Abstract
Visceral adipose tissue (VAT) is an important risk factor for obesity-related metabolic disorders. Therefore, a reduction in VAT has become a key goal in obesity management. However, VAT is correlated with intrahepatic triglyceride (IHTG) content, so it is possible that IHTG, not VAT, is a better marker of metabolic disease. We determined the independent association of IHTG and VAT to metabolic function, by evaluating groups of obese subjects, who differed in IHTG content (high or normal) but matched on VAT volume or differed in VAT volume (high or low) but matched on IHTG content. Stable isotope tracer techniques and the euglycemic–hyperinsulinemic clamp procedure were used to assess insulin sensitivity and very-low-density lipoprotein–triglyceride (VLDL-TG) secretion rate. Tissue biopsies were obtained to evaluate cellular factors involved in ectopic triglyceride accumulation. Hepatic, adipose tissue and muscle insulin sensitivity were 41, 13, and 36% lower (P < 0.01), whereas VLDL-triglyceride secretion rate was almost double (P < 0.001), in subjects with higher than normal IHTG content, matched on VAT. No differences in insulin sensitivity or VLDL-TG secretion were observed between subjects with different VAT volumes, matched on IHTG content. Adipose tissue CD36 expression was lower (P < 0.05), whereas skeletal muscle CD36 expression was higher (P < 0.05), in subjects with higher than normal IHTG. These data demonstrate that IHTG, not VAT, is a better marker of the metabolic derangements associated with obesity. Furthermore, alterations in tissue fatty acid transport could be involved in the pathogenesis of ectopic triglyceride accumulation by redirecting plasma fatty acid uptake from adipose tissue toward other tissues.
Keywords: abdominal fat, insulin resistance, NAFLD, steatosis, VLDL
Visceral adipose tissue (VAT) is an important and independent predictor of metabolic risk factors for coronary heart disease, particularly diabetes and dyslipidemia (1, 2). Moreover, data from metabolic studies conducted on human subjects (3, 4) indicate that an increase in VAT is associated with impaired glucose tolerance, insulin resistance, and increased very-low-density lipoprotein–triglyceride (VLDL-TG) secretion. These observations and the unique anatomical location of visceral fat, which releases free fatty acids (FFA) and adipokines into the portal vein for direct transport to the liver, have led to the concept that VAT is responsible for many of the metabolic abnormalities associated with abdominal obesity (5, 6). Therefore, a reduction in visceral fat has become a key therapeutic goal in the management of obesity (6, 7).
Although VAT is associated with metabolic disease, a causal link between VAT and metabolic dysfunction has not been demonstrated in humans. Recently, it has become clear that VAT correlates directly with intrahepatic triglyceride (IHTG) content (8–10), and an increase in IHTG is associated with the same metabolic abnormalities linked to an increase in VAT (9–12). Therefore, it is possible that VAT itself is not harmful, but is simply an innocent bystander that tracks with IHTG.
The mechanism(s) responsible for the interrelationship among IHTG content, insulin resistance, and hypertriglyceridemia is not known, but could involve redirecting plasma FFA uptake and intracellular triglyceride production from adipose tissue depots to other tissues, such as liver and skeletal muscle, which can impair insulin signaling (13, 14) and stimulate VLDL-TG secretion (11). Therefore, it is possible that organ-specific alterations in CD36, which regulates tissue FFA uptake from plasma (15), are involved in the pathogenesis of ectopic triglyceride accumulation and metabolic disease.
The purpose of the present study was to test the hypotheses that (i) high IHTG content, not increased VAT volume, is the primary marker of metabolic abnormalities associated with obesity and (ii) high IHTG content is associated with alterations in adipose tissue and skeletal muscle CD36 gene expression and protein content that are consistent with redirecting plasma fatty acids away from adipose tissue and toward other metabolic organs. Both in vivo and cellular metabolic assessments were conducted in obese subjects, who were carefully matched on either IHTG content or VAT volume, to help separate the potential influence of IHTG and VAT on metabolic function. Stable isotope tracer infusions in conjunction with mathematical modeling were used to evaluate hepatic, skeletal muscle and adipose tissue insulin sensitivity, and VLDL-TG secretion rate, while adipose tissue and skeletal muscle biopsies were used to determine cellular CD36 gene expression and protein content.
Results
Body Composition.
Subjects in each group were matched on age, sex, body mass index (BMI), and percentage of body fat, but differed in either IHTG content or VAT volume (Table 1). Mean IHTG content in the high-IHTG groups was 5-fold greater than in the normal-IHTG groups, and mean VAT volume in the high-VAT group was 2-fold greater than in the low-VAT group (Table 1).
Table 1.
Subject characteristics in each study group
| Variable | Group 1: matched on VAT |
Group 2: matched on IHTG content |
||
|---|---|---|---|---|
| Normal IHTG | High IHTG | Low VAT | High VAT | |
| n (M/F) | 10 (3/7) | 10 (3/7) | 10 (2/8) | 10 (2/8) |
| Age (years) | 44 ± 2 | 36 ± 3 | 40 ± 4 | 46 ± 3 |
| Body mass index (kg/m2) | 36.8 ± 1.6 | 36.3 ± 1.5 | 34.5 ± 1.4 | 37.6 ± 1.4 |
| Body fat (% body weight) | 41 ± 2 | 39 ± 2 | 42 ± 2 | 41 ± 2 |
| IHTG (% liver volume) | 3.6 ± 0.5 | 25.3 ± 3.5* | 13.2 ± 3.5 | 13.2 ± 3.3 |
| VAT volume (cm3) | 1290 ± 238 | 1335 ± 178 | 766 ± 77 | 1946 ± 319† |
| SAAT (cm3) | 3162 ± 433 | 3954 ± 371 | 3885 ± 346 | 3646 ± 342 |
Values are means ± SEM. IHTG, intrahepatic triglyceride; VAT, visceral adipose tissue; SAAT, subcutaneous abdominal adipose tissue volume.
