Abstract
Objective
To establish safe dosing protocols for the treatment of Meniere’s patients with intratympanic gentamicin.
Study Design
A validated computer model of gentamicin dispersion in the inner ear fluids was used to calculate cochlear drug levels resulting from specific clinical delivery protocols. Dosing in the cochlea was compared with changes of hearing sensitivity for 568 patients following 19 clinical protocols.
Methods
Cochlear drug levels were calculated based on the concentration and volume of gentamicin applied, the time the drug remained in the middle ear, and on the specific timing of injections. Time courses were quantified in terms of the maximum concentration (Cmax) and the area under the curve (AUC) of the drug at specific cochlear locations.
Results
Drug levels resulting from single, “one-shot” injections were typically lower than those from repeated or continuous application protocols. Comparison of hearing sensitivity changes with gentamicin dosing revealed a flat curve with a near-zero mean for lower doses, suggesting that hearing changes with doses over this range were probably unrelated to the applied drug. Higher intracochlear doses were generated with repeated or continuous delivery protocols, which in some cases caused substantial hearing losses and an increased incidence of deafened ears.
Conclusions
One-shot application protocols produce gentamicin doses in the cochlea that have minimal risk to hearing at the frequencies tested. Repeated or continuous application protocols result in higher doses that in some cases damage hearing. The high variability of hearing changes even with low gentamicin doses, calls into question the rationale for using individual hearing changes to titrate the applied dose.
Keywords: Meniere’s disease, endolymphatic hydrops, ototoxicity, intratympanic, gentamicin
Introduction
Treatments of inner ear disorders such as Meniere’s disease, idiopathic sudden sensorineural hearing loss and tinnitus are increasingly using drugs applied locally to the inner ear by intratympanic injections. The methodology is largely based on the success of local gentamicin therapy as a treatment for Meniere’s disease, which was popularized by Lange 1, 2, 3. The present dosing regimens for gentamicin were developed in many clinical studies over the past 40 years, in which suppression of vestibular symptoms or vestibular sensitivity, indicating an effective dose, was balanced against the amount of hearing loss caused by the treatment, as an index of whether overdosing had occurred. In these clinical studies, there are large variations of the applied drug concentration, of the dosing protocols (such as timing and volume injected) and in the application methods (such as single or multiple injections or continuous infusion) that make it difficult to compare patient outcome with gentamicin dosing in a quantitative way. As a result, prior reviews have not been able to compare dosing strategies quantitatively and the optimum gentamicin application protocol has yet to be established.
In recent years, we have developed a computer simulation program that calculates drug dispersal in the inner ear, taking into account scala dimensions, round window (RW) membrane permeability, drug diffusion, longitudinal perilymph flow, communications with other fluid compartments of the ear and clearance of drug to the vascular system. The model was able to closely represent the measured dispersal of ionic markers along scala tympani (ST) in guinea pigs 4, 5. Plontke et al. 6 used this model to quantify and interpret pharmacokinetic measurements of gentamicin distribution in animals, using data from samples taken from the vestibule of chinchillas 7, 8, 9. Based on the simulations, it was predicted that substantial gradients for gentamicin were likely to exist along the length of ST6. In view of this analysis, it was suggested that the lower levels of gentamicin reaching the apical regions of the cochlea could explain the preservation of hearing in humans following intratympanic application. The existence of substantial gentamicin gradients along ST perilymph has recently been confirmed experimentally, using fluid samples obtained by sequential sampling from the cochlear apex10. In conjunction with computer simulations, these sample data have allowed gentamicin distribution along the length of ST to be documented and quantified.
In the present study, we have made use of this established model of gentamicin dispersal in the cochlear fluids to estimate perilymph gentamicin levels following specific application protocols in humans. A limitation of the present analysis is that we cannot yet reliably predict drug levels in the fluid spaces of the vestibular system, due to the complex geometry of the vestibular spaces and the absence of measurements representing vestibular drug levels. The present analysis has therefore been restricted to cochlea gentamicin levels and their relationship to hearing loss. The simulations used scala dimensions and RW membrane area corresponding the human cochlea and pharmacokinetic parameters for gentamicin derived from animal studies. In the absence of suitable gentamicin measurements of human perilymph, drug concentrations predicted here are only estimates and actual values may be skewed by inter-species differences of physiology. Nevertheless, the simulations allow treatment protocols to be compared and ranked quantitatively for conditions comparable to those in the human cochlea.
Studies included in the analysis were those that provided specific details of the gentamicin protocol applied to each patient, including the concentration of applied drug, the injected volume, the duration of application of the drug (typically how long the patient remained supine) and the number of injections given. In addition, the study needed to include measures of hearing sensitivity, specifying the test frequencies used for individual patients before and after the therapy. Nineteen published reports, containing the results from 568 patients, fulfilled these requirements11,12, 13,14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29.
