SUMMARY
Otitis media (OM) is a polymicrobial disease wherein upper respiratory tract (URT) viruses compromise host airway defenses, which allows bacterial flora of the nasopharynx (NP) access to the middle ear. We have shown, in vitro, that respiratory syncytial virus (RSV), a viral co-pathogen of OM, reduces transcript abundance of the antimicrobial peptide (AP), chinchilla beta-defensin-1 (cBD-1). Here, we demonstrated that chinchillas inoculated with RSV expressed ~40% less cBD-1 mRNA and protein than did mock-challenged animals. Further, concurrent RSV infection resulted in a 10–100 fold greater recovery of nontypeable Haemophilus influenzae (NTHI) from nasopharyngeal lavage fluids, compared to chinchillas challenged with NTHI in the absence of viral co-infection. Additionally, when either: anti-cBD-1 antibody (to bind secreted AP) or recombinant cBD-1 (to increase AP concentration at the mucosal surface) were delivered to chinchillas, we demonstrated that disruption of the availability of a single AP influenced the relative load of NTHI in the URT. Collectively, our data suggested that effectors of innate immunity regulate normal bacterial colonization of the NP, and, further, virus-induced altered expression of APs can result in an increased load of NTHI within the NP which likely promotes development of OM.
Keywords: otitis media, polymicrobial disease, NTHI, antimicrobial peptide, innate immunity
INTRODUCTION
OM is one of the most frequently diagnosed pediatric illnesses (Cassell et al 1994). Approximately 83% of children less than 3 years of age will have at least one occurrence of acute OM (AOM), and 40% of children will experience greater than three episodes of OM by this age (Teele et al., 1989).
OM is a polymicrobial disease caused by viruses and bacteria commonly isolated as co-pathogens of the tympanum (Heikkinen and Chonmaitree, 2003; Bakaletz et al., 1998; Ruuskanen et al., 1989). In a study of 596 infants less than 6 months of age, a viral infection of the upper respiratory tract (URT) was the most important predictor for early acute and recurrent OM (Daly et al., 1999). Respiratory syncytial virus (RSV), rhinovirus, influenza A virus, and adenovirus, as well as several other URT viruses, can predispose children to bacterial OM (Chonmaitree et al., 2008; Heikkinen and Chonmaitree, 2003). The three main bacterial species that cause OM are Streptococcus pneumoniae, nontypeable Haemophilus influenzae (NTHI), and Moraxella catarrhalis, with NTHI predominant in cases of chronic OM and OM with effusion (Heikkinen and Chonmaitree, 2003; Monto and Ullman, 1974). The mechanisms that underlie the polymicrobial nature of OM are not completely understood, yet evidence indicates that virus-mediated damage to the mucosal epithelium that lines the uppermost airway, reduction in mucociliary clearance, and increased expression of host cell molecules to which bacteria adhere, all contribute to OM development (Bakaletz, 2002; Jiang et al., 1999; Patel et al., 1992). To better understand the polymicrobial nature of OM, we developed a chinchilla model of experimental OM wherein NTHI that colonize the nasopharynx ascend an adenovirus-compromised Eustachian tube and enter the middle ear (Bakaletz, et al., 1999). Disease modeling in this host shows that viruses co-partner with bacteria to significantly increase the percentage of OM-positive ears compared to bacterial challenge alone (Suzuki and Bakaletz, 1994; Giebink et al., 1980). Collectively, these data suggest that virus-mediated events, and specifically compromise of host airway defense mechanisms, promote development of bacterial OM.
In children, a significant increase in the nasopharyngeal bacterial load is positively correlated with an increase in middle ear infection (Faden et al., 1997; Faden et al., 1995), and otitis prone children are more heavily colonized compared to their non-otitis prone counterparts (Faden et al., 1992; Jorgensen et al., 1992). These data suggest that maintenance of a relatively low concentration of bacteria in the nasopharynx (NP) may be important to protect the middle ear. Antimicrobial peptides (APs) are key components of the innate immune response that serve to limit bacterial colonization of mucosal surfaces (Laube et al., 2006; Raqib et al., 2006; Selsted and Ouellette, 2005; Lehrer, 2004; Salzman et al., 2003). We, and others, have characterized APs, including chinchilla β-defensin-1 (cBD-1) and its greatest ortholog human β-defensin-3 (hBD-3), that kill causative agents of OM at micromolar concentrations and are operative in the tubotympanum (McGillivary et al., 2007; Song et al., 2007; Harris et al., 2004; Starner et al., 2002; Lim et al., 2000). Previously, we demonstrated, in vitro, that incubation of respiratory epithelial cells with RSV resulted in decreased expression of cBD-1 (McGillivary et al., 2007). Since other investigators have clearly shown that altered expression of even a single AP can impact the ability of bacteria to colonize a host (Chromek et al., 2006; Iimura et al., 2005; Nizet et al., 2001), we hypothesized that a virus-mediated decrease in β-defensin expression would promote increased bacterial colonization of the upper airway and thus the subsequent development of OM.
Here, we present evidence that RSV infection diminished both cBD-1 mRNA and protein abundance in the URT of the chinchilla. We also established that this decreased defensin expression resulted in an increased load of NTHI that colonized the upper airway. Further, administration of additional exogenous recombinant cBD-1 or hBD-3 to the chinchilla URT resulted in reduced NTHI colonization; whereas delivery of antibody directed against cBD-1 resulted in an increased bacterial load. Collectively our data demonstrated that infection of the mammalian airway with an URT virus downregulates expression of a β-defensin. This downregulation subsequently resulted in increased colonization of the NP by NTHI, and thus provided evidence of a novel mechanism by which URT viruses predispose to bacterial OM, by disrupting control of the load of NTHI permitted to colonize the NP.
RESULTS
RSV infection resulted in decreased cBD-1 expression in vivo
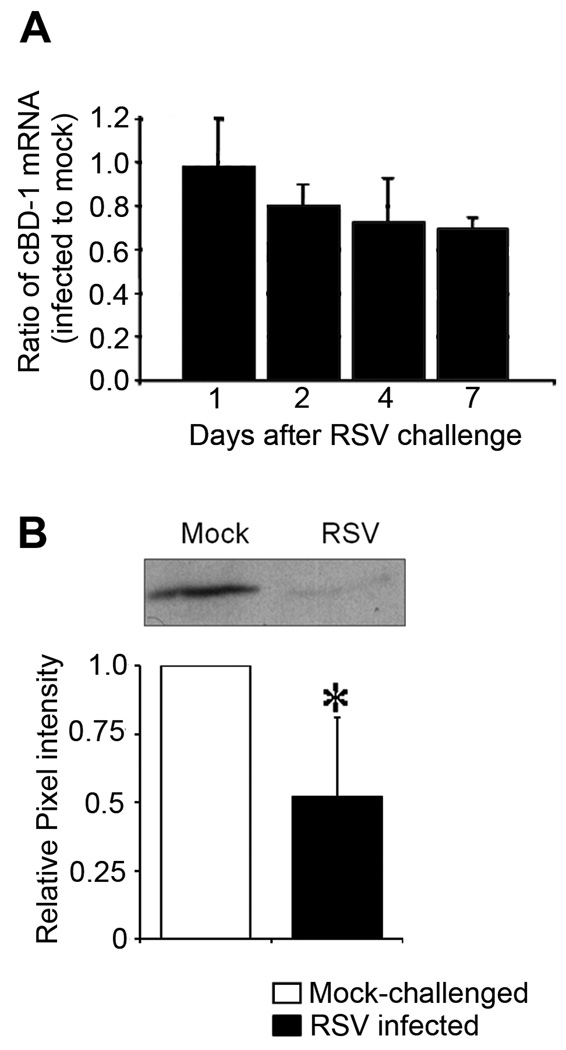
We previously demonstrated that cultured respiratory epithelial cells inoculated with RSV downregulate expression of cBD-1 transcripts in vitro (McGillivary et al., 2007). Here, we extended our study to an in vivo model and monitored cBD-1 transcript abundance in chinchilla mucosal epithelium after intranasal (IN) challenge with RSV. We observed a time-dependent decrease in the amount of cBD-1 mRNA in mucosal samples obtained from chinchillas inoculated with RSV compared to those obtained from animals that were mock-infected. A 20% reduction was noted 2 days after RSV infection, which increased to approximately 30% reduction on days 4 and 7 after viral challenge (p ≥ 0.05) (Fig. 1A). These data demonstrated that RSV infection resulted in reduced expression of cBD-1, a mucosal AP expressed in the chinchilla uppermost airway.
