Abstract
Surface seawaters are supersaturated with respect to calcite, but high concentrations of magnesium prevent spontaneous nucleation and growth of crystals. Foraminifera are the most widespread group of calcifying organisms and generally produce calcite with a low Mg content, indicating that they actively remove Mg2+ from vacuolized seawater before calcite precipitation. However, one order of foraminifera has evolved a calcification pathway, by which it produces calcite with a very high Mg content, suggesting that these species do not alter the Mg/Ca ratio of vacuolized seawater considerably. The cellular mechanism that makes it possible to precipitate calcite at high Mg concentrations, however, has remained unknown. Here we demonstrate that they are able to elevate the pH at the site of calcification by at least one unit above seawater pH and, thereby, overcome precipitation-inhibition at ambient Mg concentrations. A similar result was obtained for species that precipitate calcite with a low Mg concentration, suggesting that elevating the pH at the site of calcification is a widespread strategy among foraminifera to promote calcite precipitation. Since the common ancestor of these two groups dates back to the Cambrian, our results would imply that this physiological mechanism has evolved over half a billion years ago. Since foraminifera rely on elevating the intracellular pH for their calcification, our results show that ongoing ocean acidification can result in a decrease of calcite production by these abundant calcifyers.
Keywords: benthic foraminifera, foraminiferal evolution, ocean acidification
A large variety of organisms that form skeletons of calciumcarbonate have evolved over the last half billion years. Some groups precipitate predominantly aragonite, such as scleractinian corals (1) and calcareous chlorophytes (2), others mostly calcite, such as foraminifera (3), coccolithophores (4), and corraline Rhodophytes (2), and some a chimera of the two (5,6). The geological prevalence of the different groups is thought to be caused by successions in sea water chemistry: periods with relatively high Ca2+ concentrations and low Mg2+ concentrations (i.e., with low Mg/Ca ratios) have favored organisms precipitating calcite, while periods with relatively high Mg/Ca ratios (e.g., during the Neogene) have favored those forming aragonite (7–9). For foraminifera, the relation between ocean chemistry and their evolution is less clear (10) and possibly obscured by the existence of different calcification strategies in this group.
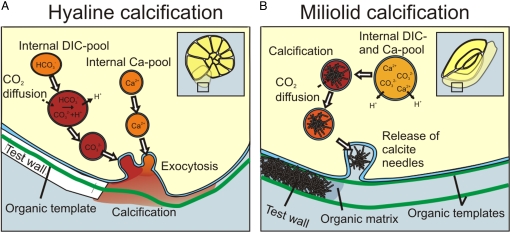
Calcifying foraminifera are commonly divided into two groups according to their test (i.e., shell) structure: miliolid and hyaline. Miliolids precipitate calcite in the form of needles with a length of 2–3 μm within cytoplasmic vesicles (11, 12) (see: 13 for the only known exception in this taxon). Before chamber formation, these needles accumulate in the cell and form a new chamber after simultaneous transport outside the test and assembly within an organic matrix (14). The needles forming the outer layer of the wall are arranged in dense rows that gives the wall of these species an opaque appearance and provided the name for the wall structure of this taxon: porcelaneous. Hyaline species (including the Rotaliids, Buliminids, and all planktonic foraminifera) store calcium and carbonate in separate intracellular pools that are used to precipitate new chambers extracellularly (15–17). Chamber formation starts with the production of a primary organic sheet (POS) in the shape of the new chamber that provides nucleation sites for the initial calcite precipitation (16, 18, 19).
Parallel to differences between their calcification pathways, composition of miliolid and hyaline calcite differs considerably (20). The needles precipitated by miliolid species contain relatively high Mg/Ca ratios (100–150 mmol/mol) (21), comparable to calcites precipitated inorganically from seawater (22). The calcite precipitated by most hyaline species has much lower Mg/Ca ratios (1–20 mmol/mol), although exceptions exist (23, 24). This low Mg calcite can only be precipitated by effective discrimination between Mg2+ and Ca2+ after seawater vacuolization. This discrimination is suggested to lead to the production of an intracellular Ca-pool with a very low Mg/Ca that is used for the precipitation of new calcite (25). This reduction in the intracellular Mg/Ca ratio may enhance calciumcarbonate precipitation, but a mechanism to elevate the carbonate concentration at the site of calcification remains unknown. Also, the additional mechanism that overcomes the inhibition of calcite precipitation by magnesium in miliolid species is not yet found.