*Value is significantly different from the corresponding value in the normal IHTG group, P < 0.01.
†Value is significantly different from the corresponding value in the low VAT groups, P < 0.01.
Plasma Metabolic Variables.
Plasma insulin concentration was almost 2-fold greater and plasma adiponectin concentration was ≈50% lower in subjects with high IHTG content than in those with normal IHTG who were matched on VAT volume (Table 2). No significant differences in metabolic variables were detected between subjects with low or high VAT volume who were matched on IHTG content (Table 2).
Table 2.
Metabolic variables and basal substrate kinetics
| Variable | Group 1: matched on VAT |
Group 2: matched on IHTG |
||
|---|---|---|---|---|
| Normal IHTG | High IHTG | Low VAT | High VAT | |
| Glucose (mg/dL) | 95 ± 2 | 97 ± 3 | 94 ± 2 | 97 ± 3 |
| Insulin (mU/L) | 12 ± 1 | 21 ± 3* | 17 ± 4 | 15 ± 2 |
| Total adiponectin (μg/mL) | 7.85 ± 1.02 | 4.25 ± 0.32* | 7.48 ± 1.47 | 9.60 ± 2.78 |
| Free fatty acids (mmol/L) | 0.43 ± 0.04 | 0.45 ± 0.03 | 0.45 ± 0.05 | 0.54 ± 0.03 |
| VLDL-triglyceride (mmol/L) | 0.55 ± 0.13 | 0.69 ± 0.13 | 0.69 ± 0.23 | 0.64 ± 0.12 |
| Total cholesterol (mg/dL) | 156 ± 13 | 163 ± 6 | 162 ± 7 | 179 ± 9 |
| LDL-cholesterol (mg/dL) | 97 ± 8 | 91 ± 7 | 86 ± 5 | 94 ± 6 |
| HDL-cholesterol (mg/dL) | 46 ± 4 | 45 ± 5 | 54 ± 3 | 52 ± 7 |
| Glucose Ra (μmol·kg FFM−1·min−1) | 14.2 ± 0.4 | 13.6 ± 0.6 | 13.8 ± 0.6 | 14.1 ± 0.8 |
| Palmitate Ra (μmol·kg FM−1·min−1) | 3.5 ± 0.4 | 3.1 ± 0.3 | 3.2 ± 0.3 | 3.2 ± 0.3 |
| Palmitate Ra (μmol·kg FFM−1·min−1) | 2.2 ± 0.2 | 2.1 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 |
Values are means ± SEM. To convert the values for glucose to millimoles per liter, multiply by 0.05551. To convert the values for insulin to picomoles per liter, multiply by 6. To convert the values for cholesterol to millimoles per liter, multiply by 0.0259. To convert the values for triglycerides to millimoles per liter, multiply by 0.0113.
IHTG, intrahepatic triglyceride; VAT, visceral adipose tissue; VLDL, very-low-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Ra, rate of appearance; FFM, fat-free mass; FM, fat mass.
*Value is significantly different from the corresponding value in the normal IHTG group, P < 0.01.
Basal Glucose and Fatty Acid Kinetics.
Basal glucose and palmitate kinetics were not different between matched subjects within any of the 2 groups (Table 2).
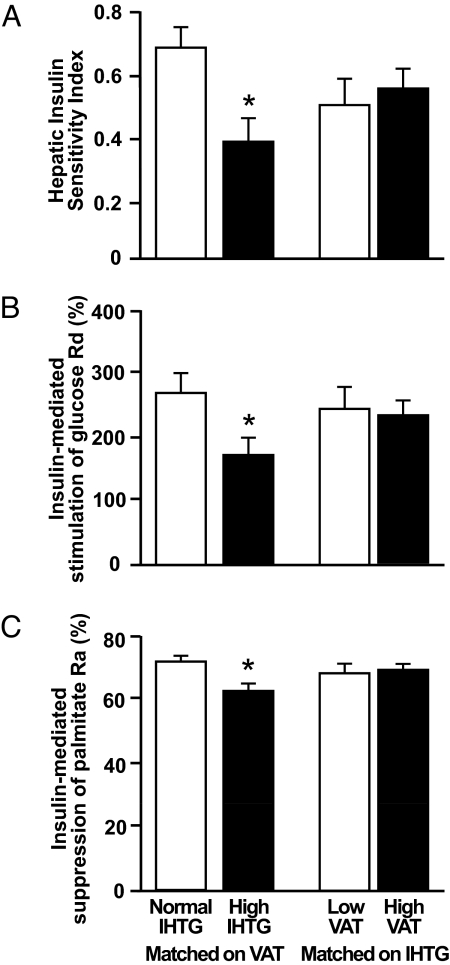
Insulin Sensitivity.