Figure 1.
Analysis of cBD-1 mRNA and protein abundance after IN challenge with RSV. 1, 2, 4, or 7 days after viral challenge, chinchillas (n = 8) were sacrificed and nasal septum mucosa was recovered for isolation of total RNA or protein. (A) qRT-PCR was used to determine abundance of cBD-1 mRNA and results were normalized to the amount of GAPDH mRNA, with values reported as the ratio of cBD-1 transcripts from infected to mock-treated samples. (B) Analysis of native cBD-1 protein expression in nasal septum mucosa seven days after RSV challenge. Mucosal homogenate proteins were fractionated by AU-PAGE, transferred to PVDF for Western analysis and a representative blot demonstrating the abundance of cBD-1 from a mock- or RSV-challenged animal is shown (top image). Quantitative densitometric analysis of cBD-1 protein abundance in mucosal extracts was reported as the ratio of the pixel intensity of samples from either mock-challenged or RSV-infected animals relative to the pixel intensity from mock-treated chinchillas (bottom graph). Asterisk denote a statistically significant (p ≤ 0.05) reduction in the abundance of cBD-1 protein in RSV-infected chinchillas compared to mock-treated animals. RSV infection resulted in time-dependent decreased expression of cBD-1 mRNA and protein in the URT of the chinchilla.
Next, we determined whether this decrease in cBD-1 mRNA resulted in a concomitant reduction in native cBD-1 protein available at the mucosal surface. RSV-infected and mock-challenged chinchillas were sacrificed 4 or 7 days after inoculation, and relative amounts of cBD-1 protein were determined by immunodetection and densitometry. Mucosal homogenates from chinchillas infected with RSV for 4 days contained 10% less native cBD-1 protein compared to samples obtained from mock-infected chinchillas (data not shown). Seven days after RSV infection (n =4 per cohort), there was ~ 50% less native cBD-1 protein in nasal septum mucosal homogenates (p = 0.04) (Fig. 1B). We further determined that cBD-1 protein abundance was decreased by 25% in a mucosal homogenate of a Eustachian tube obtained from RSV-infected chinchillas compared to that from a mock-infected animal seven days after virus challenge (data not shown). Collectively, our results indicated that URT infection with RSV resulted in a time-dependent reduction in native cBD-1 protein available at the mucosal surface in the chinchilla uppermost airway.
RSV infection resulted in increased recovery of NTHI in nasopharyngeal lavage fluids
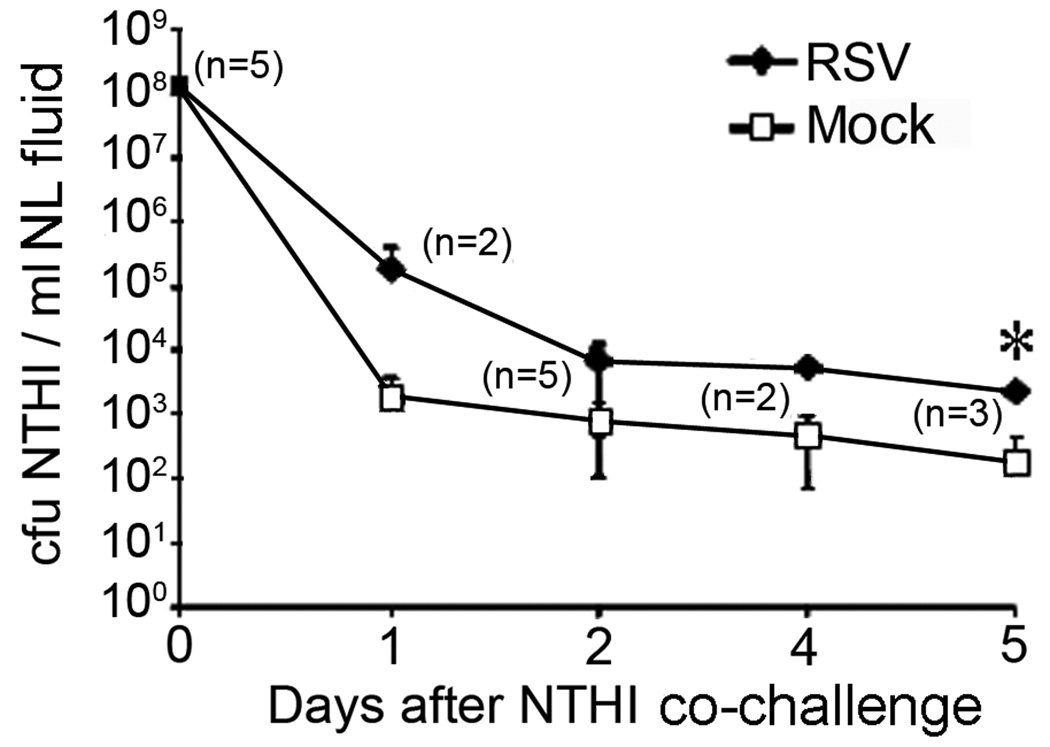
To determine whether RSV-induced dysregulation of cBD-1 expression resulted in an altered load of NTHI within the nasopharynx, chinchillas were challenged intranasally first with RSV, then with NTHI two days later. We monitored the concentration of NTHI in nasopharygeal lavage (NL) fluids obtained from virus- or mock-infected chinchillas (n = 5 per cohort) on days 1, 2, 4 or 5 after bacterial challenge. Approximately 100-fold more NTHI was obtained from RSV co-infected chinchillas 1 day after bacterial challenge, compared to animals that did not receive this virus (Fig. 2). In addition, a one-log greater NTHI concentration was maintained for up to 5 days after bacterial challenge in animals co-inoculated with RSV (p = 0.008 on day 5). Since chinchillas were inoculated with virus two days prior to bacterial challenge, these data demonstrated that the observed increase in bacterial colonization of RSV co-infected animals was due to a virus-mediated event, perhaps the co-incident reduction in cBD-1 protein available at the mucosal surfaces shown earlier (compare Fig. 1A and B with Fig. 2).
Figure 2.