A recent application of the ratiometric fluorescent probe HPTS shows its potential in visualising the intracellular pH in many foraminiferal species (26). We present results thus obtained showing that both groups are able to elevate the pH at the site of calcification and can thereby promote calcification despite low ambient carbonate concentrations and in the presence of relatively high Mg concentrations.
Results
Calcification in Hyaline Species.
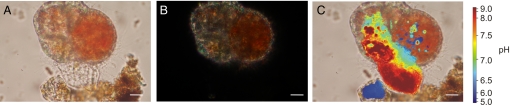
Calcifying individuals of hyaline species Cibicides lobatulus form subsequent chambers that form a trochospiral test. Like in miliolids, chamber formation by individuals of this group starts with the production of a protective cyst from debris and complete chamber formation within a few hours. During formation of the POS, a relatively large amount of cytoplasm with a pH of ≥9.0 is transported to the site of calcification (Fig. 1).
Fig. 1.
Elevated pH at the onset of calcification in Cibicides lobatulus. (A) Before calcite is precipitated, the primary organic sheet is formed that outlines the shape of the new chamber. (B) At this time, no calcite is visible under polarized light. (C) There is, however, an influx of high pH cytoplasm into the space of the new chamber. (Scale bar, 10 μm.)
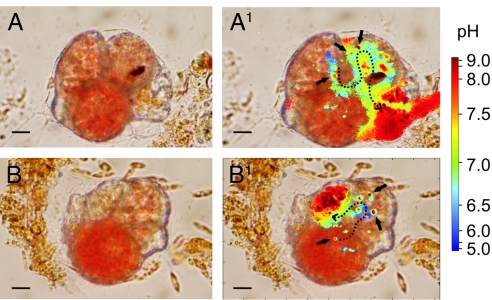
During calcite precipitation, vesicles (diameter 3–5 μm) with an elevated pH (≥9.0) are produced mainly in the penultimate chamber and transported through the ultimate chamber and its aperture toward the site of calcification (Fig. 2). The production of the vesicles, as seen in juveniles, is often related to a zone of low pH (≤6.0), suggesting that at this location the protons that are pumped out of the high pH vesicles are stored in some specialized, low pH cytosolic compartment. The arrival of numerous vesicles at the growing chamber wall often resulted in a zone of high pH, in which the new calcite is precipitated (Fig. 2).
Fig. 2.
Elevated pH during chamber formation in Cibicides lobatulus. (A and B) Vesicles with a pH ≥9.0 are produced in the chambers before the new one (black arrows), where they are often surrounded by a zone of low pH, and then transported to the site of calcification (dashed lines). The location at which the high pH vesicles are formed and the exact route they follow may vary and the arrows and dashed lines therefore depict approximations. (Scale bar, 10 μm.)
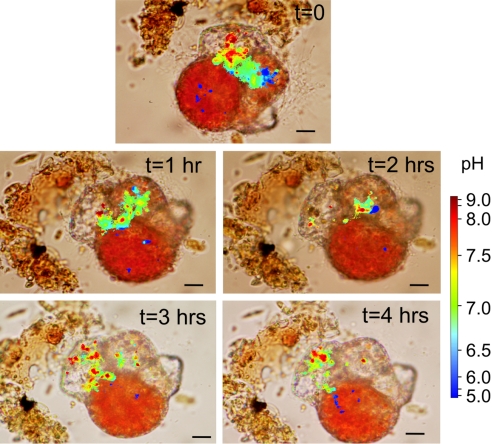
Throughout chamber formation, these high pH vesicles continue to be transported to the site of calcification. Transport of individual vesicles commonly takes less than 1 min, and the continuous production ends only after chamber formation is completed (Fig. 3).
Fig. 3.
Time lapse recording of an individual Cibicides lobatulus during chamber formation. The production of high pH vesicles in the penultimate chamber, occasionally accompanied by a low pH zone, continues until completion of a new chamber. (Scale bar, 10 μm.)
Calcification in Mililiods.