Methods
The simulation program used to calculate intracochlear gentamicin concentrations was identical to that available for download at http://oto.wustl.edu/cochlea/model/, with the exception that it was modified to include calculations of Cmax and area under the curve (AUC) for all locations along the length of ST. The physical processes underlying the spread of gentamicin in the inner ear that are incorporated into the simulations are shown in Figure 1. The parameters defining gentamicin distribution were primarily based on those derived by fitting data from experimental animals6, 10. The diffusion coefficient used for gentamicin was 0.72 × 10−9 m2/s, based on the known dependence of diffusion on molecular size and using a formula weight for gentamicin of 466. Scala dimensions as a function of distance along the human cochlea were based on a 3D imaging study30 and the area of the RW used was 2.29 mm2 31. The other parameters used (with values in parentheses) include: Permeability of the RW to gentamicin (3.0 × 10−8 m/s), clearance from the middle ear (half-time 500 min), clearance from cochlear fluids to blood (half-time 1500 min), and the rate of communication of ST with the endolymphatic space and SV (half-time 45 min). In addition, the rate of communication with other fluid spaces of the ear, including the modiolus, the organ of Corti and the spiral ligament were incorporated as a fluid space running parallel to ST with cross-sectional area 0.3 mm2 with access to ST limited by setting communication to a value of 0.25 times that of free diffusion. Selection of the rate of perilymph flow rate in the sealed, intact cochlea was problematic. In direct measurements with flow markers4, 32, very low perilymph flow rates (0.002 and 0.004 μl/min respectively) were required to account for the spread of marker. In three recent studies in which perilymph was sampled from the cochlear apex, drugs were found to spread towards the apex slightly faster than could be explained by diffusion. This was accounted for by adding apically-directed flow along ST at rates which averaged: 0.019 μl/min (TMPA)5; 0.021 μl/min (gentamicin)10 and 0.009 μl/min (dexamethasone)33. There is still some uncertainty whether these low rates of flow are real or artifactual, possibly caused by dehydration of the cochlea or of the entire animal during longer duration experiments with the cochlea exposed. In addition, the limited patency of the human cochlear aqueduct raises the possibility that perilymph flow may not occur at a similar rate in humans. We therefore calculated gentamicin distribution with zero perilymph flow (presented here) and with a flow rate of 0.022 μl/min, representing the upper limit of the mean rate seen in animals. Results at the two rates were numerically different but were highly correlated and did not affect the ranking of therapies so only the zero-flow calculations are presented here. It should also be emphasized that some of the model parameter values above represent “best estimates”, based on the limited pharmacokinetic data presently available. For example, as gentamicin clearance to blood occurs slowly it cannot be quantified in experiments of just a few hours duration10. We know that the clearance rate of 505 mins half-time derived in Plontke et al’s analysis6 of Hoffer et al.’s data7, 8, 9 is likely to be an overestimate. In these studies, drug was delivered to the RW niche in a fibrin glue base. A sustained communication between ST and the middle ear due to the presence of fibrin gel allows middle ear clearance to dominate cochlear kinetics and clearance would likely occur more slowly with an air-filled RW niche. Other studies have suggested that gentamicin clearance from cochlear tissues occurs more slowly, with half-times ranging from 360 min to over 5000 min following systemic infusions in rats34. Thus the clearance rate used (1500 min half-time) represents a balance between estimates from different sources.
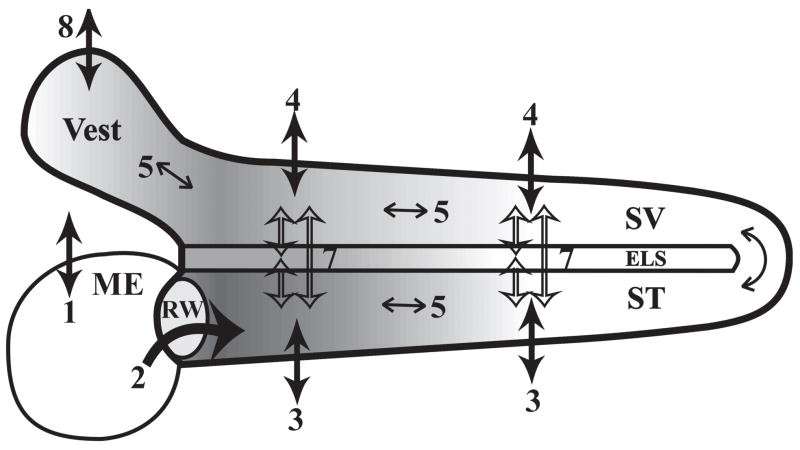
Figure 1.
Processes involved in gentamicin distribution in the inner ear (adapted from Plontke et al.6. They include 1) Clearance from the middle ear (ME); 2) Permeability of the round window (RW) membrane; 3) Clearance from scala tympani (ST) to blood; 4) Clearance from scala vestibuli (SV) to blood; 5) Diffusion along the scalae; 6) ST-SV communication through the helicotrema; 7) Inter scala communications (ST-endolymphatic space (ELS), SV-ELS, ST-SV); 8) Clearance from the vestibule (Vest) to blood.
Results
A summary of the parameters used to simulate the gentamicin application protocols in the 19 studies analyzed is shown in Table 1. The concentration of gentamicin applied varied from 10 to 80 mg/ml, with most studies using between 20 and 30 mg/ml. The duration of the application (how long drug remained on the RW membrane) was an important factor influencing the drug level achieved in perilymph. We used the time the patient was supine and discouraged from swallowing as the application time, assuming that the drug was rapidly cleared from the middle ear when the patient resumed normal activities. Clearance from the middle ear was set at 500 min half-time during the application time but as most application durations were brief, ranging from 20 – 60 min (Table 1), the applied dose was minimally affected by clearance at this rate. In the two studies in which drug was applied through an indwelling cannula (N, Q), the applied concentration was assumed to be maintained at the round window for the entire application duration. The volume of drug injected into the middle ear is shown in the table and the simulations used the stated volume but this parameter has little influence on the perilymph drug level achieved. With very small applied volumes, the amount of drug moving into the cochlea could cause the drug concentration in the RW niche to decline with time. However, given the low permeability of the RWM and the volumes typically applied (0.3 ml to 1.0 ml), the proportion of the applied drug entering the cochlea is so small that this loss has minimal influence on the drug concentration remaining in the RW niche. For example, under the protocol of Driscoll et al.12 the volumes applied ranged from 250 – 2000 μl. Changing the applied volume from 250 to 2000 μl in this protocol with all other parameters unchanged did not affect the calculated drug level at any ST location by more that 0.05%.