Effect of RSV exposure on the load of NTHI in the chinchilla URT. Animals (n = 5 per cohort) were either mock infected (open squares) or challenged with 1 × 107 pfu RSV (filled diamonds) two days prior to IN inoculation of 1 × 108 cfu NTHI strain 86-028NP. We obtained NL fluids from chinchillas 1, 2, 4, or 5 days after NTHI challenge and total bacterial cell counts were determined. Data represent the mean ± SD of bacterial counts from the number of animals shown in parenthesis on the respective day. Asterisk denote a statistically significant (p ≤ 0.05) increase in the concentration of NTHI in RSV-infected chinchillas compared to mock-treated animals. A difference of 1–2 logs was consistently seen in the concentration of NTHI resident in the nasopharnyx for a minimum of 5 days after bacterial challenge.
Reduction of native cBD-1 resulted in increased NTHI colonization in vivo
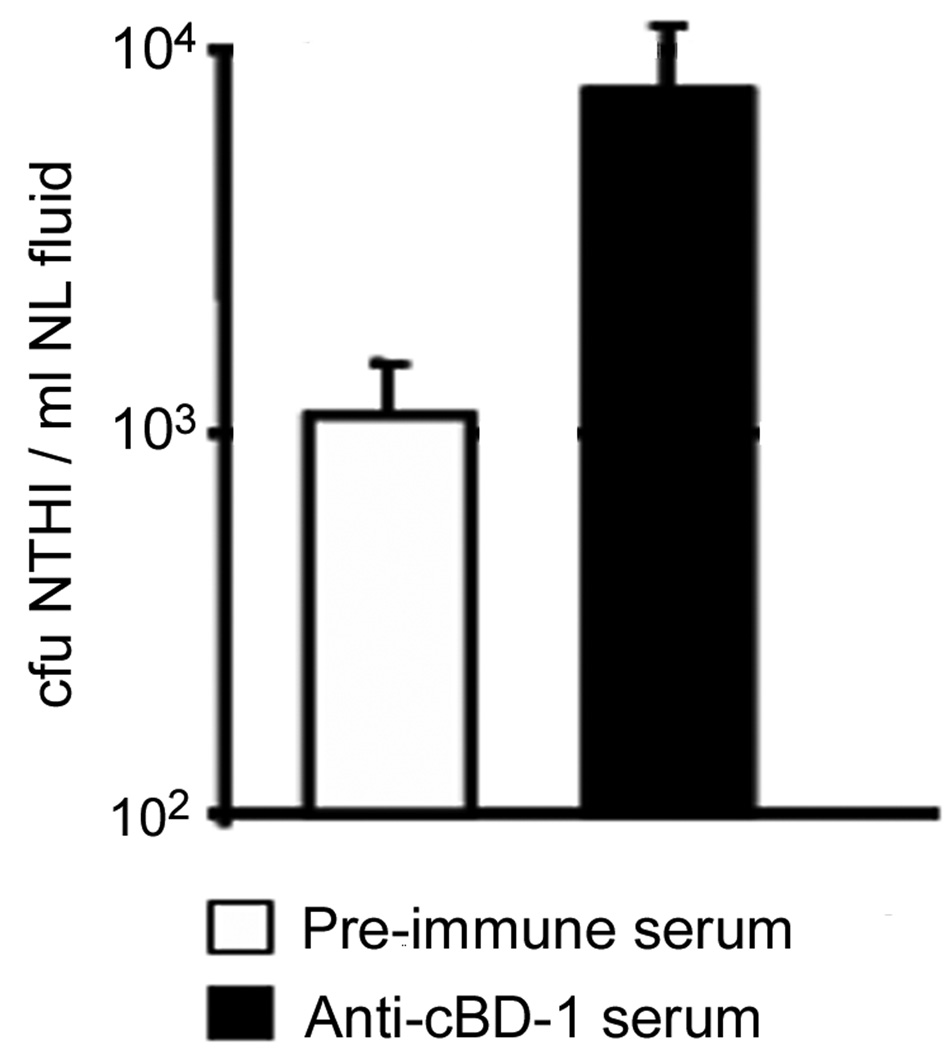
To directly demonstrate that decreased availability of native cBD-1 at the nasopharyngeal mucosal surface could alter the load of colonizing NTHI, we delivered affinity-purified anti-recombinant cBD-1 [(r)cBD-1] polyclonal antibody (or pre-immune serum as a negative control) to chinchilla nasopharynges (n = 3 per cohort) via passive inhalation, in an attempt to inhibit the activity of any available native cBD-1. Twenty minutes later, we challenged chinchillas intranasally with NTHI, then measured the relative concentration of bacteria present in NL fluids after 2 days. At this time point, animals that received anti-(r)cBD-1 antibody were colonized with approximately 104 NTHI per ml NL fluid, a log increase over those that received pre-immune serum (p ≥ 0.05) (Fig. 3). As a control, we demonstrated that the amount of polyclonal anti-(r)cBD-1 antibody used in these assays was not bactericidal against NTHI, as assessed in a standard one hour killing assay (data not shown). These results confirmed our earlier observations which suggested that inhibition of the action of a single defensin influenced colonization of the chinchilla URT by NTHI.
Figure 3.
Effect of neutralization of native cBD-1 on the load of NTHI in the chinchilla URT. Pre-immune (white bar) or anti-(r)cBD-1 serum (black bar) was administered to chinchillas (n = 3 per cohort) 20 minutes prior to challenge with 1 × 108 cfu NTHI. Enumeration of bacteria in NL fluids two days after challenge are shown as the average concentration of NTHI per ml of NP lavage fluid. Anti-(r)cBD-1 reduction of native cBD-1 protein at the mucosal surface resulted in an increased load of NTHI in the URT.
Administration of recombinant cBD-1 decreased the load of NTHI in vivo
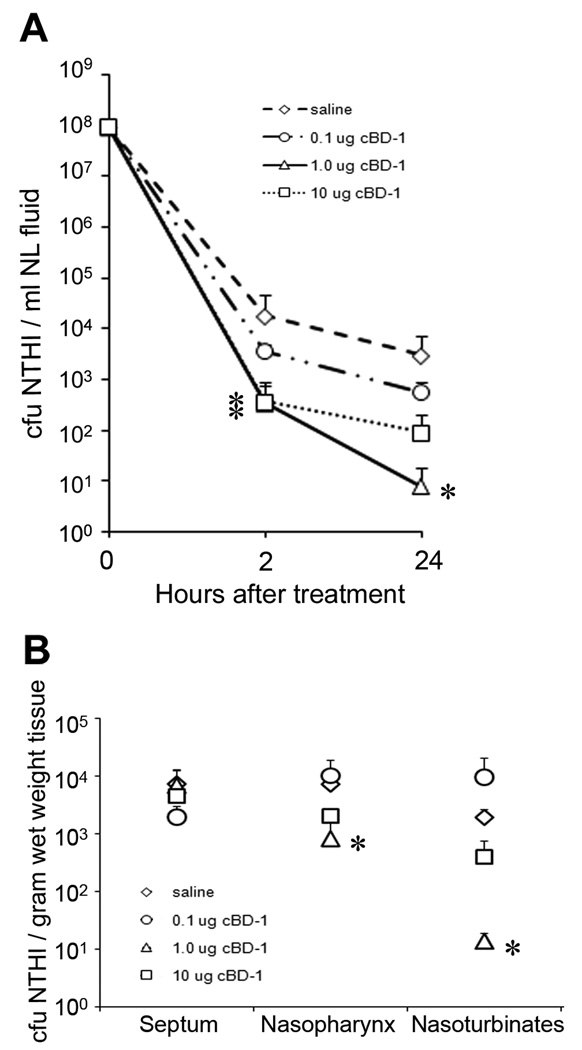
Since we had demonstrated, by two separate approaches, that reduction of available native cBD-1 at the mucosal surface augmented colonization of the chinchilla upper airway by NTHI (Fig. 2 and Fig 3), we next determined if an increase in available defensin subsequently decreased colonization by NTHI. We first challenged chinchillas (n = 3 per cohort) with NTHI followed 1 day later by delivery of a diluent control (saline) or one of three doses of (r)cBD-1 (0.1 µg, 1.0 µg and 10 µg diluted in pyrogen-free saline). Two hours after treatment, we observed a reduced concentration of NTHI present in NL fluids obtained from animals that had received any of the three doses of (r)cBD-1 compared to the saline control (p ≤ 0.05 for cohorts that received 1.0 or 10 µg cBD-1) (Fig. 4A). After 24 hours, the number of NTHI recovered from animals that received 0.1 µg (r)cBD-1 was moderately decreased when compared to the mock-treated cohort. In contrast, 2–3 logs fewer NTHI were recovered from animals that received either 1.0 µg or 10 µg (r)cBD-1 compared to animals that received saline alone (p = 0.01 for the cohort that received 1.0 µg cBD-1).
