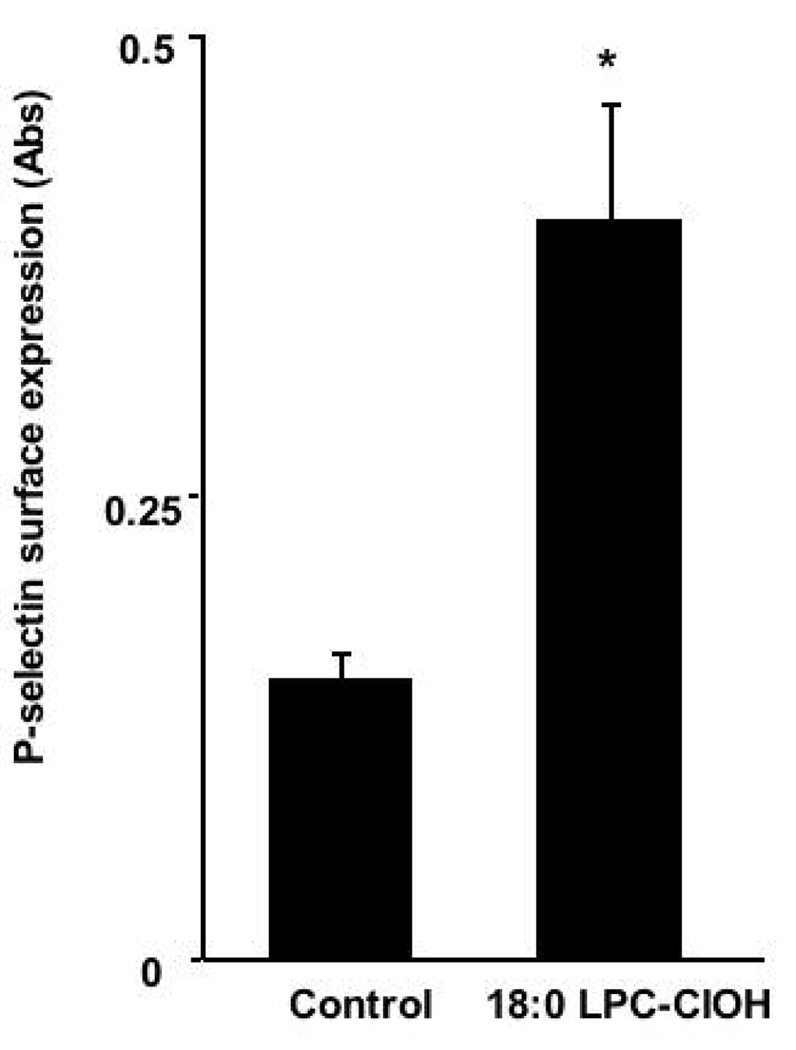
Endothelial activation, and eventual dysfunction, is recognized as a major hallmark in the development and progression of atherosclerosis (16). LPC induces a wide range of pro-atherogenic effects (17), however the effects of LPC-ClOHs are unknown. Two critical events in the development of atherosclerosis are cell-surface expression of proteins that tether blood monocytes to inflamed endothelium and release of chemotactic molecules (18). Figure 3 shows that 10 µM 18:0 LPC-ClOH elicits HCAEC surface expression of P-selectin at levels similar to that elicited by platelet activating factor (4). The results suggest that LPC-ClOH may function to recruit leukocytes to the endothelium by inducing an increase in coronary artery endothelial cell surface expression of P-selectin, an adhesion factor implicated in the development of vascular inflammation (19).
Figure 3. P-selectin is expressed on the surface of HCAEC following 18:0 LPC-ClOH treatment.
HCAEC were treated with Hank’s buffer alone (control) or buffer containing 10 µM 18:0 LPC-ClOH for 5 minutes. P-selectin surface expression was assayed and expressed as increased absorbance, p < 0.01.
Discussion
RCS attack of plasmalogens leads to the formation of LPC (6, 7) and chlorinated lipids such as 2-chloro-hexadecanal (2-ClHDA) (20, 21), and LPC-ClOH (7, 8). RCS attack of phospholipid alkene bonds occurs at a significantly lower rate than attack of protein tyrosines (22) and phospholipid vinyl ether bonds (8). ClOH formation in PCs occurs at an even lower rate than LPC (8). Additionally, with the exception of α-chloro fatty aldehydes (4), no studies to date have provided direct evidence of resident chlorinated lipids within atherosclerotic lesions. In this study, we utilized a soft ionization technique, ESI-MS/MS, and determined that human atherosclerotic tissue contains 67-fold more 16:0 and 82-fold more 18:0 LPC-ClOH than normal tissue.