Table 1.
Summary of the parameters used to simulate each study and the calculated maximum concentration (Cmax) and area under the curve (AUC) averaged for the cochlear locations corresponding to the frequencies tested. Injections separated by 3 or more days were regarded as a separate treatment, with the same Cmax but adding to the AUC as a multiple of the number of times the treatment cycle was performed. AUC values given are for a single treatment cycle.
| Letter | Authors | Applied Concentration (mg/ml) |
Applied Volume (μl) |
Application Duration ( min) |
Injection Protocol |
Number of Injections per Treatment |
Number of Treatment Cycles |
Audiometric Frequencies Tested (kHz) |
Cmax (μg/ml) |
Cmax (% of conc. applied) |
AUC for a single treatment cycle (mg·min/ml) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Nedzelski et al. (1993) | 26.7 | 1000 | 30 | 3 per day for 4 days | 12 | 1–2 | 0.5, 1, 2, 3 | 105 | 0.39 | 641 |
| B | Driscoll et al. (1997) | 40 | 250–2000 | 40 | 1 per 14 days | 1 | 1–3 | 0.5, 1, 2, 3 | 38 | 0.10 | 106 |
| C | Hirsch & Kamerer (1997) | 30 | 400 | 20 | 1 per 3 days | 1 | 1–9 | 0.5, 1, 2, 3 | 15 | 0.05 | 34 |
| D | Murofushi et al. (1997) | 30 | 750 | 60 | 1 per day for 2–5 days | 2–5 | 1–2 | 1 | 47–66 | 0.16–0.22 | 189–277 |
| E | Rauch & Oas (1997) | 40 | 500 | 60 | 1 or 2 per day | 2–7 | 1–8 | 0.5, 1, 2 | 68–130 | 0.17–0.33 | 245–3167 |
| F | McFeely et al. (1998) | 26.7 | 1000 | 30 | 3 per day for 4 days | 12 | 1 | 0.5, 1, 2, 3 | 111 | 0.42 | 424 |
| G | Pfleiderer (1998) | 26 | 650 | 30 | 3 per day for 4 days | 12 | 1–3 | 0.5, 1, 2, 4 | 108 | 0.42 | 413 |
| H | Youssef & Poe (1998) | 30 | 1000 | 45 | 1 per 7 days | 1 | 1–8 | 0.5, 1, 2, 4 | 37 | 0.12 | 94 |
| I | Minor (1999) | 26.7 | 450 | 30 | 1 per 7 days | 1 | 1–6 | 0.5, 1, 2, 3 | 19 | 0.07 | 53 |
| J | Quaranta et al. (1999) | 80 | 500 | 20 | 1 per 6 days | 1 | 2–3 | 0.5, 1, 2, 3 | 40 | 0.05 | 215 |
| K | Silverstein et al. (1999) | 26.7 | 250 | 30 | 1 per 5 days | 1 | 1–2 | 0.5, 1, 2, 3 | 19–20 | 0.07–0.08 | 52–90 |
| L | Kaplan et al. (2000) | 26.7 | 750 | 30 | 3 per day for 4 days | 12 | 1 | 0.5, 1, 2, 3 | 105 | 0.39 | 641 |
| M | Quaranta et al. (2001) | 20 | 400 | 20 | 1 per 7 days | 1 | 2–4 | 0.5, 1, 2, 3 | 10 | 0.05 | 28 |
| N | Schoendorf et al. (2001) | 40 | - | 2160–25920 | 1.5–18 days | 1 | 1 | 0.5, 1, 2, 3 | 1401–2237 | 3.50–5.59 | 5224–58484 |
| O | Abou-Halawa & Poe (2002) | 30–40 | 1000 | 45 | 1 per 7 days | 1 | 1–8 | 0.5, 1, 2, 3 | 32–48 | 0.11–0.12 | 88–117 |
| P | Wu and Minor (2003) | 26.7 | 400 | 30 | 1 per 7 days | 1 | 1–7 | 0.5, 1, 2, 3 | 19 | 0.07 | 53 |
| Q | Suryanarayanan & Cook (2004) | 10 | - | 14400 | 10 days | 1 | 1 | 0.5, 1, 2, 4 | 559 | 5.59 | 8192 |
| R | Takai et al. (2006) | 30 | 700 | 60 | 1 per 5 days | 5 | 1 | 0.5, 1, 2, 4 | 97 | 0.32 | 622 |
| S | De Beer et al. (2007) | 30 | 750 | 45 | 1 per 30 days | 1 | 1–10 | 0.5, 1, 2, 4 | 36 | 0.12 | 86 |
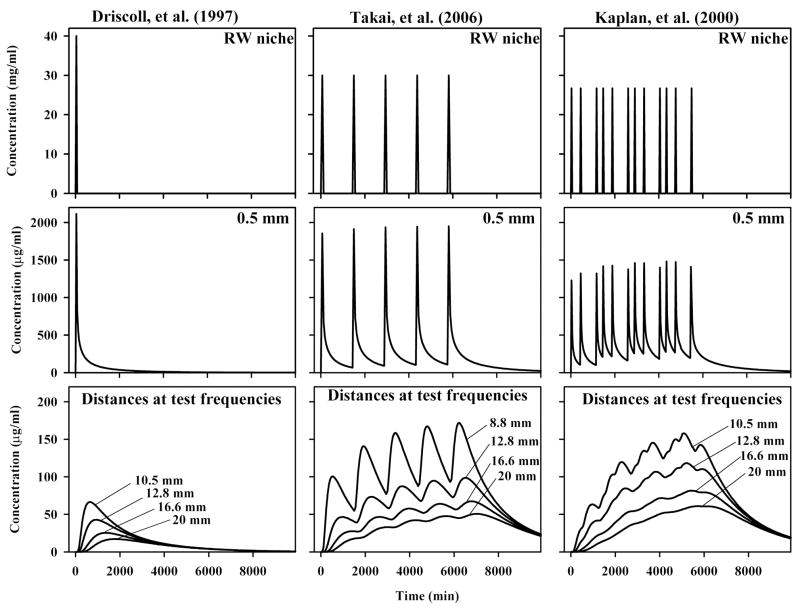
Calculations of repeated drug applications showed that injections 3 or more days apart could be regarded as independent events and in this analysis were regarded as separate treatments. For repeated injections occurring with less than 3 days separation, all injections of the series were regarded as being part of the same treatment and were included in the simulation of that treatment. Simulations of three example application protocols are shown in Figure 2. The three rows show the drug time course in the middle ear (upper row), in the perilymph of the basal turn near the round window 0.5 mm from the base of the scala (middle row), and at the scala tympani locations corresponding to the acoustic frequencies tested audiometrically (bottom row). Following a “one-shot” protocol, as used by Driscoll et al.12, the basal turn perilymph peaked at 2160 μg/ml (approximately 5% of the applied concentration) and then declined. Concentrations were calculated for the locations representing the regions tested audiometrically, based on the relationship documented by Greenwood 35, with the distance scaled to represent the 28.5 mm length of scala tympani30. The calculated doses reaching these regions are