Abstract
The orientational dynamics of water at a neutral surfactant reverse micelle interface are measured with ultrafast infrared spectroscopy of the hydroxyl stretch, and the results are compared to orientational relaxation of water interacting with an ionic interface. The comparison provides insights into the influence of a neutral vs. ionic interface on hydrogen bond dynamics. Measurements are made and analyzed for large nonionic surfactant Igepal CO-520reverse micelles (water nanopool with a 9-nm diameter). The results are compared with those from a previous study of reverse micelles of the same size formed with the ionic surfactant Aerosol-OT (AOT). The results demonstrate that the orientational relaxation times for interfacial water molecules in the two types of reverse micelles are very similar (13 ps for Igepal and 18 ps for AOT) and are significantly slower than that of bulk water (2.6 ps). The comparison of water orientational relaxation at neutral and ionic interfaces shows that the presence of an interface plays the dominant role in determining the hydrogen bond dynamics, whereas the chemical nature of the interface plays a secondary role.
Keywords: reverse micelles, ultrafast IR experiments, interfacial water, hydrogen bond dynamics
Water molecules at interfaces are involved in many processes. In biology, water is found in crowded environments, such as cells, where it hydrates membranes and large biomolecules. In geology, interfacial water molecules can control ion adsorption and mineral dissolution. Embedded water molecules can change the structure of zeolites. In chemistry, water plays an important role as a polar solvent often in contact with interfaces, for example, in ion exchange resin systems.
When water interacts with an interface, its hydrogen bonding properties are distinct from those of bulk water. Interactions with an interface influence water's ability to undergo hydrogen bond network rearrangements, which is a concerted process that involves a water molecule and its two water solvation shells (1, 2). For a water molecule to switch hydrogen bonding partners, other waters must also break and form new hydrogen bonds (1, 2). Such hydrogen bond rearrangements are necessary for both orientational and translational motions. An interface eliminates many of the pathways for hydrogen bond rearrangement that are available in bulk water. A fundamental question is whether the composition of or solely the presence of an interface plays the dominant role in affecting the hydrogen bond dynamics of interfacial water.
Ultrafast infrared (IR) spectroscopy is a valuable technique for probing the dynamics of both bulk water and water at interfaces using the hydroxyl stretching mode of water as a reporter (3–17). Because processes in water involving hydrogen bond rearrangements occur on the picosecond time scale, the femtosecond time resolution of the IR techniques makes it possible to resolve the motions of water molecules on the time scale on which they are occurring. Examples of confined or restricted environments that have been studied with ultrafast IR spectroscopy include salt water solutions (7, 18–20), zeolite cavities (21), channels of Nafion fuel cell membranes (9, 10), polyether systems (22), lipid bilayers, (23) and the interiors of reverse micelles (3–5, 8, 16, 17). It has been shown by both theoretical and experimental studies that only water molecules in the immediate vicinity of an interface or solute differ significantly from bulk water (3–5, 20, 24–27). These water molecules undergo orientational relaxation that is slower than bulk water (3–5, 8, 16, 17) because they have a reduced number of neighbors with which they can exchange hydrogen bonds (28).
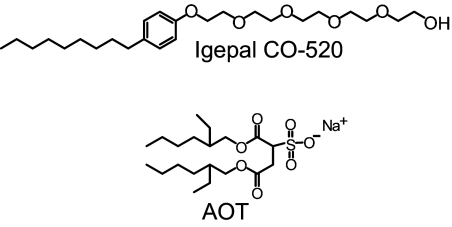
Large reverse micelles are useful for studying the behavior of water at interfaces. In a reverse micelle, the polar head groups of the surfactant molecules surround a pool of water, while the hydrophobic tails are suspended in an organic phase. A commonly used surfactant that forms reverse micelles is sodium bis(2-ethylhexyl) sulfosuccinate (AOT), which has an aliphatic tail and sulfonate head groups (Fig. 1). AOT is a well-characterized system, which forms monodispersed spherical reverse micelles in isooctane (29). Depending on the amount of water added, AOT can form water pool sizes from 1 nm (≈30 water molecules) to 14 nm (≈400,000 water molecules) (8). The number of water molecules per surfactant molecule is w0 = [H2O]/[surfactant].
Fig. 1.
Molecular structures for the surfactants Igepal CO-520 (neutral head group containing a hydroxyl) and AOT (ionic sulfonate head group with a sodium counter ion).
The water environment inside the AOT reverse micelles has been described with a two-component model (3–5, 8, 16, 17). Water inside large reverse micelles exists in two environments: a bulk-like water core and a shell of interfacial waters that hydrate the AOT head groups (3–5). The IR spectra of the OD stretch of dilute HOD in H2O inside AOT reverse micelles can be reproduced by linear combinations of the bulk water absorption spectrum and the spectrum of a very small reverse micelle (w0 = 2) in which virtually all of the waters interact with the head groups (8). In the experiments presented below, the OD stretch of dilute HOD in H2O is also used. Studying dilute HOD is important because its use eliminates vibrational excitation transfer that causes artificial decay of the orientational correlation function in either pure H2O or D2O (30, 31). MD simulations of bulk water have shown that dilute HOD in H2O does not change the behavior of water and that observations of the OD hydroxyl stretch report on the dynamics and local environment of water (32). Recently, Moilanen et al. (3–5) analyzed data for large AOT reverse micelles (w0 = 25, 37, and 46) in terms of the two-component model. The diameters of these reverse micelles are 9, 17, and 20 nm, respectively. The water pools are so large that the major portion of the water is bulk water. The frequencies associated with water molecules hydrating the interface were identified, and the experiments yielded the interfacial water vibrational lifetimes, orientational relaxation times, and the fractional population of interfacial water molecules in each micelle. In all of these large reverse micelles, the orientational relaxation time constant for water at the interface is 18 ps, and the interfacial vibrational lifetime is 4.3 ps (3).
