Abstract
Endosomes and endosomal vesicles (EVs) rapidly move along cytoskeletal filaments allowing them to exchange proteins and lipids between different endosomal compartments, lysosomes, the trans-Golgi network (TGN), and the plasma membrane. The precise mechanisms that connect membrane traffic between the TGN and perinuclear endosomal compartments with motor-protein driven transport have largely remained elusive. Here we show that Gadkin (also termed γ-BAR), a peripheral membrane protein localized to the TGN and to TGN-derived EVs, directly associates with the clathrin adaptor AP-1 and with the motor protein kinesin KIF5, thereby potentially regulating EV dynamics. Gadkin overexpression induced the dispersion of transferrin (Tf)- and Rab4-positive EVs to the cell periphery, whereas KIF5B-depleted cells displayed a perinuclear concentration. Functional experiments suggest that the role of Gadkin as a regulator of endosomal membrane traffic critically depends on complex formation with both AP-1 and KIF5. Our data thus provide a direct molecular link between TGN-derived EVs and the microtubule-based cytoskeleton.
Keywords: motor-protein driven transport, clathrin adaptor AP-1, endosomal vesicles, recycling
The endosomal system comprises a mosaic of dynamically interconnected organelles that fulfills a variety of important cell physiological functions ranging from the uptake, recycling, and degradation of nutrients, signaling molecules and cell surface receptors to the regulation of cell migration, differentiation, and morphogenesis (1–3). The endocytic pathway also intersects with the biosynthetic delivery of lysosomal enzymes at several stations, most notably at the trans-Golgi network (TGN)/endosomal boundary (1). Endosomes and TGN- or endosome-derived vesicles exhibit characteristic distribution patterns with Rab4-positive sorting endosomal vesicles (EVs) and tubular recycling endosomes (REs) typically concentrated in the pericentrosomal area (4). Early endosomes, by contrast, appear dispersed throughout the cytoplasm (5). Function and dynamics of endosomes and EVs requires a so far ill-defined interplay between organellar sorting adaptors, the cytoskeleton (6) and molecular motors (7). Here we show that γ-BAR, a recently described accessory factor of the clathrin adaptor complex AP-1 at the TGN/endosomal interface (8), modulates the dynamics of transferrin (Tf)- and Rab4-positive EVs by directly associating with AP-1 and kinesin KIF5. Because γ-BAR does not harbor a curvature-sensing BIN/amphiphysin/Rvs167 (BAR) domain, we refer to this protein as Gadkin, for γ1-adaptin and kinesin interactor. The AP-1/Gadkin/KIF5 complex identified here provides a hitherto unknown molecular link between TGN-derived EVs and the microtubule-based cytoskeleton. Our work also suggests a surprising complexity of endosomal membrane dynamics and its integration with cargo sorting.
Results
Gadkin Localizes to the TGN and to Perinuclear EVs and Regulates EV Positioning.
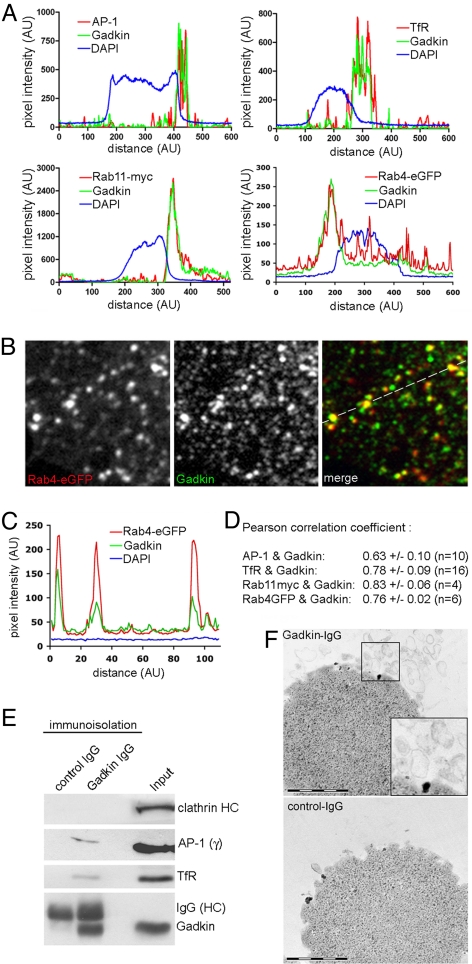
Gadkin has originally been identified as an AP-1 binding protein localized to the TGN and to mobile late endosomal vesicles (8). We used affinity-purified IgGs monospecific for Gadkin (verified by RNAi; see Fig. 1 B and C) to detect endogenous protein in HeLa cells. Gadkin was mainly concentrated within perinuclear clusters where it colocalized with the clathrin adaptor complex AP-1 (Fig. 2 A and D; and Fig. S1A) and the perinuclear endosomal marker proteins TfR, Rab11-myc, and Rab4-eGFP (Fig. 2 A and D; Fig. S1 B–D). A subpool of endogenous Gadkin was detected on peripheral vesicles where it colocalized with Rab4-eGFP (Fig. 2 B and C). These data suggest that a fraction of Gadkin is associated with perinuclear EVs formed at the TGN/endosomal boundary. In agreement with this interpretation, we observed segregation of endogenous Gadkin from the TGN marker p230 and the cis-Golgi matrix protein GM130 in cells treated with nocodazole (Fig. S2), which causes separation of Golgi/TGN from endosomal membranes.
