Abstract
Many species expanded their geographic ranges from core “refugium” populations when the global climate warmed after the Pleistocene. The bottlenecks that occur during such range expansions diminish genetic variation in marginal populations, rendering them less responsive to selection. Here, we show that range expansion also strongly depletes inbreeding depression. We compared inbreeding depression among 20 populations across the expanded range of a common European plant, and found that marginal populations had greatly reduced inbreeding depression. Similar patterns were also revealed by multilocus computer simulations. Low inbreeding depression is predicted to ease conditions for the evolution of self-fertilization, and selfing is known to be particularly frequent in marginal populations. Therefore, our findings expose a remarkable aspect of evolution at range margins, where a history of expansion can reverse the direction of selection on the mating system, providing a parsimonious explanation for the high incidence of selfing in marginal populations.
Keywords: genetic bottleneck, mating system, phenotypic performance, self fertilization, range margins
The geographic distribution of plant, animal, and microbial species is often the result of a complex history of range expansion and contraction. The occurrence of many populations at mid to high latitudes, for example, can be attributed to range expansion from core “refugium” populations at lower latitudes due to global warming after the Pleistocene (1). Because such range shifts may involve repeated population bottlenecks during colonization and population growth, marginal populations are often less variable at both neutral and nonneutral loci than populations at the core of species and will be less able to respond to natural selection if additive genetic variance is depleted (2).
Bottlenecks are also expected to erode genetic variation responsible for inbreeding depression, either through the purging of recessive deleterious mutations by selection when they are expressed in homozygotes, or through their stochastic fixation by drift (3–8). The purging of a population's load of deleterious mutations will increase its mean fitness compared with populations that have not been purged, whereas the fixation of such mutations will reduce fitness. Either way, colonization bottlenecks are expected to cause inbred and outbred progeny to resemble one another genetically, and inbreeding depression measured as the reduced fitness of inbred (e.g., self-fertilized) versus outbred progeny within a population should, therefore, be reduced in marginal populations. There is abundant evidence for the purging of inbreeding depression in artificially bottlenecked populations, and for the fixation of genetic load (9). However, we are not aware of any evidence that range expansion bottlenecks could lead to reduced inbreeding depression toward the margins of a geographic range of a species.
Whether range expansion depletes inbreeding depression in marginal populations has important implications for our understanding of the evolution of their life histories and reproductive systems. For example, in many plant species, the incidence of self-fertilization is greater in geographically marginal populations than in the core of the species (10). Similarly, the frequency of asexual reproduction in plants and animals is well known to be higher in marginal populations, a pattern referred to as “geographic parthenogenesis” (11–15). Three main hypotheses have been invoked to explain this greater frequency of uniparental reproduction in marginal populations.
First, because marginal populations may be less affected by biotic interactions with competitors, pathogens, and parasites (16), the advantages of sex and recombination in balancing the 2-fold cost of sex would be reduced (11, 17, 18). Second, asexuality (12) or self-fertilization (19) will establish preferentially in marginal populations, because uniparental reproduction prevents mating with poorly adapted migrants from core regions of the species distribution (14). And third, asexuality and self-fertilization may be common in marginal populations because of the benefits of reproductive assurance during colonization and establishment at low density (20, 21). Of these three hypotheses, putatively reduced mating opportunities at range margins are widely seen “as the crucial ecological context for the evolution of selfing” (10, 22).
The reproductive assurance hypothesis supposes that selfing evolves in marginal populations because the benefits conferred by uniparental reproduction when mating opportunities are scarce, during colonization, might outweigh the costs of producing selfed progeny with reduced fitness, i.e., inbreeding depression. Inbreeding depression is widely accepted as the principal factor regulating the evolution of mating systems and the maintenance of selfing vs. outcrossing (10, 23–26). However, patterns of variation in inbreeding depression have hitherto not been considered as a possible explanation for the higher incidence of selfing in marginal populations. If inbreeding depression is reduced by range expansion, this reduction in itself could prompt the evolution of self-fertilization in marginal populations, even in the absence of selection for reproductive assurance.
