Abstract
Objectives
To demonstrate the presence of circulating autoantibodies (Abs) from patients with chronic periodontitis (CP) that interacted with human gingival fibroblast membranes activating β1 adrenoceptors (β1-AR).
Methods
Sera and purified IgG from 25 patients with CP and 20 age-matched healthy subjects were studied by flow cytometry, ELISA and DNA synthesis. Human gingival fibroblast membranes and/or synthetic peptides with amino acid sequences identical to human β1-AR were used as antigens.
Results
By flow cytometry and ELISA procedures, we proved that the serum-purified IgG fraction from patients with CP reacted with the fibroblast surface and to the β1 synthetic peptide. The corresponding affinity-purified anti-β1 peptide Abs displayed agonist-like activity associated with specific receptor activation, inhibiting the DNA synthesis of human gingival fibroblasts.
Conclusions
This study demonstrates that β1-AR autoantibodies are elevated in patients with CP. These autoantibodies were targeted to the fibroblasts, and specifically to the β1-AR, and has receptor-like activity inhibiting DNA synthesis.
Keywords: Cell proliferation, Cytokines, Autoantibodies, Antigens/peptides, Signal transduction, Gingival fibroblasts
INTRODUCTION
Data from epidemiologic studies have suggested that periodontal diseases are multifactorial.1,2 Patient variables, such as age, race, smoking, and stress or local hyperactivation of the autonomic adrenergic system are important cofactors which contribute to both the prevalence of disease and the incidence of disease progression.3 Moreover, immunologic factors associated with infections caused by selected organisms within the sub-gingival plaque are essential and dominant risk factors for modelling periodontal diseases severity.4
The cellular and molecular events of pathogenesis consider that both the effects of serum on bacteria and neutrophil-bacterial interactions are associated with the acute inflammatory response5 that ultimately results in bone resorption and loss of connective tissue support.6 The pathogenesis of periodontal disease includes locally synthesized biological products, such as enzymes produced by fibroblasts or bone cells, bacterial-specific immunoglobulin (Ig) secretion, and soluble inflammatory mediators, including cytokines and prostaglandins and cellular or tissue degradation products.6,7 Although many products have been associated with the presence of inflammation,8 few have been demonstrated to be associated with a progressive autoimmune disorder.
The autoimmune concept established the foundation of a paradigm of disease susceptibility and progression which emphasize not only the virulence of the microbial pathogens, but also considers the role of the host response in regulating and limiting both the composition of the local flora and the magnitude of the tissue destruction. Thus, in periodontal disease during the process of combating pathogenic invasion, the immune system may cause localized tissue damage9 and activate the systemic humoral immune response.10
Detection of elevated immunity to type I collagen in sera of patients with periodontal disease led to the suggestion that autoimmunity may play a role in periodontal disease.11 This was supported by Anusaksathien et al12 who demonstrated that the levels of antibodies to collagen type I in periodontal tissues were above the levels detectable in serum from the same patients, suggesting autoantibody production occurs predominantly at the sites of disease. Local production of antibodies to autoantigens in granulomatous tissues contained within the periodontal lesion has been reported.13
Furthermore, the autonomic adrenergic system is an important regulator of the immune response14 and modified fibroblast DNA synthesis.15 On the basis of the autoimmune hypothesis of periodontal disease,16–21 we focused our research on the possibility of a gingival fibroblast specific antigen-antibody interaction in the disease. We investigated the adrenergic system, by screening sera of patients with periodontal disease for autoantibodies against β-adrenergic receptors (β1-AR). Thus, we studied the molecular interactions between circulating antibodies from sera of patients with chronic periodontitis (CP) and human β1-AR positive fibroblasts, pointing to the role of the second extracellular loop of the receptors as the main target of human antibody-mediated biological effects.
The aim of this work was to analyze the presence of circulating autoantibodies from CP patients which interact with gingival fibroblasts and activate β1-AR. The results demonstrated that these autoantibodies were targeted to the fibroblasts, and specifically to the β1-AR. The autoantibodies exhibited adrenergic agonistic activity by inhibiting DNA synthesis measured by 3H-thymidine incorporation.