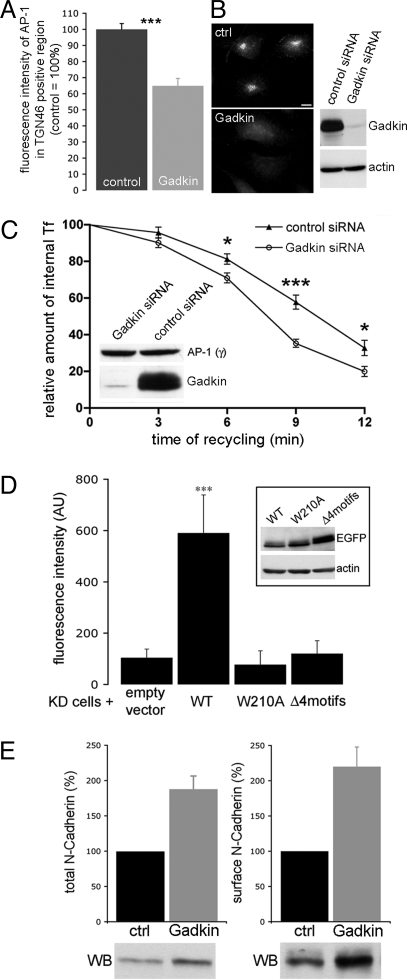
Fig. 1.
Gadkin modulates endosomal recycling. (A) Perinuclear AP-1 is reduced in Gadkin knockdown cells. Sum fluorescence intensity of AP-1 within the TGN46-positive area of control or Gadkin siRNA-treated (B) HeLa cells. (C) Knockdown of Gadkin facilitates Tf recycling. siRNA-transfected HeLa cells were analyzed for their ability to recycle internalized 125I-Tf. Data represent mean values ± SEM (n = 11 values for each data point; five independent experiments; *, P < 0.05; ***, P < 0.001; Student's t test). Knockdown was verified by immunoblot analysis (Inset). (D) Effect of Gadkin WT or mutant expression in cells depleted of endogenous Gadkin on Tf recycling. siRNA- and DNA-transfected cells were allowed to internalize Alexa568-labeled Tf, followed by a 30 min chase. Remaining Tf was quantified by fluorescence microscopy. Bars represent mean values ± SEM (n = 20–25 cells for each sample; ***, P < 0.001; one-way ANOVA followed by Tukey's Multi Comparison Test). (Inset) Gadkin-eGFP expression in Gadkin-knockdown HeLa cells. (E, Left) Total N-cadherin levels in Gadkin-depleted Cos7 cells. (E, Right) Surface levels of N-cadherin in Gadkin-depleted Cos7 cells assayed by surface biotinylation. Signals were quantified with ImageJ. Immunoblot analysis of samples representative of at least three independent experiments is shown below the bar diagram.
Fig. 2.
Gadkin associates with AP-1- and TfR-containing endosomal organelles. (A–D) Colocalization of endogenous Gadkin with AP-1, TfR, Rab11-myc, and Rab4-eGFP in HeLa cells. (A) Fluorescence intensity profiles of HeLa cells immunostained for endogenous Gadkin and TfR, AP-1, transfected Rab11-myc, or Rab4-eGFP. (B) Enlarged view of the peripheral area of a HeLa cell expressing Rab4-eGFP (depicted in Fig. S1D) immunostained for endogenous Gadkin. (C) Line profile taken at the indicated position in B. (D) Pearson correlation coefficients for the colocalization between Gadkin and AP-1, TfR, Rab11-myc, or Rab4-eGFP. (E) Immunoblot analysis of immunoisolated Gadkin-containing organelles. Shown are representative immunoblots (three independent experiments) using control IgGs or affinity-purified IgGs monospecific for Gadkin (see also Fig. S3G). Input, 50 μg starting material. Samples were analyzed by SDS-PAGE and immunoblotting for CHC, AP-1γ, TfR, and Gadkin. (F) Thin-section electron microscopy of organelles immunoisolated using Gadkin-specific (Top) or control IgG (Bottom) as described in E. The dark material seen in the images represents Dynal (M-280) magnetic beads used for immunoisolation. Inset, 2-fold magnified image of boxed area. (Scale bar, 1 μm.)
To confirm the physical association of Gadkin with EVs, we immunoisolated Gadkin-containing organelles from light rat brain membrane fractions using affinity-purified antibodies linked to magnetic beads. When viewed by thin-section electron microscopy, Gadkin-immunobead-associated membranes appeared as pleiomorphic vesicular, often invaginated structures with diameters of 100–400 nm (Fig. 2F). In addition to Gadkin, the immunoisolated structures also contained AP-1, the perinuclear endosomal marker protein TfR, as well as small amounts of the axonally targeted synaptic vesicle membrane protein synaptotagmin I (Fig. S3G), but no detectable clathrin (Fig. 2E), AP-3, or β- and δ-COP (Fig. S3G). All of these proteins were absent from negative control samples. Our results demonstrate that a significant pool of Gadkin associates with AP-1- and TfR-positive EVs.