A fundamental question is the relative importance of the presence of an interface as opposed to the chemical composition of the interface. AOT has ionic head groups. To address the question of the role of the chemical nature of the interface, we compared water orientational relaxation at the AOT interface to orientational relaxation at a neutral interface in reverse micelles of Igepal CO-520 (Igepal). The structure of Igepal is shown in Fig. 1. Igepal, like AOT, forms well-characterized, monodispersed, spherical reverse micelles (33). Igepal is a nonionic surfactant with a head group terminated by an alcohol hydroxyl. Therefore, the interactions of water with AOT and Igepal interfaces will be very different.
The role of the chemical composition of the interface on water dynamics was addressed previously by Moilanen et al. (11) in relatively small (4-nm diameters) AOT and Igepal reverse micelles. The experiments showed that the orientational relaxation anisotropy decay curves were qualitatively very similar. However, the analysis did not separate the interfacial orientational relaxation times from the orientational relaxation times of water in the cores (water not at the interfaces) nor did it account for the contribution to the signal from the Igepal alcohol head groups. In addition, it was assumed that all of the water in the reverse micelles comprised a signal ensemble with a single orientational anisotropy decay. However, recent experiments on 4-nm (w0 = 10) AOT reverse micelles demonstrated that this size reverse micelle has both core and interfacial water with distinct dynamics and that the core water molecules are not bulk-like (5). Therefore, in the earlier study of AOT and Igepal reverse micelles, the orientational relaxation times of water at the interfaces of the two types of reverse micelles were not determined and compared.
To separate the interfacial dynamics from the core dynamics, it is necessary to know the vibrational lifetime and orientational relaxation time of the core (3, 5, 22). For the large reverse micelles studied here, the core has bulk water dynamics; therefore, the necessary dynamical parameters are known. The study is performed on w0 = 20 Igepal reverse micelles with a nanopool diameter of 9 nm, which is the same size as the water nanopool in w0 = 25 AOT reverse micelles. The difference in w0 for the same size water nanopool is caused by the different aggregation numbers of the two surfactants. A water pool size of 9 nm is more than sufficient to have distinct interfacial and bulk-like core subensembles of water molecules (5). The method for extracting the interfacial water dynamics from the data (3) is modified to account for the contribution to the signal from the Igepal head group alcohol hydroxyls. The results show that the orientational relaxation time of water at the Igepal interface (13 ps) is very similar but probably not identical to water at the AOT interface (18 ps; see the discussion of error bars under “Population Relaxation and Orientational Relaxation”). Thus, the presence of an interface is the dominant factor in determining interfacial hydrogen bond dynamics, but the chemical composition of the interface may matter to some extent.
Results and Discussion
Absorption Spectroscopy.
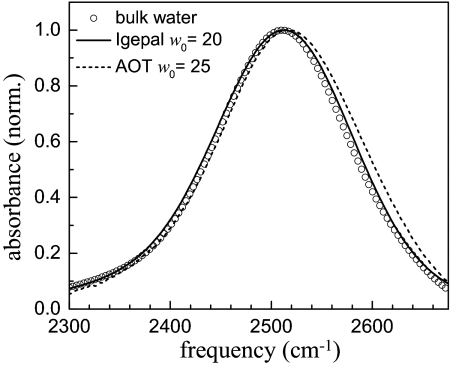
Fig. 2 displays Fourier transform (FT)–IR spectra of the OD stretch of dilute HOD in H2O in three different environments: pure water, w0 = 20 Igepal reverse micelles, and w0 = 25 AOT reverse micelles. Both reverse micelle spectra are slightly blue-shifted from bulk water, which peaks at 2,509 cm−1. The AOT and Igepal spectra peak at 2,519 and 2,514 cm−1, respectively.
Fig. 2.
IR absorption spectra of the OD stretch of 5% HOD in H2O for Igepal w0 = 20, AOT w0 = 25, and bulk water.
The AOT spectrum for w0 = 25 and other sizes can be fit as a linear combination of the bulk water spectrum and the spectrum of a w0 = 2 AOT reverse micelle, which peaks at 2,565 cm−1 (3, 8). The w0 = 2 spectrum is akin to the interfacial water spectrum because essentially all of the water molecules are associated with the AOT sulfonate head groups (3, 8). The equivalent analysis is not possible for the Igepal reverse micelles. The sizes of smaller Igepal reverse micelles (under ≈w0 = 10) are not well-characterized, so it is not possible to have a well-defined small reverse micelle to use as a model for the interfacial water spectrum (33). The Igepal surfactant has hydroxyl head groups that will exchange deuterium atoms with the HOD molecules in the water nanopool. The surfactant's deuterated hydroxyls will contribute to the spectrum. For a very small Igepal reverse micelle (little water), the head group hydroxyls will make a major contribution to the spectrum, preventing the spectrum from being used to represent interfacial water.