Figure 4.
Influence of (r)cBD-1 on the load of NTHI in the chinchilla upper airway. (A) Chinchillas (n = 3 per cohort) were challenged with NTHI 86-028NP, and 20 hours later, were administered saline or one of three doses of (r)cBD-1. NL fluids were obtained 2 or 24 hours after animals received either saline or the AP, and bacterial counts were determined. (B) After conducting nasopharyngeal lavages, we recovered mucosa from the chinchilla URT and tissue homogenates were plated for determination of the adherent population of NTHI after AP treatment. (r)cBD-1 decreased the load of NTHI in NL fluids and at the mucosal surface of the upper airway. Asterisks denote a statistically significant (p ≤ 0.05) decrease in the concentration of NTHI in chinchillas that received (r)cBD-1 compared to animals that received saline alone.
After we obtained NL fluids from animals that had been administered (r)cBD-1 24 hours earlier, we removed and homogenized several tissues from the chinchilla upper airway to determine the relative adherent populations of NTHI, in contrast to that population of NTHI that were retrievable by lavage of the NP and thereby were not likely adherent to mucosal epithelial cells. The concentration of NTHI that we recovered from nasal septum mucosa was not significantly different among animals that received the saline control or any of the three doses of (r)cBD-1 (Fig. 4B). In contrast, we showed a 10-fold decrease in the concentration of NTHI cultured from nasopharyngeal mucosal homogenates using tissues recovered from animals that had received either 1.0 or 10.0 µg (r)cBD-1, as compared to the mock cohort (p = 0.045 for the cohort that received 1.0 µg cBD-1). We further observed a 100-fold decrease in the load of NTHI adherent to nasoturbinate mucosa isolated from chinchillas that received 1.0 µg (r)cBD-1, when compared to animals that received saline alone (p = 0.009). These results demonstrated that intranasal administration of (r)cBD-1 resulted in a statistically significant reduction in the number of NTHI retrievable by NL, and also in those bacteria adhering adherent to the mucosal surface.
Administration of recombinant human β-defensin-3 decreased the load of NTHI in vivo
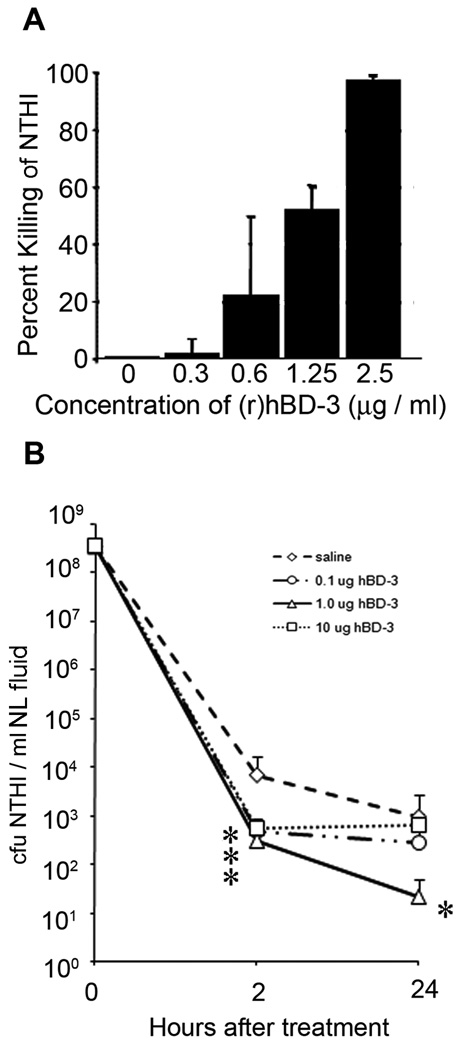
Chinchilla BD-1 shares significant homology with human β-defensin-3 (77% nucleotide identity) (Harris et al., 2004), and thereby we tested the ability of this xenobiotic AP to also alter colonization of the chinchilla URT by NTHI. We first determined the ability of (r)hBD-3 to kill NTHI 86-028NP and showed that a concentration of 1.25 µg recombinant hBD-3 [(r)hBD-3]/ml killed greater than 50% of a 1 × 105 cfu inoculum of NTHI (Fig. 5A). We then intranasally delivered 0.1 µg, 1.0 µg, or 10.0 µg (r)hBD-3 to chinchillas (n = 5 per cohort) that were already colonized with NTHI and demonstrated that, within 2 hours, all three doses of (r)hBD-3 decreased the load of NTHI recoverable by NL compared to the mock-treated cohort (p ≤ 0.05) (Fig. 5B). After 24 hours, the number of NTHI recovered from animals that received 1.0 µg (r)hBD-3 was decreased by 100-fold compared to animals that received saline alone (p = 0.01). The concentration of NTHI present in NL fluids from animals that were administered 0.1 µg or 10 µg (r)hBD-3 for 24 hours was not statistically different from the number of NTHI recovered from the mock cohort. These data suggested that, of those tested, the 1.0 µg dose of (r)hBD-3 was optimal for mediating a reduction in the load of NTHI resident within the chinchilla nasopharynx. This result was thus highly similar to that observed following exogenous delivery of cBD-1 (compare Fig 4A and Fig 5B).
Figure 5.
Influence of (r)hBD-3 on the load of NTHI in the chinchilla upper airway. (A) To determine the ability of (r)hBD-3 to kill NTHI in vitro (prior to initiation of animal studies), NTHI 86-028NP was incubated with increasing concentrations of (r)hBD-3 for 1 hour, and the number of surviving colony forming units were determined. (B) Chinchillas (n = 5 per cohort) were challenged with NTHI 86-028NP, and 20 hours later, were administered saline or one of three doses of (r)hBD-3. NL fluids were obtained 2 or 24 hours after animals received saline or the AP, and bacterial counts were determined. Asterisks denote a statistically significant (p ≤ 0.05) decrease in the concentration of NTHI in (r)hBD-3-treated chinchillas compared to saline-treated animals.