LPC is elevated in atherosclerotic lesions (4) and in sera of people with atherosclerosis (23). In cultured endothelial cells, depending on concentration and cell culture conditions, LPC increases permeability (24, 25), induces chemotactic factors (26, 27), enhances production of ROS (28–30), decreases the synthesis of tissue factor pathway inhibitor (31) increases the secretion of matrix metalloproteinase 2 (32) and leads to death (33, 34). The LPC from oxidized LDL has been shown to impair endothelium-dependent relaxation in aortic ring preparations from several mammals (35, 36), and only long-chain LPCs are effective in impairing endothelium-dependent relaxation (37). The effect of LPC in certain cases, however, may be protective. LPC serum levels decrease in patients with advanced cancer (38) and sepsis (39), and administration of LPC led to decreased mortality in rodent models of sepsis from both Gram-positive and Gram-negative bacteria (40, 41). Additionally, it has been shown that extracellular application of LPC suppressed tissue factor expression in human monocytes (42) and prevented injury by activated neutrophils in isolated perfused lungs (43).
RCS attack of plasmenylcholine leads to the production of a chlorinated aldehyde (2-ClHDA), LPC, and LPC-ClOH (7–9, 20). Previous studies have demonstrated that 2-ClHDA is a potent neutrophil chemoattractant, while unsaturated LPC increases P-selectin surface expression on HCAEC (4, 6). The results from this study show that 18:0 LPC-ClOH also leads to increased P-selectin surface expression on HCAEC, which may function to recruit more MPO-containing leukocytes to sites of inflammation. RCS attack of plasmenylcholine may lead to the production of molecular species that lead to both leukocyte attraction and adhesion. In conclusion, the studies herein demonstrate that lysophosphatidylcholine-chlorohydrins are present in human atherosclerotic plaques. These data also explore the potential biological roles of LPC-ClOH, showing increased P-selectin surface expression on HCAEC, and the data provide additional evidence for the involvement of MPO in atherosclerosis. Further studies will be essential to delineate the complex biological properties of the chlorinated lipidome.
Abbreviations
- RCS
reactive chlorinating species
- LPC
lysophosphatidylcholine
- ClOH
chlorohydrin
- LPC-ClOH
lysophosphatidylcholine-chlorohydrin
- PC-ClOH
phosphatidylcholine-chlorohydrin
- FFA-ClOH
free fatty acid chlorohydrin
- HCAEC
human coronary artery endothelial cells
- MPO
myeloperoxidase
- GC–MS
gas chromatography–mass spectrometry
- ESI-MS
electrospray ionization-mass spectrometry
References
- 1.Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 2.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thukkani AK, McHowat J, Hsu FF, Brennan ML, Hazen SL, Ford DA. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 2003;108:3128–3133. doi: 10.1161/01.CIR.0000104564.01539.6A. [DOI] [PubMed] [Google Scholar]
- 5.Vance JE. Lipoproteins secreted by cultured rat hepatocytes contain the antioxidant 1-alk-1-enyl-2-acylglycerophosphoethanolamine. Biochim Biophys Acta. 1990;1045:128–134. doi: 10.1016/0005-2760(90)90141-j. [DOI] [PubMed] [Google Scholar]
- 6.Thukkani AK, Hsu FF, Crowley JR, Wysolmerski RB, Albert CJ, Ford DA. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J Biol Chem. 2002;277:3842–3849. doi: 10.1074/jbc.M109489200. [DOI] [PubMed] [Google Scholar]
- 7.Lessig J, Schiller J, Arnhold J, Fuchs B. Hypochlorous acid-mediated generation of glycerophosphocholine from unsaturated plasmalogen glycerophosphocholine lipids. J Lipid Res. 2007;48:1316–1324. doi: 10.1194/jlr.M600478-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Messner MC, Albert CJ, Hsu FF, Ford DA. Selective plasmenylcholine oxidation by hypochlorous acid: formation of lysophosphatidylcholine chlorohydrins. Chem Phys Lipids. 2006;144:34–44. doi: 10.1016/j.chemphyslip.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Panasenko OM, Vakhrusheva T, Tretyakov V, Spalteholz H, Arnhold J. Influence of chloride on modification of unsaturated phosphatidylcholines by the myeloperoxidase/hydrogen peroxide/bromide system. Chem Phys Lipids. 2007;149:40–51. doi: 10.1016/j.chemphyslip.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Hazen SL, Hsu FF, Duffin K, Heinecke JW. Molecular chlorine generated by the myeloperoxidase-hydrogen peroxide-chloride system of phagocytes converts low density lipoprotein cholesterol into a family of chlorinated sterols. J Biol Chem. 1996;271:23080–23088. doi: 10.1074/jbc.271.38.23080. [DOI] [PubMed] [Google Scholar]