The small blue-shift and broadening on the blue side of the w0 = 20 Igepal reverse micelle OD stretch spectrum and the fact that a blue-shift has been observed in many systems of confined or hydrating water (3, 10, 11, 18, 19, 22) indicate that the frequencies associated with interfacial water are on the blue side of the spectrum. As in the AOT studies, it will be shown below that interfacial water population relaxation and orientational relaxation for the Igepal reverse micelle system can be obtained from measurements on the blue side of the spectrum.
Population Relaxation and Orientational Relaxation.
Polarization selective pump–probe measurements yield both the population relaxation (vibrational lifetimes) and decay of the anisotropy (orientational relaxation parameters). Vibrational lifetimes are very sensitive to the details of the local environment of the vibrational oscillator, which in this case is the OD stretching mode (8, 34, 35). As discussed briefly in the Introduction, orientational relaxation of water occurs by concerted hydrogen-bond switching, which is referred to as jump reorientation (1, 2, 9). Orientational relaxation is strongly influenced by the local details of the hydrogen bonding network.
Both the population relaxation and the anisotropy decay are obtained from measurements of the pump–probe signals with the probe pulse polarization parallel and perpendicular to the pump pulse, denoted as I‖; and I⊥, respectively. The population relaxation, P(t), is
The anisotropy decay is
where C2(t) is the second Legendre polynomial orientational correlation function for the OD stretch transition dipole (36).
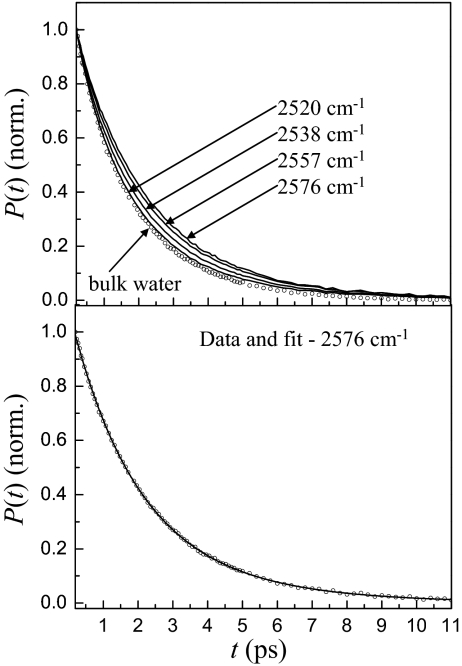
Fig. 3A displays population relaxation data for four frequencies in the Igepal w0 = 20 reverse micelle and bulk water. As the frequency is increased, the data decay more slowly. The decays are not exponential. Fig. 3B shows the data for 2,576 cm−1 (circles) and a biexponential fit to the data (solid curve). In the fit, one component has its lifetime fixed at 1.8 ps, the value measured for bulk water (3). The amplitudes and the second decay time are allowed to float. However, if the core water component is also allowed to vary, the fit again yields 1.8 ps for the major component of the decay. To determine the other decay component, a number of decay curves for a range of wavelengths on the blue side of the line were fit simultaneously. The results of this fit yield an interfacial water vibrational lifetime of ≈3.6 ± 0.3 ps and fractions ranging from 0.96 bulk water for 2,520 cm−1 to 0.78 bulk water for 2,595 cm−1. The apparent slowing of the decays as the frequency is increased is caused by a larger fraction of the decay coming from the slower decaying interfacial component.
Fig. 3.
Vibrational population relaxation data. (Upper) Data for the OD stretch of HOD in H2O in the water nanopools of w0 = 20 Igepal reverse micelle at four frequencies and bulk water. (Lower) The vibrational population relaxation data for 2,576 cm−1 and a biexponential fit.
In the recent study of large AOT reverse micelles, including w0 = 25, very similar biexponential population decays were observed with the core (bulk water) component having a vibrational lifetime of 1.8 ps (3). The second component is assigned to the OD stretch of water molecules at the AOT interface. Here the situation is more complicated. In the population decays for the Igepal reverse micelles (Fig. 3), the 1.8 ps component is also the core (bulk water) component. The other component is associated with interfacial ODs. However, the Igepal head group has an alcohol hydroxyl group. Because the head group hydroxyls will exchange deuterium atoms with the water HODs, 5% of the head group alcohol hydroxyls will be ODs. Thus, there are two types of interfacial OD hydroxyls that will contribute to the slow component of the population decay. Therefore, the population decay is described with three components, a bulk water-like core (1.8 ps decay), an interfacial water shell, and the Igepal hydroxyls,
where the ai and T1i are the amplitudes and vibrational lifetimes of the three components, respectively. cw stands for core water; iw stands for interfacial water; and ia stands for interfacial alcohol. As discussed below, the fact that the population relaxation data fit so well to a biexponential decay arises because aia ≪ aiw, and T1iw and T1ia are similar in value.