much lower (note the axis scaling is expanded) and occur with substantially slower time courses. The peak concentrations (with times of peaks in parentheses) were 66.5 μg/ml (640 min), 41.7 μg/ml (960 min), 25.7 μg/ml (1360 min) and 17.1 μg/ml (1800 min) for locations in scala tympani 10.5, 13, 16.5 and 20 mm from the base respectively, corresponding to test frequencies at 3, 2, 1 and 0.5 kHz. Cmax and AUC were calculated for the 4 curves and averaged, in this case resulting in an average Cmax of 38 μg/ml and AUC of 106 mg·min/ml. This Cmax represented 0.1 % of the applied concentration, a figure that was calculated for all studies and is given in Table 1. Concentration time courses calculated for a protocol of daily injections as used by Takai et al.28 are shown in the middle column. The peaks calculated for the basal turn at 0.5 mm each appear of similar height, with little apparent accumulation of drug as the injections are repeated. In contrast, at the distances corresponding to the frequencies tested, the gentamicin concentration can be seen to build up with each additional injection. Thus, with repeated injections higher doses are obtained at the more-apical cochlear locations. In this case Cmax and AUC at the tested locations were 97 μg/ml and 622 mg·min/ml respectively, with Cmax corresponding to 0.32% of the applied concentration. The importance of application protocol is demonstrated further by the calculated levels when drug is applied 3 times a day (right column) following the protocol described by Kaplan et al.22. The increase in the basal turn for each application is a little lower, as the application duration is shorter with this protocol, but the drug level builds up to a greater degree. The gentamicin levels at distances representing the frequency regions tested are seen to increase progressively throughout the application period. Cmax and AUC were 105 μg/ml and 641 mg·min/ml respectively, with Cmax corresponding to 0.39 % of the applied concentration. These calculations demonstrate that the drug levels reaching the cochlear locations important for hearing are highly dependent on application protocol. The calculated Cmax and AUC values for all of the studies are shown in Table 1.
Figure 2.
Calculated gentamicin dosing for 3 protocols, specifically a single injection (Driscoll et al.12), daily injections (Takei et al.28) and 3 times daily injections (Kaplan et al.22). In each case, the dose applied to the RW niche is given (top row) and the level produced in the basal turn of scala tympani near the RW membrane, 0.5 mm from the base (middle row). The bottom row shows concentration time courses at the cochlear locations corresponding to the frequencies tested, which are (with distances from the base of ST shown in parentheses): 4000 Hz (8.8 mm), 3000 Hz (10.5 mm), 2000 Hz (12.8 mm), 1000 Hz (16.6 mm), 500 Hz (20 mm). Cmax and AUC were calculated for each of the four curves and the four values were averaged for comparison with hearing sensitivity changes. For the repeated application protocols there is little accumulation of drug in the basal turn (0.5 mm), but there is a pronounced cumulative effect for cochlear locations more distant (10 – 20 mm) from the application site.
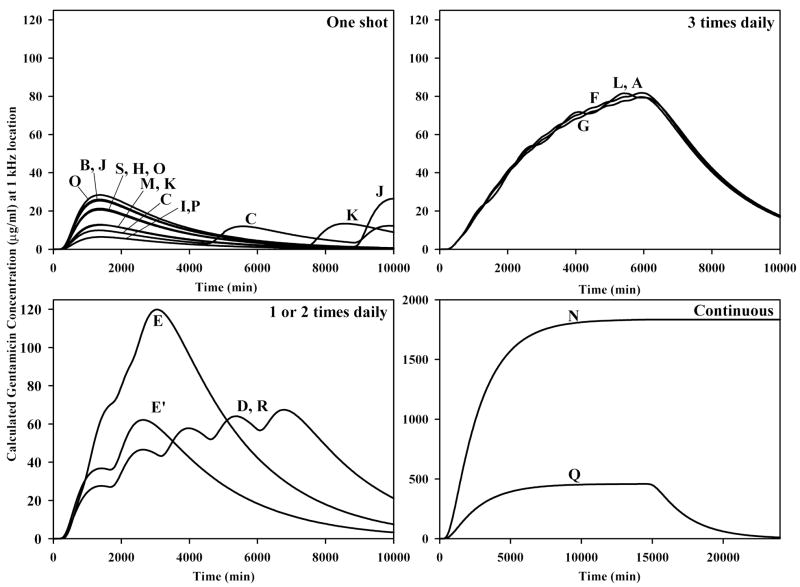
A comparison of the drug dosing reaching the 1 kHz region of the cochlea (16.6 mm from the base of ST) for all 19 studies analyzed is shown in Figure 3. The protocols have been grouped in terms of “one-shot” applications (upper left), applications 1 or 2 times daily (lower left), applications 3 times daily (upper right) and continuous delivery (lower right). The letters refer to the specific studies as indicated in Table 1. The curves show that there is virtually no accumulation when applications are given separated by 3 or more days (4320 min). When the drug application is repeated daily, there is a slow build-up of drug. The build-up is progressively greater with twice daily, 3 times daily and continuous applications. This analysis confirms the important role of the application protocol in determining how much gentamicin reaches regions of the cochlear important for hearing.