- 11.Thomas EL, Fishman M. Oxidation of chloride and thiocyanate by isolated leukocytes. J Biol Chem. 1986;261:9694–9702. [PubMed] [Google Scholar]
- 12.Dittmer JC, Douglas MG. Quantitative determination of phosphoinositides. Ann N Y Acad Sci. 1969;165:515–525. [PubMed] [Google Scholar]
- 13.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purificationq. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 14.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 15.Panasenko OM, Spalteholz H, Schiller J, Arnhold J. Myeloperoxidase-induced formation of chlorohydrins and lysophospholipids from unsaturated phosphatidylcholines. Free Radic Biol Med. 2003;34:553–562. doi: 10.1016/s0891-5849(02)01358-8. [DOI] [PubMed] [Google Scholar]
- 16.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 17.Kougias P, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Lysophosphatidylcholine and secretory phospholipase A2 in vascular disease: mediators of endothelial dysfunction and atherosclerosis. Med Sci Monit. 2006;12:RA5–RA16. [PubMed] [Google Scholar]
- 18.Ley K, Reutershan J. Leucocyte-endothelial interactions in health and disease. Handb Exp Pharmacol. 2006:97–133. doi: 10.1007/3-540-36028-x_4. [DOI] [PubMed] [Google Scholar]
- 19.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24:2166–2179. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J Biol Chem. 2001;276:23733–23741. doi: 10.1074/jbc.M101447200. [DOI] [PubMed] [Google Scholar]
- 21.Albert MA, Danielson E, Rifai N, Ridker PM, Investigators P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 22.Pattison DI, Hawkins CL, Davies MJ. Hypochlorous acid-mediated oxidation of lipid components and antioxidants present in low-density lipoproteins: absolute rate constants, product analysis, and computational modeling. Chem Res Toxicol. 2003;16:439–449. doi: 10.1021/tx025670s. [DOI] [PubMed] [Google Scholar]
- 23.Lavi S, McConnell JP, Rihal CS, Prasad A, Mathew V, Lerman LO, Lerman A. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115:2715–2721. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 24.Leung YM, Xion Y, Ou YJ, Kwan CY. Perturbation by lysophosphatidylcholine of membrane permeability in cultured vascular smooth muscle and endothelial cells. Life Sci. 1998;63:965–973. doi: 10.1016/s0024-3205(98)00354-3. [DOI] [PubMed] [Google Scholar]
- 25.Huang F, Subbaiah PV, Holian O, Zhang J, Johnson A, Gertzberg N, Lum H. Lysophosphatidylcholine increases endothelial permeability: role of PKCalpha and RhoA cross talk. Am J Physiol. 2005;289:L176–L185. doi: 10.1152/ajplung.00003.2005. [DOI] [PubMed] [Google Scholar]
- 26.Murugesan G, Sandhya Rani MR, Gerber CE, Mukhopadhyay C, Ransohoff RM, Chisolm GM, Kottke-Marchant K. Lysophosphatidylcholine regulates human microvascular endothelial cell expression of chemokines. J Mol Cell Cardiol. 2003;35:1375–1384. doi: 10.1016/j.yjmcc.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Takahara N, Kashiwagi A, Maegawa H, Shigeta Y. Lysophosphatidylcholine stimulates the expression and production of MCP-1 by human vascular endothelial cells. Metabolism. 1996;45:559–564. doi: 10.1016/s0026-0495(96)90024-4. [DOI] [PubMed] [Google Scholar]
- 28.Takeshita S, Inoue N, Gao D, Rikitake Y, Kawashima S, Tawa R, Sakurai H, Yokoyama M. Lysophosphatidylcholine enhances superoxide anions production via endothelial NADH/NADPH oxidase. J Atheroscler Thromb. 2000;7:238–246. doi: 10.5551/jat1994.7.238. [DOI] [PubMed] [Google Scholar]