MATERIALS AND METHODS
Patients
The study group consisted of 25 adult patients with CP who were attending the Periodontology Clinics from the metropolitan area of Buenos Aires. There were 20 males and 5 females, with a mean age of 49 years and a range of 42–62 years. Healthy subjects were used as controls (20 normal subjects [17 males and 3 females]), with mean age of 47 years and a range of 40–60 years. The characteristic clinical signs of CP included the following: loss of clinical attachment, horizontal or/and angular alveolar bone loss, periodontal pocket formation, and gingival inflammation. To be included in the study, at least six sites with ongoing periodontal disease were required. Clinical measurements on patients with cPD included the following: sites with alveolar bone loss > 2 mm and pocket depth > 5 mm with bleeding and attachment loss > 3 mm. In healthy subjects (control group), the probing depth was < 3 mm and the attachment loss was < 2 mm. None of the subjects (patients in group 1 and controls in group 2) had systemic illnesses and they were not smokers. The patients with cPD had not received periodontal treatment or antibiotics within the preceding 5 months or any antiinflammatory drugs 3 weeks prior to the study. All of the patients consented to participate in the study and the investigation was conducted according to the tenets of the Declaration of Helsinki.
Cell Culture
Human gingival fibroblasts were taken from a medically healthy donor, who was clinically free of periodontal disease. The healthy tissue appeared firm, non-erythematous, non-edematous, and was non-bleeding. Samples were obtained from donors who gave informed consent for this study. The gingival tissue was surgically excised from the maxilla and washed six times with normal saline to remove blood from the surface and stored in pre-weighed containers in liquid nitrogen. Gingival tissue was subsequently minced in small pieces and seeded in well plates using Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, and penicillin (100 U/ml) and streptomycin (100 Ug/ ml) in a 5% CO2 environment at 37°C, as described by Varani et al.22 The medium was replenished every 3–4 days. Confluent cells were sub-cultured by detaching the monolayer with 0.25% trypsin in phosphate buffered saline solution (PBS). Cells were used between passages 6–8.
Human sera and IgG purification
Sera and the corresponding IgG were obtained from groups 1 and 2. Six ml of blood was obtained by venipuncture and allowed to clot at room temperature, serum separated by centrifugation at 2000 g, and stored at −20°C until used in assays. The IgG was obtained by precipitation with ammonium sulphate at 50%, followed by three washes and re-precipitation with 33% ammonium sulphate. The resulting precipitate was submitted to chromatography on DEAE-cellulose, equilibrated with 10 mM phosphate buffer (pH 8). The eluted peaks were concentrated by ultrafiltration to 10 mg protein/ml. Control immune electrophoresis with goat anti-human total serum and goat non-specific anti-human IgG showed only one precipitin line.
Purification of antipeptide antibodies by affinity chromatography
The IgG fraction of 25 patients with CP was independently subjected to affinity chromatography on the synthesized peptide covalently linked to Affi-Gel 15 gel (Bio-Rat, Richmond, CA, USA). The IgG fraction was loaded on the affinity column equilibrated with PBS, and the non-peptide fraction was first eluted with the same buffer. Specific anti-peptide autoantibodies were then eluted with 3 M KSCN and 1 M NaCl, followed by immediate extensive dialysis against PBS. The IgG concentration of both non-antipeptide antibodies and specific anti-β1-AR peptide antibodies were determined by radial immunodiffusion assay, and their immunological reactivity against the β1-AR peptide was evaluated by ELISA.14
Flow cytometry procedure
Fibroblasts were obtained as described above. After washing cells with PBS, pellets were resuspended in PBS containing IgG (1x10−9 M) from patients with CP or normal subjects as negative controls. After a 1 hour incubation at 4oC, cells were washed and incubated for 30 min with rabbit anti-human IgG FITC-conjugated F(ab’)2 fragments (1:100). Cells were then fixed with 1% paraformaldehyide and analyzed by flow cytometry in a FACScan cytofluorometer (Becton Dickinson, Mountain View, CA, USA). An argon laser operating at 488 nm exited fluorescence attributable to FITC-conjugated antibodies. Emission from fluorescein was measured using bandpass filters at 525 nm. Appropriate settings of forward and side scatter gates were use to examine 10,000 cells per experiment. The percentage of positive cells was determined by the thresholds set using isotopic controls. The numbers of fluorescent molecules per cell were indirectly measured by assessing the mean intensity of arbitrary units of fluorescence of cells.
Measurement of DNA synthesis
DNA synthesis was estimated by measurement of [3H]-thymidine incorporation into trichloroacetic acid (TCA)–precipitable material. The β adrenergic actions of IgG were evaluated in cells (1x106 cell/ ml) that had been serum-starved for 8 hours. Each concentration of IgG was in contact with the cells for 8 hours. When β1 adrenergic antagonistic drugs were used, they were added 10 min before IgG. The [3H]-thymidine (0.1μCi/ml) was added during the last 2 hours. Cells were precipitated twice with ice-cold 10% TCA and fibroblasts were then removed with trypsin/PBS and radioactivity was determined by liquid scintillation counting. Cells that had been serum-starved for 8 hours without drugs were used as controls (basal value).