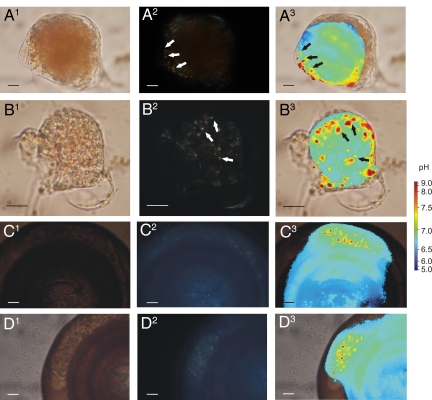
Needles formed in the miliolid species Quinqueloculina yabei and Cyclogyra planorbis are precipitated at a pH of ≈9.0 (Fig. 4). The longevity of the high pH vesicles could not be determined accurately, mainly because these vesicles move around in the cytoplasm relatively fast and seemingly undirected. During transport of the vesicles containing the calcitic needles out of the test to form a chamber wall (in Q. yabei) or to elongate the continuous spiral test (in C. planorbis), however, the pH in these vesicles was considerably lower (7.5–8.0). Chamber formation in Q. yabei is completed within a few hours, and the cytoplasm of this species is then virtually devoid of calcitic needles.
Fig. 4.
Occurrence of calcite needles and correlated high pH vesicles in juvenile specimens of Quinqueloculina yabei and adults of Cyclogyra planorbis. Pictures of all series are taken at the same time. (A and B) 1: Bright field microscopical pictures of juvenile Q. yabei, 2: Polarized light indicating the calcite crystallites inside the cell (white arrows), and 3: Intracellular pH distribution, superimposed on bright field picture (1) containing high pH vesicles correlated to the location of the crystallites (black arrows). (C and D) Same series of pictures for C. planorbis. (Scale bar, 10 μm.)
Discussion
Effects of an Elevated pH for Calcification.
Elevating the pH at the site of calcification has been suggested for foraminifera (25) and in other organisms inferred from boron isotopic measurements [i.e., in corals (27)], but not directly measured in other unicellular calcifyers. The effect of pH elevation on calcite precipitation is two-fold. It overcomes inhibition by Mg2+ on calcite precipitation that prevents spontaneous crystal nucleation and growth in seawater with modern day Mg/Ca ratios. To accomplish calcite growth without altering this Mg/Ca ratio, the pH of the surrounding medium should be 9.8 or higher (25). An elevated pH also promotes the conversion from bicarbonate into carbonate (28). At modern surface seawater pH (8.2), ≈90% of the dissolved inorganic cabron (DIC) is present in the form of HCO3−, while elevating the pH with one unit results in ≈90% being CO32−. This conversion alone (neglecting the foraminiferal control on [Ca2+] and absolute [CO32−]) results in a 9-fold increase of the calcite saturation state (Ω) and thereby approximately doubles the precipitation rate (29).
The elevation of the pH at the site of calcification in the hyaline foraminifera is not an adaptation (solemnly) to overcome the inhibition by Mg2+ of calcite growth, since they precipitate their calcite from a fluid with a very low Mg/Ca ratio. Moreover, inorganic precipitation experiments show that an increasing pH also increases the incorporation of Mg into calcite (30). Alternatively, the rise in pH during calcification may be largely explained by the conversion of bicarbonate into carbonate. For both groups, the rise in pH has the alternative effect that any CO2(aq) present in the cytosol surrounding these vesicles, diffuses into the high pH vesicles and increases the carbonate ion concentration. This in turn, provides a mechanism by which metabolic carbon dioxide can enter the calcification pathway, often invoked to explain δ13C-depleted foraminiferal calcium carbonate (31).
Taxonomic Diversity and Calcification Pathways.
The hyaline and miliolid species adopt a similar physiological strategy to precipitate calcite (see Fig. 5 for a schematic overview), even though they 1) have a different calcification pathway, 2) do not form a monophyletic group (32), and 3) have evolved their pathways at periods with contrasting seawater chemistries (8, 33). Calcification in foraminifera was likely invented before or during the Cambrian radiation, when miliolid and the agglutinating foraminifera separated from each other (32). The agglutinating foraminifera secrete an organic matrix holding together a suite of particles, silicates or carbonates, of various sizes and shapes. Although this group is not commonly regarded as calcifying, some agglutinating foraminifera (e.g., Valvulina oviedoiana) produce a low Mg calcitic matrix consisting of crystallites arranged in rods, comparable to the needles secreted by miliolids (34, 35). Another feature that underscores the common decent of hyaline and miliolid foraminifera is the recognition that both produce calcite that can consist of small, globular crystallites (36).