DISCUSSION
It is well established that URT viruses play a significant role in the development of bacterial OM, thereby making this a truly polymicrobial disease. The mechanisms that underlie the synergistic relationship between URT viruses and bacteria in the OM disease course are not completely understood, but are predicated on impairment of host airway defenses. We hypothesized that dysregulation of expression of antimicrobial peptide(s) by a preceding URT virus infection would similarly facilitate the development of OM. Herein, we used a chinchilla model of the highly prevalent pediatric disease OM to show that RSV infection resulted in reduced expression of an effector of innate immunity, cBD-1. Further, we demonstrated that this decrease in cBD-1 protein resulted in an increased load of NTHI in the chinchilla nasopharynx, a classic clinical feature of pending development of OM (Faden et al., 1997; Faden et al., 1995).
By qRT-PCR, we showed that the amount of cBD-1 transcripts was diminished in time-dependent manner in mucosal epithelium recovered from RSV-infected chinchillas, with a maximal decrease observed at the final time point evaluated (7 days after RSV infection). We further demonstrated that expression of native cBD-1 protein was reduced by more than half in mucosa recovered from animals infected with RSV, compared to tissues from chinchillas that received only viral diluent. Since we analyzed cBD-1 transcript abundance as reflected by analysis of the entire recovered tissue sample, it is likely that the downregulation of cBD-1 mRNA is even more pronounced within specific anatomical regions of the uppermost airway or by individual RSV-infected cells. It would be interesting to more specifically determine the effect of RSV infection on expression of cBD-1 in individual cells of the Eustachian tube as we have obtained evidence that a gradient of cBD-1 mRNA production existed within this tubal organ (author’s unpublished data). RSV-mediated downregulation of cBD-1 at the nasopharyngeal orifice of the Eustachian tube, the site where cBD-1 expression was greatest, could thus weaken the effectiveness of this primary defense organ of the middle and thus promote retrograde movement of bacteria resident within the nasopharynx into the middle ear space. Indeed, we have shown by immunohistochemistry that intranasal challenge of chinchillas with RSV results in foci of viral replication throughout the upper airway including the Eustachian tube (Jurcisek et al., unpublished).
RSV infection induces increased expression of specific innate immune mediators (Janssen et al., 2007; Thompson et al., 2006) and downregulates expression of others (Polack et al., 2005; Griese, 2002) to induce a complex transcriptional response within a given host (Janssen et al., 2007; Zhang et al., 2005; Zhang et al., 2001). So what is the net collateral effect of RSV-induced altered expression of host cell molecules on the bacterial load present in the upper airway? We utilized a polymicrobial infection model to demonstrate that RSV challenge prior to inoculation with NTHI, resulted in a consistent 1–2 log increase in the concentration of NTHI in NL fluids, compared to a mock cohort. In addition, we demonstrated that this increased load of NTHI within the chinchilla URT was observed for at least 5 days after bacterial challenge, a time frame in which we also showed that the amount of cBD-1 mRNA and protein within URT mucosa was diminished. Previously, we demonstrated in vitro that incubation of chinchilla middle ear epithelial cells with NTHI 86-028NP moderately decreases cBD-1 mRNA abundance. We have observed that a similar downregulation of cBD-1 transcripts (~25%) also occurred in nasal septum mucosae 4 days after chinchillas were challenged with NTHI alone (data not shown). Collectively, our data suggested that, among many potential mechanisms by which upper respiratory tract viral infection predisposes to bacterial otitis media, RSV-mediated decrease in cBD-1 expression likely contributed to the observed enhanced colonization of the nasopharynx by NTHI, an event known to precede development of OM in children.
Whereas the observed increase in colonization of the URT by NTHI after RSV infection may have also been influenced by increased expression of host cell receptors to which NTHI bind, such as ICAM-1, CEACAM-1, or PAF-r (Avadhanula et al., 2006), we provided evidence for the direct impact of defensin-mediated bactericidal activity, or lack thereof, on the relative concentration of NTHI within the NP. In support of our hypothesis that virus-mediated altered expression of APs contributes to dysregulation of the load of NTHI residing within the NP, we showed that delivery of anti-(r)cBD-1 to the chinchilla nasal cavity effectively increased the relative cfu NTHI ml−1 recovered from NL fluids by 10-fold, compared to animals that received pre-immune serum. These results showed that inactivation of native cBD-1 augmented the load of NTHI within the upper airway. We also provided evidence that IN delivery of exogenous (r)cBD-1 to the chinchilla URT decreased both the concentration of NTHI recovered by NL and the number of bacteria that were adherent to nasopharngeal and nasoturbinate mucosae. A single dose of either (r)cBD-1 or (r)hBD-3 reduced the load of NTHI in the URT within two hours of administration of the APs, compared to animals that received saline. Further, we demonstrated that chinchillas treated with 1.0 µg of (r)cBD-1 or (r)hBD-3 yielded 100-fold fewer NTHI in NL fluids than did mock-treated animals. The mechanism that underlies the greater effectiveness of 1.0 µg of either (r)cBD-1 or (r)hBD-3, compared with 10 µg of the respective AP, to decrease the concentration of NTHI in the chinchilla URT is not currently understood. However, others have similarly reported that beyond a specific optimal dose, delivery of additional AP to an animal does not always result in greater efficacy, as measured by the relative concentration of bacteria at a specific anatomical site (Sharma et al., 2001; Welling et al., 1998). Despite some remaining unanswered questions, we have, nonetheless, clearly demonstrated that altered expression of a single defensin impacted the load of NTHI in the upper airway, an important contributing factor in development of the prevalent pediatric disease OM.
An important question that remains is whether the decreased expression of defensins provides a benefit to RSV. It is known that production of cytokines, such as IFN-γ, affect the Th1/Th2 balance that develops during a given immune response and the resulting character of that immune response can influence the outcome and severity of RSV infection (Castilow et al., 2008; Durbin et al., 2002). In addition, it has been reported that IFN-γ induces expression of hBD-3 (Joly et al., 2005), an observation which provides additional support to the intimation that this AP may play a pivotal role in the host response to RSV. In further support of this hypothesis, we have demonstrated that both hBD-3 and cBD-1 inhibited the ability of RSV to induce the formation of syncytia in epithelial cells in vitro (data not shown). Collectively, our data suggested that RSV-induced downregulation of hBD-3 mRNA and protein abundance likely also occurs in the human upper airway and may represent a mechanism used by the virus to evade inactivation by this AP.
The importance of carefully regulated AP expression was recently demonstrated by Raqib and colleagues, who showed that LL-37 was downregulated in patients infected with Shigella and that augmentation of LL-37 expression, by addition of butyrate, could reduce the severity of shigellosis (Raqib et al., 2006). Similarly, we have provided evidence that RSV-induced downregulation of cBD-1 expression influenced colonization of the NP by NTHI. These data have provided the first evidence that virus-induced dysregulation of antimicrobial peptide expression contributes to the increased bacterial colonization known to precede development of OM. Moreover, they provide a basis upon which we can continue to elucidate the role of innate immunity in the polymicrobial disease OM, and continue to devise novel therapeutic strategies that intervene at an early step in the course of this highly prevalent pediatric disease.