Hepatic (Fig. 1A), skeletal muscle (Fig. 1B), and adipose tissue (Fig. 1C) insulin sensitivity was lower in subjects with high than in those with normal IHTG content. However, no differences in insulin sensitivity measures were observed between subjects with low or high VAT volume, when matched on IHTG content (Fig. 1).
Fig. 1.
Hepatic (A), skeletal muscle (B), and adipose tissue (C) insulin sensitivity in subjects matched on visceral adipose tissue (VAT) volume with either normal or high intrahepatic triglyceride (IHTG) content and subjects matched on IHTG content who had either low or high VAT volume. AU, arbitrary units. Values are means ± SEM. *, value is significantly different from the corresponding value in the normal IHTG group, P < 0.05.
VLDL-TG Kinetics.
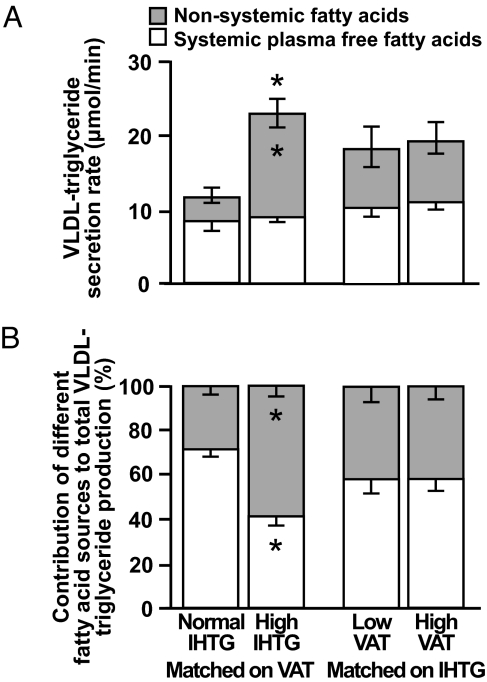
Hepatic VLDL-TG secretion rate was almost double in subjects with high than in those with normal IHTG content (23 ± 2 and 12 ± 1 μmol/min, respectively; P < 0.001), when matched on VAT volume (Fig. 2A). However, VLDL-TG secretion rate was not different in subjects with low or high VAT volume, when matched on IHTG content (Fig. 2A).
Fig. 2.
Very-low-density lipoprotein–triglyceride (VLDL-TG) secretion rate (A) and the relative contribution of systemic (generated primarily by lipolysis of s.c. adipose tissue triglycerides) and nonsystemic fatty acids (generated primarily by lipolysis of intrahepatic triglycerides) to VLDL-TG production (B) in subjects matched on visceral adipose tissue (VAT) volume with either normal or high intrahepatic triglyceride (IHTG) content and in subjects matched on IHTG content who had either low or high VAT volume. Values are means ± SEM. *, value is significantly different from corresponding value in the normal IHTG group, P < 0.001.
The relative contribution of nonsystemic fatty acids incorporated into newly secreted VLDL-TG (presumably derived from lipolysis of intrahepatic and i.p. triglyceride, hepatic lipolysis of circulating triglyceride, and de novo hepatic fatty acid synthesis) was much greater in subjects with high than with normal IHTG content (58 ± 4% and 28 ± 4%, respectively; P < 0.001) (Fig. 2B). The secretion rate of VLDL-TG composed of nonsystemic fatty acids was 4-fold greater in subjects with high than with normal IHTG content (14 ± 2 and 3 ± 1 μmol/min, respectively; P < 0.001) and accounted for their increase in total VLDL-TG secretion rate (Fig. 2A). In contrast, the absolute secretion rate of VLDL-TG composed of systemic plasma FFA was similar in both the high and the normal IHTG groups (8 ± 1 and 9 ± 1 μmol/min, respectively) (Fig. 2A). The relative contribution of fatty acids from different sources to total VLDL-TG production was not different between subjects with either low or high VAT volume, when matched on IHTG (Fig. 2B).
Predictors of Insulin Sensitivity and VLDL-TG Kinetics.
Multivariate linear regression analyses, which included age, sex, BMI, percentage of body fat, IHTG, VAT, and s.c. abdominal adipose tissue volumes as independent variables, indicated that IHTG content was the best predictor of insulin action in liver, skeletal muscle, and adipose tissue and of VLDL-TG secretion rate, accounting for 21, 45, 38, and 21%, respectively, of the variability (P ≤ 0.01 for each model). VAT was not a predictor of any of the dependent variables.
Muscle and Adipose Tissue Regulation of Fatty Acid Trafficking.