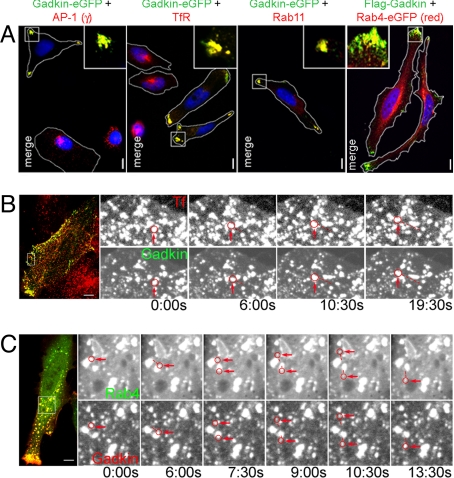
To obtain further insights into the function of Gadkin at EVs, Gadkin fusion proteins were ectopically expressed in HeLa cells. This resulted in a dramatic redistribution of AP-1, and the perinuclear endosomal marker proteins TfR, Rab11, as well as a major fraction of Rab4-eGFP (Fig. 3A and Fig. S1 E–H) to discrete polarized regions within the cell periphery. C-terminally eGFP-tagged and N-terminally FLAG-tagged Gadkin exhibited identical phenotypes (Fig. 3A and Fig. S1 E–H). Redistribution of EVs from a perinuclear to a peripheral location was seen in >80% of all Gadkin-eGFP-positive cells (Fig. 4A) and appeared to be accompanied by an elongated, sometimes spindle-like cell shape (Figs. 3A and Fig. S1 E–H). Live-cell confocal imaging revealed a population of highly mobile Gadkin-eGFP or -mRFP-positive vesicles that contained internalized Tf (Fig. 3B; Movie S1) and Rab4-eGFP (Fig. 3C; Movie S2). The localization of the plasmalemmal clathrin adaptor AP-2, early endosomal antigen EEA1, or the cis-Golgi marker GM130 was not affected by overexpressed Gadkin-eGFP (Fig. S4 A, F, and G). The localization of noncycling TGN markers such as p230 and Golgin-97 also remained largely unaffected, whereas a fraction of the cycling TGN/endosomal proteins TGN46 (2), or the mannose-6-phosphate receptor MPR46 (Fig. S4 B–E) as well as a pool of clathrin (Fig. S5D), colocalized with Gadkin-eGFP in peripheral membrane spots. Gadkin did not exhibit any obvious effects on the localization pattern of mitochondria or LAMP1-positive late endosomes/lysosomes (Fig. S4 H and I). These data indicate that Gadkin specifically participates in regulating the positioning of perinuclear, presumably TGN-derived Rab4- and Tf-positive EVs. The view that Gadkin operates on TGN-derived neosynthesized, as well as on recycling TfR, is supported by our observation that colocalization of endogenous Gadkin with TfR was reduced, but not abolished, in internalization-defective cells treated for 6 h with the dynamin inhibitor dynasore (Fig. S3H).
Fig. 3.
Ectopically expressed Gadkin induces the dispersion of EVs. (A) Overexpression of Gadkin-eGFP or Flag-Gadkin causes peripheral dispersion of AP-1- and TfR-containing Rab11- and Rab4-positive EVs. HeLa cells expressing Gadkin-eGFP or Flag-Gadkin (24 h posttransfection) were analyzed by indirect immunofluorescence microscopy with antibodies against AP-1γ, TfR, and Rab11. Rab4 was detected via its eGFP-tag. Cell boundaries are outlined in white. Blue, DAPI-stained nuclei. Insets, 3× magnified views of boxed areas. (Scale bar, 10 μm.) (B and C) A subpool of motile Gadkin vesicles is positive for Tf and Rab4. (B) HeLa cells expressing Gadkin-eGFP loaded with Alexa568-Tf (1 h at 37 °C) analyzed by live-cell confocal imaging at 37 °C. Shown are still images (enlargements of the boxed area of the cell depicted on the Left) extracted from Movie S1 at the indicated time points. (C) Still confocal images (enlargements of the boxed area of the cell depicted on the Left) extracted from Movie S2 of a Gadkin-mRFP and Rab4-eGFP coexpressing HeLa cell. Examples of motile vesicles positive for both markers are circled in red and pointed at. The distances traveled by the vesicles are indicated by dotted lines. (Scale bar, 10 μm.)
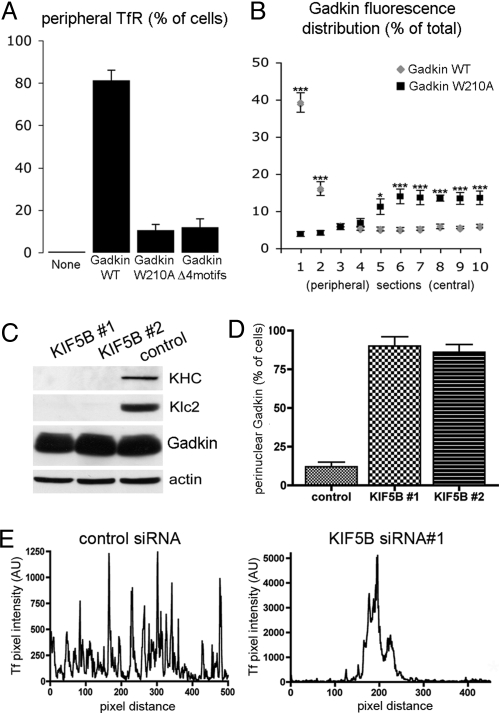
Fig. 4.