In the AOT system, the orientational decay involved the core water with an orientational relaxation time of 2.6 ps and interfacial water with one unknown orientational relaxation time. Fitting the data gave 18 ps for the interfacial water orientational relaxation time. Here, there are three orientational relaxation times. The core value is that of bulk water, and the orientational relaxation time of the alcohol head group can be taken to be infinitely long on the time scale of the experiments. Therefore, there is again one unknown orientational relaxation time. Nonetheless, the presence of the third component, the head group alcohol hydroxyls, complicates the data analysis and increases the error bars on the results. The anisotropy for the three component system is
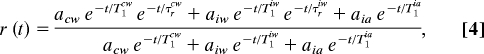 |
where the ai and Ti are the same as those in Eq. 3, and the τri are the orientational relaxation times. Because the alcohol hydroxyl will not reorient on the time scale of the experiments, the third term in the numerator does not contain an orientational relaxation exponential. We know most of the parameters in Eq. 4. T1cw and τrcw are the bulk water values, 1.8 and 2.6 ps, respectively. At a given wavelength, we know acw from fitting the population decay curve. We also know that aiw + aia = 1 − acw. Furthermore we know that at each wavelength aiw e−t/T1iw+ai e−t/T1ia must have the appearance of a single exponential decay, and the two terms combine to give the time constant and amplitude of the slow component of the population decay curve. To make additional progress, we need to address the issue of the relative sizes of aiw and aia.
Without the terms with aia, Eq. 4 is the same equation that was used to fit the AOT reverse micelle data (3–5). As has been discussed in detail previously (3, 8, 16), r(t) does not necessarily decay monotonically for a two-component system. For systems that have a bulk water component and water interacting with an interface or solutes, it has been measured that both the lifetime and the orientational relaxation of water interacting with other species are slower than those of bulk water (9, 10, 18, 19, 22, 37–39). In these situations, a two-component system will display an anisotropy decay that is fast initially, then levels off to a plateau or even increases for a time before finally decaying with the orientational relaxation time of the slow component (3, 6, 22, 39). Plots of this type are presented in figure 5 of ref. 3. This is the situation for water in large AOT reverse micelles, such as w0 = 25, which is being used for comparison to the Igepal reverse micelles studied here. Because of the limitation imposed by the vibrational lifetimes, it is not possible to observe the full r(t) curve. However, in the AOT systems, with the bulk water parameters known and the interfacial water lifetime determined from the population relaxation curve, the form of the anisotropy data around the plateau and the plateau level are very sensitive to the interfacial orientational relaxation time.
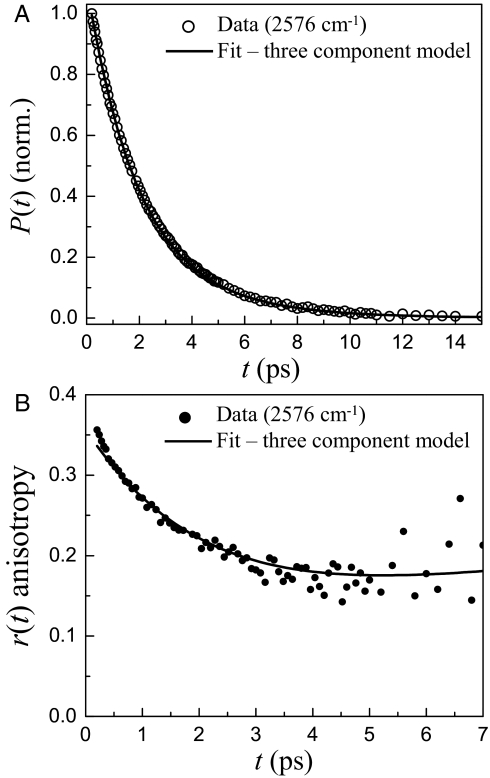
Fig. 5.
Data analysis with three component model. (A) Fit to population relaxation data (2,576 cm−1). (B) Fit to anisotropy data (2,576 cm−1).
The analysis of the Igepal data are similar to the method used for AOT except there are the additional terms in Eq. 4 to account for the nonreorienting alcohol hydroxyls. The term in the numerator contributes a time-independent anisotropy that decays with the Igepal surfactant hydroxyl lifetime. This constant anisotropy term will contribute to the level of the plateau. Therefore, to fit the data we need a reasonable estimate of the size of this term.