EXPERIMENTAL PROCEDURES
Microbial strains and culture conditions
NTHI strain 86-028NP has been described previously and was grown on chocolate II agar (Fisher Scientific, Pittsburgh, PA) or on chocolate agar prepared in house supplemented with 15 µg ampicillin/ml to minimize growth of contaminating normal flora present in NL fluids (Bakaletz et al., 2005; Harrison et al., 2005; Mason et al., 2005; Novotny et al., 2002). RSV A2 was purchased from the American Type Culture Collection (Manassas, VA) and grown in HeLa cells. Three days after RSV inoculation, HeLa cells were scraped from tissue culture dishes, vortexed, and pelleted at 1,500 rpm for 5 minutes. The supernatant was centrifuged for 90 minutes at 20,000 × g, and the virus pellet was rinsed with Hanks’ Balanced Salt Solution (HBSS) (Invitrogen, Carlsbad, CA) supplemented with 25 mM HEPES (Fisher Scientific).
Animals
Healthy adult or juvenile chinchillas (Chinchilla lanigera), purchased from Rauscher’s Chinchilla Ranch (LaRue, OH), were used for these studies, after allowing them to acclimate to the vivarium for 7 to 10 days. Juvenile chinchillas (approximately 3 months of age) were used due to their greater permisssivity to RSV infection than adult chinchillas (Gitiban et al., 2005). Chinchillas were anesthetized with xylazine (2 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA) and ketamine (10 mg/kg, Phoenix Scientific Inc., St. Joseph, MO), and NL fluids were obtained by passive inhalation of 500 µl of pyrogen-free sterile saline into one naris with recovery of lavage fluid from the contralateral naris as liquid was exhaled (Mason et al., 2005). For recovery of airway tissues, animals were anesthetized, sacrificed, and tissues of interest were dissected and snap frozen in liquid nitrogen. Mucosal samples were then stored at −80°C until needed.
Assessment of RSV-induced dysregulation of cBD-1 mRNA expression in vivo
Two cohorts of 8 juvenile chinchillas each were inoculated IN with 1 × 107 pfu RSV or viral suspension buffer (HBSS supplemented with 25 mM HEPES), diluted to 200 µl with sterile saline. Virus was administered by passive inhalation of droplets of viral suspension delivered to the nares of anesthetized chinchillas. On days 1, 2, 4, or 7 after viral challenge, 2 animals were sacrificed per cohort and selected URT chinchilla mucosal samples were recovered. Total RNA was prepared essentially as described previously (McGillivary et al., 2007), except that tissues were homogenized in 1 ml of Trizol solution (Invitrogen, Carlsbad, CA). Two nanograms of total RNA was used in each qRT-PCR reaction with primers designed based on the cBD-1 and chinchilla glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA sequences (McGillivary et al., 2007). Values reported are the geometric mean ratios (± standard error of the mean) of cBD-1 transcript abundance of RSV-infected to mock-infected samples normalized against transcripts from the GAPDH gene. Statistical significance was calculated using a Student's T-test and was significance was accepted at a p-value of ≤0.05.
Determination of RSV-mediated alteration of cBD-1 protein expression
Juvenile chinchillas were inoculated with 1 × 107 pfu RSV or viral suspension buffer, as described above. On days 4 (2 animals total) or 7 (n = 4 per cohort) after viral challenge, animals inoculated with RSV and that received viral diluent were sacrificed, and selected URT mucosal samples were recovered. Native cBD-1 was isolated from URT mucosa based upon methods used to purify human defensin-5 from intestinal tissues and this procedure utilized extraction of protein in ice-cold 20% acetic acid (Ghosh et al., 2002).
Twenty micrograms of acid-extracted protein from URT mucosa was separated, in duplicate, by 12.5% AU-PAGE based upon a published protocol (Wang et al., 1997). The Coomassie Plus reagent (Pierce, Rockford, Il) was used to determine protein concentration of samples, and gels were stained with the SilverSNAP® stain kit II (Pierce) to confirm that equivalent protein amounts were loaded. A Western blot of the duplicate gel was performed as previously described (Porter et al., 1998). A 1:5000 dilution of rabbit anti-(r)cBD-1 (see below) was used, and antibody bound to the membrane was visualized with a 1:10,000 dilution of HRP-conjugated goat anti-rabbit IgG (Invitrogen) followed by chemiluminescent detection (GE healthcare, Piscataway, NJ). In our analysis, we could not provide a blot of a standard reference protein such as glyceraldehyde-3-phopsphate dehydrogenase since the protein samples used were purified by acid extraction. A GS-800 calibrated densitometer with Quantity One® 1-D analysis software (Bio-Rad, Hercules, CA) was used for densitometric analysis of native cBD-1 protein in mucosal extracts, and values were reported as the ratio of the pixel intensity of samples from RSV-infected chinchillas relative to the pixel intensity from mock-infected tissues. Statistical significance was calculated using a Student's T-test and significance was accepted at a p-value of ≤0.05.
Influence of RSV infection on the ability of NTHI to colonize the chinchilla URT
Two cohorts of 5 juvenile chinchillas each were inoculated IN with RSV or viral diluent only, prior to challenge two days later with 1 × 108 cfu NTHI 86-028NP. Nasopharyngeal lavages were performed at 1, 2, 4, or 5 days after bacterial challenge, and the concentration of NTHI was determined by dilution of the NL fluids and plate counts on chocolate agar with 15 µg ampicillin/ml. T-tests were performed separately for each day to compare means of the two cohorts. Due to the sample sizes within each group, normality assumptions were not met and bonferroni methods were used to adjust for multiple comparisons. A bonferroni adjusted p-value ≤ 0.05 was considered significant.
Production of anti-(r)cBD-1
We prepared and purified (r)cBD-1 based upon previously published methods with minor changes (Harris et al., 2004). Purity of the preparation was confirmed by Coomassie brilliant blue stained SDS-PAGE, and three 60 µg doses of peptide were used to generate rabbit polyclonal antiserum (Spring Valley Laboratories, Sykesville, MD). To minimize any contribution that non-specific, NTHI-cross reactive antibodies present in anti-(r)cBD-1 serum would have on the ability of NTHI to colonize the chinchilla (see below), rabbits were pre-screened to ensure minimal pre-existing antibodies to NTHI 86-028NP outer membrane proteins (OMP) (Sirakova et al., 1994) prior to immunization with (r)cBD-1.
Antibody-induced neutralization of native cBD-1 in vivo
A HiTrap™ protein G HP column (GE healthcare, Pittsburgh , PA) was used to affinity purify total IgG from rabbit anti-(r)cBD-1 and the cognate pre-immune serum. One milliliter of serum was dialyzed [3.5 kDa molecular weight cutoff (MWCO), EMD Chemicals Inc., San Diego, CA] at 4°C against 20 mM sodium phosphate buffer, pH 7.0. A syringe was used to apply sera to a column equilibrated with sodium phosphate buffer, non-specifically bound protein was washed from the HiTrap™ apparatus, and immunoglobulins were eluted from the affinity matrix with 0.1 M glycine-HCl, pH 2.7. One ml fractions were collected into eppendorf tubes that contained 200 µl of 1.0 M Tris-HCl, pH 9.0, to neutralize the acidic elution conditions. Samples that contained the greatest amount of protein (fractions 1 and 2) were pooled and dialyzed overnight at 4°C against sterile saline. Protein concentrations of the anti-(r)cBD-1 and the pre-immune serum were determined as described above.