Gadkin induced peripheral accumulation of TfR depends on the ability of Gadkin to directly associate with both AP-1 and kinesin. (A) HeLa cells expressing Gadkin-eGFP WT, W210A, or the AP-1-binding-deficient (Δ4motifs) mutant were fixed 24 h posttransfection and quantitatively analyzed by indirect immunofluorescence microscopy for the distribution of endogenous TfR. Cells displaying a strong accumulation of TfR in the cell periphery were counted (n = 25 from two independent experiments). Data are given as mean values ± SEM. (B) Fluorescence intensity distribution of Gadkin WT or W210A. Two line intensity profiles were taken along the longest cell axis (n = 10 cells each) and divided into 20 segments. Fluorescence intensities were pooled in pairwise combinations starting from the two most peripheral segments (section 1) proceeding toward the cell center (i.e., section 2 = pooled intensities in the two second-most peripheral segments, etc.). Data are given as mean values ± SEM (*, P < 0.05; ***, P < 0.0001; Student's t test). (C) Immunoblot analysis of HeLa cell extracts of cells treated with two different siRNAs (#1, #2) against KIF5B or control siRNA. (D) Distribution of Gadkin-eGFP-containing endosomes in HeLa cells after knockdown of KIF5B. Cells displaying a perinuclear pool of Gadkin-eGFP (as depicted in B) were quantified (n = 50 cells for each condition). Gadkin-eGFP-expressing cells transfected with control siRNA are shown for comparison. Data are given as mean values ± SEM. (E) Fluorescence intensity profile of Alexa568-Tf in control siRNA-treated (Left) or KIF5B knockdown cells (Right).
To mechanistically dissect how Gadkin regulates trafficking of TGN-derived EVs, we next performed an in-depth molecular characterization of its protein binding properties.
Gadkin Directly Associates with AP-1 and Kinesin KIF5 Via Distinct Sites.
The fact that overexpression of Gadkin induces the coredistribution of AP-1 into peripheral clusters (see Fig. 3A) may reflect its direct binding to the AP-1γ ear (8). We tested this possibility using cellulose-bound peptide SPOT arrays in combination with site-directed mutagenesis and direct protein binding assays. In addition to three putative AP-1γ ear-binding motifs characterized previously, we identified a nonconventional AP-1 binding site within Gadkin with the sequence 260WENDF264 (Fig. S3E). To assess the contribution of these motifs to Gadkin association with AP-1, we generated Gadkin mutant proteins in which the WENDF alone or in combination with the three conventional Øxx[Ø/L/M]-type interaction motifs had been inactivated (Fig. 5A). WT or mutant Gadkin were transiently expressed in Cos7 cells and subjected to immunoprecipitation. Mutational inactivation of the nonconventional WENDF motif considerably reduced but did not abolish AP-1 binding. Mutation of all four motifs completely abrogated AP-1 association of Gadkin in living cells (Fig. 5A).
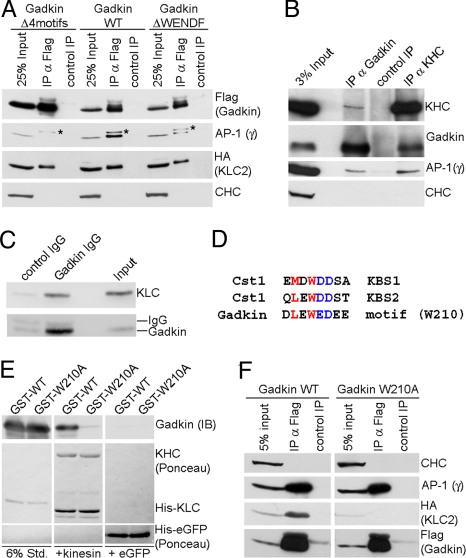
Fig. 5.
