Abstract
The endothelial glycocalyx is believed to play a major role in capillary permeability by functioning as a macromolecular sieve overlying the intercellular junction. Little is known about the three-dimensional organization of the glycocalyx, nor which constituents contribute to its overall structure-function relationship. We applied fluorescence correlation spectroscopy (FCS) to evaluate albumin diffusion and concentration profiles directly within the glycocalyx overlying the intercellular junctions of lung capillary endothelial cells. FCS data were obtained before and after enzymatic digestion of the glycocalyx with pronase, heparinase, or hyaluronidase. FCS revealed a structure interacting with albumin located from 1.0 to 2.0 μm above the cell membrane; this structure was capable of reducing albumin diffusion by 30% and increasing local albumin concentration by 5-fold. Digestion of the glycocalyx with pronase or heparinase resulted in only modest changes of albumin diffusion and concentration. Hyaluronidase digestion completely eliminated albumin-glycocalyx interactions. Based on these data, the biophysical structure of lung capillary glycocalyx appears like a dense canopy approximately 1.0 μm in thickness located well above the cell surface. These data also suggest that hyaluronan is a major determinant for albumin interactions with the lung endothelial glycocalyx structure. Confocal images of heparan sulfate and hyaluronan confirm a cell-surface layer 2-3 μm in thickness, thus validating FCS-derived measurements. In summary, we have used FCS to probe the extra-cellular structure of the endothelial glycocalyx and further our understanding of the structure-function relationship.
Keywords: endothelial cells, glycocalyx, lung, fluoresence correlation spectroscopy
INTRODUCTION
The endothelial glycocalyx is a complex three-dimensional matrix composed of polymers of glycoproteins, glycolipids, and glycosaminoglycans found on the luminal surface of endothelial cells. These entangled polymers form the scaffolding upon which serum proteins assemble and form an immobile plasma layer adjacent to the capillary wall1. Such a multifunctional protein-matrix layer has been hypothesized to influence inflammation2-4, coagulation 5, rheology 5-7, solute permeability8-12, and endothelial mechanotransduction13, 14. Its role in capillary barrier function has been the most studied attribute and the general consensus is that the glycocalyx forms a molecular filter overlying the intercellular junction, creating the primary determinant for both water and solute flux into the cell-cell junction.15, 16 Support for the molecular filter hypothesis developed from the observation that serum protein concentration influenced ferritin penetration into the glycocalyx and reduced the loading of luminal vesicles with ferritin.17 These observations were explained by serum proteins adsorbing onto the glycocalyx and forming a protein-matrix barrier to both diffusion and convection of macromolecules to the cell surface. Based on these observations, it was assumed that the glycocalyx could sieve macromolecules from entering the intercellular junction.
Fluid filtration is also modulated by the physical structure of the glycocalyx. Numerous studies have shown that capillary hydraulic conductivity is strongly dependent on perfusate protein concentration, suggesting that protein adsorption onto the glycocalyx decreases transendothelial fluid flux in vivo18-20 and in vitro21,22. Interventions that directly alter the structure of the glycocalyx, such as enzymatic degradation, result in increased hydraulic conductivity, adding additional support to the fiber-matrix hypothesis.15,16 More recently, in vivo studies examining the exclusion of macromolecules within the glycocalyx have conclusively demonstrated the sieving ability of this important cell-surface layer.10
Curry FE and Michel CC (1980)23 proposed the first quantitative model of the glycocalyx, so-called the “fiber matrix model,” based on estimated fiber diameters and fibers arrangements obtained from transmission electron microscopy (TEM). More detailed structural models have been developed to explain the influence of the glycocalyx on water and solute transport across the capillary wall.24,25 Thus far, the most detailed description of the glycocalyx structure has been put forth by Squires JM, et al (2001)26, who used TEM micrographs and subsequent Fourier transform to uncover a quasi-periodic structure of the fiber arrangement that predicted sieving capabilities. All of these models describe the glycocalyx as a thick porous layer residing directly on the endothelial surface.
In this paper, we obtained novel biophysical determinants of the glycocalyx structure using fluorescence correlation spectroscopy (FCS)27 which measured the diffusivity and local concentration of human serum albumin directly within the glycocalyx above the intercellular junctions of cultured lung microvascular endothelial cells. These data provide evidence of a different structure regarding the three-dimensional organization of the glycocalyx. We call this new structural model the “canopy model”. The methodologies presented herein provide the highest spatial and temporal resolution to date for examining, in vitro, macromolecular solute diffusion within the glycocalyx and furthers our understanding of the role of the glycocalyx in endothelial barrier function.
MATERIALS AND METHODS
Materials
Bovine serum albumin (30% BSA solution), Type XIV bacterial pronase from Streptomyces griseus (EC 3.4.24.31), heparin lyase III from Flavobacterium heparinum (heparinase III, EC 4.2.2.8), and hyaluronate lyase from Streptomyces hyalurolyticus (hyaluronidase, EC 4.2.2.1) were obtained from Sigma Chemical. Other materials included fraction V, fatty acid free human serum albumin (HSA) from ICN Biomedicals (Mp Biomedicals, Irvine, CA), AlexaFluor 532 carboxylic acid succinimidyl ester (AF532, Molecular Probes, Eugene, OR). HSA was labeled with AlexaFluor 532 carboxylic acid succinimidyl ester to a near 1:1 molar protein/fluorophore ratio.
Methods
Cell Culture
Bovine lung microvascular endothelial cells (BLMVEC) and MCDB-131 complete cell medium were from Vec Technologies (Rensselaer, NY). Clean round glass coverslips (1 in. diameter, 1 1/2 thickness) were coated with 0.2% type B bovine gelatin for one hour at 37° C, followed by exposure to 30 μg/mL bovine fibronectin solution in serum-free MCDB-131 for another hour. Cells were used at 7-days post-plating and measurements were made from confluent monolayers.