Fig. 5.
Similarities and differences in foraminiferal calcification pathways. (A) Increased pH in the carbonate pool and possible diffusion of metabolic CO2 during chamber formation in a generalized hyaline foraminifer. Basic scheme modfied from (16). (B) Elevation of the pH during calcite precipitation and subsequent decerase during chamber formation in the miliolid foraminifers. Basic scheme modfied from (12).
Precipitation of calcite in all groups and elevation of the pH at the site of calcification despite different calcification pathways suggest that this physiological strategy was invented before the radiation of foraminiferal into different orders and remained used by a large variety of species. The evolution of the miliolid calcification pathway puts an upper limit to the time calcification was invented. The test morphology of Cyclogyra planorbis represents that of the common miliolid (32, 37) and agglutinating (38) ancestor, dating back to the Cambrian. Since this species elevates the pH during calcification (Fig. 4), this suggests that these early miliolids already adopted this physiological strategy to precipitate calcite.
Implications for Ocean Acidification.
With rising levels of atmospheric CO2, the consequent rise in oceanic inorganic carbon levels may enhance calcification in foraminifera by supplying them with extra bicarbonate. The accompanying decrease in seawater pH by an estimated 0.3–0.4 units for the coming century (39, 40) may well countereffect the extra supply of inorganic carbon and result in a net decreased growth rate. For coccolithophores it has been suggested that future ocean acidification will result in a reduced net calcification (41–43), but for foraminifera data from micro- or mesocosm experiments are scarce. It has been shown that shell weights in the planktonic species Orbulina universa decrease with decreased carbonate ion concentration (44), possibly reflected by shifts in formainiferal shell weights over glacial-interglacial cycles and consequent shifts in oceanic pH (45). The high Mg content of the calcite produced by miliolids in particular, may easily be susceptible to dissolution with decreasing oceanic calcite saturation state in the near future (46).
The modification of the pH at the site of calcification in foraminifera may explain why the total amount of calciumcarbonate they will precipitate in an ocean subjected to ongoing acidification, may decrease. Assuming that the difference between seawater pH and that in the calcifying vesicles determines the energy spend by foraminifera on calcification, a lower oceanic pH will increase the cost for producing the same amount of calcite by one individual. If foraminifera increase the pH to maintain a fixed difference between seawater and their calcifying vesicles, decreasing oceanic pH will result in calcification at a lower pH that will in turn decrease the carbonate available for calcite precipitation. Both of these possibilities will have the same result: More energy needs to be spent by foraminifera to produce the same amount of calcite and, therefore, likely less calcite precipitation in an acidified ocean.
Methods
Specimens of the benthic nonsymbiotic, hyaline species Cibicides lobatulus and of the miliolid species Quinqueloculina yabei and Cyclogyra planorbis were used in this study. Specimens of C. lobatulus were picked from coralline algae living at the intertidal rocky shores near Hayama, Kanagawa, Japan. The sediment and coralline algal samples were regularly collected once a month from September 2007 to April 2008. Coralline algae and sediment were transported to the laboratory and kept at a constant temperature of 17 °C at daily light cycles for several days. Living specimens were isolated and cleaned from an excess of sediment and debris, transferred to filtered seawater and fed freeze-dried cells of the algae Chlorella sp. Every 2 days, the seawater was replaced and every week the food was renewed.
At various moments during this period, adult individuals were incubated in HPTS dissolved in seawater and allowed to take up the fluorescent probe for 2 days. The individuals were then rinsed several times and placed in filtered seawater and observed under an inverted fluorescent microscope [see (26) for detailed description of the methods]. Occasionally, individuals reproduced that resulted in 50–150 new individuals (30–50 μm in diameter) that consisted of two chambers. Those juveniles were also placed in HPTS+seawater and photographed within 2 days of incubation. These juveniles produce calcite walls through which movement of particles and vesicles can be observed easily.