Figure 3.
Calculated concentrations at the 1 kHz region of the cochlea (16.6 mm from the base of ST) for all the application protocols analyzed in this study. The letter on each curve refers to the specific study indicated in Table 1. With one-shot protocols repeated at 3 or more days apart (C, K, J) there is negligible accumulation of drug at this location so each injection is regarded as an independent treatment. With daily dosing (E′, D, R) there is a slow build up of concentration with each injection. The rate of build up is greater with twice daily injections (E) and 3 times daily injections (A, F, G, L). With continuous application protocols, much higher doses reach this region (N, Q). Note the 16x difference in axis scaling used for the continuous injection protocol plots.
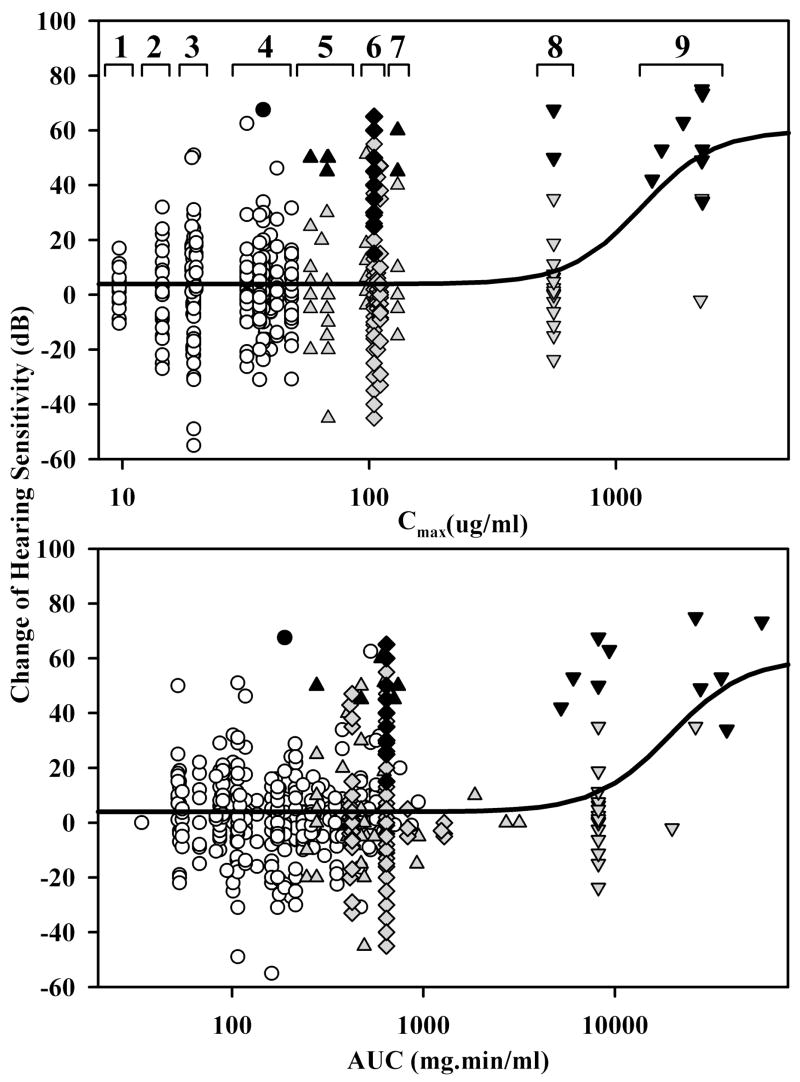
The relationship between calculated gentamicin concentrations, quantified as Cmax or AUC averaged for the cochlear locations corresponding to the frequencies tested, and the measured functional changes reported for the 568 patients in 19 studies are summarized in Figure 4. Since the calculated Cmax remains unchanged with repeated treatment cycles, the specific studies included in each dosage group have been indicated on this plot. The curves shown are Hill functions that were fitted to the data, with an EC50 for Cmax of 1267 μg/ml and EC50 for AUC of 19,403 mg·min/ml respectively. It is apparent that for the highest doses of gentamicin, the risk to hearing and incidence of deafness (as indicated by black symbols) after the treatment are increased. It is also notable that for one-shot treatments (open circles) the risk to hearing, as indicated by the average change of PTA at the specified frequencies, is minimal. The mean hearing change for those one-shot protocols with the lowest doses (Figure 4, groups 1, 2 and 3; all of which generated Cmax < 20 μg/ml) was 2.2 dB (n=127). The variation was large (SD 16.2 dB). However, these were the only groups with no deafened patients. Calculated AUC values compared similarly with the hearing losses (Figure 4, lower panel), with no indication that Cmax or AUC provides a better indication of the toxic level.
Figure 4.