Membrane preparation
Membranes were prepared as previously described.23 In brief, the cells (2x106 cell/ml) were homogenized in an Ultraturrax at 4°C in 6 volumes of potassium phosphate buffer, 1 mM MgCI2, 0.25 M sucrose (pH 7.5) supplemented with 0.1 mM phenylmethyl sulphonyl fluoride (PMSF), 1 mM EDTA, 5 μg ml−1 leupeptin, 1 μM bacitracin, and 1 μM pepstatin A. The homogenate was centrifuged twice for 10 min at 3000 g, then at 10,000 g and 40,000 g at 4°C for 15 and 90 min, respectively. The resulting pellets were suspended in 50 mM phosphate buffer fortified with the same protease inhibitors (pH 7.5).
ELISA assay
Fifty ml of peptide solution (20 μg/ml) or gingival fibroblast-purified membranes (50 μg/ml) in 0.1 M Na2CO3 buffer (pH 9.6) was used to coat microtiter plates at 4°C overnight. After blocking the wells with 2% bovine serum albumin in PBS for 1 hour at 37°C, 100 ml of a 1/30 dilution of sera of different concentrations or purified IgG from groups 1 and 2 were added in triplicate and allowed to react with the peptide for 2 hours at 37°C. After thoroughly washing the wells with 0.05% Tween 20 in PBS, 100 μl of 1:6000 goat anti-human IgG alkaline phosphate conjugate antibodies were added and incubated for 1 hour at 37°C. After extensive washing, p-nitrophenylphosphate (1 mg/ml) was added as the substrate and the reaction was stopped after 30 min. Optical density (OD) were measured at 405 nm with an ELISA reader. As a negative control, non-antigen paired wells with M1 cholinergic peptide and wells with no primary antiserum were also conducted. The results for each sample were expressed as the mean ± s.e.m. of triplicate values.
Drugs
Stock solutions of atenolol and butoxamine were freshly prepared before each experiment. The β1 peptide corresponds to the sequence of the second extracellular loop of the human β1-AR (HWWRA ESDEA RRCYN DPKCC DFVTN RC). A control, unrelated peptide derived from the second extracellular loop of the human M1 cholinoreceptor (ERTLA GQCYI QFLSQ PIITF GTAM) was used. Radioactive material, synthetic peptides, and β adrenergic antagonists were from Dupont/New England Nuclear (Boston, MA, USA), Sigma Genosys (St. Louis, MO, USA) and Sigma Chemical Company (St. Louis, MO, USA), respectively.
Statistical analysis
Student’s t-test for unpaired values was used to determine the levels of significance. Analysis of variance (ANOVA) and a post hoc test (Dunnett’s method β1 and Student-Newman-Keuls test) were employed when pairwise multiple comparison procedures were necessary. Differences between means were considered significant at a P<.05.
RESULTS
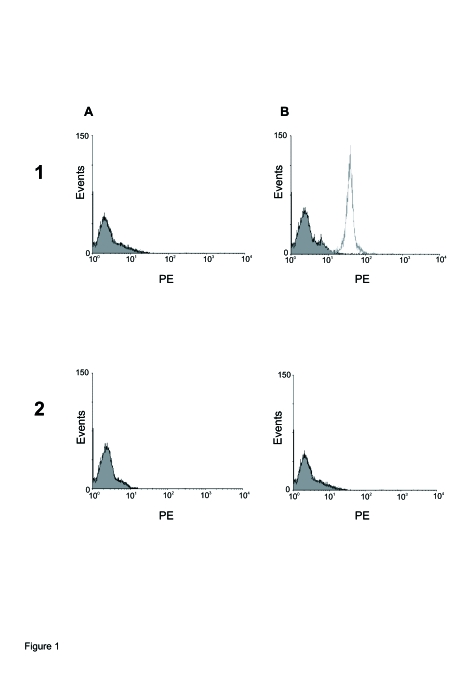
The binding capacity of human normal IgG (Figure 1A) and autoantibodies of patients with CP (Figure 1B) incubated with fibroblast cells is shown. Control IgG presented low binding (1A: 48 ± 6) and IgG from patients with CP showed high binding in fibroblast cells (1B: 229 ± 18). Moreover, when β1 synthetic peptide corresponding to the second extracellular loop of human β1-AR were included in the reaction, the binding capacity of normal control IgG was not modified (2A: 43 ± 6), while the autoantibodies of patients with CP were inhibited (2B: 53 ± 5).
Figure 1.
Representative flow cytometry analysis of binding IgG. Culture fibroblasts were incubated with A: normal human IgG (1x10−9 M) or B: CP IgG (1x10−9 M) alone (1). The same concentration of normal (2 A) or CP (2 B) IgGs preincubated during 40 min with 1x10−5 M β1 synthetic peptide is also shown. Figure is representative of separated assays using antibody from 7 patients with CP and 6 healthy subjects with similar results.