The focal plane of the microscope results in observations with a field depth of 1–2 μm, although the fluorescent signal from below and above the depth at which the objective lens is focused, may cause additional fluorescence to decrease the sharpness of the obtained pictures. Since the total amount of fluorescent intracellular material is relatively small in juveniles, these individuals generally reveal more intracellular structures when using traditional fluorescence microscopy.
In addition to the methods described in (26), we conducted three experiments to analyze possible distortions or attenuations caused by the calcitic foraminiferal tests. Firstly, to test autofluorescence by organic compounds in the foraminiferal calcite we photographed large empty tests of specimens of Ammonia tepida that were not incubated with HPTS. With the relatively short shutter time (1.0 s) and without usage of either of the two neutral density filters we used for photographing living specimens, no autofluorescence was detectable in our specimens. To quantify attenuation of the calcitic test, we incubated large, empty tests in HPTS dissolved in seawater (20 μM) at a pH of 8.2 and excited these at the two excitation wavelengths (Fig. 6). The obtained pictures were processed as the ones from the living individuals [see (26) for detailed description]. Finally, incubated empty tests were rinsed with seawater so that the HPTS was confined to the space inside the tests and subsequently photographed (Fig. 6).
Fig. 6.
The effect of foraminiferal calcite on the emitted fluorescence signal after subsequent excitations. (A) Example of a specimen incubated in HPTS dissolved in seawater and photographed 1: with transmitted light, 2: at the first excitation optimum, and 3: at the second excitation optimum. 4: The resulting pH-image after dividing the images from (2) and (3). (B) Example of an empty test incubated with HPTS, rinsed with seawater, and photographed in the same order as done for (A). (Scale bar, 100 μm.)
The resulting (overlaid) pH-pictures show that the attenuation of the emitted wavelengths caused by the calcitic test is negliable. For both the tests in HPTS dissolved in seawater and those rinsed with seawater, the shift in pH is less than 0.1 pH unit. These small shifts are furthermore confined to the sutures and edges of the chambers. The shifts are smaller than the uncertainties rising from the calibration (26) and are therefore not accounted for in our results and subsequent discussion.
Acknowledgments.
We thank R.E. Zeebe, G.J. Reichart, J. Bijma, and M. Tsuchiya for comments that greatly improved earlier versions of this manuscript. This work was sponsored by the Japanese Society for the Promotion of Science and Japanese Ministry of Education, Culture, Sports, Science and Technology Grants P07701 (to L.N.), 20740301 (to T.T.), and 17204046 (to H.K.)
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Cuif J-P, Dauphin Y, Doucet J, Salome M, Susini J. XANES mapping of organic sulphate in three scleractinian coral skeletons. Geochim Cosmochim Acta. 1999;67:75–83. [Google Scholar]
- 2.Chave KE. Physics and chemistry of biomineralization. Ann Rev Earth Planet Sci. 1984;12:293–305. [Google Scholar]
- 3.Todd R, Blackmon P. Calcite and aragonite in foraminifera. J Paleontol. 1956;30:217–219. [Google Scholar]
- 4.Brownlee C, Taylor A. In: Coccolithophores: From molecular processes to global impact. Thierstein HR, Young JR, editors. Berlin: Springer; 2004. pp. 31–50. [Google Scholar]
- 5.Carter JG. In: Skeletal growth of aquatic organisms. Rhoads DC, Lutz RA, editors. New York: Plenum Press; 1980. pp. 69–113. [Google Scholar]
- 6.Kennedy WJ, Taylor JD, Hall A. Environmental and biological controls on bivalve shell mineralogy. Biol Rev. 2008;44:499–530. doi: 10.1111/j.1469-185x.1969.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson BH. Biominerallization, paleoceanography, and the evolution of calcareous marine organisms. Geology. 1979;7:524–527. [Google Scholar]