Gadkin directly associates with AP-1 and KIF5 via distinct sites. (A) Coimmunoprecipitation of AP-1/Gadkin complexes. Lysates of Cos7 cells (200 μg protein) expressing HA-Klc-2 and FLAG-Gadkin WT, ΔWENDF, or Δ4motifs were subjected to immunoprecipitation using anti-FLAG (IP α-FLAG) or anti-c-myc (control IP) antibodies. Samples were analyzed by SDS-PAGE and immunoblotting for AP-1γ, CHC, HA, or FLAG. Asterisk, nonspecific band recognized by AP-1γ antibody. (B) Endogenous kinesin-1 and AP-1 coimmunoprecipitate with Gadkin from brain. Control, nonspecific rabbit IgG. Input, 3% of the total material used for immunoprecipitation. Samples were analyzed by immunoblotting for Gadkin, KHC, AP-1γ, and CHC. (C) Immunoblot analysis of immunoisolated Gadkin-containing organelles as shown in Fig. 2E. Input, 50 μg starting material. Samples were analyzed by SDS-PAGE and immunoblotting for Klc2 and Gadkin. (D) Sequence comparison between the kinesin-binding segments (KBS) 1 and 2 of Calsyntenin-1 (Cst1) and a similar motif found in Gadkin. Residues shown to be important for Klc binding (22) are colored. (E) Recombinant kinesin heterotetramers directly bind to purified Gadkin (Δ51), but not to its W210A mutant. Four micrograms purified His10-kinesin heterotetramers or His6-eGFP (control) immobilized on beads were incubated with 2.5 μg GST-Gadkin (Δ51) WT or W210A mutant. Samples were subjected to SDS-PAGE and immunoblotting for Gadkin. Six percent Std, 6% of total amount of purified GST-tagged proteins used. (F) HA-Klc2 coimmunoprecipitates with FLAG-Gadkin WT, but not Gadkin W210A in transfected Cos7 cells (1 mg lysate). Anti-FLAG (α-FLAG IP) or anti-c-myc (control IP) antibodies were used. Samples were analyzed by immunoblotting for HA, FLAG, AP-1γ, and CHC.
In Cos7 cells expressing Gadkin-eGFP for ≈12 h (a time point at which EVs had not yet been redistributed to the cell periphery) at low levels, we noticed that mobile puncta displayed two types of motility: Rapid short-range motion and long-range, sometimes bidirectional movement along stable, presumably microtubular tracks (Movie S3). We hypothesized that plus-end-directed transport along microtubules might underlie the dispersion of AP-1- and TfR-positive EVs seen in Gadkin-eGFP expressing cells. Hence, we searched for Gadkin-associated motor proteins using large-scale affinity chromatography from brain extracts in conjunction with MALDI-TOF mass spectrometry. GST-Gadkin (Δ51, a truncation to render the protein soluble) specifically retained AP-1 and a protein of ≈130 kDa identified as KIF5C, the neuron-specific heavy chain of classical kinesin (Fig. S3A). Importantly, both Gadkin and AP-1 coimmunoprecipitated with native endogenous KIF5 from CHAPS-extracted rat brain membrane fractions (Fig. 5B), indicating that Gadkin, kinesin, and AP-1 form a complex in vivo. Endogenous kinesin was also present on Gadkin-containing organelles immunoisolated from neonatal rat brain (Fig. 5C). Further delineation of the underlying mechanism showed that Gadkin directly associates with the kinesin light chain (KLC) (Fig. S3 B and D) via a motif similar to the KLC binding sites identified within Calsyntenin-1 (Cst1; Fig. 5D). Indeed, mutation of the highly conserved residue W210 abolished the ability of Gadkin to associate with KLC in coimmunoprecipitation (Fig. 5F, Fig. S4, and Fig. S3F) or direct binding experiments using purified kinesin heterotetramers (Fig. 5E). Gadkin complex formation with AP-1 was not affected by the mutation (Fig. 5F and Fig. S3F). These data identify Gadkin as an adaptor that links KIF5-based plus-end-directed microtubular transport to AP-1-dependent membrane traffic.
Gadkin-Induced Redistribution of TfR-Containing Endosomal Vesicles Is Dependent on Its Association with AP-1 and KIF5.
Given the direct binding of Gadkin to both AP-1 and KIF5, we hypothesized that the Gadkin-induced redistribution of EVs to the periphery of HeLa cells might depend on either or both of these interactions. Whereas Gadkin-eGFP WT caused the peripheral accumulation of TfR-containing Gadkin-positive EVs, TfR localized to dispersed puncta scattered throughout the cytoplasm in cells expressing kinesin- (W210A) or AP-1-binding-defective (Δ4 motifs) Gadkin (Fig. 4 A and B and Fig. S6A). As expected, Gadkin-eGFP (W210A)-positive puncta also contained AP-1 (Fig. S6B), MPR46, and TGN46, but not GM130 or Golgin-97 (Fig. S4 A–E). These puncta were also accessible to externally added Tf or endocytosed MPRs but not to internalized dextrans (Fig. S4 J–L).
Overexpressing WT Gadkin in AP-1 knockdown cells phenocopied the expression of the AP-1-binding-defective Gadkin mutant (Fig. S6C), whereas knockdown of clathrin did not produce any overt phenotypic change (Fig. S5D). These data are consistent with a role for kinesin-dependent microtubular transport in regulating the dynamics of perinuclear endosomes (9). In agreement with this, depletion of the ubiquitous kinesin isoform KIF5B by two different siRNA oligonucleotides (Fig. 4C) caused peripheral Gadkin-eGFP-containing EVs to become concentrated perinuclearly (Fig. 4D and Fig. S5 A). Confocal imaging revealed a striking perinuclear clustering of Tf-labeled endosomes in KIF5B-depleted HeLa cells expressing endogenous levels of Gadkin (Fig. 4E and Fig. S5B). This defect could be rescued by expression of KIF5C-HA resistant to the siRNA treatment (Fig. S5C). Thus, KIF5B is required for Gadkin-induced translocation of EVs to the cell periphery and for positioning Tf-containing perinuclear endosomes in general.