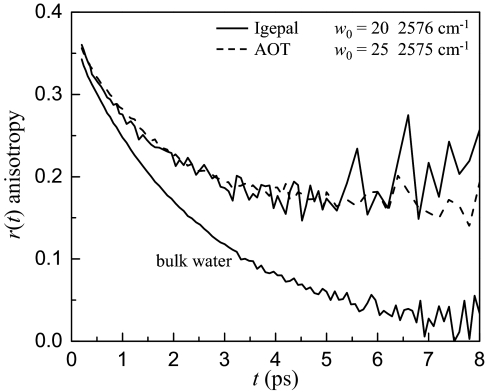
To illustrate the procedure for extracting the interfacial water dynamics, data at the specific frequency 2,576 cm−1 will be discussed. Fig. 4 shows the anisotropy data for the w0 = 20 Igepal system (solid curve) and the w0 = 25 AOT system (dashed curve), and bulk water. [For comparison to the Igepal curve, the AOT curve was shift down by 0.03 because it has a smaller ultrafast (<200 fs) inertial drop (12).] First, it is obvious that the Igepal and AOT data are quite similar in shape, but they are not identical. The AOT data displays a plateau at long time, whereas the Igepal data turns up slightly. In addition, the analysis of the Igepal data needs to include the constant anisotropy and lifetime terms that come from the alcohol head groups. Fitting the population relaxation for Igepal at 2,576 cm−1 to a biexponential decay gives a lifetime of 1.8 ps and an amplitude of 0.74 for the core bulk water component and an amplitude and time constant of 0.26 and 3.2 ps for the second component. This second time constant is essentially a weighted average of the lifetimes of the interfacial water and Igepal hydroxyls and is consistent with the global fit described previously. The 0.26 fraction is the sum of the amplitudes for the interfacial water (iw) and alcohol head group (ia) components.
Fig. 4.
Anisotropy data for bulk water and Igepal w0 = 20 and AOT w0 = 25, both with water nanopool diameters of 9 nm. The decays are quite similar but not identical.
The fractions aiw and aia (Eqs. 3 and 5) can be estimated from the molar ratios of water and Igepal surfactant molecules and the physical geometry of the water nanopool. The diameter of the water nanopool is 9 nm. Experimental and theoretical results have shown that only the first hydration layer around an interface or solute is substantially perturbed (20, 24–27), so we can assume that the interfacial water is located primarily within a shell having a thickness of 0.28 nm (3), which is the approximate diameter of a water molecule. Calculating the volume of this shell and dividing it by the total volume of the micelle interior yields a fraction of 0.18. In the w0 = 20 Igepal reverse micelle, there are ≈12,800 water molecules; 18% of them will be at the interface. There are two hydroxyls per water molecules, yielding ≈4,500 interfacial water hydroxyls in total. Approximately 600 Igepal surfactant molecules form the w0 = 20 reverse micelles, and each surfactant has a single alcohol hydroxyl in the head group. Therefore, there are ≈4,500/600 or ≈7.5 water hydroxyls per Igepal hydroxyl head group. To reflect this ratio, we can assign a fraction of 0.23 and 0.03 to the interfacial water hydroxyls and Igepal alcohol hydroxyls, respectively. Their sum equals 0.26, the fraction of non-bulk hydroxyls. We can now fix aiw and aia to these fractions. Because the contributions to the signal depend on the transition dipoles as well as the population fractions, we assume that the transition dipoles of the two species at the same frequency are the same. Recently it was shown that the interfacial water and the core water in AOT reverse micelles have the same transition dipoles at the same frequency within experimental error (3). These transition dipoles also have the same frequency dependence. It is known for bulk water that both the hydrogen bond strength and the transition dipole vary together with frequency (40). Here the signals from the three types of hydroxyls are detected at the same frequency, so the assumption that their transition dipoles are the same may be reasonable. To account for error in our estimate of the fractions and possible differences in the transition dipoles, we varied the fractions around the calculated values in the data fits. There are three remaining parameters, the lifetimes T1iw and T1ia, and the interfacial water rotation time, τriw. T1iw and T1ia are highly constrained because they must yield the slow component of the population decay. For a given choice of aiw and aia, the T1iw and T1ia values have little room to vary.
Fig. 5 shows the population relaxation data and the anisotropy data at 2,576 cm−1 along with the fits to the data. In the fits, the following parameters have fixed input parameters: acw = 0.74, T1cw = 1.8, τrcw = 2.6, aiw = 0.23, and aia = 0.03. The fits return the following values: T1iw = 3.0 ps, T1ia = 4.9 ps, and τriw = 16 ps. As mentioned above, the values obtained from the geometric considerations for the interfacial water fraction and the interfacial alcohol fraction were varied. aiw was varied from 0.20 to 0.25 with aia varied correspondingly. Outside of this range, the fits were clearly poor. For each pair of values, the data were fit. Any set of fit parameters that could not reproduce both the population and anisotropy data were rejected. In the end, ranges of acceptable values of the parameters were obtained. The ranges were used to assign the error bars. The results are given in Table 1. Fractions for aiw between 0.21 and 0.23 gave the best fits. The T1iw varied over a range of 2.8 ps to 3.5 ps, and T1ia varied between 4.4 and 5 ps. The T1iw values are reasonable for the OD stretch of HOD in water in confined or restricted environments (3, 8, 22).
Table 1.
Parameters for population and orientational relaxation for water in w0 = 20 Igepal reverse micelles (2,576 cm−1)
| Bulk water | Interfacial water | Alcohol head group | |||
|---|---|---|---|---|---|
| acw | 0.74* | aiw | 0.22 ± 0.01 | aia | 0.04 ± 0.01 |
| T1cw | 1.8 ps* | T1iw | 3.2 ± 0.3 ps | T1ia | 4.7 ± 0.3 ps |
| τrcw | 2.6 ps* | τriw | 13 ± 4 ps |
ai, fractional populations; T1, lifetimes; τr, orientational relaxation times;
*, fixed parameters.