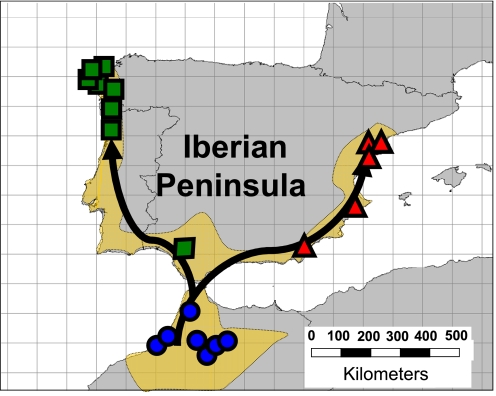
Here, we test the hypothesis that range expansion depletes inbreeding depression by comparing the fitness of selfed versus outcrossed progeny within each of 20 hexaploid populations of the European plant Mercurialis annua. Hexaploid M. annua expanded its geographic range from a North African refugium into the Iberian Peninsula along two separate corridors in the east and west, probably after the Pleistocene glaciation (Fig. 1) (27). Range expansion of M. annua diminished both neutral genetic diversity (27) and additive genetic variation for key life-history traits (2). Thus, we hypothesized that if range expansion also reduced variation caused by deleterious recessive mutations, then inbreeding depression would be lower in northern populations compared with those in the south. To evaluate the pattern of variation in inbreeding depression observed along the corridors of range expansion, we compared our results with those produced by multilocus computer simulations of inbreeding depression during, and subsequent to, a range expansion similar to that hypothesized for M. annua.
Fig. 1.
Map showing the coastal distribution of hexaploid M. annua in the Iberian Peninsula and North Africa (ochre area) and the location of study populations. Blue circles, red triangles, and green squares represent, respectively, North African, eastern Iberian, and western Iberian populations. Arrows indicate putative routes of range expansion out of Africa.
Results
Inbreeding Depression in Populations of M. annua.
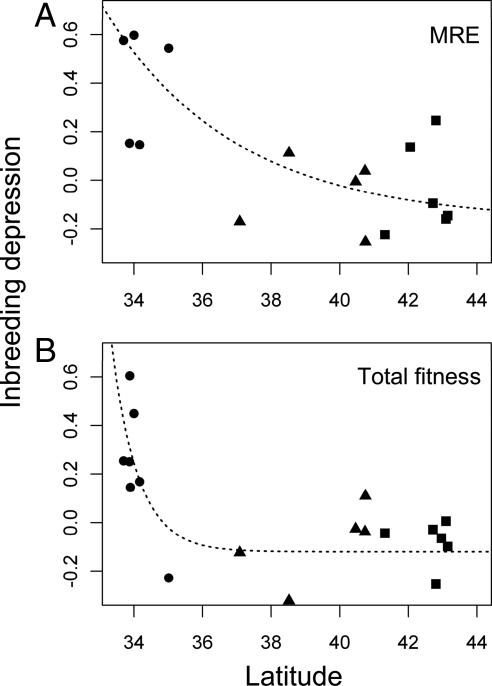
We plotted components of inbreeding depression (δ) for each population against latitude (Fig. 2). As predicted, we found that δ, measured in terms of both male reproductive effort (MRE) and plant size (estimated by both height and biomass), declined with latitude (linear regressions for MRE: r2 = 19.5%, P = 0.029; height: r2 = 46%, P = 0.001; biomass: r2 = 19.7%, P = 0.028). Interestingly, we found no relation between inbreeding depression in female reproductive effort (FRE) and either latitude or neutral diversity (P = 0.44 and 0.60, respectively). MRE is a key life-history trait that is expected to correlate closely with fitness in wind-pollinated plants such as M. annua (28–30), and reproductive success typically increases with size in plants (31). As expected, we found that total δ also declined with latitude (Fig. 2B).
Fig. 2.
Decline with latitude of inbreeding depression measured in terms of MRE (A), and in terms of an estimate of total fitness for 20 hexaploid populations of M. annua (B). Circles, triangles, and squares, respectively, represent North African, eastern Iberian, and western Iberian populations. Asymptotic exponential curves of the form y = a − bexp(−cx − m) were fitted to the data, where a, b, and c were parameters estimated by least squares nonlinear regression, and m was the latitude of the southern-most population sampled; parameters a, b, and c (± SEs) were estimated, for MRE and total fitness, respectively, as (−0.169 ± 0.240, −0.120 ± 0.047), (−0.752 ± 0.225, −0.540 ± 0.134), and (0.260 ± 0.252, 1.302 ± 0.989).