Fluorescence correlation spectroscopy
A 532 nm laser beam (Nd:YAG 75 mW laser (CrystaLaser Inc., Reno, NV) was collimated and directed into the epi-port of a Nikon Diaphot 200 inverted microscope with a Nikon 100x, 1.4 NA oil Plan Apochromat objective and a dichroic filter (Chroma Q565LP, Chroma, Rockingham, VT). Emitted fluorescence was collected by the same objective, passed through a 532 nm notch filter (Kaiser Optical, Ann Arbor, MI) and imaged onto a pinhole positioned at the objective conjugate plane. Fiber optic (OZ optics, Carp, Ontario) collected the photons passing through the pinhole. The fiber optic split the collected light evenly and sent it to two avalanche photodiodes (APD) (Perkin-Elmer, Wellesley, MA), thus enabling cross-correlation analysis. Single photons created transistor-transistor logic (TTL) pulses were registered by Flex99/160 hardware correlator (Correlator.com). FCS samples were placed on an MFC-2000 (ASI, Eugene, OR) xyz microscope stage with a fine focus controlled by a computer.
Cell monolayers were washed in phenol red-free DMEM, covered with AF532-HSA solution (1.24 μg/mL), and sealed into a custom-made perfusion chamber. A z-axis FCS scan was performed by stepping the focus in 100 nm increments: a typical z-axis profile was carried out over a cell-cell junction starting from the coverslip, through the cell, and into the solution above the cell (n = 6 to 9 different cells for each run).
Two separate sets of FCS data were acquired:
by measuring competitive displacement of AF532-HSA using excess of unlabeled bovine serum albumin (BSA), and
by measuring interactions of AF532-HSA within the glycocalyx after three different enzymatic degradations of the glycocalyx.
For excess BSA experiments, 5 mg/mL BSA solution in DMEM was added to the cells 30 minutes prior to FCS data collection. In the enzyme experiments, cell monolayers were first treated with the enzyme solution prior to DMEM wash, addition of AF532-HSA and FCS data collection. Pronase, a broad spectrum protease, was used at 0.01 mg/mL in MCDB-131 for 5 minutes because longer exposure times caused the cells to detach from the glass coverslip. Heparanase III was diluted to 15 mU/mL (Sigma Units) and hyaluronidase was diluted to 50 IU/mL, both in MCDB-131. Each enzyme solution was placed on the cells for one hour. In controls, z-axis profiling was performed on cells with no AF532-HSA and on coverslips without cells in the presence of AF532-HSA.
Characteristic fluorescence correlation function, G3D(τ), for a diffusion in a volume illuminated with Gaussian shaped excitation intensity is given by:
| (1) |
where N is the average number of fluorescent molecules in the detection volume, τD is the characteristic time of their diffusion, and G∞ is equal to one. S equals the ratio of axial (ωz) and radial (ωr) distances at which the intensity of the Gaussian excitation falls to 1/e2 of its maximum. S was determined experimentally to be approximately 10 using diffusion of Rhodamine-6G (D = 2.8×10-6 cm2/s28). Experimental FCS data were fitted to equation (1) using Levenberg-Marquardt non-linear least squares optimization in Igor Pro (WaveMetrics Inc., Lake Oswego, OR). Each fit resulted in a pair of parameters, N and τD. These were averaged for every z-distance and the standard error of the mean was computed.
Confocal Microscopy
Cells were cultured on glass coverslips as described above. Monolayers were washed 3 times with phosphate buffer saline (PBS) and fixed with 2% paraformaldehyde for 30 minutes at room temperature. Heparan sulfates were immunostained with HSS-1 (US Biologicals, Swampscott, MA) at a concentration of 9.5 μg/mL for 1 hour at room temperature, washed with PBS, and incubated with labeled secondary antibody (Jackson ImmunoResearch, West Grove, PA). Hyaluronan was localized using the combination of biotinylated hyaluronan-binding protein (Associates of Cape Cod, East Falmouth, MA) and FITC-labeled anti-biotin Ab (Jackson ImmunoResearch, West Grove, PA). Images were obtained using a Fluoview 300 confocal microscope (Olympus, Melville, NY) equipped with a PLAPON 60x oil objective. 3D rendering and image analysis was performed using Volocity (Improvision Inc., Lexington, MA).
RESULTS
Control experiments
The z-profile for AF523-HSA concentration, N(z)aqsol, for an aqueous protein solution with no cells, subtracted from the AF523-HSA concentration profile, N(z)cells, in the presence of the cell monolayer is shown in Figure 1A (ΔN(z) = N(z)cells - N(z)aqsol). One notes the increase of ΔN(z) occurring between 1.5 and 2.5 μm distance measured above the glass surface. The characteristic diffusion time of AF523-HSA, τD(z), is shown in Figure 1B. In the presence of the cell monolayer, τD(z) values increased over the same distance range (i.e. from 1.5 to 2.5 μm above the glass interface) where ΔN(z) increased (Fig. 1A), suggesting the presence of a structure that both concentrates albumin molecules and slows their diffusion. Note that at distances below 1.5 μm, τD(z)cell value was not different than τD(z)aqsol. Furthermore, N and τD increased below 1 μm distance for both the control (coverslip without cells) and cell monolayer sample, indicating that the glass interface also accumulates albumin by non-specific adsorption and thus slows its diffusion.
Figure 1. Control cell AF532-HSA concentration (ΔN) and diffusion rate, τD, vs. distance above the glass coverslip.