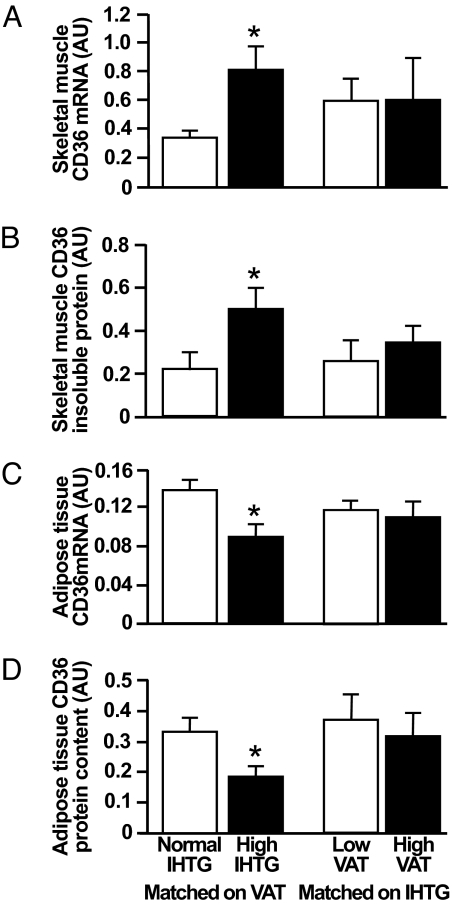
Skeletal muscle CD36 gene expression was almost 3-fold greater in subjects with high IHTG than in those with normal IHTG content, matched on VAT volume (0.82 ± 0.16 and 0.34 ± 0.05 arbitrary units; P < 0.05) (Fig. 3A). Total and detergent-soluble muscle CD36 protein content was not different between any of our subject groups, but detergent-insoluble CD36 content was 2-fold greater in subjects with high IHTG than in those with normal IHTG content (Fig. 3B). In contrast, abdominal s.c. adipose tissue CD36 gene expression was 35% lower in the high IHTG than in the normal IHTG group (0.09 ± 0.01 and 0.14 ± 0.01 AU; P < 0.05) (Fig. 3C), and adipose tissue CD36 protein content was 43% lower in subjects with high IHTG than in those with normal IHTG (0.19 ± 0.03 and 0.33 ± 0.05 AU; P < 0.05) (Fig. 3D). Plasma insulin concentration was inversely correlated with adipose tissue CD36 expression (r = 0.56, P < 0.01) and directly correlated with muscle insoluble CD36 content (r = 0.58, P = 0.02). No differences in CD36 adipose tissue gene expression and protein content and in muscle gene expression were detected between subjects with low or high VAT volume, when matched on IHTG content (Fig. 3).
Fig. 3.
Skeletal muscle (A) and s.c. abdominal adipose tissue (C) CD36 gene expression and skeletal muscle (B) and adipose tissue CD36 protein content (D) in subjects matched on visceral adipose tissue (VAT) volume with either normal or high intrahepatic triglyceride (IHTG) content and in subjects matched on IHTG content who had either low or high VAT volume. Values are means ± SEM. *, value is significantly different from corresponding value in the normal IHTG group, P < 0.05.
Discussion
Increased IHTG and VAT levels are important risk factors for the metabolic complications associated with obesity, particularly insulin resistance and dyslipidemia (1–4). However, it is not known whether IHTG or VAT is independently associated with metabolic risk because both are strongly correlated with each other (8–10). In the present study, we were able to determine the independent association of IHTG and VAT with insulin action and hepatic VLDL-TG metabolism by evaluating metabolic function in obese, nondiabetic, subjects who were separated into distinct groups on the basis of IHTG content and VAT volume. We found that insulin action in liver, skeletal muscle, and adipose tissue was impaired and hepatic VLDL-TG secretion rate was increased in subjects with high IHTG content, but not in those with high VAT volume if IHTG content was matched for. In addition, adipose tissue CD36 mRNA and protein content were decreased, whereas skeletal muscle CD36 mRNA and membrane-associated protein content were increased, in subjects with high IHTG content. These data demonstrate that IHTG content, not VAT volume, is a marker of obesity-related metabolic dysfunction. However, our data are not able to determine whether this association is a cause-and-effect relationship, and other mechanisms for insulin resistance have also been proposed (13, 14). In addition, insulin resistance could be a cause, rather than a consequence, of IHTG accumulation (16–18). Furthermore, the CD36 profile observed in different tissues and subject groups suggests that alterations in cellular fatty acid uptake could be involved in the pathogenesis of ectopic triglyceride accumulation in subjects with nonalcoholic fatty liver disease (NAFLD), by redirecting plasma FFA uptake from adipose tissue toward other tissues.
The results of the present study contradict the prevailing dogma that VAT has deleterious metabolic effects (5, 6). Data from both large epidemiological surveys and small physiological studies have shown an association between increased VAT and many of the metabolic complications of obesity, particularly insulin resistance, diabetes, and dyslipidemia (1–4). It has been hypothesized that the mechanism responsible for the adverse effects of VAT is related to the release of FFA and inflammatory adipokines from VAT directly into the portal vein, where they are delivered to the liver and affect glucose (6) and VLDL-TG (19) metabolism. However, a causal relationship between VAT and metabolic disease has never been established. In fact, only ≈20% of portal vein FFA delivered to the liver and 14% of systemic FFA delivered to skeletal muscle are derived from lipolysis of VAT in obese subjects (20, 21). In addition, in vivo secretion rates of most adipokines from VAT are probably not greater than the secretion rates from s.c. adipose tissue (22). Therefore, the majority of adipokines in the portal vein are derived from s.c. fat, which releases adipokines into the systemic circulation that enter the portal vein through the splanchnic bed. We were able to separate the effect of VAT from the potentially confounding influences of total and regional adiposity and ectopic fat distribution on metabolic function, by matching obese subjects with low and high VAT volume on BMI, percentage of body fat, abdominal s.c. adipose tissue, and IHTG content. In subjects matched for IHTG content, a 2-fold difference in VAT volume between low and high VAT groups was not associated with detectable differences in insulin sensitivity or VLDL-TG secretion rate. In contrast, subjects with high IHTG content had impaired insulin action in liver, adipose tissue, and skeletal muscle and increased hepatic VLDL-TG secretion rate, independently of VAT. Moreover, IHTG content was the best independent predictor of increased VLDL-TG secretion rate and insulin resistance in all tissues, whereas VAT did not help explain any of the metabolic outcomes. These data demonstrate that VAT is not an important contributor to the metabolic complications associated with obesity and suggest that the reported association between VAT and metabolic disease is because of the direct correlation between VAT volume and IHTG content.