Hence, one would expect that Gadkin by associating with AP-1 and KIF5 selectively targets TGN-derived EVs to the plus-end of microtubules. This should have most dramatic consequences in primary neurons where microtubules are oriented exclusively with their plus-ends toward the distal part of the neurite in axons but display mixed polarity in dendrites. In agreement with this proposal and with the presence of synaptotagmin I on Gadkin-positive organelles (Fig. S3G), we observed that overexpression of Gadkin-eGFP targets EVs containing endogenous KIF5, TfR, and AP-1 to axonal clusters including growth cones. No such phenotype was seen for Gadkin mutants defective in binding to AP-1 (Δ4 motifs) or KIF5 (W210A) (Fig. S7).
Gadkin Regulates Endosomal Membrane Traffic Via Association with AP-1 and KIF5.
Our data suggest that Gadkin directly associates with both AP-1 and KIF5, thereby potentially regulating formation and/or dynamics of Tf- and Rab4-positive EVs formed at the TGN/endosomal boundary. To test this hypothesis directly, we silenced Gadkin expression by siRNA-mediated knockdown. HeLa cells depleted of Gadkin by >95% (Fig. 1B) appeared morphologically normal, but displayed reduced rates of cell division (Fig. S8B). TfR was concentrated in the perinuclear area in both control and Gadkin knockdown cells (Fig. S8A). Total levels of cell surface TfR were reduced by ≈20% in Gadkin-knockdown cells (Fig. S8C), consistent with a minor fraction of TfR being recycled to the plasmalemma via perinuclear endosomes. This small difference in TfR surface pools, however, did not affect the amount or kinetics of 125I-Tf internalization (see below). Similar results were obtained with different siRNA oligonucleotides. Although Gadkin-depleted cells did not display any overt changes in the distribution of MPR46 or AP-1 (Fig. S8A), quantitative image analysis revealed a significant reduction in perinuclearly concentrated AP-1 (Fig. 1A), in agreement with previous studies (8).
Cycling surface receptors such as the TfR are initially internalized into EEA1-positive early endosomes from where they can return directly to the cell surface via a fast actin-dependent pathway. As an alternative route, Tf/TfR can undergo sorting to Rab4- and Rab11-positive TGN-apposed perinuclear endosomes from where it can be recycled on a slow pathway to the plasmalemma (2, 10, 11). If Gadkin via direct binding to AP-1 and KIF5 regulates the dynamics of Tf- and Rab4-positive EVs, perturbation of Gadkin function might inhibit sorting of cargo including Tf/TfR to perinuclear endosomes. Under these conditions increased fractions of Tf would be expected to recycle via a fast early endosomal pathway, resulting in altered kinetics of Tf recycling. We tested this possibility by quantifying uptake and recycling of 125I-Tf in control or Gadkin knockdown cells using a pulse/chase assay. Silencing of Gadkin expression indeed moderately but significantly accelerated the half-time of Tf recycling from 10.04 ± 0.51 min in control cells to 8.01 ± 0.27 min in Gadkin knockdown cells (n = 11; P = 0.0019; Student's t test) analyzed in parallel (Fig. 1C), whereas initial rates of 125I-Tf internalization were identical (Fig. S8D). These data are consistent with a role for Gadkin in regulating the dynamics of Tf- and Rab4-positive EVs and with the reported phenotype of Rab4-depleted cells (12). Gadkin knockdown did not affect constitutive secretory traffic of vesicular stomatitis virus G protein (VSVG) from the TGN to the surface (Fig. S9) or retrograde trafficking of Shiga toxin from endosomes to the Golgi (Fig. S10).
To address whether direct interactions between Gadkin and AP-1 and/or KIF5 contribute to the regulation of endosomal recycling, we expressed siRNA-resistant versions of Gadkin WT, or kinesin (W210A)- or AP-1 (Δ4 motifs)-binding-defective mutants in cells depleted of endogenous Gadkin. eGFP-transfected cells were taken as control. Cells were allowed to internalize Alexa568-Tf, before undergoing a chase, during which Alexa568-Tf is recycled into the medium. As expected, Gadkin-depleted control cells displayed rapid and near complete recycling of Tf into the medium, whereas a significant fraction of Tf was retained intracellularly in cells expressing siRNA-resistant Gadkin WT. Gadkin mutants defective in associating with either AP-1 (Δ4 motifs) or KIF5 (W210A) did not cause intracellular retention of Tf (Fig. 1D), although they were expressed at least at the same level as the WT protein (Fig. 1D, Inset). These results indicate that complex formation of Gadkin with AP-1 and KIF5 is required for its regulatory function in endosomal recycling of Tf. Finally, because Gadkin and AP-1 have both been reported to regulate endosomal sorting of lysosomally targeted proteins (8), we analyzed cell surface expression of N-cadherin, a protein known to partition between fast early endosomal- (13) or slow perinuclear recycling (14) and degradative lysosomal sorting pathways (15). Gadkin-depleted cells contained elevated levels of N-cadherin in total cell lysates, and this correlated with increased plasmalemmal pools of N-cadherin (Fig. 1E), consistent with a shift in N-cadherin sorting away from a degradative lysosomal toward a recycling route. These results are compatible with a model whereby Gadkin regulates the partitioning between degradative lysosomal sorting (8) and endosomal recycling pathways.