- 8.Stanley SM, Hardie LA. Secular oscillations in the carbonate mineralogy of reef-building and sediment-producing organisms driven by tectonically forced shifts in seawater chemistry. Palaeogeogr Palaeoclimatol Palaeoecol. 1998;144:3–19. [Google Scholar]
- 9.Stanley SM. Influence of seawater chemistry on biomineralization throughout phanerozoic time: Paleontological and experimental evidence. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;232:214–236. [Google Scholar]
- 10.Martin RE. Cyclic and secular variation in microfossil biomineralization: clues to the biogeochemical evolution of Phaenerozoic oceans. Global Planet Change. 1995;11:1–23. [Google Scholar]
- 11.Berthold W-U. Biomineralisation bei milioliden Foraminiferen und die Matritzen-Hypothese. Naturwissenschaften. 1976;63:196–197. [Google Scholar]
- 12.Hemleben CH, Anderson OR, Berthold W, Spindler M. In: Biomineralization in lower plants and animals. Leadbeater BSC, Riding R, editors. Clarendon: Systematics Association special volume 30; 1986. pp. 237–249. [Google Scholar]
- 13.Habura A, Goldstein SL, Parfrey LW, Bowser SS. Phylogeny and ultrastructure of Miliammina fusca: Evidence for secondary loss of calcification in a miliolid foraminifer. J Eukaryot Microbiol. 2006;53:204–210. doi: 10.1111/j.1550-7408.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 14.Angell RW. Test morphogenesis (chamber formation) in the foraminifer Spiroloculina hyalina schulze. J Foraminifer Res. 1980;10:89–101. [Google Scholar]
- 15.Anderson OR, Faber WW. An estimation of calcium carbonate deposition rate in a planktonic foraminifer Globigerinoides sacculifer using 45Ca as a tracer: A recommended procedure for improved accuracy. J Foraminifer Res. 1984;14:303–308. [Google Scholar]
- 16.Erez J. In: Reviews in mineralogy and geochemistry. Dove PM, De Yoreo JJ, Weiner S, editors. vol. 54. Washington, DC: Mineralogical Society of America; 2003. pp. 115–149. [Google Scholar]
- 17.Toyofuku T, De Nooijer LJ, Yamamoto H, Kitazato H. Real-time visualization of calcium ion activity in shallow benthic foraminiferal cells using the fluorescent indicator Fluo-3 AM. Geochem Geophys Geosyst. 2008;9:Q05005. doi: 10.1029/2007GC001772. [Google Scholar]
- 18.Angell RW. Calcification during chamber development in Rosalina floridana. J Foraminifer Res. 1979;9:341–353. [Google Scholar]
- 19.Hemleben CH, Bé AWH, Anderson OR, Tuntivate S. Test morphology, organic layers, and chamber formation of the planktonic foraminifer Globorotalia menardii. J Foraminifer Res. 1977;7:1–25. [Google Scholar]
- 20.Bentov S, Erez J. Impact of biomineralization processes on the Mg content of foraminiferal shells: A biological perspective. Geochem Geophys Geosyst. 2006;7:Q01P08. doi: 10.1029/2005GC001015. [Google Scholar]
- 21.Toyofuku T, Kitazato H, Kawahata H, Tsuchiya M, Nohara N. Evaluation of Mg/Ca thermometry in foraminifera: Comparison of experimental results and measurements in nature. Paleoceanography. 2000;15:456–464. [Google Scholar]
- 22.Morse JW, et al. Calcium carbonate formation and dissolution. Chem Rev. 2007;107:342–381. doi: 10.1021/cr050358j. [DOI] [PubMed] [Google Scholar]
- 23.Raja R, Saraswati PK, Rogers K, Iwao K. Magnesium and strontium compositions of recent symbiont-bearing benthic foraminifera. Mar Micropaleontol. 2005;58:31–44. [Google Scholar]
- 24.Toyofuku T, Kitazato H. Micromapping of Mg/Ca values in cultured specimens of the high-magnesium benthic foraminifera. Geochem Geophys Geosyst. 2005;6:Q11P05. doi: 10.1029/2005GC000961. [Google Scholar]
- 25.Zeebe RE, Sanyal A. Comparison of two potential strategies of planktonic foraminifera for house building: Mg2+ or H+ removal? Geochim Cosmochim Acta. 2002;66:1159–1169. [Google Scholar]