The mechanism responsible for excessive triglyceride accumulation in nonadipose tissues (i.e., ectopic fat) is not known. This issue has important physiological and clinical implications because of the association between ectopic fat and metabolic dysfunction (23). It has been hypothesized that ectopic fat accumulation is caused by an inadequate capacity of adipose tissue to store triglyceride (17, 24). However, it is unlikely that the small amount of triglyceride that accumulates in “ectopic” organs, such as liver and skeletal muscle, cannot be accommodated by the large adipose tissue mass in obese persons. For example, the amount of IHTG in our subjects with high (25%) IHTG content represented ≈1% of the total triglyceride present in adipose tissue; on average, these subjects had ≈0.4 kg of triglyceride in the liver and ≈40 kg of triglyceride in adipose tissue. Our data suggest a more plausible mechanism for ectopic fat accumulation, which involves alterations in the regulation of FFA uptake from plasma by CD36.
The importance of CD36 in FFA transport and ectopic triglyceride accumulation has been demonstrated by observations in human subjects indicating that increased skeletal muscle plasmalemma CD36 content is associated with increased muscle FFA uptake and intramyocellular triglyceride accumulation (25). We found that adipose tissue CD36 expression and protein content were lower, while skeletal muscle CD36 expression and detergent-insoluble protein content were higher in subjects who had high IHTG content than in those who had normal IHTG content. In skeletal muscle, CD36 moves between intracellular stores and the cell membrane (26). Cell membrane CD36 is mostly localized to lipid rafts/caveolae, which are detergent-resistant membrane domains that are important in CD36-facilitated fatty acid uptake (27, 28). Therefore, CD36 presence in detergent-insoluble cell components is an indicator of membrane-associated CD36 available for fatty acid uptake. The mechanism responsible for differences in tissue CD36 content is not known, but could be related to circulating insulin, because plasma insulin concentration was inversely correlated with adipose tissue CD36 expression and directly correlated with muscle insoluble CD36 content in our subjects.
Although we did not determine CD36 gene expression or protein content in the liver, data from other studies suggest that hepatic CD36 expression was likely increased in our subjects with high IHTG content, because hepatic CD36 expression is directly correlated with liver fat in human subjects (29). Our data suggest that alterations in cellular fatty acid transport are involved in the pathogenesis of ectopic fat distribution by diverting the accumulation of triglyceride away from adipose tissue and toward other key metabolic organs.
Alterations in tissue CD36 activity can also help explain the close link between ectopic fat accumulation and tissue insulin resistance (9, 14). The pattern of CD36 expression and protein content in adipose tissue and skeletal muscle in our subjects with high IHTG content indicate an increase in muscle, and presumably liver, uptake of plasma FFA. Intramyocellular and intrahepatocellular fatty acids that are not oxidized or exported as VLDL-TG are esterified to triglyceride or metabolized to intermediates that can impair insulin signaling (13, 14). The potential importance of CD36 in regulating insulin sensitivity is supported by studies conducted in animal models and in obese and diabetic human subjects that found increased plasmalemma skeletal muscle CD36 was associated with increased FFA uptake and insulin resistance (25, 30, 31) (even though total muscle CD36 protein was not different among obese, diabetic, and lean subjects) (25, 32, 33), whereas pharmacological stimulation of adipose tissue CD36 expression and FFA uptake by peroxisome proliferator-activated receptor-γ agonist therapy in patients with type 2 diabetes (34) is associated with a reduction in IHTG content and improvements in insulin sensitivity (17).
A decrease in adipose tissue CD36 gene expression and protein content could also have contributed to steatosis and insulin resistance by affecting adiponectin metabolism. Adiponectin is the most abundant secretory protein produced by adipose tissue and has important beneficial metabolic effects. Adiponectin administration decreases IHTG (35), increases hepatic insulin sensitivity (36), and reverses obesity-related skeletal muscle insulin resistance (37). Adipose tissue adiponectin gene expression is decreased in CD36-null mice, demonstrating that adipose tissue CD36 is directly involved in the regulation of adiponectin production (38). Therefore, it is possible that decreased CD36 activity in adipose tissue in our subjects with high IHTG content led to a decrease in adiponectin secretion and low plasma adiponectin concentration that we observed in these subjects.
The data from the present study support the notion that IHTG could be directly involved in the pathogenesis of dyslipidemia associated with obesity (11). Most triglycerides in plasma are a component of circulating VLDL. Therefore, VLDL-TG metabolism is involved in the regulation of plasma triglyceride concentrations, and increased VLDL-TG secretion rate can increase plasma triglyceride concentration. The rate of hepatic VLDL-TG secretion was 2-fold greater in subjects with high IHTG content than in those with normal IHTG, independent of VAT volume. Moreover, the increase in VLDL-TG secretion rate was entirely because of an increase in the contribution of nonsystemic fatty acids. Our data suggest that the increase in VLDL-TG secretion rate in obese subjects who have NAFLD is primarily caused by the availability of nonsystemic fatty acids derived from lipolysis of IHTG, not VAT, because VAT volume was the same in both groups and de novo lipogenesis accounts for up to 20% of the fatty acids secreted in VLDL-TG in subjects with NAFLD (39, 40). Therefore, it is possible that excessive IHTG is not only a marker of metabolic dysfunction, but also contributes to the increase in VLDL-TG secretion rate and plasma triglyceride concentration observed in obese persons with NAFLD.