The key parameter of interest is the interfacial water reorientation time. The fits yield τriw = 13 ± 4 ps. Moilanen et al. (3) found the interfacial reorientation time in large AOT reverse micelles, including w0 = 25, to be 18 ±3 ps. The error bars reflect estimates of the maximum absolute errors, which depend on the data analysis model. Precision and reproducibility of the data are much better. The error bars for τriw in Igepal and AOT overlap to some extent. The orientational relaxation time of water at the neutral interface of Igepal is at most modestly shorter than it is at the ionic interface of AOT.
A good deal is known about the AOT interface, for example, the surface area per head group and how it changes with reverse micelle size (41). Little is known about the nature of the Igepal interface. The alcohol hydroxyls that terminate the Igepal surfactant molecules do not have sufficient area to solely comprise the interface. It is likely that there are also ether oxygens, which can hydrogen-bond water, and possibly methylenes at the interface. Therefore, water molecules will have a variety of types of interfacial interactions. Although AOT in some sense has a more chemically homogeneous interface, it will also be inhomogeneous in the details of the water-head group interactions. Therefore, the interfacial orientational relaxation times of water at the Igepal and AOT interfaces should be considered average values for the interfacial water dynamics.
In the analysis, exchange of population between interfacial water and the core water was not included, although possible exchange was considered in connection with the previous experiments on AOT reverse micelles (3). For exchange to occur, water must move away from the interface into the core and vice versa. Such translational motion requires hydrogen bond rearrangements, which involve large amplitude angular jumps from one hydrogen bond acceptor to another (1, 2). Thus, reorientation must occur for exchange to occur. The converse is not true. As discussed briefly above, there are multiple water binding sites at the interfaces. Reorientation may take place by forming a new hydrogen bond at a different interfacial binding site without exchanging away from the interface. It would be unphysical for a water molecule to diffuse away from the interface into the bulk-like core without rotating. Furthermore, both the lifetime and orientational relaxation time of water at the reverse micelle interfaces are slower than those of bulk water. Exchange between interfacial water and the bulk-like core water would result in apparent vibrational lifetimes and orientational relaxation times of the core water being slower than those of bulk water (38). However, the data for all large AOT reverse micelles (3) and the w0 = 20 Igepal reverse micelles studied here were fit very well using the bulk water lifetime and orientational relaxation time. Based on these considerations and the experimental results of chemical exchange in concentrated salt solutions (38), the orientational relaxation time of reverse micelle interfacial water can be taken as a lower bound for the exchange time.
Concluding Remarks
The results presented here demonstrate that water at the neutral Igepal interface undergoes orientational relaxation (13 ps) that is much slower than that of bulk water (2.6 ps) and possibly somewhat faster than water at the ionic AOT interface (18 ps). Orientational relaxation of water requires concerted hydrogen bond rearrangement (1, 2). The presence of an interface blocks many of the pathways that are needed for the hydrogen bond rearrangements that give rise to jump reorientation. The results show that the main influence on interfacial hydrogen bond dynamics and orientational relaxation is the presence of the interface rather than its chemical make-up, ionic head groups (AOT) vs. neutral head groups (Igepal).
The possibly faster orientational relaxation at the neutral interface compared to the ionic interface seems reasonable in light of recent experiments on the exchange time for a water hydroxyl hydrogen-bonded to an anion to become hydrogen-bonded to another water molecule. Ultrafast 2D IR vibrational echo chemical exchange experiments were used to directly measure time for hydrogen bond switching between waters bound to tetrafluoroborate anions and water hydrogen-bonded to other water molecules (38). The time for a water to go from being bound to an anion to being bound to another water is 7 ps (38). The equivalent number for water is ≈2 ps. Therefore, interaction with an ion slows hydrogen-bond switching in a concentrated salt solution but not to a great extent. For water at the interfaces of ionic and nonionic reverse micelles, a similar trend is observed in the orientational relaxations times, which depend on hydrogen-bond switching. The difference in the time for hydrogen-bond switching from ion to water vs. water to water is similar to the difference between the orientational relaxation times at the ionic interface vs. the nonionic hydrophilic interface in reverse micelles. Although the orientational relaxation times at the two types of interfaces are similar, the chemical compositions of the interfaces may have some influence on the dynamics.
Materials and Methods
Igepal CO-520, n-hexane, cyclohexane, H2O, and D2O (Sigma-Aldrich) were used as received. A 0.3 M stock solution of Igepal CO-520 in a 50/50 weight percent mixture of n-hexane and cyclohexane was prepared. Enough water (5% HOD in H2O) was added to obtain w0 = 20 Igepal reverse micelles with a water pool diameter of 9 nm (33). The water content of the reverse micelles was verified by Karl Fischer titration. The optical density of the sample was ≈0.6.
The pump–probe measurements were made with a Ti:Sapphire oscillator/regenerative amplifier operating at 1 kHz that pumped an optical parametric amplifier to produce ≈70 fs mid-IR pulses at ≈4 μm (2,500 cm−1). The mid-IR light was beam-split into weak probe and intense pump pulses with horizontal polarization. The pump pulse was rotated by 45° immediately before the sample. After the beams cross in the sample, the probe was resolved parallel and perpendicular to the pump by a polarizer on a computer-controlled rotation stage before it was dispersed by a spectrograph onto a 32-element HgCdTe detector. The experimental system was purged with CO2 and H2O-scrubbed air.