- 29.Zhou L, Shi M, Guo Z, Brisbon W, Hoover R, Yang H. Different cytotoxic injuries induced by lysophosphatidylcholine and 7-ketocholesterol in mouse endothelial cells. Endothelium. 2006;13:213–226. doi: 10.1080/10623320600780926. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe N, Zmijewski JW, Takabe W, Umezu-Goto M, Le Goffe C, Sekine A, Landar A, Watanabe A, Aoki J, Arai H, Kodama T, Murphy MP, Kalyanaraman R, Darley-Usmar VM, Noguchi N. Activation of mitogen-activated protein kinases by lysophosphatidylcholine-induced mitochondrial reactive oxygen species generation in endothelial cells. Am J Pathol. 2006;168:1737–1748. doi: 10.2353/ajpath.2006.050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato N, Kokame K, Shimokado K, Kato H, Miyata T. Changes of gene expression by lysophosphatidylcholine in vascular endothelial cells: 12 up-regulated distinct genes including 5 cell growth-related, 3 thrombosis-related, and 4 others. J Biochem (Tokyo) 1998;123:1119–1126. doi: 10.1093/oxfordjournals.jbchem.a022051. [DOI] [PubMed] [Google Scholar]
- 32.Inoue N, Takeshita S, Gao D, Ishida T, Kawashima S, Akita H, Tawa R, Sakurai H, Yokoyama M. Lysophosphatidylcholine increases the secretion of matrix metalloproteinase 2 through the activation of NADH/NADPH oxidase in cultured aortic endothelial cells. Atherosclerosis. 2001;155:45–52. doi: 10.1016/s0021-9150(00)00530-x. [DOI] [PubMed] [Google Scholar]
- 33.Sata M, Walsh K. Endothelial cell apoptosis induced by oxidized LDL is associated with the down-regulation of the cellular caspase inhibitor FLIP. J Biol Chem. 1998;273:33103–33106. doi: 10.1074/jbc.273.50.33103. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi M, Okazaki H, Ogata Y, Takeuchi K, Ikeda U, Shimada K. Lysophosphatidylcholine induces apoptosis in human endothelial cells through a p38-mitogen-activated protein kinase-dependent mechanism. Atherosclerosis. 2002;161:387–394. doi: 10.1016/s0021-9150(01)00674-8. [DOI] [PubMed] [Google Scholar]
- 35.Kugiyama K, Kerns SA, Morrisett JD, Roberts R, Henry PD. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990;344:160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- 36.Hirata K, Miki N, Kuroda Y, Sakoda T, Kawashima S, Yokoyama M. Low concentration of oxidized low-density lipoprotein and lysophosphatidylcholine upregulate constitutive nitric oxide synthase mRNA expression in bovine aortic endothelial cells. Circ Res. 1995;76:958–962. doi: 10.1161/01.res.76.6.958. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Liang B, Froese DE, Liu S, Wong JT, Tran K, Hatch GM, Mymin D, Kroeger EA, Man RY, Choy PC. Oxidative modification of low density lipoprotein in normal and hyperlipidemic patients: effect of lysophosphatidylcholine composition on vascular relaxation. J Lipid Res. 1997;38:546–553. [PubMed] [Google Scholar]
- 38.Taylor LA, Arends J, Hodina AK, Unger C, Massing U. Plasma lysophosphatidylcholine concentration is decreased in cancer patients with weight loss and activated inflammatory status. Lipids Health Dis. 2007;6:17. doi: 10.1186/1476-511X-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drobnik W, Liebisch G, Audebert FX, Frohlich D, Gluck T, Vogel P, Rothe G, Schmitz G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J Lipid Res. 2003;44:754–761. doi: 10.1194/jlr.M200401-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 41.Murch O, Collin M, Sepodes B, Foster SJ, Mota-Filipe H, Thiemermann C. Lysophosphatidylcholine reduces the organ injury and dysfunction in rodent models of gram-negative and gram-positive shock. Br J Pharmacol. 2006;148:769–777. doi: 10.1038/sj.bjp.0706788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engelmann B, Zieseniss S, Brand K, Page S, Lentschat A, Ulmer AJ, Gerlach E. Tissue factor expression of human monocytes is suppressed by lysophosphatidylcholine. Arterioscler Thromb Vasc Biol. 1999;19:47–53. doi: 10.1161/01.atv.19.1.47. [DOI] [PubMed] [Google Scholar]
- 43.Lin P, Welch EJ, Gao XP, Malik AB, Ye RD. Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. J Immunol. 2005;174:2981–2989. doi: 10.4049/jimmunol.174.5.2981. [DOI] [PubMed] [Google Scholar]