In summary, increased IHTG is an independent indicator of multiorgan insulin resistance and increased hepatic secretion of VLDL-TG. In addition, fatty acids released from lipolysis of IHTG might stimulate hepatic VLDL-TG production, demonstrating that IHTG in itself could be directly involved in the pathogenesis of dyslipidemia associated with NAFLD (11). Our data refute the notion that increased VAT causes metabolic abnormalities associated with obesity and suggest the commonly observed relationship between increased VAT and metabolic disease (1–4) is because of the correlation between VAT and IHTG (8–10). Alterations in CD36 content in adipose tissue, muscle, and presumably liver might contribute to the association between ectopic fat distribution and obesity-related metabolic disease, by redirecting fatty acid uptake from adipose tissue toward other metabolic organs. These data underscore the importance of increased IHTG content as a marker of the metabolic complications associated with obesity.
Materials and Methods
Subjects.
A total of 42 obese men and women were screened for this study. We found that 31 subjects from this group (74% of the screened population; mean BMI 35.7 ± 0.8 kg/m2) could be separately matched on visceral or liver fat and therefore participated in this study. Subjects were distributed among 2 groups on the basis of IHTG content and VAT volume to help separate the interrelationships between IHTG, VAT, and metabolic function; 9 subjects were assigned to more than 1 group to maximize appropriate matching within groups. Group 1 subjects (n = 20) were matched on VAT volume and had either high (>10% of liver volume) (n = 10) or normal (≤5.5% of liver volume) (n = 10) IHTG content (Table 1) (41). The range in VAT volume was similar in both the normal (VAT volume: 689–3,088 cm3) and the high (VAT volume: 638–2,702 cm3) IHTG groups. Each subject with normal IHTG and a given VAT volume was matched with a subject from the high IHTG group on VAT (within ≈20% of VAT volume of the normal IHTG group). Group 2 subjects (n = 20) were matched on IHTG content and had either low (n = 10) or high (n = 10) VAT volume (Table 1). Subjects were separated into low and high VAT volume groups by using the median value of all subjects (1,100 cm3) as the cut point for low and high VAT volumes. Subjects within groups were matched on age, sex, BMI, and percentage of body fat. We did not have knowledge of any outcome measures when the matches were performed.
All subjects completed a comprehensive medical evaluation, which included a 2-h oral glucose tolerance test. No subject had any history or evidence of liver disease other than NAFLD, took medications that can affect metabolism or cause hepatic abnormalities, consumed >20 g/day of alcohol, or had diabetes. Subjects gave their written informed consent before participating in this study, which was approved by the Human Research Protection Office of Washington University School of Medicine, St. Louis, MO.
Body Composition Analyses.
Body fat mass (FM) and fat-free mass (FFM) were determined by using dual-energy x-ray absorptiometry (Delphi-W densitometer, Hologic). Intraabdominal and abdominal s.c. adipose tissue volumes were quantified by magnetic resonance imaging (Siemens; ANALYZE 7.0 software, Mayo Foundation) (9) and IHTG content was measured by using proton magnetic resonance spectroscopy (Siemens) as we have previously described (42).
Hyperinsulinemic–Euglycemic Clamp Procedure.
Subjects were admitted to the Intensive Research Unit at Washington University School of Medicine on the evening before the clamp procedure. At 0500 hours the following morning, after subjects fasted for 12 h overnight, a 2-stage hyperinsulinemic–euglycemic clamp procedure was started and continued for 9 h. Insulin was infused at a rate of 20 mU·m−2 body-surface area (BSA)·min−1 during stage 1 (3–6 h) and at a rate of 50 mU·m−2 BSA·min−1 during stage 2 (6–9 h) of the clamp procedure (9, 43). [6,6-2H2]glucose, [2,2-2H2]palmitate, and 20% dextrose enriched to 2.5% with [6,6-2H2]glucose were infused to determine hepatic, skeletal muscle, and adipose tissue insulin sensitivity. Tissue samples were obtained from s.c. abdominal adipose tissue and from the quadriceps femoris muscle 60 min after starting the glucose tracer infusion during the basal stage. A detailed description of the infusion protocol and of collection of tissues and blood samples is available in supporting information (SI) Materials and Methods.
VLDL-TG Kinetics Study.
One week after the hyperinsulinemic–euglycemic clamp procedure, subjects were readmitted to the Intensive Research Unit on the evening before the VLDL kinetics study. At 0600 hours the following morning, after subjects fasted for 12 h overnight, a bolus of [1,1,2,3,3-2H5]glycerol was injected, and a constant infusion of 2,2-2H2]palmitate was started and maintained for 12 h. Blood samples were obtained at regular time points throughout the study to determine VLDL-TG kinetics (a description of the procedure is available in SI Materials and Methods).
Analyses of Samples.