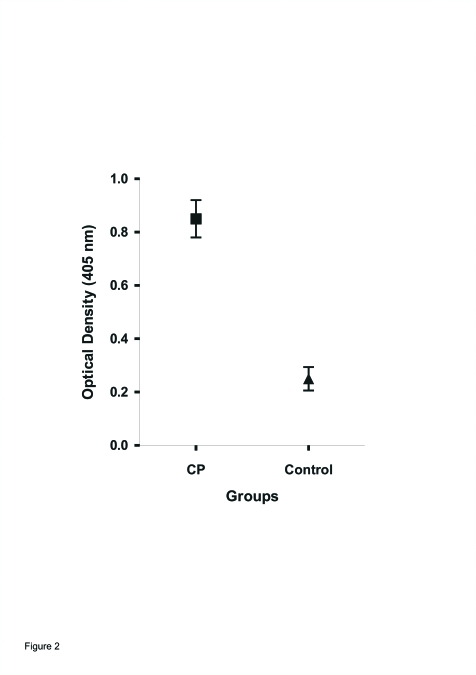
Figure 2 shows the immune reactivity of sera from different groups against human gingival fibroblast membranes. The optical density (OD) values for sera from CP were significantly higher than that from healthy individuals (control).
Figure 2.
Immunoreactivity (ELISA) of anti-membrane human gingival fibroblast antibodies of individual sera from groups: (▪) 25 patients with CP and (▴) 20 healthy subjects, control. Sera (1/50 dilution) was assayed on sensitized microplates with 50 μg/ml of human gingival fibroblast membranes. Values are means ± SEM. P<.001 between CP and control.
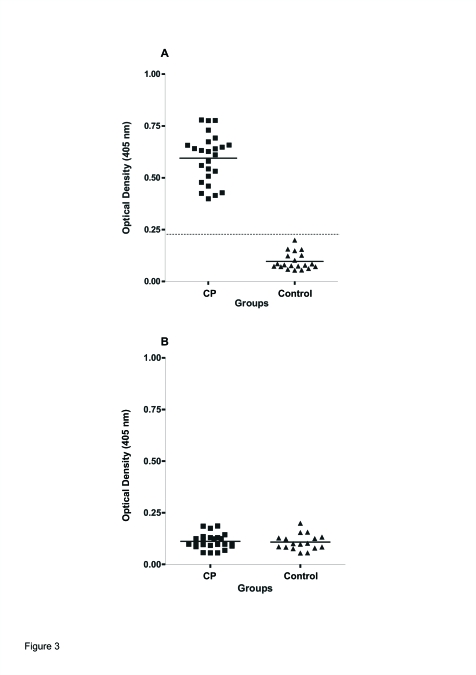
To determine the molecular interaction between IgG and human β1-AR, OD values for each of the 45 subjects studied against β1 synthetic peptide as coating antigen are shown in the scatterogram (Figure 3A). The immune reactivity of sera from CP was significantly higher than that from control individuals (P<.001). The OD of sera from CP was always at least 3 SD from that of sera from healthy individuals (control). On the other hand, when unrelated peptide (M1 cholinoreceptor peptide) was used as a coating antigen, both the IgG from patients with CP and IgG from healthy controls gave negative results (Figure 3B).
Figure 3.
Scatterogram showing immune reactivity (ELISA) of sera IgG antibodies against the second extracellular loop of A: β1-AR and B: M1 cholinoreceptor tested by ELISA. Shown are the individual optical density (OD) values each sera samples (1/50 dilution) from 25 patients with CP (▪) and 20 healthy volunteers, control (▴). Datted/dashed line, cutoff value 0.242 (mean optical density ± SEM for control; solid lines, median optical density value. P<.001 between CP and healthy subjects (control).