A) Concentration profiles (ΔN) of AF532-HSA vs. distance (μm) above glass-cell interface. Curve shows ΔN (concentration of albumin over cell layer minus concentration of albumin in aqueous solution without cells). ΔN increase nearly 5-fold between 1.5 and 2.5 μm above the glass coverslip. B) Characteristic diffusion times, τD, of AF532-HSA vs. distance above a control cell (control cell) and in aqueous solution over a coverslip without cells (solution). Increases in τD, indicative of slowing of AF532-HSA, can be seen from approximately 1.5 to 2.5 μm above the glass coverslip containing a confluent endothelial monolayer. The combined observation of increases in ΔN and τD(z), occurring at the same distance above the cell surface, identifies the location of a structure that sieves albumin, consistent with the function of the glycocalyx.
Competitive displacement of AF532-HSA using excess of unlabeled BSA
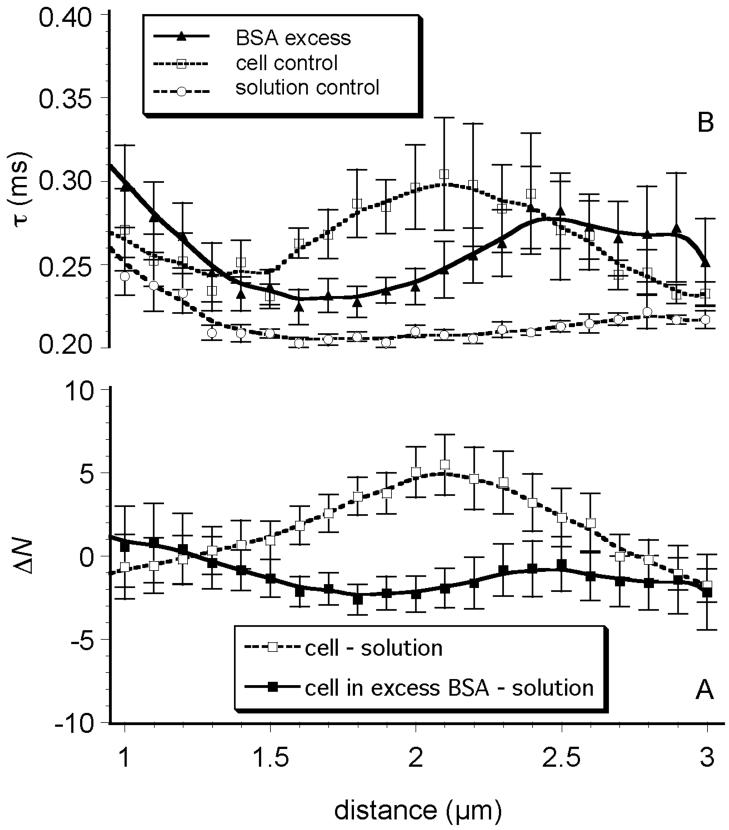
To test whether the changes in ΔN(z) and τD(z) shown in Figure 1 were due to interactions of AF523-HSA with the glycocalyx we applied an excess (0.5 mg/mL) of unlabeled BSA to the cells prior to adding AF532-HSA. The effects of excess unlabeled albumin are shown in Figure 2A. Two curves are shown: ΔN(z) (i.e., the difference between N(z)cell and N(z)aqsol and the difference between N(z)cell+BSA and N(z)aqsol). It is evident that the ΔN(z) profile without excess albumin is very similar to the control shown in Figure 1A and that the saturation of glycocalyx with unlabeled albumin caused ΔN(z) to drop close to or below zero, i.e. that the number of labeled molecules above the cell was smaller than the number of the same molecules in solution control where no excess of BSA has been added.
Figure 2. Effects of saturating concentrations of unlabeled albumin.
A) Change in albumin concentration (ΔN) vs. distance above glass-cell interface, in a glycocalyx saturated with unlabeled albumin (cell in excess BSA - solution) relative to a control glycocalyx bathed in aqueous media without albumin (cell - solution). In the presence of excess unlabeled albumin, AF532-HSA is excluded (concentration is lower than solution) at the depth where the glycocalyx is believed to exist. B) Diffusion time, τD(z), of AF532-HSA vs. depth in a glycocalyx saturated with unlabeled albumin, relative to solution control (no cell) and control cells. The presence of the excess unlabeled albumin shifts the τD(z) profile approximately 0.5 μm to the right, i.e. further above the cell membrane.
Excess of unlabeled albumin also altered AF532-HSA characteristic diffusion time, τD(z), within the glycocalyx (Fig. 2B). AF532-HSA diffusion was somewhat faster inside the glycocalyx saturated with unlabelled BSA compared to control cells, indicating that BSA has displaced glycocalyx-associated AF532-HSA. In addition, the shallow maximum of τD(z) was shifted approximately 0.5 μm further out from the cell membrane, into the region 2.5 to 3.0 μm. This suggests that excess BSA concentration swelled the glycocalyx and thus caused an outward shift. The actual AF532-HSA fluorescence counts were approximately 16% lower in the systems saturated with BSA, relative to the respective values for AF532-HSA in controls with cell monolayer but no BSA (data not shown).