Plasma glucose concentration was measured by using an automated glucose analyzer (Yellow Spring Instruments). Plasma insulin concentration was measured by using a chemiluminescent immunoassay method (Immulite 1000, Diagnostic Products). Plasma adiponectin concentration was measured by using an ELISA kit (Linco Research). Plasma FFA concentration was determined by using gas chromatography (44). Plasma VLDL was prepared as previously described (45, 46) and VLDL-TG concentration was determined by using an enzymatic spectrophotometric kit (Sigma). Plasma glucose, palmitate, and glycerol tracer-to-tracee ratios (TTRs) in plasma and in VLDL-TG were determined by using electron impact ionization gas chromatography–mass spectrometry, as previously described (45–47).
CD36 gene expression was determined by using quantitative real-time PCR. Total RNA was isolated from muscle and adipose tissues by using either RNAzol B (muscle, Tel-Test) or TRIzol (adipose, Invitrogen). RNA was quantified by using spectrophotometry (NanoDrop 1000) and cDNA was synthesized using a Taqman Reverse Transcription Kit (Applied Biosystems). cDNA samples were then amplified by using SYBR Green PCR Master Mix (Applied Biosystems) on the ABI 7500 Real-Time PCR System (Applied Biosystems). Results were analyzed by comparing the threshold crossing (Ct) of each sample after normalization to the housekeeping 36B4 gene (ΔCt). The changes in the threshold crossing (ΔCt) were used to calculate the relative levels of each mRNA compared to the control gene from the various samples, using the formula 2−ΔCt. Primer pairs used for transcript detection were CD36, (forward) GAGACCTGCTTATCCAGAAGACAAT and (reverse) TTCTGTGCCTGTTTTAACCCAATTTTT, and 36B4, (forward) GTGATGTGCAGCTGATCAAGACT and (reverse) GATGACCAGCCCAAAGGAGA.
Total adipose tissue CD36 protein and muscle detergent-soluble and detergent-insoluble CD36 protein (generated from muscle lysates containing 1% Triton) were determined by Western blot (48) and protein detection by infrared imaging technology (LI-COR Biosciences). β-Αctin was used to normalize CD36 protein intensity levels.
Calculations.
Glucose and palmitate kinetics.
Isotopic steady-state conditions were achieved during the final 30 min of the basal period and stages 1 and 2 of the clamp procedure, so Steele's equation for steady-state conditions was used to calculate substrate kinetics (49). It was assumed that glucose rate of disappearance (Rd) from plasma was equal to glucose rate of appearance (Ra) during basal conditions; during the clamp procedure glucose Rd was assumed to be equal to the sum of endogenous glucose Ra and the rate of infused glucose. Palmitate kinetics were expressed in micromoles per kilogram of FM per minute to provide an index of adipose tissue lipolytic activity in relation to the amount of endogenous fat stores and in micromoles per kilogram of FFM per minute to provide an index of FFA availability for lean tissues that use fatty acids for fuel.
VLDL-TG kinetics.
The fractional turnover rate (FTR) of VLDL-TG (in pools per hour) and hepatic VLDL-TG secretion rate into plasma (in micromoles per minute) were calculated by fitting the glycerol TTR in plasma and VLDL-TG to a multicompartmental model as previously described (47, 50). The proportions of fatty acids within VLDL-TG derived from systemic plasma FFA (generated by lipolysis of s.c. adipose tissue triglyceride) and nonsystemic fatty acids (generated by lipolysis of intrahepatic and i.p. triglyceride, hepatic lipolysis of circulating triglyceride, and/or de novo hepatic fatty acid synthesis) were calculated by accounting for isotopic dilution between plasma and VLDL-TG palmitate by using a multicompartmental model (11).
Insulin sensitivity.
Hepatic insulin sensitivity was determined by calculating the reciprocal of the Hepatic Insulin Resistance Index (defined as the product of basal endogenous glucose production rate, in micromoles per kilogram FFM per minute, and fasting plasma insulin concentration in milliunits per liter) (51). Adipose tissue and skeletal muscle insulin sensitivity were assessed as the relative decrease in palmitate Ra during stage 1 and the relative increase in glucose Rd during stage 2 of the euglycemic–hyperinsulinemic clamp procedure, respectively (9).
Statistical Analyses.
All data sets were normally distributed according to the Kolmogorov–Smirnov test, so comparisons between subjects within each grouping (i.e., between subjects who had the same VAT volume but either normal or high IHTG content and subjects who had the same IHTG content but different VAT volume) were performed by using parametric procedures. Levene's test was used to assess the equality of group variances on each dependent variable, and Student's t test for unpaired samples was used to compare results between groups. Multiple stepwise linear regression analysis (with age, sex, BMI, percentage of body fat, IHTG content, VAT, and s.c. abdominal adipose tissue volumes as independent variables) was performed to identify significant independent predictors of metabolic outcomes. Results are presented as means ± SEM. A P-value ≤0.05 was considered statistically significant. Analyses were performed by using SPSS 16.0.
Supplementary Material
Acknowledgments.
The authors thank Dr. Ken Schechtman for assistance in statistical analyses; Jennifer McCrea, Freida Custodio, and Jennifer Shew for their technical assistance; the staff of the Clinical Research Unit for their help in performing the studies; and the study subjects for their participation. This work was supported by National Institutes of Health grants DK 37948, DK60022, DK33301, DK 56341 (Clinical Nutrition Research Unit), UL1 RR024992 (Clinical and Translational Science Award), and RR-00954 (Biomedical Mass Spectrometry Resource).