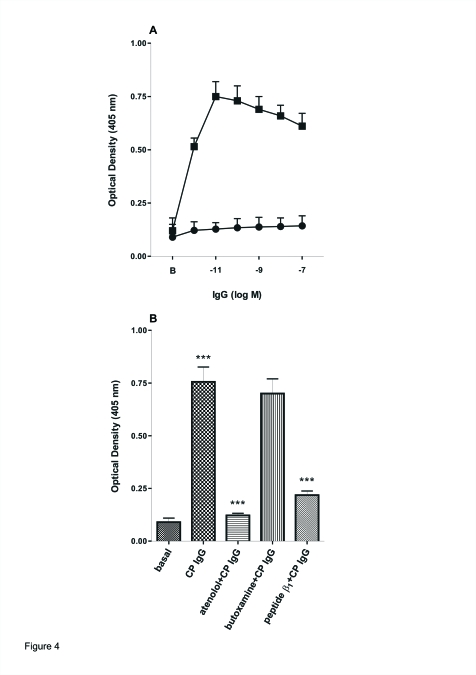
Figure 4 shows the concentration-dependent increase in OD values with affinity-purfied anti-β1 peptide IgG compared with the non-antipeptide IgG fraction eluted from the column from CP patients when the β1-AR synthetic peptide was used as the coating antigen. The OD values of the anti-β1 synthetic peptide IgG were always greater than 3 SD of those of the non-antipeptide IgG (Figure 4A). This reaction was blocked by the β1 synthetic peptide (10-fold concentration) IgG when the IgG from patients with CP was incubated for 30 min at 37°C and then added together in the microtiter plates (Figure 4B). Also, atenolol (1x10−7 M), a specific β1-AR antagonist, but not butoxamine (1x10−6 M), a specific β2-AR antagonist, blocked the increase of OD values triggered by IgG from CP (Figure 4B). The concentration of affinity-purified anti-β1 synthetic peptide IgG (1x10−9 M) that maximally increased OD values corresponded to a 1x10−8 M total IgG concentration. The non-antipeptide IgG fraction eluted from the column showed OD values similar to normal IgG (0.122 ± 0.02, n=20). As expected, the IgG fraction from normal subjects (control) purified by affinity chromatography with the β1 synthetic peptide gave negative results (data not shown).
Figure 4.
Recognition of synthetic β1 peptide by patients with CP. A: anti β1 peptide IgG (▪) and non-anti β1 fraction eluted from the column from group 1 (●) were incubated at different concentrations in multiwell plates sensitized with β1 peptide as described in the Material and Methods section. b: Basal values without IgG . B: 1x10−9 M anti-β1 peptide IgG from CP in the absence or in the presence of 1x10−7 M atenolol or 1x10−6 M butoxamine or 1 μg of β1 synthetic peptide. Basal values are also shown. Values are means ± SEM of 10 patients in each group. ***:P<.001 between basal vs. CP IgG; ***:P<.001 anti-β1 peptide or atenolol+CP IgG vs. CP IgG.
As already shown, the serum IgG from patients with CP was able to react with the β1-AR human fibroblast membrane; we studied the β1-AR-mediated effect of IgG from patients with CP patients on human gingival fibroblast proliferation using the incorporation of tritiated thymidine.
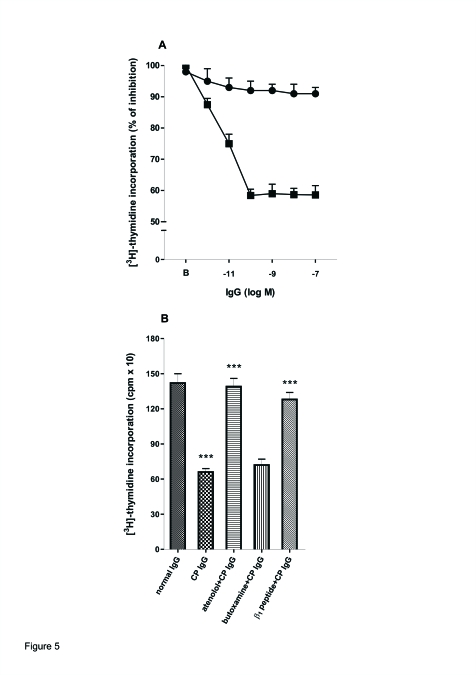
Figure 5A shows that the antipeptide IgG from patients with CP decreased DNA synthesis in a concentration-dependent manner. Atenolol (1x10−7 M) and β1 synthetic peptide (10-fold concentration), but not butoxamine (1x10−6 M), prevented the inhibitory effect of IgG upon DNA synthesis of human gingival fibroblasts (Figure 5B). Normal IgG was without effect on the study system.
Figure 5.
Inhibition of [3H]-Thymidine Incorporation of human gingival fibroblast by IgG from patients with CP. A: Increasing concentrations of anti-β1 peptide IgG (▪) and non-anti-β1 peptide IgG fraction (●) from CP patients were incubated with 1x106 cells and DNA synthesis was measured as described in the Material and Methods section. b: basal values without IgG. B: 1x10−9 M anti-β1 peptide IgG from patients with CP in the absence or in the presence of 1x10−7 M atenolol and 1x10−6 M butoxamine or 1 μg of β1 synthetic peptide. Normal IgG treated as IgG from patients with CP was ineffective in the studied system. Values are means ± SEM of 10 patients in each group performed by triplicate. ***:P<.001 between β1 peptide or atenolol+CP IgG; ***:P<.001 between CP IgG vs. normal IgG.