A nonlinear quadratic model provided a better fit for the relation between total δ and latitude (r2 = 45.9%; P = 0.002) than a linear model (r2 = 32.4%; P = 0.005). This nonlinearity is reflected by the fact that although the drop in δ between populations in the putative refugium in north Africa and those in the expanded range of the species in the Iberian Peninsula was highly significant (r2 = 44.8%; P = 0.001), there was no significant decline in δ with latitude among the Iberian populations themselves; indeed, there was no significant difference between the fitness of selfed versus outcrossed progeny in Iberian populations, whether the Iberian populations were analyzed as a single group of populations (Iberian Peninsula: P = 0.978) or as two separate groups distributed along the eastern and western corridors, respectively (eastern Iberia: P = 0.905; western Iberia: P = 0.912). The steep initial decline and subsequent shallower decline in inbreeding depression were well described by asymptotic exponential curves (Fig. 2).
Computer Simulations of Inbreeding Depression During a Range Expansion.
The pattern of a single sudden drop in inbreeding depression between North Africa and Iberia would seem to require an explanation invoking a single severe colonization bottleneck, for example, across the Strait of Gibraltar between Africa and Spain, followed by a range expansion in the Iberian Peninsula with less severe bottlenecks. Alternatively, the pattern might still be consistent with a scenario of range expansion along corridors of colonization characterized by repeated bottlenecks of similar intensity. To assess this possibility, we ran computer simulations of a range expansion from a single outcrossing refugium population and measuring inbreeding depression as a function of distance from the source of origin. We first allowed the source population to reach an equilibrium between the influx of partially recessive deleterious mutations and their removal by selection, and we then allowed the species to expand its range along a 1D array of habitat patches by stepping-stone migration and exponential population growth to a local carrying capacity, assuming identical per-individual migration rates between neighboring patches.
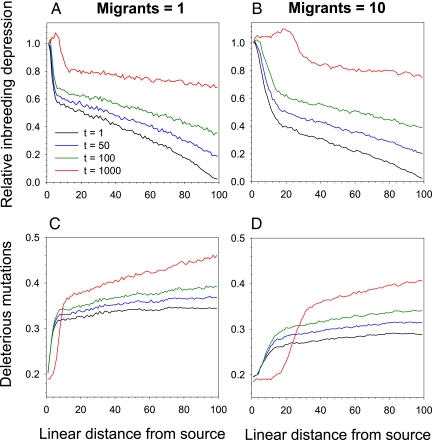
Our simulations revealed a pattern of inbreeding depression among populations similar to that observed for M. annua: There was a sharp decline in inbreeding depression between the source and the first populations along the expansion corridor, followed by a markedly shallower decline (Fig. 3). Although the absolute decline was reduced to some extent when bottlenecks were less severe, the nonlinear pattern remained (compare Fig. 3 A and B). When all habitat patches were first fully occupied by the range expansion, populations at the extreme margin showed almost no inbreeding depression compared with those at the source. With the passage of time, inbreeding depression in these marginal populations gradually increased as a result of both new deleterious mutations and the migration of mutations along the expansion corridor from the source (Fig. 3). However, even with relatively high migration rates, the shape of decline in inbreeding depression from source to marginal populations was maintained for long periods of time. We also simulated various scenarios involving a single severe bottleneck close to the refugium, and the results were qualitatively similar to those found for the simple model invoking uniform spread. Accordingly, although it is possible, and even likely, that the reduced inbreeding depression in Iberian populations is a result of a single severe bottleneck close to the refugium, there is no compelling reason to reject a model assuming equivalent bottlenecks along the length of the expansion corridors.
Fig. 3.
Simulated values of relative inbreeding depression (A and B) and the number of deleterious mutations per genome (C and D) in demes plotted against their position in a linear array of 100 habitat patches from the source of range expansion (situated at zero). Curves, which link points representing an average over 20 independent simulations, are shown for several times after the full array was filled by the range expansion (indicated), with t measured in generations. For details, see main text.