Footnotes
Conflict of interest: The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904944106/DCSupplemental.
References
- 1.Gastaldelli A, et al. Metabolic effects of visceral fat accumulation in type 2 diabetes. J Clin Endocrinol Metab. 2002;87:5098–5103. doi: 10.1210/jc.2002-020696. [DOI] [PubMed] [Google Scholar]
- 2.Vega GL, et al. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 3.Adiels M, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 4.Despres JP. The insulin resistance-dyslipidemic syndrome of visceral obesity: Effect on patients' risk. Obes Res. 1998;6(Suppl 1):8S–17S. doi: 10.1002/j.1550-8528.1998.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 5.Bergman RN, et al. Why visceral fat is bad: Mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(Suppl 1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 6.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25:431–438. doi: 10.2337/diacare.25.3.431. [DOI] [PubMed] [Google Scholar]
- 8.Jakobsen MU, Berentzen T, Sorensen TI, Overvad K. Abdominal obesity and fatty liver. Epidemiol Rev. 2007;29:77–87. doi: 10.1093/epirev/mxm002. [DOI] [PubMed] [Google Scholar]
- 9.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang JH, et al. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab. 2007;293:E1663–E1669. doi: 10.1152/ajpendo.00590.2006. [DOI] [PubMed] [Google Scholar]
- 11.Fabbrini E, et al. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchesini G, et al. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 13.Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am. 2008;37:635–646. viii-ix. doi: 10.1016/j.ecl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res. 2009;50(Suppl):S86–S90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ota T, et al. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology. 2007;132:282–293. doi: 10.1053/j.gastro.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Belfort R, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 18.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein S. The case of visceral fat: Argument for the defense. J Clin Invest. 2004;113:1530–1532. doi: 10.1172/JCI22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 23.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S12–S21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 24.Kim JY, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonen A, et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004;18:1144–1146. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- 26.Koonen DP, Glatz JF, Bonen A, Luiken JJ. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim Biophys Acta. 2005;1736:163–180. doi: 10.1016/j.bbalip.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Ehehalt R, et al. Uptake of long chain fatty acids is regulated by dynamic interaction of FAT/CD36 with cholesterol/sphingolipid enriched microdomains (lipid rafts) BMC Cell Biol. 2008;9:45. doi: 10.1186/1471-2121-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pohl J, et al. Long-chain fatty acid uptake into adipocytes depends on lipid raft function. Biochemistry. 2004;43:4179–4187. doi: 10.1021/bi035743m. [DOI] [PubMed] [Google Scholar]
- 29.Greco D, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–G1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 30.Luiken JJ, et al. Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem. 2001;276:40567–40573. doi: 10.1074/jbc.M100052200. [DOI] [PubMed] [Google Scholar]
- 31.Luiken JJ, et al. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes. 2002;51:3113–3119. doi: 10.2337/diabetes.51.10.3113. [DOI] [PubMed] [Google Scholar]
- 32.Pelsers MM, et al. Skeletal muscle fatty acid transporter protein expression in type 2 diabetes patients compared with overweight, sedentary men and age-matched, endurance-trained cyclists. Acta Physiol (Oxf) 2007;190:209–219. doi: 10.1111/j.1748-1716.2007.01698.x. [DOI] [PubMed] [Google Scholar]
- 33.Bruce CR, et al. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab. 2003;88:5444–5451. doi: 10.1210/jc.2003-030791. [DOI] [PubMed] [Google Scholar]
- 34.Kolak M, et al. Effects of chronic rosiglitazone therapy on gene expression in human adipose tissue in vivo in patients with type 2 diabetes. J Clin Endocrinol Metab. 2007;92:720–724. doi: 10.1210/jc.2006-1465. [DOI] [PubMed] [Google Scholar]
- 35.Xu A, et al. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi T, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 38.Hajri T, et al. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes. 2007;56:1872–1880. doi: 10.2337/db06-1699. [DOI] [PubMed] [Google Scholar]
- 39.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non-esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 40.Donnelly KL, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szczepaniak LS, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 42.Frimel TN, Deivanayagam S, Bashir A, O'Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 43.Klein S, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 44.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 45.Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab. 2003;284:E549–E556. doi: 10.1152/ajpendo.00379.2002. [DOI] [PubMed] [Google Scholar]
- 46.Magkos F, Patterson BW, Mittendorfer B. Reproducibility of stable isotope-labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. J Lipid Res. 2007;48:1204–1211. doi: 10.1194/jlr.D600048-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res. 2002;43:223–233. [PubMed] [Google Scholar]
- 48.Smith J, Su X, El-Maghrabi R, Stahl PD, Abumrad NA. Opposite regulation of CD36 ubiquitination by fatty acids and insulin: Effects on fatty acid uptake. J Biol Chem. 2008;283:13578–13585. doi: 10.1074/jbc.M800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187:15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- 50.Magkos F, Wright DC, Patterson BW, Mohammed BS, Mittendorfer B. Lipid metabolism response to a single, prolonged bout of endurance exercise in healthy young men. Am J Physiol Endocrinol Metab. 2006;290:E355–3E62. doi: 10.1152/ajpendo.00259.2005. [DOI] [PubMed] [Google Scholar]
- 51.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.