Our collective data hence show that Gadkin connects AP-1-mediated vesicular traffic to KIF5-dependent EV dynamics and thereby regulates cargo flux through the TGN/endosomal system.
Discussion
Here we identify the AP-1 binding peripheral membrane protein Gadkin as a receptor for KIF5 on TGN-derived EVs. We find that TfR- and AP-1-positive EVs undergo a dramatic KIF5-dependent dispersion into the cell periphery in transfected fibroblasts and to axonal growth cones in primary neurons overexpressing Gadkin. Knockdown of Gadkin is associated with increased rates of Tf recycling, presumably caused by impaired retrieval of Tf from fast recycling early endosomes to perinuclear TGN-derived EVs. Similar phenotypes are also observed in cells depleted of the perinuclear EV marker Rab4 (12) or the AP-1 binding partner aftiphilin (16). Reexpression of Gadkin (WT) but not of AP-1- or KIF5-binding-defective mutants delays Tf recycling in cells lacking the endogenous protein. Gadkin thus represents a protein connecting AP-1-dependent trafficking of TGN-derived EVs with KIF5-dependent plus-end-directed microtubule-based organellar motility. Based on electron microscopic analysis of Gadkin-positive endosomal structures in situ (8) and of immunoisolated organelles (Fig. 2F), the Rab4- and Tf-positive late EVs trafficked by Gadkin-KIF5 are nonidentical to bona fide recycling endosomal tubules that have been reported to undergo myosin Vb- and KIF3B-dependent movements (17, 18). It is possible that additional factors other than Gadkin provide partially overlapping functions in the regulation of KIF5-dependent endosomal dynamics, as suggested by the dramatic pericentrosomal accumulation of Tf-positive endosomes in KIF5-depleted cells. This might reflect redundancy among endosomal adaptors or, more likely, the presence of functionally distinct subpopulations of EVs, each connected to KIF5 via different adaptors. We thus favor a tentative model whereby Gadkin facilitates the AP-1-dependent formation and KIF5-dependent transport of a subpopulation of TGN-derived EVs positive for Rab4 and Tf. In agreement with this proposal, a complex between AP-1 and KIF13A that is physically and functionally distinct from AP-1/Gadkin/KIF5 has previously been shown to regulate cell surface traffic of MPRs (19), underlining the concept that functionally distinct vesicles use different adaptor/motor protein complexes for transport.
Together with recent reports from others (5), our work thus strengthens the emerging paradigm that membrane traffic within the endosomal system is fine-tuned by a delicate balance between plus- and minus-end-directed endosomal motor proteins. The AP-1/Gadkin/KIF5 complex described here may serve as an important regulator of the dynamics of TGN-derived EVs and thus could be involved in diverse cell physiological processes known to involve perinuclear endosomes ranging from the regulation of cell signaling (3) to morphogenesis (20) and long-term synaptic plasticity (21). Addressing these possible roles in vivo in an organismic context will remain one of the challenges for future studies.
Materials and Methods
Cell Culture and Transfections.
HeLa or Cos7 cells were cultured in DMEM (Gibco BRL) supplemented with FCS and antibiotics. Transfections were done using Lipofectamine 2000 (Gibco BRL) for plasmids or Oligofectamine (Gibco BRL) for siRNAs.
Affinity Chromatography and Immunoprecipitation Experiments.
GST-fusion proteins were purified from bacterial lysates using GST-bind resin (Novagen) according to standard protocols. For immunoprecipitation experiments, antibodies were immobilized on Protein A/G agarose beads (Santa Cruz Biotechnology) and incubated with either rat brain extracts or with cleared cell extracts (total protein concentration: 0.5–1 mg/mL) in lysis buffer (20 mM HEPES, pH 7.4, 2 mM MgCl2, 100 mM NaCl, 1% Triton X-100, 1 mM PMSF plus protease inhibitor mixture) for 4 h at 4 °C. Washed beads were eluted with sample buffer. Samples were analyzed by SDS/PAGE and immunoblotting. For immunoprecipitations from neonatal rat brain (P0–P3), V1 fractions [in 75 mM NaCl, 5 mM EDTA, 5 mM NEM, 10 mM CHAPS, 50 mM Tris-HCl, pH 7.5,1 mM PMSF plus protease inhibitor mixture (Sigma)] were incubated with antibody-coupled protein A/G-Sepharose essentially as above.
Expression and Purification of Recombinant Kinesin Heterotetramers.
His10-tagged Drosophila kinesin-1 heterotetramers were purified from Escherichia coli BL21 (DE3) by Ni-NTA affinity chromatography.
Fluorescence Microscopy.
Images were acquired on a Zeiss Axiovert 200M equipped with the Stallion System (3i Inc.). Live-cell confocal imaging was performed with a Zeiss Axiovert 200M equipped with the Perkin-Elmer Ultra View ERS system and a Hamamatsu C9100 EM-CCD camera under control of Volocity software (Perkin-Elmer).
Microscopic Tf Recycling Assay and Quantification.