Discussion
Inbreeding depression within populations declines dramatically between North African populations of hexaploid M. annua in its putative Pleistocene refugium and Iberian populations north of the Strait of Gibraltar, but there is relatively little change in inbreeding depression with latitude further north. Our computer simulations indicate that this pattern is consistent with a range expansion due to recurrent bottlenecks of similar size along a 1D corridor north. However, although the observed pattern does not require an explanation invoking a single severe bottleneck followed by spread by, for example, diffusion (32), neither can we rule out an expansion through a single bottleneck, which would also be consistent with our observations and is perhaps even more likely. Either way, our results indicate that range expansions can have a dramatic effect not only on patterns of neutral and additive genetic variance (and a corresponding ability to respond to selection) (2, 33), but also on inbreeding depression, a key factor affecting the evolution of mating and sexual systems (10, 23–26, 34).
Transitions from outcrossing to self-fertilization are expected to be selected when inbreeding depression is low (10, 23–26). Therefore, one might expect populations in which inbreeding depression has been reduced by range expansion to be more susceptible to the invasion and spread of mutations that increase the selfing rate (7). Marginal populations might then evolve self-fertilization in response to reduced inbreeding depression rather than, or in addition to, selection for reproductive assurance. The raised incidence of self-fertilization in marginal populations is well known and is widely attributed to selection for reproductive assurance in populations where mate or pollinator availability is low (10, 22). Our study prompts a new hypothesis that the association between the mating system and geographic marginality may be due to selection under evolved low levels of inbreeding depression brought about by expansion bottlenecks.
It would be difficult to test our hypothesis using species that have in fact undergone a shift from outcrossing to selfing between core and marginal populations. In such cases, an association between phylogeography and inbreeding depression would be confounded by variation in the mating system: Reduced inbreeding depression could be the result either of range expansion, as hypothesized here, or of purging after a transition to self-fertilization precipitated by other causes. In contrast, although M. annua has undergone several shifts in its sexual system between combined and separate sexes in various parts of its geographic range (30), its mating system is not confounded with the observed geographic patterns in inbreeding depression. Eppley and Pannell (35) inferred low selfing rates of <0.2 for high-density populations of M. annua sampled from its northern range margin, similar to those estimated for populations in the southern core of the range of the species (36). Because the floral morphology and sex allocation of monoecious individuals of M. annua are largely invariant across the species range (2), the observed reduction in inbreeding depression with latitude is more likely to be a consequence of range expansion, as exposed by our simulations, than of purging after a shift from outcrossing to selfing, which has not occurred.
We emphasize that, although range expansion can evidently reduce within-population inbreeding depression in marginal populations, this reduction does not imply that marginal populations are in any way “fitter” as a result. On the contrary, the repeated genetic bottlenecks associated with a range expansion are likely to fix deleterious mutations in marginal populations, reducing their fitness (Fig. 3 C and D) (7, 8). Although marginal populations may suffer lower absolute fitness, critically natural selection may still favor mutations that alter the selfing rate, because both selfed and outcrossed progeny will have a similar, lowered, fitness.
Geographically marginal populations of many species are the result of relatively recent range expansions, particularly those at high latitudes (1). In cases where such populations also display elevated selfing rates, our study indicates that the reduction of inbreeding depression by range expansion could facilitate an evolutionary transition from outcrossing, irrespective of local mate or pollinator availability. Thus, our findings expose a remarkable aspect of evolution at range margins, where a history of expansion can reverse the direction of selection on a key predictor of the mating system. They further imply that uniparental reproduction will have evolved more frequently at species range margins that have been established by a recent range expansion than at those that have not.
Materials and Methods
Generation of Experimental Populations.
Because M. annua is wind-pollinated and produces its flowers indeterminately, we could not self and outcross different flowers on the same plants. Thus, we created a pool of selfed and outcrossed progeny for each of the 20 populations sampled, and estimated inbreeding depression for each population by comparing then. For each population, outcrossed progeny were generated by sampling seeds from parents grown together in groups of 50 at high density; selfed progeny were generated by pooling seeds sampled from each of three plants isolated from each other and other plants using screens in the same greenhouse. Twenty selfed and 20 outcrossed progenies were then grown for each population in the same glasshouse at the same time, distributed randomly on the bench. Fitness was assayed when plants were 6 weeks old.
Measures.
We measured inbreeding depression by comparing fitness components of selfed with outcrossed progeny within each population separately. In particular, we estimated the total fitness of the ith individual in terms of its relative contribution to the population's production of seeds and pollination cloud: wi = si + pis̄/p̄, where s̄ and p̄ are the mean seed production and mean pollen production of all outcrossed individuals in the respective population. For every plant, we recorded the dry weight of male flowers, seeds, above-ground parts, and height. MRE and FRE were calculated as the ratio of male and female allocations, respectively, to above-ground plant biomass.