Dependence of reported hearing sensitivity changes on the calculated gentamicin dose at the locations along scala tympani corresponding to the auditory frequencies tested. Dose was quantified either as the maximum concentration (Cmax: upper plot) or as the area under the curve (AUC: lower plot) averaged for specific locations in each study. The symbols represent the four delivery protocols depicted in Figure 3 (circles: one-shot; triangles: one or two times daily; diamonds: 3 times daily; inverted trangles: continuous). Open circles highlight the one shot protocol, while multiple injection (diamonds, triangles) and continuous protocols (inverted triangles) are shown shaded. Solid black symbols indicate patients who were deaf after the gentamicin treatment. Details of the fitted Hill functions are given in the text. In the calculation of Cmax, the studies from Table 1 within each of the numbered groups were 1: M; 2: C; 3: I, K, P; 4: B, H, J, O, S; 5: D, E; 6: A, F, G, L, R; 7: E; 8: Q; 9: N.
Discussion
Computer simulations of clinical treatment protocols allow drug dosing to be compared with patient outcome. By analyzing a disparate range of protocols, the analysis permits a larger range of dosing to be represented than would be possible for a single study or single delivery protocol. It is demonstrated that continuous delivery or multiple-injection protocols are expected to produce higher gentamicin concentrations in the regions of the cochlea related to hearing and are shown here to be associated with increased risk of hearing loss and deafness. Conversely, the one-shot protocol, in which the amount of gentamicin reaching auditory regions is expected to be substantially lower, is associated with a lower risk to the hearing of patients. The simulations demonstrate that the steepest basal-apical gradients of gentamicin occur after a one-shot application, while repeated or prolonged applications allow levels in the important auditory regions to build up, resulting in lower basal-apical drug gradients. In view of this dependence, there appears no logical rationale for rapidly-repeated or continuous applications of gentamicin in the treatment of Meniere’s disease. If the intended goal of the therapy was to apply drug throughout the length of the cochlea (such as in the use of a locally-applied steroid to treat acute sudden sensorineural hearing loss) then repeated or continuous delivery protocols may be more appropriate. In the treatment of Meniere’s disease with gentamicin, where the goal is to preserve hearing by minimizing the drug level in the higher regions of the cochlea, then the one-shot application protocol is demonstrated here to be preferable.
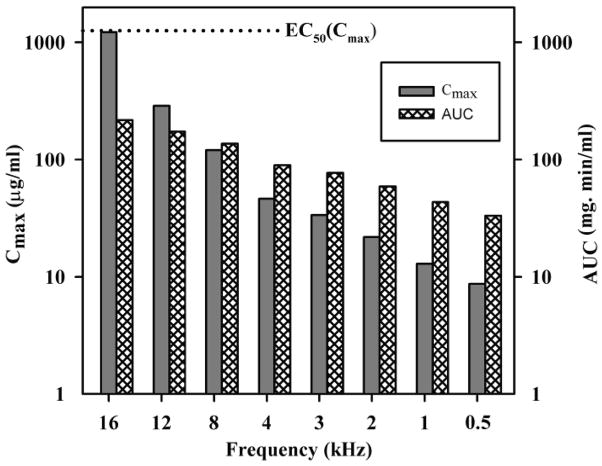
Based on the guidelines of the AAOHNS, Committee on Hearing and Equilibrium36, the changes of hearing sensitivity resulting from treatments for Meniere’s disease are commonly measured as a pure tone average of the hearing levels at 0.5, 1, 2 and 3 kHz. Based on the expected distribution of gentamicin along scala tympani we would expect the highest drug levels to occur at the base of the scala and affect sensitivity to the highest acoustic frequencies first6, 10. Estimation of gentamicin levels at scala tympani locations corresponding to higher frequency regions is shown in Figure 5. Due to the higher drug levels at more basal sites, damage to hearing may be better detected using high frequency audiometry. Hirsch and Kamerer reported fewer improvements and more patients unchanged or worse when hearing sensitivity was measured at 8 kHz13.
Figure 5.
Calculation of Cmax and AUC for frequency locations corresponding to distances along scala tympani following a one shot injection according to the protocol of Minor19, a protocol that is calculated to produce low drug levels in the cochlea (from Group 3 in Figure 4). Even though the gentamicin dose reaching regions corresponding to frequencies of 4 kHz and below is well below the EC50 (shown as a dotted line), higher frequency regions, such as 16 kHz may be exposed to higher drug levels approaching the EC50. Calculated AUC levels also increase for higher frequencies but do not come close to the EC50 for the AUC of 19,403 mg·min/ml (substantially exceeding the upper limit of the graph scale).
It is important that the limitations of the present analysis are appreciated. The concentrations estimated here were based on model parameters derived from animal studies and may differ numerically from the actual levels generated in humans. Furthermore, inter-patient variations in cochlear dimensions, RW membrane permeability or other parameters cannot be incorporated into the calculations. Animal studies have shown substantial variations in intracochlear gentamicin concentration7–10 and in intracochlear concentration gradients for gentamicin10 after local applications to the RW membrane. Nevertheless, as gentamicin distribution in the fluids of the ear has been shown to be dominated by passive diffusion6, 10, the primary observations of the study, specifically the ranking of the protocols, are believed to be robust.
For much of the range of gentamicin dosing (Figure 4) the changes of hearing show a mean of just a few dB, but with large variation, with gains and losses sometimes over 50 dB. This raises the question of whether the changes to hearing are caused directly by the gentamicin treatment or whether they could arise from other sources. As substantial variation occurs with the lowest dosing levels (Figure 4, groups 1 –3), it is more probable that the changes, most notably the improvements, originate from fluctuations associated with the natural history of the disease. If this is true, then there are significant implications for both management of patients and for the reporting of hearing changes in this type of patient. First, the observed large variation makes it extremely difficult to use an individual’s hearing change as an indicator to terminate or otherwise modify the treatment.