Six adult chinchillas were administered 270 µg anti-cBD-1 or pre-immune rabbit immunoglobulin, in a volume of 80 µl, similar to what we have reported previously (Bookwalter et al., 2007). Antisera were administered by passive inhalation of droplets of the solution delivered to the nares of anesthetized chinchillas. Animals were then placed in a prone position for 20 minutes prior to IN challenge with approximately 1 × 108 cfu NTHI in a 100 µl volume. Nasopharyngeal lavages were performed two days after NTHI challenge, and bacterial counts were determined by dilution plating of bacteria on chocolate agar supplemented with 15 µg ampicillin/ml.
Antimicrobial Assays
To determine the ability of (r)hBD-3 to kill NTHI 86-028NP, we utilized a microbiocidal assay that has been previously described (Harris et al., 2004).
Ability of (r)cBD-1 to reduce the load of NTHI in the chinchilla URT
Recombinant cBD-1 was prepared based upon previously published methods (Harris et al., 2004). To remove residual LPS from the protein preparation prior to administration of the AP to chinchillas, (r)cBD-1 was further purified on an EndoTrap® red column (Fisher) according to the suppliers suggestions. This purification step resulted in a (r)cBD-1 solution that contained less than 0.5 endotoxin units/ml as determined by the chromo-LAL assay (Associates of Cape Cod, East Falmouth, MA). The AP (0.1 µg, 1 µg, or 10 µg) was prepared in saline to a total volume of 80 µl. Four cohorts of 3 chinchillas each were challenged with 1 × 108 cfu NTHI 86-028NP, and colonization of the URT was allowed to proceed for twenty hours, wherein animals are typically colonized with 1 × 103-1 × 104 cfu NTHI/ml (Mason et al., 2005). Animals were then administered saline or one of the three amounts of (r)cBD-1, and lavaged 2 or 24 hours after treatment. The concentration of NTHI in NL fluids was determined by dilution plating of bacteria on chocolate agar supplemented with 15 µg ampicillin/ml. At each time point, bacterial counts from the cohorts that received (r)cBD-1 were compared to the concentration of NTHI in NL fluids recovered from animals that received saline. After the final lavage, chinchillas were sacrificed and mucosae from the nasal septum, nasoturbinate, and nasopharynx were also recovered, weighed, homogenized in 1.0 ml sterile pyrogen-free saline using a tissue grinder, and plated to determine cfu NTHI/gram tissue. For statistical analysis, original count data was log transformed and a bonferroni adjusted p-value ≤ 0.05 was considered significant.
Ability of (r)hBD-3 to reduce the load of NTHI in the chinchilla URT
Recombinant hBD-3 (PeptroTech Inc., Rocky Hill, NJ) was solubilized in 10 mM acetic acid to a concentration of 0.5 µg/µl, and the solution was dialyzed (3.5 kDa MWCO) against pyrogen-free sterile saline. The AP (0.1 µg, 1 µg, or 10 µg) was prepared in saline to a total volume of 80 µl and the (r)hBD-3 samples contained less than 0.1 ng endotoxin per µg recombinant peptide. Four cohorts of 5 chinchillas each were challenged with 1 × 108 cfu NTHI 86-028NP, and analysis of the concentration of NTHI in NL fluids 2 or 24 hours after administration of saline or one of the three amounts of the recombinant protein was determined as above. For statistical analysis, original count data was log transformed and a bonferroni adjusted p-value ≤ 0.05 was considered significant.
ACKNOWLEDGEMENTS
This work was supported by R01 DC005847 from NIDCD/NIH to LOB. We thank Jennifer Neelans for manuscript preparation and Molly Bruggeman for technical assistance.
REFERENCES
- Avadhanula V, Rodriguez CA, Ulett GC, Bakaletz LO, Adderson EE. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infection & Immunity. 2006;74:830–838. doi: 10.1128/IAI.74.2.830-838.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO. Otitis Media. Washington DC: ASM Press; 2002. [Google Scholar]
- Bakaletz LO, White GJ, Post JC, Ehrlich GD. Blinded multiplex PCR analyses of middle ear and nasopharyngeal fluids from chinchilla models of single- and mixed-pathogen-induced otitis media. Clinical & Diagnostic Laboratory Immunology. 1998;5:219–224. doi: 10.1128/cdli.5.2.219-224.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO, Baker BD, Jurcisek JA, Harrison A, Novotny LA, Bookwalter JE, et al. Demonstration of Type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infection & Immunity. 2005;73:1635–1643. doi: 10.1128/IAI.73.3.1635-1643.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookwalter JE, Jurcisek JA, Gray Owen SD, McGillivary G, Bakaletz LO. CEACAM1 homologue plays a pivotol role in nontypeable Haemophilus influenzae colonization of the chinchilla nasopharynx via the OMP P5-homologous adhesin. Infection & Immunity. 2007;76:48–55. doi: 10.1128/IAI.00980-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilow EM, Legge KL, Varga SM. Cutting edge: Eosinophils do not contribute to respiratory syncytial virus vaccine-enhanced disease. J Immunol. 2008;181:6692–6696. doi: 10.4049/jimmunol.181.10.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonmaitree T, Revai K, Grady JJ, Clos A, Patel JA, Nair S, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46:815–823. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- Daly KA, Brown JE, Lindgren BR, Meland MH, Le CT, Giebink GS. Epidemiology of otitis media onset by six months of age. Pediatrics. 1999;103:1158–1166. doi: 10.1542/peds.103.6.1158. [DOI] [PubMed] [Google Scholar]
- Durbin JE, Johnson TR, Durbin RK, Mertz SE, Morotti RA, Peebles RS, Graham BS. The role of IFN in respiratory syncytial virus pathogenesis. J Immunol. 2002;168:2944–2952. doi: 10.4049/jimmunol.168.6.2944. [DOI] [PubMed] [Google Scholar]
- Faden H, Bernstein J, Brodsky L, Stanievich J, Ogra PL. Effect of prior antibiotic treatment on middle ear disease in children. Annals of Otology, Rhinology & Laryngology. 1992;101:87–91. doi: 10.1177/000348949210100119. [DOI] [PubMed] [Google Scholar]
- Faden H, Duffy L, Williams A, Krystofik DA, Wolf J. Epidemiology of nasopharyngeal colonization with nontypeable Haemophilus influenzae in the first 2 years of life. Journal of Infectious Diseases. 1995;172:132–135. doi: 10.1093/infdis/172.1.132. [DOI] [PubMed] [Google Scholar]
- Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. Journal of Infectious Diseases. 1997;175:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J, et al. Paneth cell trypsin is the processing enzyme for human defensin-5. Nature Immunology. 2002;3:583–590. doi: 10.1038/ni797. [see comment] [DOI] [PubMed] [Google Scholar]
- Giebink GS, Berzins IK, Marker SC, Schiffman G. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infection & Immunity. 1980;30:445–450. doi: 10.1128/iai.30.2.445-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitiban N, Jurcisek JA, Harris RH, Mertz SE, Durbin RK, Bakaletz LO, Durbin JE. Chinchilla and murine models of upper respiratory tract infections with respiratory syncytial virus. Journal of Virology. 2005;79:6035–6042. doi: 10.1128/JVI.79.10.6035-6042.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griese M. Respiratory syncytial virus and pulmonary surfactant. Viral Immunology. 2002;15:357–363. doi: 10.1089/08828240260066279. [DOI] [PubMed] [Google Scholar]