Analysis.
For each population, components of δ were calculated as δ = 1 − wself/wout for wout > wself, δ = wout/wself − 1 for wout < wself, where wout and wself are the mean fitnesses of selfed and outcrossed progeny, respectively. Estimates of inbreeding depression were plotted against the latitudes of the 20 populations, and linear and second-order polynomial regression models were compared. We also fitted three-parameter asymptotic exponential curves to our data, using nonlinear regression. All analyses were conducted in R (www.R-project.org).
Simulations.
We simulated the effects of a range expansion on inbreeding depression by allowing a single source population to expand its range along a 1D corridor of length 100. Each population had a carrying capacity of 1,000 individuals, and individual fitness was reduced by mutations to deleterious alleles occurring at each of 100 independently segregating loci. We first allowed the source population at one end of the array to reach an equilibrium between mutation and selection. We then allowed range expansion to fill the array by stepping-stone colonization. We allowed the array to evolve for a further 1,000 generations, and recorded inbreeding depression and the number of deleterious mutations accumulated within each population (for detailed description of the model, see SI Materials and Methods).
Supplementary Material
Acknowledgments.
We thank D. Obbard, D. Charlesworth, S. Barrett, and C. Haag for helpful comments on the manuscript. D. Obbard, R. Buggs, and G. Campbell helped with population sampling. B.P. was supported by a Natural Environment Research Council grant awarded to J.R.P.; S.-R.Z. was supported by a Royal Society China Fellowship grant awarded to J.R.P and by the National Science Foundation of China Grant 30670314; and J.S.V. was supported by a postdoctoral grant from the Fundación Caixa Galicia, Spain.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.S.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902257106/DCSupplemental.
References
- 1.Hewitt G. The genetic legacy of the Quaternary ice ages. Nature. 2000;405:907–913. doi: 10.1038/35016000. [DOI] [PubMed] [Google Scholar]
- 2.Pujol B, Pannell JR. Reduced responses to selection following species range expansion. Science. 2008;321:96. doi: 10.1126/science.1157570. [DOI] [PubMed] [Google Scholar]
- 3.Kimura M, Maruyama T, Crow JF. The mutation load in small populations. Genetics. 1963;48:1303–1312. doi: 10.1093/genetics/48.10.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett SCH, Charlesworth D. Effects of a change in the level of inbreeding on the genetic load. Nature. 1991;352:522–524. doi: 10.1038/352522a0. [DOI] [PubMed] [Google Scholar]
- 5.Husband BC, Schemske DW. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution. 1996;50:54–70. doi: 10.1111/j.1558-5646.1996.tb04472.x. [DOI] [PubMed] [Google Scholar]
- 6.Byers DL, Waller DM. Do plant populations purge their genetic load? Effects of population size and mating history on inbreeding depression. Annu Rev Ecol Syst. 1999;30:479–513. [Google Scholar]
- 7.Bataillon T, Kirkpatrick M. Inbreeding depression due to mildly deleterious mutations in finite populations: Size does matter. Genet Res. 2000;75:75–81. doi: 10.1017/s0016672399004048. [DOI] [PubMed] [Google Scholar]
- 8.Glemin S. How are deleterious mutations purged? Drift versus nonrandom mating. Evolution. 2003;57:2678–2687. doi: 10.1111/j.0014-3820.2003.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 9.Crnokrak P, Barrett SCH. Purging the genetic load: A review of the experimental evidence. Evolution. 2002;56:2347–2358. doi: 10.1111/j.0014-3820.2002.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 10.Barrett SCH. The evolution of plant sexual diversity. Nat Rev Genet. 2002;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- 11.Bell G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. Berkeley: Univ of California Press; 1982. [Google Scholar]
- 12.Peck JR, Yearsley JM, Waxman D. Explaining the geographic distributions of sexual and asexual populations. Nature. 1998;391:889–892. [Google Scholar]