Specifically, titration protocols in which widely-spaced gentamicin treatments are terminated if the individual patient experiences hearing loss of greater than 15 dB must be questioned. Under these protocols, it is likely that therapy of some patients will be terminated due to the random nature of hearing fluctuations. For the individual patient experiencing a decline of hearing sensitivity following treatment, a pause of further treatments may seem logical, but the issue of whether the hearing declined as a direct result of the gentamicin therapy remains uncertain based on this analysis. In addition, the AAOHNS guidelines36 suggest that an elevation of hearing thresholds following gentamicin treatment by 10 dB should be considered clinically significant. This guideline makes the implicit assumption that test-retest variability of the patients is low and that hearing threshold elevations are primarily caused by toxic effects of the gentamicin treatment. On this basis, 30% (38/127) of the patients in groups 1–3 would be classified as having clinically-significant hearing loss (10 dB or greater) related to the gentamicin treatment. Given the large variations of both improvements and losses and the mean loss for the group of just 2.2 dB, this classification appears to grossly overestimate the number of patients with hearing loss directly caused by gentamicin toxicity to the cochlea.
Our analysis of hearing changes is largely in agreement with the conclusions drawn from a metaanalysis by Chia et al. 37. This study reported that the titration method of gentamicin delivery demonstrated significantly better complete (81.7%, p = 0.001) and effective (96.3%, p < 0.05) vertigo control compared with other methods while low-dose methods of delivery demonstrated significantly worse complete vertigo control (66.7%, p < 0.001) and trended toward worse effective vertigo control (86.8%, p = 0.05). The weekly method of delivery also trended toward less overall hearing loss (13.1%, p = 0.08), and the multiple daily method demonstrated significantly more overall hearing loss (34.7%, p < 0.01) compared with other groups. No significant difference in profound hearing loss was found between groups. The degree of vestibular ablation after gentamicin therapy was not found to be significantly correlated with the resulting vertigo control or hearing loss status.
Conclusions
An analysis of gentamicin delivery protocols in humans demonstrates that one-shot application protocols produce gentamicin levels in the cochlea that have minimal risk to hearing at the frequencies tested. Protocols in which gentamicin is given repeatedly or by continuous application protocols are calculated to produce higher doses in the cochlea that in some cases may be sufficient to damage hearing. The observed high variability of hearing changes with lowest gentamicin dosing suggests that the hearing changes may not be a direct result of gentamicin toxicity and calls into question the rationale for using individual hearing changes to titrate the applied dose.
Acknowledgments
This study was supported by research grant RO1 DC01368 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
Abbreviations
- AUC
area under the curve
- RW
round window
- ST
scala tympani
References
- 1.Lange G. The intratympanic treatment of Meniere’s disease with ototoxic antibiotics. A follow-up study of 55 cases. Laryngol Rhinol Otol. 1977;56:409–414. [PubMed] [Google Scholar]
- 2.Lange G. Gentamicin and other ototoxic antibiotics for the transtympanic treatment of Meniere’s disease. Arch Otorhinolaryngol. 1989;246:269–270. doi: 10.1007/BF00463571. [DOI] [PubMed] [Google Scholar]
- 3.Lange G. 27 years experiences with transtympanic aminoglycoside treatment of Meniere’s disease. Laryngorhinootologie. 1995;74:720–723. doi: 10.1055/s-2007-997832. [DOI] [PubMed] [Google Scholar]
- 4.Salt AN, Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hear Res. 2001;154:88–97. doi: 10.1016/s0378-5955(01)00223-4. [DOI] [PubMed] [Google Scholar]
- 5.Mynatt R, Hale SA, Gill RM, et al. Demonstration of a longitudinal concentration gradient along scala tympani by sequential sampling of perilymph from the cochlear apex. J Assoc Res Otolaryngol. 2006;7:182–193. doi: 10.1007/s10162-006-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plontke SK, Wood AW, Salt AN. Analysis of gentamicin kinetics in fluids of the inner ear with round window administration. Otol Neurotol. 2002;23:967–974. doi: 10.1097/00129492-200211000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Hoffer ME, Balough B, Henderson J, et al. Use of sustained release vehicles in the treatment of Meniere’s disease. Otolaryngol Clin North Am. 1997;30:1159–1166. [PubMed] [Google Scholar]
- 8.Balough BJ, Hoffer ME, Wester D, et al. Kinetics of gentamicin uptake in the inner ear of Chinchilla langier after middle-ear administration in a sustained-release vehicle. Otolaryngol Head Neck Surg. 1998;119:427–431. doi: 10.1016/S0194-5998(98)70097-X. [DOI] [PubMed] [Google Scholar]
- 9.Hoffer ME, Balough B, Kopke RD, et al. Morphologic changes in the inner ear of chinchilla laniger after middle ear administration of gentamicin in a sustained-release vehicle. Otolaryngol Head Neck Surg. 1999;120:643–648. doi: 10.1053/hn.1999.v120.a91762. [DOI] [PubMed] [Google Scholar]
- 10.Plontke SK, Mynatt R, Gill RM, et al. Concentration gradient along scala tympani following the local application of gentamicin to the round window membrane. Laryngoscope. 2007;117:1191–1198. doi: 10.1097/MLG.0b013e318058a06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nedzelski JM, Chiong CM, Fradet G, et al. Intratympanic gentamicin instillation as treatment of unilateral Meniere’s disease: update of an ongoing study. Am J Otol. 1993;3:278–282. [PubMed] [Google Scholar]