Acknowledgments.
This work was supported by Department of Energy Grant DE-FG03-84ER13251, National Institutes of Health Grant 2-R01-GM061137-09, and National Science Foundation Grant DMR 0652232. E.E.F. was supported by a Stanford Graduate Research Fellowship.
Footnotes
The authors declare no conflict of interest.
References
- 1.Laage D, Hynes JT. On the molecular mechanism of water reorientation. J Phys Chem B. 2008;112:14230–14242. doi: 10.1021/jp805217u. [DOI] [PubMed] [Google Scholar]
- 2.Laage D, Hynes JT. A molecular jump mechanism of water reorientation. Science. 2006;311:832–835. doi: 10.1126/science.1122154. [DOI] [PubMed] [Google Scholar]
- 3.Moilanen DE, Fenn EE, Wong DB, Fayer MD. Water dynamics at the interface in AOT reverse micelles. J Phys Chem B. 2009;113:8560–8568. doi: 10.1021/jp902004r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moilanen DE, Fenn EE, Wong DB, Fayer MD. Geometry and nanolength scales vs. Interface interactions: Water dynamics in AOT lamellar structures and reverse micelles. J Am Chem Soc. 2009;131:8318–8328. doi: 10.1021/ja901950b. [DOI] [PubMed] [Google Scholar]
- 5.Moilanen DE, Fenn EE, Wong DB, Fayer MD. Water dynamics in large and small reverse micelles: From two ensembles to collective behavior. J Chem Phys. 2009;131 doi: 10.1063/1.3159779. 014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dokter AM, Woutersen S, Bakker HJ. Anomalous slowing down of the vibrational relaxation of liquid water upon nanoscale confinement. Phys Rev Lett. 2005;94:178301. doi: 10.1103/PhysRevLett.94.178301. [DOI] [PubMed] [Google Scholar]
- 7.Park S, Moilanen DE, Fayer MD. Water dynamics-the effects of ions and nanoconfinement. J Phys Chem B. 2008;112:5279–5290. doi: 10.1021/jp7121856. [DOI] [PubMed] [Google Scholar]
- 8.Piletic IR, Moilanen DE, Spry DB, Levinger NE, Fayer MD. Testing the core/shell model of nanoconfined water in reverse micelles using linear and nonlinear IR spectroscopy. J Phys Chem A. 2006;110:4985–4999. doi: 10.1021/jp061065c. [DOI] [PubMed] [Google Scholar]
- 9.Moilanen DE, Piletic IR, Fayer MD. Water dynamics in Nafion fuel cell membranes: The effects of confinement and structural changes on the hydrogen bond network. J Phys Chem C. 2007;111:8884–8891. doi: 10.1021/jp067460k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moilanen DE, Piletic IR, Fayer MD. Tracking water's response to structural changes in Nafion membranes. J Phys Chem A. 2006;110:9084–9088. doi: 10.1021/jp0623084. [DOI] [PubMed] [Google Scholar]
- 11.Moilanen DE, Levinger N, Spry DB, Fayer MD. Confinement or properties of the interface? Dynamics of nanoscopic water in reverse micelles. J Am Chem Soc. 2007;129:14311–14318. doi: 10.1021/ja073977d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moilanen DE, et al. Water inertial reorientation: Hydrogen bond strength and the angular potential. Proc Natl Acad Sci USA. 2008;105:5295–5300. doi: 10.1073/pnas.0801554105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fecko CJ, Loparo JJ, Roberts ST, Tokmakoff A. Local hydrogen bonding dynamics and collective reorganization in water: Ultrafast infrared spectroscopy of HOD/D2O. J Chem Phys. 2005;122 doi: 10.1063/1.1839179. 054506. [DOI] [PubMed] [Google Scholar]
- 14.Asbury JB, et al. Dynamics of water probed with vibrational echo correlation spectroscopy. J Chem Phys. 2004;121:12431–12446. doi: 10.1063/1.1818107. [DOI] [PubMed] [Google Scholar]
- 15.Steinel T, et al. Water dynamics: Dependence on local structure probed with vibrational echo correlation spectroscopy. Chem Phys Lett. 2004;386:295–300. doi: 10.1063/1.1818107. [DOI] [PubMed] [Google Scholar]
- 16.Dokter AM, Woutersen S, Bakker HJ. Inhomogeneous dynamics in confined water nanodroplets. Proc Natl Acad Sci USA. 2006;103:15355–15358. doi: 10.1073/pnas.0603239103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cringus D, et al. Ultrafast energy transfer in water-AOT reverse micelles. J Phys Chem B. 2007;111:14193–14207. doi: 10.1021/jp0723158. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Fayer MD. Hydrogen bond dynamics in aqueous NaBr solutions. Proc Natl Acad Sci USA. 2007;104:16731–16738. doi: 10.1073/pnas.0707824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakker HJ, Kropman MF, Omata Y, Woutersen S. Hydrogen-bond dynamics of water in ionic solutions. Phys Scr. 2004;69:C14–C24. [Google Scholar]