Pronase treatment
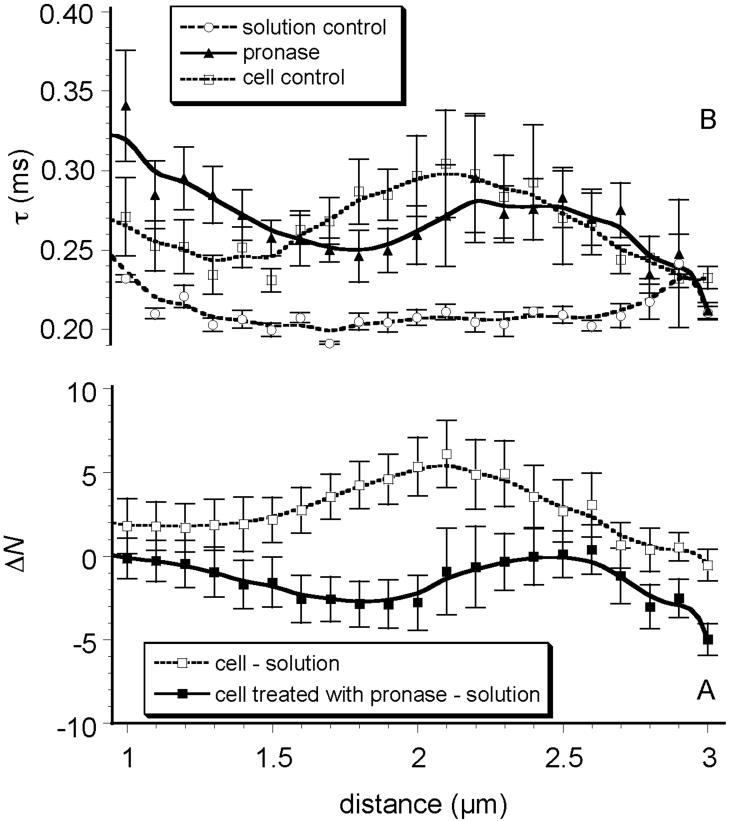
Enzymatic digestion of the glycocalyx with pronase resulted in moderate changes in the AF532-HSA concentration z-profile; N(z) was below the level seen in controls (no pronase) and was also reduced to a level lower than N(z) of the aqueous control (Fig. 3A). The point at which AF532-HSA concentration in the glycocalyx decreased below the level seen in the solution controls was shifted 0.5 μm closer to the cell membrane, i.e. between 1.0 and 2.0 μm distance from glass interface (Fig. 3A). The fluorescence counts from AF532-HSA in the pronase-treated glycocalyx were approximately 16% lower than in control experiments (untreated glycocalyx) (data not shown). Variation of the characteristic diffusion times for AF532-HSA within the pronase-treated glycocalyx (Fig. 3B) was not much different than in controls except that a slightly shorter τD(z) was observed and the pronase flattened the τD(z) profile.
Figure 3. Pronase Treatment.
A) Difference in albumin concentration (ΔN) vs. distance in a glycocalyx enzymatically digested with pronase, relative to untreated cell controls. Pronase digestion reduced ΔN at all distances above the cell surface, consistent with alteration(s) in glycocalyx structure. B) Diffusion time, τD(z), of AF532-HSA vs. distance in a glycocalyx digested with pronase, relative to solution and cell controls. Pronase digestion results in slowing of AF532-HSA diffusion at distances less than 2.0 μm above the cell membrane.
Heparanase treatment
Enzymatic digestion of the glycocalyx with heparanase III resulted in an increase of N closer to the cell surface compared to the untreated cells but also eliminated the N maximum at distances further above the cell membrane (at 2.0 to 2.5 μm), relative to untreated cells (Fig. 4A). The concentration of AF532-HSA in the heparanase-treated glycocalyx was approximately 25% higher than that of AF532-HSA in solution between 0.5 and 2.0 μm, while approximately 15% lower than that of HSA in an untreated glycocalyx between 2.0 and 2.5 μm. The characteristic diffusion times for AF532-HSA in the heparanase-treated glycocalyx (Fig. 4B) were slightly longer than in the solution control but heparanase reduced the maximum in τD, making the τD(z) profile flat and similar to pronase treatment.
Figure 4. Heparanase treatment.
A) Difference in AF532-HSA concentration (ΔN) vs. distance in a glycocalyx enzymatically digested with heparanase III relative to cell controls. Heparanase digestion results in an increase of ΔN at distances <1.5 μm above the glass-cell interface and a reduction in ΔN, relative to control cells, at distances beyond 1.5 μm. B) Diffusion time, τD(z), of AF532-HSA vs. distance in a glycocalyx degraded with heparinase, relative to solution and cell controls. Heparanase treatment increased τD(z) at distances closer to the cell membrane and flattened the τD(z) vs. distance profile.
Hyaluronidase treatment
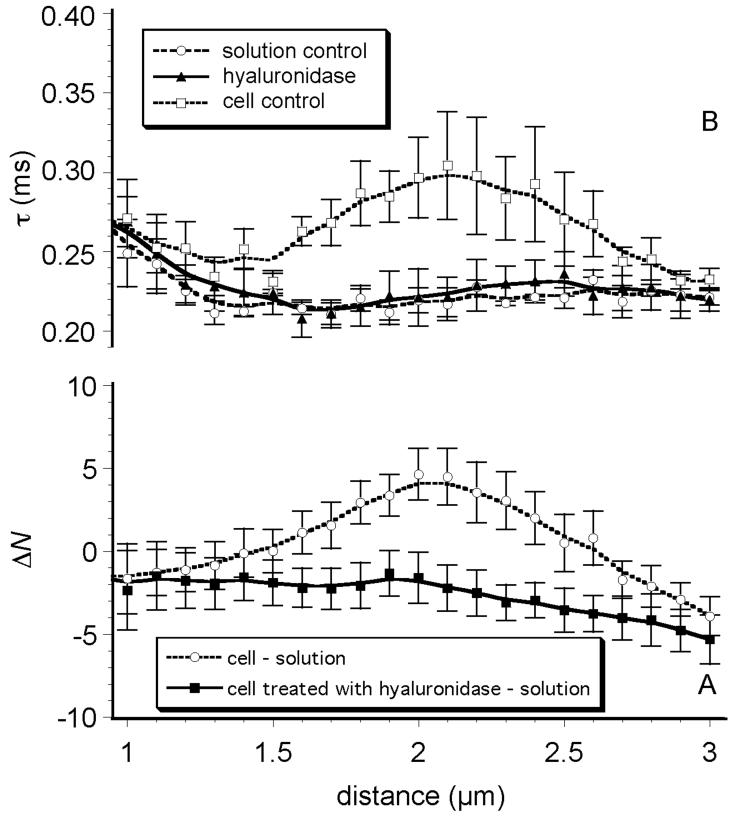
Digestion of the glycocalyx with hyaluronidase resulted in large changes in both N(z) and τD(z). Hyaluronidase treatment completely eliminated any variation in N(z) and τD(z) over the entire range of z-distances above the cell surface. N was reduced to the levels smaller than found in the protein solution controls at all distances measured (ΔN(z) < 0, Fig. 5A). The characteristic diffusion times after hyaluronidase treatment, τD(z), were no different than the τD's found for the aqueous solution of AF532-HSA with no cells (Fig. 5B).