- 26.De Nooijer LJ, Toyofuku T, Oguri K, Nomaki H, Kitazato H. Intracellular pH distribution in foraminifera determined by the fluorescent probe HPTS. Limnol Oceanogr-Meth. 2008;6:610–618. [Google Scholar]
- 27.Blamart D, et al. Correlation of boron isotopic composition with ultrastructure in the deep-sea coral Lophelia pertusa: Implications for biomineralization and paleo-pH. Geochem Geophy Geosy. 2007;8:Q12001. doi: 10.1029/2007GC001686. [Google Scholar]
- 28.Zeebe RE, Wolf-Gladrow D. CO2 in seawater: Equilibrium, kinetics and isotopes, Elsevier Oceanographic Series. Amsterdam: Elsevier; 2001. [Google Scholar]
- 29.Lopez O, Zuddas P, Faivre O. The influence of temperature and seawater composition on calcite crystal growth mechanisms and kinetics: Implications for Mg incorporation in calcite lattice. Geochim Cosmochim Acta. 2009;73:337–347. [Google Scholar]
- 30.Burton EA, Walter LM. The effects of PCO2 and temperature on magnesium incorporation in calcite in seawater and MgCl2-CaCl2 solutions. Geochim Cosmochim Acta. 1991;55:777–785. [Google Scholar]
- 31.Grossman EL. Stable isotopes in modern benthic foraminifera: A study of vital effect. J Foraminifer Res. 1987;17:48–61. [Google Scholar]
- 32.Pawlowski J, et al. The evolution of early foraminifera. Proc Natl Acad Sci USA. 2003;100:11494–11498. doi: 10.1073/pnas.2035132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardie LA. Secular variation in seawater chemistry: An explanation for the coupled secular variation in the mineralogies of marine limestones and potash evaporites over the past 600 m. y. Geology. 1996;24:279–283. [Google Scholar]
- 34.Bender H, Hemleben C. Constructional aspects in test formation of some agglutinated foraminifera. Abh Geol B. 1988;41:13–21. [Google Scholar]
- 35.Bender H, Hemleben C. Calcitic cement secreted by agglutinated foraminifers grown in laboratory culture. J Foraminifer Res. 1988;18:42–45. [Google Scholar]
- 36.Debenay JP, Guillou J-J, Lesourd M. Colloidal calcite in foraminiferal tests: Crystallization and texture of the test. J Foraminifer Res. 1996;26:277–288. [Google Scholar]
- 37.Arnold ZM. Biological clues to the origin of miliolidean foraminifera. J Foraminifer Res. 1979;9:302–321. [Google Scholar]
- 38.Culver SJ. Early Cambrian Foraminifera from West Africa. Science. 1991;254:689–691. doi: 10.1126/science.254.5032.689. [DOI] [PubMed] [Google Scholar]
- 39.Wolf-Gladrow DA, Riebesell U, Burkhardt S, Bijma J. Direct effects of CO2 concentration on growth and isotopic composition of marine plankton. Tellus B Chem Phys Meterol. 1999;51:461–476. [Google Scholar]
- 40.Caldeira K, Wickett ME. Anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- 41.Riebesell U, et al. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature. 2000;407:364–367. doi: 10.1038/35030078. [DOI] [PubMed] [Google Scholar]
- 42.Sciandra A, et al. Response of coccolithophorid Emiliania huxleyi to elevated partial pressure of CO2 under nitrogen limitation. Mar Ecol Prog Ser. 2003;261:111–122. [Google Scholar]
- 43.Delille B, et al. Response of primary production and calcification to changes of pCO2 during experimental blooms of the coccolithophorid Emiliania huxleyi. Global Biogeochem Cycles. 2005;19 doi: 10.1029/2004GB002318. [Google Scholar]
- 44.Bijma J, Spero HJ, Lea DW. In: Use of proxies in paleoceanography. Fischer, Wefer, editors. Berlin: Springer; 1999. pp. 489–512. [Google Scholar]
- 45.Barker S, Elderfield H. Foraminiferal calcification response to glacial-interglacial changes in atmospheric CO2. Science. 2002;297:333–336. doi: 10.1126/science.1072815. [DOI] [PubMed] [Google Scholar]
- 46.Andersson AJ, Mackenzie FT, Bates NR. Life on the margin: Implications of ocean acidification on Mg-calcite, high latitude, and cold-water marine calcifiers. Mar Ecol Prog Ser. 2008;373:365–273. [Google Scholar]