- Harris RH, Wilk D, Bevins CL, Munson RS, Jr, Bakaletz LO. Identification and characterization of a mucosal antimicrobial peptide expressed by the chinchilla (Chinchilla lanigera) airway. Journal of Biological Chemistry. 2004;279:20250–20256. doi: 10.1074/jbc.M400499200. [DOI] [PubMed] [Google Scholar]
- Harrison A, Dyer DW, Gillaspy A, Ray WC, Mungur R, Carson MB, et al. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. Journal of Bacteriology. 2005;187:4627–4636. doi: 10.1128/JB.187.13.4627-4636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T, Chonmaitree T. Importance of respiratory viruses in acute otitis media. Clinical Microbiology Reviews. 2003;16:230–241. doi: 10.1128/CMR.16.2.230-241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, Kagnoff MF. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. Journal of Immunology. 2005;174:4901–4907. doi: 10.4049/jimmunol.174.8.4901. [DOI] [PubMed] [Google Scholar]
- Janssen R, Pennings J, Hodemaekers H, Buisman A, van Oosten M, de Rond L, et al. Host transcription profiles upon primary respiratory syncytial virus infection. J Virol. 2007;81:5958–5967. doi: 10.1128/JVI.02220-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Nagata N, Molina E, Bakaletz LO, Hawkins H, Patel JA. Fimbria-mediated enhanced attachment of nontypeable Haemophilus influenzae to respiratory syncytial virus-infected respiratory epithelial cells. Infection & Immunity. 1999;67:187–192. doi: 10.1128/iai.67.1.187-192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly S, Organ CC, Johnson GK, McCray PB, Jr, Guthmiller JM. Correlation between beta-defensin expression and induction profiles in gingival keratinocytes. Mol Immunol. 2005;42:1073–1084. doi: 10.1016/j.molimm.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Jorgensen F, Andersson B, Larsson S, Nylen O. Nasopharyngeal bacterial flora in otitis prone children treated with immunoglobulin. Acta Oto-Laryngologica. 1992;112:530–538. doi: 10.3109/00016489209137436. [DOI] [PubMed] [Google Scholar]
- Laube DM, Yim S, Ryan LK, Kisich KO, Diamond G. Antimicrobial peptides in the airway. Curr Top Microbiol Immunol. 2006;306:153–182. doi: 10.1007/3-540-29916-5_6. [DOI] [PubMed] [Google Scholar]
- Lehrer RI. Primate defensins. Nat Rev Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- Lim DJ, Chun YM, Lee HY, Moon SK, Chang KH, Li JD, Andalibi A. Cell biology of tubotympanum in relation to pathogenesis of otitis media - a review. Vaccine. 2000;19 Suppl 1:S17–S25. doi: 10.1016/s0264-410x(00)00273-5. [DOI] [PubMed] [Google Scholar]
- Mason KM, Munson RS, Jr, Bakaletz LO. A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infection & Immunity. 2005;73:599–608. doi: 10.1128/IAI.73.1.599-608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillivary G, Ray WC, Bevins CL, Munson RS, Jr, Bakaletz LO. A member of the cathelicidin family of antimicrobial peptides is produced in the upper airway of the chinchilla and its mRNA expression is altered by common viral and bacterial co-pathogens of otitis media. Molecular Immunology. 2007;44:2446–2458. doi: 10.1016/j.molimm.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227:164–169. [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Novotny LA, Pichichero ME, Denoel PA, Neyt C, Vanderschrick S, Dequesne G, Bakaletz LO. Detection and characterization of pediatric serum antibody to the OMP P5-homologous adhesin of nontypeable Haemophilus influenzae during acute otitis media. Vaccine. 2002;20:3590–3597. doi: 10.1016/s0264-410x(02)00306-7. [DOI] [PubMed] [Google Scholar]
- Patel J, Faden H, Sharma S, Ogra PL. Effect of respiratory syncytial virus on adherence, colonization and immunity of non-typable Haemophilus influenzae: implications for otitis media. International Journal of Pediatric Otorhinolaryngology. 1992;23:15–23. doi: 10.1016/0165-5876(92)90075-z. [DOI] [PubMed] [Google Scholar]
- Polack FP, Irusta PM, Hoffman SJ, Schiatti MP, Melendi GA, Delgado MF, et al. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8996–9001. doi: 10.1073/pnas.0409478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter EM, Poles MA, Lee JS, Naitoh J, Bevins CL, Ganz T. Isolation of human intestinal defensins from ileal neobladder urine. FEBS Letters. 1998;434:272–276. doi: 10.1016/s0014-5793(98)00994-6. [DOI] [PubMed] [Google Scholar]
- Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9178–9183. doi: 10.1073/pnas.0602888103. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuskanen O, Arola M, Putto-Laurila A, Mertsola J, Meurman O, Viljanen MK, Halonen P. Acute otitis media and respiratory virus infections. Pediatric Infectious Disease Journal. 1989;8:94–99. [PubMed] [Google Scholar]
- Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [see comment] [DOI] [PubMed] [Google Scholar]
- Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- Sharma S, Verma I, Khuller GK. Therapeutic potential of human neutrophil peptide 1 against experimental tuberculosis. Antimicrobial Agents & Chemotherapy. 2001;45:639–640. doi: 10.1128/AAC.45.2.639-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirakova T, Kolattukudy PE, Murwin D, Billy J, Leake E, Lim D, et al. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infection & Immunity. 1994;62:2002–2020. doi: 10.1128/iai.62.5.2002-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Chae SW, Woo JS, Lee HM, Jung HH, Hwang SJ. Differential expression of human beta defensin 2 and human beta defensin 3 in human middle ear cholesteatoma. Annals of Otology, Rhinology & Laryngology. 2007;116:235–240. doi: 10.1177/000348940711600312. [DOI] [PubMed] [Google Scholar]
- Starner TD, Swords WE, Apicella MA, McCray PB., Jr Susceptibility of nontypeable Haemophilus influenzae to human beta-defensins is influenced by lipooligosaccharide acylation. Infect Immun. 2002;70:5287–5289. doi: 10.1128/IAI.70.9.5287-5289.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Bakaletz LO. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infection & Immunity. 1994;62:1710–1718. doi: 10.1128/iai.62.5.1710-1718.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. Journal of Infectious Diseases. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- Thompson L, Turko I, Murad F. Mass spectrometry-based relative quantification of human neutrophil peptides 1, 2, and 3 from biological samples. Mol Immunol. 2006;43:1485–1489. doi: 10.1016/j.molimm.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Wang MS, Pang JS, Selsted ME. Semidry electroblotting of peptides and proteins from acid-urea polyacrylamide gels. Analytical Biochemistry. 1997;253:225–230. doi: 10.1006/abio.1997.2347. [DOI] [PubMed] [Google Scholar]
- Welling MM, Hiemstra PS, van den Barselaar MT, Paulusma-Annema A, Nibbering PH, Pauwels EK, Calame W. Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leukocyte accumulation. Journal of Clinical Investigation. 1998;102:1583–1590. doi: 10.1172/JCI3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Parente J, Harris SM, Woods DE, Hancock RE, Falla TJ. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrobial Agents & Chemotherapy. 2005;49:2921–2927. doi: 10.1128/AAC.49.7.2921-2927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Luxon BA, Casola A, Garofalo RP, Jamaluddin M, Brasier AR. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. Journal of Virology. 2001;75:9044–9058. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]