Figure 5. Hyaluronidase treatment.
A) Difference in AF532-HSA concentration (ΔN) vs. distance in a glycocalyx enzymatically digested with hyaluronidase. Hyaluronidase abolished albumin accumulation within the glycocalyx. B) Hyaluronidase reduced τD(z) to values characteristic of aqueous solution, suggesting a complete removal of albumin-glycocalyx interaction.
Confocal microscopy
Immunofluorescent imaging of heparan sulfates (HS) revealed a dense HS layer across the surface of the cell (Fig. 6A). The thickness of the heparan sulfate layer was 2.82 ± 0.49 μm (n=10). Hyaluronan localization also revealed a dense layer of staining across the surface of the cell (Fig. 6B). The thickness of the hyaluronan layer was 3.06 ± 0.37 μm (n=10). Confocal-derived glycocalyx thickness is nearly identical to FCS measurements of the glycocalyx.
Figure 6. Confocal Images of glycocalyx.
A) 3D image of heparan sulfate layer on the surface of lung capillary endothelial monolayer. Images show a field of approximately 10 cells (*), covered with a heparan sulfate layer measuring 2.82 ± 0.49 μm (n =10). 1 unit = 7.2 μm. B) 3D image of hyaluronan layer on the surface of lung capillary endothelial cells. The hyaluronan layer measured 3.06 + 0.37 μm (n=10). 1 unit = 7.2 μm.
DISCUSSION
The goal of this study was to characterize the contribution of major constituents to the three-dimensional structure of the glycocalyx, using albumin as a dynamic probe-molecule of the glycocalyx. We expected FCS to provide spatial and temporal information about the three-dimensional organization of the glycocalyx. Furthermore, we utilized three different enzymes to differentiate between the contributions of specific glycosaminoglycans to the structural organization of glycocalyx and their interactions with albumin. In summary, we attempted to demonstrate the utility of FCS/albumin combination for probing extra-cellular structure and showed that: 1) cultured endothelial cells possess a glycocalyx capable of limiting the diffusion of albumin and increasing local concentrations by 5-fold, 2) heparan sulfates contribute modestly to the structure-albumin binding function of the glycocalyx, 3) hyaluronan is a crucial component of the three-dimensional organization of the glycocalyx responsible for albumin binding, 4) there is strong correlation between measured glycocalyx thickness using FCS and confocal microscopy, and 5) lung capillary glycocalyx, in vitro, is more like a dense canopy located well above the cell surface, rather than a brush-like structure on the cell surface, as previous models have hypothesized.
The FCS spatial resolution is defined by the dimensions of the observation volume as defined by the microscope optics. This volume, with an ellipsoidal shape extending approximately 1.0 μm in the z axis and 0.2 μm in the x and y direction, is stepped incrementally in z-direction through the cell and into the solution above the cell. Since the coverslip surface is the only fixed reference in the z-direction, one had to establish where along the z-axis the cell membrane exists and where the glycocalyx begins. Pesen and Hoh29 used atomic force microscopy (AFM) and confocal fluorescence microscopy (CFM) to study live bovine pulmonary artery endothelial cells (BPAEC), in vitro, and found that the thickness of these cells at the cell-cell junctions was approximately 0.5 μm. The fluorescence collected below this z-level originates from both the glass-cell interface and from the cell body volume. At distances greater than 1.5 μm from the coverslip surface (i.e. 0.5 μm of cell thickness + 1 μm of optical resolution in z-direction) any changes in AF532-HSA concentration (N) or characteristic diffusion time (τD) are then attributed to structures that are above the cell membrane.