DISCUSSION
From the clinical point of view, the course of exacerbation and remissions, genetic vulnerability, and environmental factors17 hint at parallels between periodontal disease and autoimmune diseases.24 Moreover, autoimmune systemic diseases, such as rheumatoid arthritis and adult periodontitis, share common pathogenic mechanisms and immunologic and pathogenic findings.25
The aim of our study was to examine the possible role of altered humoral immunity, exploring the β adrenergic activity of IgG from patients with CP. By flow cytometry and ELISA assays, we have provided evidence that certain components of the serum IgG fraction from patients with CP can recognize human gingival fibroblast membranes. The titer of serum antibodies in individuals with periodontal disease are often elevated as compared to healthy individuals,10 but others26,27 did not detect a rise in antibody levels. Furthermore, following disease activity episodes, the increase in IgG antibody often shows a shift in microbial specificity.28 The level of antibodies to collagen type I was significantly higher in the patients with periodontitis than in healthy controls.12 In contrast to these observations. Hirsch et al29 reported that anti-collagen-producing cells are rarely detected in peripheral blood and the levels of anti-collagen antibodies in serum are low. Thus, serum antibody detection could indicate the chronic exposure of antigen challenge and the presence of exacerbation of the disease.
The autonomic adrenergic system is an important regulator of the immune response.14,30 Therefore, in an attempt to elucidate the nature of the sympathetic mechanism involved, we characterized the participation of the β adrenergic system on the effect of serum antibodies. In this sense, we demonstrated that patients with periodontal disease have functional serum IgG autoantibodies that interact with the β1-AR to human gingival fibroblasts. The high prevalence of these anti-β1-AR autoantibodies in periodontal disease patients provides new evidence in interaction with other causes to be considered in the immunopathology of the disease. Antibodies against β1-AR have also been found in the sera of patients with other autoimmune disorders.31,32 The antibodies are able to interact with the second extracellular loop of the human β1-AR, which is the main immunogenic region of these receptors.14 The pathogenic properties of anti-β1-AR autoantibodies have been ascribed to their potency to continuously stimulate the sympathetic system. Thus, β1-AR autoantibodies could trigger two important mechanisms. On the one hand, autoantibodies, by targeting cardiac β1-AR, altered the physiologic behavior of the myocardium,31 and on the other hand, it may exacerbate or maintain chronic inflammatory heart disease32 through the induction of an immune suppression response.14 In fact, it has been proposed that during sympathetic hyperactivity there is a dysregulation of the pro- versus anti-inflammatory cytokines and T helper (Th1) versus TH2 lymphocytes balance. Thus, catecholamines and PGE2 up-regulate Th2 lymphocytes, associated with increase in humoral immunity and decrease in Th1 with down-regulation of cell-mediate immunity (CMI).33 Then, the β1-AR autoantibodies could exacerbate the course of the disease by altered focus of immune function with an up-regulation of its own production and suppression of CMI. Although, the regulatory mechanism of autoantibodies in CP has not been clarified, further studies are needed to provide insight not only into the role of β1-AR autoantibodies in the pathogenesis of periodontitis, but also into the possible link between periodontitis and heart disease.
The most important feature of this study relating to the autoimmune nature of CP was the presence of IgG in total sera, acting and fixing on β1-AR, resulted in a primary cell-specific growth inhibition. In fact, IgG from these patients were able to inhibit DNA synthesis of human gingival fibroblasts. The specificity of this interaction was assessed by inhibiting the IgG effect by human β1-AR synthetic peptide and with a β1-AR specific antagonist (atenolol). Ligand interactions of β1- AR IgG from CP with membrane receptors are the initial triggers for altering fibroblast cell function. Deficiencies or modifications in such receptors or inappropriate coupling to second messengers can result in dysfunctional gingival fibroblasts that contribute not only to connective tissue degradation, but also have been associated with progressive loss of tooth attachment.27 The fact that IgG from patients with CP IgG inhibited DNA synthesis indicates the possibility that IgG fixation to fibroblast β1-AR could cause loss of both connective and bone tissue, accompanied by loss of tooth attachment. The β1-AR are present in human fibroblasts where they modulate the proliferation with an increase in cAMP production.34
CONCLUSIONS
The findings of this study support the autoimmune process against β1-AR from fibroblasts. It can be speculated that the damage to periodontal tissues can expose self antigens to β1-AR. Although molecular mimicry may also play a role in this situation but more importantly it may unravel indirect pathogenic mechanisms which interfere with the effects of catecholamines on periodontal tissues.