- 20.Smith JD, Saykally RJ, Geissler PL. The effects of dissolved halide anions on hydrogen bonding in liquid water. J Am Chem Soc. 2007;129:13847–13856. doi: 10.1021/ja071933z. [DOI] [PubMed] [Google Scholar]
- 21.Kubota J, et al. Vibrational lifetimes of surface hydroxyl groups of zeolites by picosecond IR pulses. Chem Phys Lett. 1993;204:272–276. [Google Scholar]
- 22.Fenn EE, Moilanen DE, Levinger NE, Fayer MD. Water dynamics and interactions in water-polyether binary mixtures. J Am Chem Soc. 2009;131:5530–5539. doi: 10.1021/ja809261d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao W, Moilanen DE, Fenn EE, Fayer MD. Water at the surface of aligned phospholipid multibilayer model membranes probed with ultrafast vibrational spectroscopy. J Am Chem Soc. 2008;130:13927–13937. doi: 10.1021/ja803252y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhide SY, Berkowitz ML. The behavior of reorientational correlation functions of water at the water-lipid bilayer interface. J Chem Phys. 2006;105 doi: 10.1063/1.2337623. 094713. [DOI] [PubMed] [Google Scholar]
- 25.Bagchi B. Water dynamics in the hydration layer around proteins and micelles. Chem Rev. 2005;105:3197–3219. doi: 10.1021/cr020661+. [DOI] [PubMed] [Google Scholar]
- 26.Jana B, Pal S, Bagchi B. Hydrogen bond breaking mechanism and water reorientational dynamics in the hydration layer of lysozyme. J Phys Chem B. 2008;112:9112–9117. doi: 10.1021/jp800998w. [DOI] [PubMed] [Google Scholar]
- 27.Halle B, Davidovic M. Biomolecular hydration: From water dynamics to hydrodynamics. Proc Natl Acad Sci USA. 2003;100:12135–12140. doi: 10.1073/pnas.2033320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laage D, Stirnemann G, Hynes JT. Why water reorientation slows without iceberg formation around hydrophobic solutes. J Phys Chem B. 2009;113:2428–2435. doi: 10.1021/jp809521t. [DOI] [PubMed] [Google Scholar]
- 29.Zulauf M, Eicke H-F. Inverted micelles and microemulsions in the ternary system water/aerosol-OT/isooctane as studied by photon correlation spectroscopy. J Phys Chem. 1979;83:480–486. [Google Scholar]
- 30.Woutersen S, Bakker HJ. Resonant intermolecular transfer of vibrational energy in liquid water. Nature. 1999;402:507–509. [Google Scholar]
- 31.Gaffney KJ, Piletic IR, Fayer MD. Orientational relaxation and vibrational excitation transfer in methanol–carbon tetrachloride solutions. J Chem Phys. 2003;118:2270–2278. [Google Scholar]
- 32.Corcelli S, Lawrence CP, Skinner JL. Combined electronic structure/molecular dynamics approach for ultrafast infrared spectroscopy of dilute HOD in liquid H2O and D2O. J Chem Phys. 2004;120:8107. doi: 10.1063/1.1683072. [DOI] [PubMed] [Google Scholar]
- 33.Lipgens S, et al. Percolation in nonionic water-in-oil-microemulsion systems: A small angle neutron scattering study. Langmuir. 1998;14:1041–1049. [Google Scholar]
- 34.Kenkre VM, Tokmakoff A, Fayer MD. Theory of vibrational relaxation of polyatomic molecules in liquids. J Chem Phys. 1994;101:10618. [Google Scholar]
- 35.Egorov SA, Berne BJ. Vibrational energy relaxation in the condensed phases: Quantum vs. Classical bath for multiphonon processes. J Chem Phys. 1997;107:6050–6061. [Google Scholar]
- 36.Tokmakoff A. Orientational correlation functions and polarization selectivity for nonlinear spectroscopy of isotropic media. 1. Third order. J Chem Phys. 1996;105:1–12. [Google Scholar]
- 37.Piletic IR, Moilanen DE, Levinger NE, Fayer MD. What nonlinear-IR experiments can tell you about water that the IR spectrum cannot. J Am Chem Soc. 2006;128:10366–10367. doi: 10.1021/ja062549p. [DOI] [PubMed] [Google Scholar]
- 38.Moilanen DE, Wong DB, Rosenfeld DE, Fenn EE, Fayer MD. Ion-water hydrogen bond switching observed with 2D IR vibrational echo chemical exchange spectroscopy. Proc Natl Acad Sci USA. 2009;106:375–380. doi: 10.1073/pnas.0811489106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rezus YLA, Bakker HJ. Observation of immobilized water molecules around hydrophobic groups. Phys Rev Lett. 2007;99:148301–148304. doi: 10.1103/PhysRevLett.99.148301. [DOI] [PubMed] [Google Scholar]
- 40.Corcelli SA, Skinner JL. Infrared and Raman line shapes of dilute HOD in liquid H2O and D2O from 10 to 90 C. J Phys Chem A. 2005;109:6154–6165. doi: 10.1021/jp0506540. [DOI] [PubMed] [Google Scholar]
- 41.Eicke H-F, Rehak J. On the formation of water/oil-microemulsions. Helv Chim Acta. 1976;59:2883–2891. [Google Scholar]