FCS measured diffusional dynamics of serum albumin within and above the lung capillary endothelial cell monolayer. The FCS z-profiles demonstrated that albumin diffusion, τD(z), and its concentration, N, both increased within the same region located at a distance of 1.5 to 2.5 μm above the glass-cell interface. We attribute these effects to the glycocalyx structure: it was capable of reducing albumin characteristic diffusion times by 30% and increasing local concentrations of AF532-HSA by 5-fold. The reduction of albumin diffusivity and increase of its concentration are consistent with the so-called “sieving effect.” The variations of N(z) and τD(z) were wide and symmetrical as expected by the broadening caused by 1 μm optical z-resolution. This optical broadening prevented us from determining the exact thickness of the glycocalyx structure; however, its location well above the cell surface was not affected by optics. Assuming the cell thickness was 0.5 μm, the structure responsible for albumin interactions was located between 1.0 and 2.0 μm above the endothelial cell membrane. This physical interpretation of glycocalyx is quite different than previous models25, 26 that described the glycocalyx as residing directly on the cell surface. The experimental results (Fig. 1A) also indicated that albumin diffusion was unhindered between the cell surface (at 0.5 μm from the glass-cell interface) up to a distance of 1.5 μm. These results also rule out the possibility that the observed effect was due to albumin diffusion inside cytoplasmic vesicles.30
In vivo, the glycocalyx is exposed to albumin concentrations that average 40-50 mg/mL and it has been well established that albumin concentration has important affects on endothelial permeability.16 Furthermore, the three-dimensional structure of the glycocalyx may be altered31 and its barrier properties may be more restrictive in the presence of saturating protein concentrations.21, 32 The presence of excess of unlabeled BSA (5 mg/mL) reduced AF532-HSA concentration within the glycocalyx to the levels slightly below those found in the aqueous AF532-HSA solution and reduced τD(z) over the range of 1.5 to 2.5 μm above the glass surface. Between 2.5 - 3.0 μm, however, τD(z) increased slightly. Taken together, these observations may be explained as: 1) the longer τD(z) and higher N within the glycocalyx of control cells (with no excess of unlabeled albumin) was due to AF532-HSA binding to the glycocalyx, and/or 2) excess concentrations of albumin alters the structure of the glycocalyx, which more effectively excludes AF532-HSA. The measured reduction in τD(z) in the presence of excess unlabeled albumin is most easily explained by a reduction in available binding sites. In other words, the higher τD(z) seen in controls is most likely due to AF532-HSA binding to the structure of glycocalyx and not due to simple or even spatially restricted diffusion. The shift of higher τD(z) to greater distances above the cell surface, in the presence of excess albumin, suggests that saturating albumin concentration swells the glycocalyx, making it thicker and extending it over a greater distance above the cell surface.
The primary goal of enzymatic degradation experiments was to identify structural components of the glycocalyx that contributed to the three-dimensional organization responsible for the mass transport behavior of albumin. Relatively little is known about the structural determinants of the glycocalyx, although heparan sulfate (HS) and hyaluronan are believed to be important components. We have previously shown that syndecan, a major HS proteoglycan on endothelial cells, is localized to the cell periphery33 and could spatially contribute to barrier properties of the glycocalyx over the cell-cell junction. Hyaluronan has been shown to significantly influence permeation of the glycocalyx to fluorescently-labeled dextrans11, suggesting that it may also play a major structural role. Therefore, we examined the effects of three enzymes: pronase, heparanase III, and hyaluronidase on FCS-observed glycocalyx-albumin interactions.
Pronase (protease E), a broad spectrum protease, has been used to digest the capillary glycocalyx in vivo where it significantly increased hydraulic conductivity but without altering the dimensions of the intercellular junction15, suggesting that the change in permeability was due solely to structural alterations of the glycocalyx. Chang YS (1998)34 tested the effect of various concentrations of pronase on cultured endothelial cell permeability and reported that hydraulic conductivity (Lp) was increased (3-fold) with low concentrations of pronase (0.10 - 0.125 mg/mL) while albumin diffusion was unaffected; at higher concentrations (0.20 mg/mL), both Lp and albumin diffusion increased. Collectively, these data suggest that a graded removal of the glycocalyx could be accomplished and that this cell surface layer provides significant resistance for both water and protein entrance into the cell-cell junction.
Pronase treatment reduced AF532-HSA concentration at distances of 1.25 to 2.0 μm to a level slightly below that found in protein solution. Pronase also slightly affected τD(z); however, these effects were barely outside of the measurement errors (Fig. 3). The uncertainty about the effects of pronase treatment is most likely due to the inability of using higher enzyme concentrations or longer treatment times. Attempts to use higher concentrations of pronase caused the cells to detach from the glass coverslip, even at relatively short incubation times. Therefore, we selected a lower enzyme concentration (0.01 mg/mL) and an exposure shorter than for other enzymes (5 minutes), and the minimal alterations in FCS parameters likely reflect limited removal of glycocalyx structure.
Heparanase III digestion of the glycocalyx reduced the maximum in ΔN(z) and shifted the ΔN(z) profile closer to the cell membrane (Fig. 4). The τD(z) profile was flattened and also shifted leftward. There was a notable increase in τD(z) from 1.0 to 1.5 μm distance, indicating that albumin diffusion was slower at distances closer to the cell surface compared to controls. It appears that heparanase III digestion reduced the ability of the glycocalyx to exclude albumin above the cell surface and allowed more AF532-HSA into lower regions of the glycocalyx. The heparanase-induced increase of τD(z) closer to the cell surface suggests that the glycocalyx may have partially collapsed into this region or the loss of heparan sulfates unmasked previously inaccessible binding sites for albumin.
Digestion of the glycocalyx with hyaluronidase had the most marked effect on FCS-derived parameters. Hyaluronidase reduced ΔN(z) to values below that of the protein solution (Fig. 5), suggesting a complete abolition of albumin accumulation over the entire z-axis. In addition, hyaluronidase treatment reduced τD(z) to the characteristic diffusion times found in aqueous solution, suggesting that hyaluronidase treatment disrupted all albumin binding and/or eliminated hindrance to albumin diffusion at all distances above the cell membrane. In fact, the data suggested that hyaluronidase completely eliminated albumin-glycocalyx interactions: as far as albumin is concerned, hyaluronan appeared to be a major structural component of the lung capillary glycocalyx, in vitro.
In order to validate the biophysical measurements of the glycocalyx thickness derived by FCS/albumin profiling, we obtained 3D confocal images by immunostaining for heparan sulfate and hyaluronan on the endothelial surface. Immunostaining for both glycosaminoglycans revealed a dense surface layer that was approximately 3.0 μm in thickness, consistent with our FCS measurements.