- 12.Driscoll CL, Kasperbauer JL, Facer GW, et al. Low-dose intratympanic gentamicin and the treatment of Meniere’s disease: preliminary results. Laryngoscope. 1997;107:83–89. doi: 10.1097/00005537-199701000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch BE, Kamerer DB. Intratympanic gentamicin therapy for Meniere’s disease. Am J Otol. 1997;18:44–51. [PubMed] [Google Scholar]
- 14.Murofushi T, Halmagyi GM, Yavor RA. Intratympanic gentamicin in Meniere’s disease: results of therapy. Am J Otol. 1997;18:52–57. [PubMed] [Google Scholar]
- 15.Rauch SD, Oas JG. Intratympanic gentamicin for treatment of intractable Meniere’s disease: a preliminary report. Laryngoscope. 1997;107:49–55. doi: 10.1097/00005537-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 16.McFeely WJ, Singleton GT, Rodriguez FJ, et al. Intratympanic gentamicin treatment for Meniere’s disease. Otolaryngol Head Neck Surg. 1998;118:589–596. doi: 10.1177/019459989811800505. [DOI] [PubMed] [Google Scholar]
- 16.Pfleiderer AG. The current role of local intratympanic gentamicin therapy in the management of unilateral Meniere’s disease. Clin Otolaryngol Allied Sci. 1998;23:34–41. doi: 10.1046/j.1365-2273.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 18.Youssef TF, Poe DS. Intratympanic gentamicin injection for the treatment of Meniere’s disease. Am J Otol. 1998;19:435–442. [PubMed] [Google Scholar]
- 19.Minor LB. Intratympanic gentamicin for control of vertigo in Meniere’s disease: vestibular signs that specify completion of therapy. Am J Otol. 1999;20:209–219. [PubMed] [Google Scholar]
- 20.Quaranta A, Aloisi A, De Benedittis G, et al. Intratympanic therapy for Meniere’s disease. High-concentration gentamicin with round-window protection. Ann N Y Acad Sci. 1999;884:410–424. doi: 10.1111/j.1749-6632.1999.tb08658.x. [DOI] [PubMed] [Google Scholar]
- 21.Silverstein H, Arruda J, Rosenberg SI, et al. Direct round window membrane application of gentamicin in the treatment of Meniere’s disease. Otolaryngol Head Neck Surg. 1999;120:649–655. doi: 10.1053/hn.1999.v120.a91763. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan DM, Nedzelski JM, Chen JM, et al. Intratympanic gentamicin for the treatment of unilateral Meniere’s disease. Laryngoscope. 2000;110:1298–1305. doi: 10.1097/00005537-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Quaranta A, Scaringi A, Aloidi A, et al. Intratympanic therapy for Meniere’s disease: effect of administration of low concentration of gentamicin. Acta Otolaryngol. 2001;121:387–392. doi: 10.1080/000164801300102879. [DOI] [PubMed] [Google Scholar]
- 24.Schoendorf J, Neugebauer P, Michel O. Continuous intratympanic infusion of gentamicin via a microcatheter in Meniere’s disease. Otolaryngol Head Neck Surg. 2001;124:203–207. doi: 10.1067/mhn.2001.112310. [DOI] [PubMed] [Google Scholar]
- 25.Abou-Halawa AS, Poe DS. Efficacy of increased gentamicin concentration for intratympanic injection therapy in Meniere’s disease. Otol Neurotol. 2002;23:494–502. doi: 10.1097/00129492-200207000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Wu IC, Minor LB. Long-term hearing outcome in patients receiving intratympanic gentamicin for Meniere’s disease. Laryngoscope. 2003;113:815–820. doi: 10.1097/00005537-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Suryanarayanan R, Cook JA. Long-term results of gentamicin inner ear perfusion in Meniere’s disease. J Laryngol Otol. 2004;118:489–495. doi: 10.1258/0022215041615083. [DOI] [PubMed] [Google Scholar]
- 28.Takai Y, Murofushi T, Ushio M, Iwasaki S. Recovery of subjective visual horizontal after unilateral vestibular deafferentation by intratympanic instillation of gentamicin. J Vestib Res. 2006;16:69–73. [PubMed] [Google Scholar]
- 29.De Beer L, Stokroos R, Kingma H. Intratympanic gentamicin therapy for intractable Meniere’s disease. Acta Otolaryngol. 2007;127:605–612. doi: 10.1080/00016480600951475. [DOI] [PubMed] [Google Scholar]
- 30.Thorne M, Salt AN, DeMott JE, et al. Cochlear fluid space dimensions for six species derived from reconstructions of 3-D magnetic resonance images. Laryngoscope. 1999;109:1661–1668. doi: 10.1097/00005537-199910000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Okuno H, Sando I. Anatomy of the round window. A histopathological study with a graphic reconstruction method. Acta Otolaryngol. 1988;106:55–63. doi: 10.3109/00016488809107371. [DOI] [PubMed] [Google Scholar]
- 32.Ohyama K, Salt AN, Thalmann R. Volume flow rate of perilymph in the guinea pig cochlea. Hear Res. 1988;35:119–130. doi: 10.1016/0378-5955(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 33.Plontke SK, Biegner T, Kammerer B, Delabar U, Salt AN. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otology Neurotology. doi: 10.1097/MAO.0b013e318161aaae. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran Ba Huy P, Bernard P, Schacht J. Kinetics of gentamicin uptake and release in the rat. Comparison of inner ear tissues and fluids with other organs. J Clin Invest. 1986;77:1492–1500. doi: 10.1172/JCI112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenwood DD. A cochlear frequency-position function for several species--29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 36.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière’s disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. . Otolaryngol Head Neck Surg. 1995;113:181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 37.Chia SH, Gamst AC, Anderson JP, Harris JP. Intratympanic gentamicin therapy for Ménière’s disease: a meta-analysis. Otol Neurotol. 2004;25:544–552. doi: 10.1097/00129492-200407000-00023. [DOI] [PubMed] [Google Scholar]