ACKNOWLEDGEMENTS
The authors thank also, to Mrs. Elvita Vannucchi and Fabiana Solari for their expert technical assistance. This work was supported by UBACyT O 003, CONICET (PIP 5680), PICT 02120 and PICT 01647 grants from Buenos Aires University, Argentine Research Council and National Agency of Scientific and Technology Promotion respectively.
REFERENCES
- 1.Oliver RC, Brown IJ, Löe H. Periodontal disease in the United State population. J Periodontol. 1998;69:269–278. doi: 10.1902/jop.1998.69.2.269. [DOI] [PubMed] [Google Scholar]
- 2.Beck JD, Koch GG, Zambon JJ, Genco RJ, Tudor GE. Evaluation of oral bacteria as risk indicators for periodontitis in older adults. J Periodontol. 1992;63:93–99. doi: 10.1902/jop.1992.63.2.93. [DOI] [PubMed] [Google Scholar]
- 3.Bonchard P, Boutouyrie P, Mattout C, Bourgeois D. Risk assessment for severe clinical attachment loss in an adult population. J Periodontol. 2006;77:479–489. doi: 10.1902/jop.2006.050128. [DOI] [PubMed] [Google Scholar]
- 4.Listgarten MA, Loomer PM. Microbial identification in the management of periodontal diseases. A systemic review. Ann Periodontol. 2003;8:182–192. doi: 10.1902/annals.2003.8.1.182. [DOI] [PubMed] [Google Scholar]
- 5.Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Levels of interleukin-1 beta, -8 and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol. 2000;71:1535–1545. doi: 10.1902/jop.2000.71.10.1535. [DOI] [PubMed] [Google Scholar]
- 6.Holmlund A, Hänström L, Lerner UH. Bone resorbing activity and cytokine levels in gingival crevicular fluid befoire and after treatment of periodontal disease. J Clin Periodontol. 2004;31:475–482. doi: 10.1111/j.1600-051X.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 7.Heasman PA, Collins JG, Offenbacher S. Changes in crevicular fluid levels in IL-1β, LTB4, PGE2, TXB4 and TNFα in experimental gingivitis in humans. J Periodontal Res. 1993;28:241–247. doi: 10.1111/j.1600-0765.1993.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 8.Lamster IB, Oshrain RL, Celenti RS, Find JB, Grbic JT. Indicators of the acute inflammatory and humoral immune responses in gingival crevicular fluid: relationship to active periodontal disease. J Periodontal Res. 1991;26:261–263. doi: 10.1111/j.1600-0765.1991.tb01653.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith MA, Braswell LD, Boyd DL, Collins JG, Jeffcoat MK, Reddy M. Changes in inflammatory mediators in experimental periodontitis in the rhesus money. Infec Immun. 1993;61:453–1459. doi: 10.1128/iai.61.4.1453-1459.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenkein HA. Host responses in maintaining periodontal health and determining periodontal disease. Periodontology. 2000;40:77–93. doi: 10.1111/j.1600-0757.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- 11.Ftis A, Singh G, Dolby AE. Antibody to collagen type I in periodontal disease. J Periodontol. 1986;57:693–698. doi: 10.1902/jop.1986.57.11.693. [DOI] [PubMed] [Google Scholar]
- 12.Anusaksathien O, Singh G, Matthews N, Dolby AE. Autoimmunity to collagen in adult periodontal disease; immunoglobulin classes in sera and tissue. J Periodontal Res. 1992;27:55–61. doi: 10.1111/j.1600-0765.1992.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 13.Rajapakse PS, Dolby AE. Evidence for local production of antibodies to auto and non-self antigens in periodontal disease. Oral Dis. 2004;10:99–105. doi: 10.1046/j.1354-523x.2003.00961.x. [DOI] [PubMed] [Google Scholar]
- 14.Sterin-Borda L, Gorelik G, Postam M, Gonzalez Cappa S, Borda E. Alterations in cardiac beta adrenergic receptor in chagasic mice and their association with circulating beta adrenoceptor related antibodies. Cardiov Res. 1999;41:116–125. doi: 10.1016/s0008-6363(98)00225-9. [DOI] [PubMed] [Google Scholar]
- 15.Furlan C, Sterin-Borda L, Borda E. Activation of β3 adrenergic receptor decreases DNA synthesis in human skin fibroblasts via cyclic GMP/nitric oxide pathway. Cell Physiol Biochem. 2005;16:175–182. doi: 10.1159/000089843. [DOI] [PubMed] [Google Scholar]
- 16.Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol. 2005;32:87–107. doi: 10.1111/j.1600-051X.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharma CG, Pradeep AR. Anti-neutrophil cytoplasmic autoantibodies: a renewed paradigm in periodontal disease. J Periodontol. 2006;77:13–17. doi: 10.1902/jop.2006.050308. [DOI] [PubMed] [Google Scholar]