There has been considerable debate about the existence, structure, and function of the glycocalyx found on cultured endothelial cells and whether it is comparable to the glycocalyx found in vivo. Our results, utilizing FCS and confocal microscopy, demonstrate that 1) cultured endothelial cells possess a significant glycocalyx layer, 2) the glycocalyx is at least as thick as the endothelial surface layer (ESL) measured in vivo10, 35, 3) functionally, the in vitro glycocalyx can effectively sieve albumin to increase its concentration 5-fold, and 4) heparan sulfates and hyaluronan are present as a 2-3 μm thick layer covering much of the cell surface.
Previous structural models of the glycocalyx are largely based on images obtained by transmission electron microscopy (TEM), which show the glycocalyx as a dense brush-like layer arising directly from the endothelial cell surface. The results of the present study suggest otherwise, that the bulk of the glycocalyx is located well above the cell surface and is more akin to a dense canopy rather than a brush-like surface layer. Therefore, we propose the name “Canopy Model” to describe our view of the glycocalyx based on this FCS study.
The major problem in obtaining faithful structural information about the glycocalyx from TEM micrographs is, we believe, fixation artifacts related to tissue processing. The glycocalyx is assumed to be composed of highly hydrated glycoproteins and glycosaminoglycans. When subjected to the dehydration during electron microscopy pre-processing, the glycocalyx likely collapses onto the cell surface. Several methods have been proposed that claim to preserve the structural integrity of the glycocalyx during the fixation and dehydration process36, 37; however, these methods have not been validated since no established technique exists for comparison. Another troubling aspect of some glycocalyx TEM images is the morphology of the brush-like structure which does not correlate well with any known structures of glycoproteins or glycosaminoglycans.
In this study we have used albumin as a dynamic molecular probe of glycocalyx structure, hence our model of the glycocalyx is mechanistically-based on those structural attributes that affect albumin diffusion and local concentration. Since albumin is the major serum protein and it is responsible for 70% of the oncotic pressure of plasma, we believe that the choice of albumin as a probe is important: any characterization of the structure-function relationship of endothelial glycocalyx must account for its ability to sieve and restrict albumin diffusion. FCS provided the highest spatial and temporal resolution possible to make detailed measurements of fluorescently labeled analytes using live cells. However, the optical resolution of FCS in z-direction either needs to be improved or the experimental data must be de-convoluted to eliminate the broadening effects of relatively poor z-resolution. We acknowledge that there is likely a fine structure to glycocalyx that exists between the cell surface and the region where FCS effects were observed, a region that does not hinder albumin diffusion and, therefore, was not measurable in our experiments. Studies are currently underway to examine dynamic behavior of a wider range (in terms of molecular sizes) of fluorescently-labeled proteins within the glycocalyx region.
CONCLUSIONS
In this study we have validated the utility of FCS to measure diffusivity and local concentration of albumin above the surface of a live cell and within the structure of the glycocalyx. Specifically, we examined the region overlying the cell-cell junctions, since this area is likely to have the most influence on permeability. Our results indicate that the bulk of the glycocalyx is positioned well above the cell membrane, at distances of 1-2 μm above the cell surface. Interestingly, above the cell membrane but below 1 μm distance, albumin diffusion appeared relatively unhindered. Degradation of the glycocalyx by highly specific enzymes demonstrated that hyaluronan is a major structural component, while heparan sulfates appear less influential in affecting albumin diffusion and its local concentration. Studies are currently underway to examine the behavior of a wider range of serum proteins within the glycocalyx.
ACKNOWLEDGEMENTS
The authors would like to thank the University of Utah Cell Imaging Core Facility for their assistance with 3D reconstruction of the confocal images.
Funding: NIH K08 Award Grant # HL0680683-05 “The Glycocalyx and Endothelial Barrier Regulation” P.I.: Randal O. Dull
University of Utah Seed Grant “Biophysical Indices of the Endothelial Surface Layer (ESL) and Permeability” P.I.: Randal O. Dull
REFERENCES
- 1.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000 Sep;440(5):653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 2.Dull RO, Dinavahi R, Schwartz L, Humphries DE, Berry D, Sasisekharan R, Garcia JG. Lung endothelial heparan sulfates mediate cationic peptide-induced barrier dysfunction: a new role for the glycocalyx. Am J Physiol Lung Cell Mol Physiol. 2003 Nov;285(5):L986–995. doi: 10.1152/ajplung.00022.2003. [DOI] [PubMed] [Google Scholar]
- 3.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002 Oct;283(4):H1282–1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 4.Henry CB, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000 Dec;279(6):H2815–2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- 5.Pries AR, Secomb TW. Rheology of the microcirculation. Clin Hemorheol Microcirc. 2003;29(34):143–148. [PubMed] [Google Scholar]
- 6.Pries AR, Secomb TW, Jacobs H, Sperandio M, Osterloh K, Gaehtgens P. Microvascular blood flow resistance: role of endothelial surface layer. Am J Physiol. 1997 Nov;273(5 Pt 2):H2272–2279. doi: 10.1152/ajpheart.1997.273.5.H2272. [DOI] [PubMed] [Google Scholar]
- 7.Desjardins C, Duling BR. Microvessel hematocrit: measurement and implications for capillary oxygen transport. Am J Physiol. 1987 Mar;252(3 Pt 2):H494–503. doi: 10.1152/ajpheart.1987.252.3.H494. [DOI] [PubMed] [Google Scholar]