- 13.Vandel A. “Geographic parthenogenesis: a contribution to the biological and cytological study of natural parthenogenesis” (Translated from French) Bull Biol Belg. 1928;62:164–281. [Google Scholar]
- 14.Lynch M. Destabilizing hybridization, general-purpose genotypes and geographic parthenogenesis. Q Rev Biol. 1984;59:257–290. [Google Scholar]
- 15.Haag CR, Ebert D. A new hypothesis to explain geographic parthenogenesis. Ann Zool Fenn. 2004;41:539–544. [Google Scholar]
- 16.Engelkes T, et al. Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature. 2008;456:946–948. doi: 10.1038/nature07474. [DOI] [PubMed] [Google Scholar]
- 17.Glesener RR, Tilman D. Sexuality and components of environmental uncertainty: Clues from geographic parthenogenesis in terrestrial animals. Am Nat. 1978;112:659–673. [Google Scholar]
- 18.Levin DA. Pest pressure and recombination systems in plants. Am Nat. 1975;109:437–457. [Google Scholar]
- 19.Antonovics J. Evolution in closely adjacent plant populations: V Evolution of self-fertility. Heredity. 1968;23:219–238. doi: 10.1038/sj.hdy.6800835. [DOI] [PubMed] [Google Scholar]
- 20.Bierzychudek P. Patterns in plant parthenogenesis. Experientia. 1985;41:1255–1264. doi: 10.1007/978-3-0348-6273-8_9. [DOI] [PubMed] [Google Scholar]
- 21.Stebbins GL. Longevity, habitat, and release of genetic variability in the higher plants. Cold Spring Harbor Symp Quant Biol. 1958;23:365–378. doi: 10.1101/sqb.1958.023.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Schoen DJ, Morgan MT, Bataillon T. How does self-pollination evolve? Inferences from floral ecology and molecular genetic variation. Philos Trans R Soc London B. 1996;351:1281–1290. [Google Scholar]
- 23.Lande R, Schemske DW. The evolution of self-fertilization and inbreeding depression in plants: I. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 24.Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst. 1987;18:273–288. [Google Scholar]
- 25.Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating systems in plants: Occurrence, theoretical explanations, and empirical evidence. Annu Rev Ecol Evol Syst. 2005;36:47–79. [Google Scholar]
- 26.Herlihy CR, Eckert CG. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature. 2002;416:320–323. doi: 10.1038/416320a. [DOI] [PubMed] [Google Scholar]
- 27.Obbard DJ, Harris SA, Pannell JR. Sexual systems and population genetic structure in an annual plant: Testing the metapopulation model. Am Nat. 2006;167:354–366. doi: 10.1086/499546. [DOI] [PubMed] [Google Scholar]
- 28.Klinkhamer PGL, de Jong TJ, Metz H. Sex and size in cosexual plants. Trends Ecol Evol. 1997;12:260–265. doi: 10.1016/s0169-5347(97)01078-1. [DOI] [PubMed] [Google Scholar]
- 29.Charlesworth D, Morgan MT. Allocation of resources to sex functions in flowering plants. Philos Trans R Soc London B. 1991;332:91–102. [Google Scholar]
- 30.Pannell JR, Dorken ME, Pujol B, Berjano R. Gender variation and transitions between sexual systems in Mercurialis annua (Euphorbiaceae) Int J Plant Sci. 2008;169:129–139. [Google Scholar]
- 31.Harper JL. Population Biology of Plants. London: Academic; 1977. [Google Scholar]
- 32.Ibrahim KM, Nichols RA, Hewitt GM. Spatial patterns of genetic variation generated by different forms of dispersal during range expansion. Heredity. 1996;77:282–291. [Google Scholar]
- 33.Obbard DJ, Harris SA, Buggs RJA, Pannell JR. Hybridization, polyploidy, and the evolution of sexual systems in Mercurialis (Euphorbiaceae) Evolution. 2006;60:1801–1815. [PubMed] [Google Scholar]
- 34.Charlesworth D, Charlesworth B. A model for the evolution of dioecy and gynodioecy. Am Nat. 1978;112:975–997. [Google Scholar]
- 35.Eppley SM, Pannell JR. Density-dependent self-fertilization and male versus hermaphrodite siring success in an androdioecious plant. Evolution. 2007;61:2349–2359. doi: 10.1111/j.1558-5646.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- 36.Dorken ME, Pannell JR. Density-dependent regulation of the sex ratio in an annual plant. Am Nat. 2008;171:824–830. doi: 10.1086/587524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.