Transfected HeLa cells were serum-starved for 1 h before adding Alexa Fluor568-Tf (25 μg/mL) for 20 min at 37 °C. Cells were placed on ice, washed three times with ice-cold buffer, and either directly fixed (uptake) or “chased” with prewarmed medium containing 10% FCS/1 mg/mL Tf (Sigma) for 30 min at 37 °C to allow for recycling. Sum fluorescence intensities were determined using the masks function of Slidebook 4.1 software after correction for background. Values from up to 10 frames for each condition (20–25 cells each) were averaged, plotted as fluorescence intensities (a.u.) (± SEM), and analyzed statistically (one-way ANOVA followed by Tukey's Multi Comparison Test).
[125I]-Tf Assays.
Serum-starved (2 h) cells were chilled on ice before medium containing 20 μg/mL Tf and 300 ng/mL [125I]-labeled Tf (specific activity: 0.3–1.0 μCi/μg) was added. Cells were incubated at 4 °C (ctrl) or 37 °C for different time intervals (uptake) or Tf internalization was allowed for 30 min (recycling). Plates were chilled on ice and washed three times with ice-cold 0.5% BSA in PBS. For uptake assays, plates were kept on ice in PBS plus 0.1% BSA. For recycling, prewarmed medium containing 100-fold excess of holo-Tf (2 mg/mL) was added, and the plates were incubated at 37 °C. Plates were removed at different time points and chilled on ice. Surface-Tf was removed by acidic washes in 0.1% BSA/PBS/25 mM acetic acid, pH 4.2. Internal 125-Tf was determined by liquid scintillation counting after cell lysis. cpm values were normalized to the initial uptake (recycling) or to the last uptake point of the control cells (uptake).
Supplementary Methods.
SI Materials and Methods available online includes plasmids, mutagenesis, siRNAs, antibodies; floatation, immunoisolation, electron microscopy; Shiga toxin trafficking assay; Tf and antibody uptake assays; detailed affinity chromatography and immunoprecipitation protocol; biotinylation.
Supplementary Material
Acknowledgments.
We thank Drs. Stefan Höning (University of Cologne, Germany), Rainer Pepperkok (European Molecular Biology Laboratory, Heidelberg, Germany), Jonathon Howard (Max-Planck-Institut, Dresden, Germany), Ludger Johannes (Institute Curie, Paris, France), and Xiao-Jiang Li (Emory University, Atlanta, GA) for reagents and Dr. Dorothea Lorenz and Martina Ringling (Leibniz Institute for Molecular Pharmacology, Berlin), Christiane Landgraf, Isabelle Grass, Inge Walther, and York Posor for experimental help. This work was supported by Grants from the German funding agency Deutsche Forschungsgemeinschaft (HA2686/1–1&1–2, SFB 449/A11 to V.H.). M.R.S. was a student of the International MSc/PhD Program Molecular Biology at the University of Göttingen (Germany) and acknowledges support from the Lichtenberg Foundation (Niedersachsen, Germany).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904268106/DCSupplemental.
References
- 1.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 2.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 3.Miaczynska M, Pelkmans L, Zerial M. Not just a sink: Endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins CR, Gibson A, Shipman M, Strickland DK, Trowbridge IS. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoepfner S, et al. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell. 2005;121:437–450. doi: 10.1016/j.cell.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- 7.Miki H, Okada Y, Hirokawa N. Analysis of the kinesin superfamily: Insights into structure and function. Trends Cell Biol. 2005;15:467–476. doi: 10.1016/j.tcb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Neubrand VE, et al. Gamma-BAR, a novel AP-1-interacting protein involved in post-Golgi trafficking. EMBO J. 2005;24:1122–1133. doi: 10.1038/sj.emboj.7600600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin SX, Gundersen GG. Maxfield FR Export from pericentriolar endocytic recycling compartment to cell surface depends on stable, detyrosinated (glu) microtubules and kinesin. Mol Biol Cell. 2002;13:96–109. doi: 10.1091/mbc.01-05-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dam EM, Ten Broeke T, Jansen K, Spijkers P, Stoorvogel W. Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2002;277:48876–48883. doi: 10.1074/jbc.M206271200. [DOI] [PubMed] [Google Scholar]
- 12.Deneka M, et al. Rabaptin-5alpha/rabaptin-4 serves as a linker between rab4 and gamma(1)-adaptin in membrane recycling from endosomes. EMBO J. 2003;22:2645–2657. doi: 10.1093/emboj/cdg257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulrich F, et al. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant DM, et al. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J Cell Sci. 2007;120:1818–1828. doi: 10.1242/jcs.000653. [DOI] [PubMed] [Google Scholar]
- 16.Hirst J, Borner GH, Harbour M, Robinson MS. The aftiphilin/p200/gamma-synergin complex. Mol Biol Cell. 2005;16:2554–2565. doi: 10.1091/mbc.E04-12-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J Biol Chem. 2002;277:50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- 18.Schonteich E, et al. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci. 2008;121:3824–3833. doi: 10.1242/jcs.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagawa T, et al. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell. 2000;103:569–581. doi: 10.1016/s0092-8674(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 20.Pelissier A, Chauvin JP, Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr Biol. 2003;13:1848–1857. doi: 10.1016/j.cub.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Park M, et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konecna A, et al. Calsyntenin-1 docks vesicular cargo to kinesin-1. Mol Biol Cell. 2006;17:3651–3663. doi: 10.1091/mbc.E06-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.