- 8.van Haaren PM, VanBavel E, Vink H, Spaan JA. Charge modification of the endothelial surface layer modulates the permeability barrier of isolated rat mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2005 Dec;289(6):H2503–2507. doi: 10.1152/ajpheart.00587.2005. [DOI] [PubMed] [Google Scholar]
- 9.Huxley VH, Williams DA. Role of a glycocalyx on coronary arteriole permeability to proteins: evidence from enzyme treatments. Am J Physiol Heart Circ Physiol. 2000 Apr;278(4):H1177–1185. doi: 10.1152/ajpheart.2000.278.4.H1177. [DOI] [PubMed] [Google Scholar]
- 10.Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000 Jan;278(1):H285–289. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- 11.Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol. 1999 Aug;277(2 Pt 2):H508–514. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- 12.Schneeberger EE, Hamelin M. Interaction of serum proteins with lung endothelial glycocalyx: its effect on endothelial permeability. Am J Physiol. 1984 Aug;247(2 Pt 2):H206–217. doi: 10.1152/ajpheart.1984.247.2.H206. [DOI] [PubMed] [Google Scholar]
- 13.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003 Nov 14;93(10):e136–142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 14.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol. 2003 Aug;285(2):H722–726. doi: 10.1152/ajpheart.00691.2002. [DOI] [PubMed] [Google Scholar]
- 15.Adamson RH. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J Physiol. 1990 Sep;428:1–13. doi: 10.1113/jphysiol.1990.sp018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamson RH, Clough G. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J Physiol. 1992 Jan;445:473–486. doi: 10.1113/jphysiol.1992.sp018934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loudon MF, Michel CC, White IF. The labelling of vesicles in frog endothelial cells with ferritin. J Physiol. 1979 Nov;296:97–112. doi: 10.1113/jphysiol.1979.sp012993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michel CC, Phillips ME. The effects of bovine serum albumin and a form of cationised ferritin upon the molecular selectivity of the walls of single frog capillaries. Microvasc Res. 1985 Mar;29(2):190–203. doi: 10.1016/0026-2862(85)90016-0. [DOI] [PubMed] [Google Scholar]
- 19.Mason JC, Curry FE, Michel CC. The effects of proteins upon the filtration coefficient of individually perfused frog mesenteric capillaries. Microvasc Res. 1977 Mar;13(2):185–202. doi: 10.1016/0026-2862(77)90084-x. [DOI] [PubMed] [Google Scholar]
- 20.Levick JR, Michel CC. The effect of bovine albumin on the permeability of frog mesenteric capillaries. Q J Exp Physiol Cogn Med Sci. 1973 Jan;58(1):87–97. doi: 10.1113/expphysiol.1973.sp002194. [DOI] [PubMed] [Google Scholar]
- 21.Dull RO, Jo H, Sill H, Hollis TM, Tarbell JM. The effect of varying albumin concentration and hydrostatic pressure on hydraulic conductivity and albumin permeability of cultured endothelial monolayers. Microvasc Res. 1991 May;41(3):390–407. doi: 10.1016/0026-2862(91)90037-c. [DOI] [PubMed] [Google Scholar]
- 22.McCandless BK, Powers MR, Cooper JA, Malik AB. Effect of albumin on hydraulic conductivity of pulmonary artery endothelial monolayers. Am J Physiol. 1991 Jun;260(6 Pt 1):L571–576. doi: 10.1152/ajplung.1991.260.6.L571. [DOI] [PubMed] [Google Scholar]
- 23.Curry FE, Michel CC. A fiber matrix model of capillary permeability. Microvasc Res. 1980 Jul;20(1):96–99. doi: 10.1016/0026-2862(80)90024-2. [DOI] [PubMed] [Google Scholar]
- 24.Fu BM, Weinbaum S, Tsay RY, Curry FE. A junction-orifice-fiber entrance layer model for capillary permeability: application to frog mesenteric capillaries. J Biomech Eng. 1994 Nov;116(4):502–513. doi: 10.1115/1.2895802. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Weinbaum S. A new view of Starling's hypothesis at the microstructural level. Microvasc Res. 1999 Nov;58(3):281–304. doi: 10.1006/mvre.1999.2177. [DOI] [PubMed] [Google Scholar]
- 26.Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel C. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol. 2001 Dec;136(3):239–255. doi: 10.1006/jsbi.2002.4441. [DOI] [PubMed] [Google Scholar]
- 27.Magde D, Elson EL, Webb WW. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- 28.Rigler R, Mets Ü, Widengren J, Kask P. Fluorescence correlation spectroscopy with high count rate and low background: analysis of translational diffusion. Eur Biophys J. 1993 August;22(3):169–175. [Google Scholar]
- 29.Pesen D, Hoh JH. Micromechanical architecture of the endothelial cell cortex. Biophys J. 2005 Jan;88(1):670–679. doi: 10.1529/biophysj.104.049965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida N, Kinjo M, Tamura M. Microenvironment of endosomal aqueous phase investigated by the mobility of microparticles using fluorescence correlation spectroscopy. Biochem Biophys Res Commun. 2001 Jan 12;280(1):312–318. doi: 10.1006/bbrc.2000.4115. [DOI] [PubMed] [Google Scholar]
- 31.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huxley VH, Curry FE. Albumin modulation of capillary permeability: test of an adsorption mechanism. Am J Physiol. 1985 Feb;248(2 Pt 2):H264–273. doi: 10.1152/ajpheart.1985.248.2.H264. [DOI] [PubMed] [Google Scholar]
- 33.Dull RO, Garcia JG. Leukocyte-induced microvascular permeability: how contractile tweaks lead to leaks. Circ Res. 2002 Jun 14;90(11):1143–1144. doi: 10.1161/01.res.0000023047.87638.76. [DOI] [PubMed] [Google Scholar]
- 34.Chang Y. The mechanism of shear-stress induced increases in endothelial transport properties. University Park, Penn State University; 1998. [Google Scholar]
- 35.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996 Sep;79(3):581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- 36.van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003 Apr 4;92(6):592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- 37.Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvasc Res. 1997 Jan;53(1):1–13. doi: 10.1006/mvre.1996.1987. [DOI] [PubMed] [Google Scholar]