- 18.Ye P, Simonian M, Nakarni MA, Decarlo AA, Chapple CC, Hunter N. Identification of epithelial auto antigens associated with periodontal disease. Clin Exp Immunol. 2005;139:328–337. doi: 10.1111/j.1365-2249.2005.02692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anusaksathien O, Dolby AE. Autoimmunity in periodontal disease. J Oral Pathol Med. 1991;20:101–107. doi: 10.1111/j.1600-0714.1991.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 20.Clagett JA, Page RC. Insoluble immune complexes and chronic periodontal disease in man and the dog. Arch Oral Biol. 1978;23:153–165. doi: 10.1016/0003-9969(78)90211-x. [DOI] [PubMed] [Google Scholar]
- 21.Novo E, Viera N. Antineutrophil cytoplasmic antibodies: a missing link in the pathogenesis of periodontal disease? J Periodontal Res. 1996;31:365–368. doi: 10.1111/j.1600-0765.1996.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 22.Varani J, Mitra RS, Gibbs D, Phan SH, Dixit DM, Mitra R, Jr, Wang T, Siebert KJ, Nickoloff BJ, Voorhees JJ. All-trans retinoic acid stimulates growth an extracellular matrix production in growth-inhibited cultured human skin fibroblasts. J Invest Dermatol. 1990;94:717–723. doi: 10.1111/1523-1747.ep12876294. [DOI] [PubMed] [Google Scholar]
- 23.Goin JC, Perez Leiros C, Borda E, Sterin-Borda L. Interaction of human chagasic IgG with the second extracellular loop of the human heart muscarinic acetylcholine receptor: functional and pathological implications. FASEB J. 1997;10:77–83. doi: 10.1096/fasebj.11.1.9034169. [DOI] [PubMed] [Google Scholar]
- 24.Colburn KK, Green LM, Wong AK. Circulating antibodies to guanocine in systemic lupus erythematosus: correlation with nephritis and polyserositis by acute and longitudinal analyses. Lupus. 2000;10:410–418. doi: 10.1191/096120301678646155. [DOI] [PubMed] [Google Scholar]
- 25.Rosenstein ED, Greenwald RA, Kushner LJ, Weismann G. The humoral immune response to oral bacteria provides a stimulus for the development rheumatoid arthritis. Inflammation. 2005;28:311–318. doi: 10.1007/s10753-004-6641-z. [DOI] [PubMed] [Google Scholar]
- 26.Haffajee AD, Socransky SS, Dzink JL, Taubman MA, Ebersole JL, Smith DJ. Clinical, microbiological and immunological features of subjects with destructive periodontal diseases. J Clin Periodontol. 1988a;15:255–262. doi: 10.1111/j.1600-051x.1988.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 27.Haffajee AD, Socransky SS, Dzink JL, Taubman MA, Ebersole JL, Smith DJ. Clinical, microbiological and immunological features of subjects with destructive periodontal diseases. J Clin Periodontol. 1988b;51:240–246. doi: 10.1111/j.1600-051x.1988.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 28.Sorkin E, del Rey A, Besedovsky HO. Adrenergic system in immune response. In: Steimberg CM, Lefkovitz I, editors. The immune system. Vol. 1. Basel S. Karger; Switzerland: 1981. pp. 340–348. [Google Scholar]
- 29.Hirsch HZ, Tarkowsky A, Miller EJ, Gay S, Koopma W, Mestecky J. Autoimmunity to collagen in adult periodontal disease. J Oral Pathol. 1988;17:456–459. doi: 10.1111/j.1600-0714.1988.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 30.Felten DL, Felten SY, Carlson SL, Olschowk J, Aionat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755S–765S. [PubMed] [Google Scholar]
- 31.Borda ES, Pascual J, Cossio PM, Vega M, Arana RM, Sterin-Borda L. A circulating IgG in Chagas disease which binds to β adrenoceptor of myocardium and modulates its activity. Clin Exp Immunol. 1984;57:679–686. [PMC free article] [PubMed] [Google Scholar]
- 32.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, Lohsc MJ. Direct evidence for a beta 1 adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–1429. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakier Smith L. Overtraining, excessive exercise and altered immunity: is this a T-helper-1 versus T-helper-2 lymphocyte response? Sports Med. 2003;33:347–364. doi: 10.2165/00007256-200333050-00002. [DOI] [PubMed] [Google Scholar]
- 34.Coofey R, Hadden JW. Neurotransmitters hormones and cyclic nucleotides in lymphocytes regulation. Fed Proc. 1985;44:112–117. [PubMed] [Google Scholar]