Abstract
Signal peptides of membrane proteins are cleaved by endoplasmic reticulum-resident signal peptidase, and thus, are not present on mature membrane proteins. Here, we report that, contrary to the paradigm, the signal peptide of ruminant CD18, the β-subunit of β2-integrins, is not cleaved. Intriguingly, the intact signal peptide of CD18 is responsible for the susceptibility of ruminant leukocytes to Mannheimia (Pasteurella) haemolytica leukotoxin (Lkt). Inhibition of Lkt-induced cytolysis of ruminant leukocytes by CD18 peptide analogs revealed that the Lkt-binding site is formed by amino acids 5–17 of CD18, which, surprisingly, comprise most of the signal sequence. Flow cytometric analysis of ruminant leukocytes indicated the presence of the signal peptide on mature CD18 molecules expressed on the cell surface. Analysis of transfectants expressing CD18 containing the FLAG epitope at the putative cleavage site confirmed that the signal peptide of bovine CD18 is not cleaved. Analysis of the signal sequence of CD18 of eight ruminants and five nonruminants revealed that the signal sequence of CD18 of ruminants contains “cleavage-inhibiting” Q, whereas that of nonruminants contains “cleavage-conducive” G at position −5 relative to the cleavage site. Site-directed mutagenesis of Q to G at position −5 of the signal peptide of bovine CD18 resulted in the cleavage of the signal peptide and abrogation of cytolysis of transfectants expressing bovine CD18 carrying the Q(−5)G mutation. We propose that engineering cattle and other ruminants to contain this mutation would provide a novel technology to render them less susceptible to pneumonic pasteurellosis and concomitant economic losses.
Keywords: receptor, cloning, site-directed mutagenesis
The nascent membrane protein contains a signal sequence that directs the protein/ribosome to the endoplasmic reticulum (ER) membrane (1–3). The signal peptide binds to the signal-recognition particle (SRP), which in turn, binds to the SRP receptor on the ER membrane and helps in the translocation of the protein into the lumen of the ER. The signal peptide is cleaved from the protein by the ER-resident signal peptidase while it is still growing on the ribosome. Thus, the signal peptide is not present on the mature protein that reaches the plasma membrane after posttranslational modifications. Our studies aimed at mapping the Mannheimia (Pasteurella) haemolytica leukotoxin (Lkt) binding site on its receptor CD18 have led to the unexpected finding that the signal peptide of ruminant CD18 remains intact on the mature CD18 molecule on the leukocytes of ruminants and renders these cells susceptible to cytolysis by Lkt.
M. haemolytica is the most important bacterial pathogen of respiratory disease in cattle and other domestic and wild ruminants (4–7). This disease, commonly known as pneumonic pasteurellosis or shipping fever in cattle, has been estimated to cost >$1 billion to the cattle industry of the United States alone (8). M. haemolytica is a Gram-negative coccobacillus commonly found as a commensal in the tonsillar crypts and upper respiratory tract of healthy ruminants (9). In conjunction with active viral infection and stress factors, the organism multiplies rapidly, reaches the lungs, and causes an acute fibrinonecrotic pleuropneumonia (10).
This organism produces several virulence factors, which include the capsule, outer membrane proteins, adhesins, neuraminidase, lipopolysaccharide, and Lkt (11). Based on the observation that Lkt-deletion mutants of M. haemolytica cause reduced mortality and much milder lung lesions than the WT organisms, Lkt is accepted as the most important virulence factor of this organism (12–15). M. haemolytica Lkt belongs to the family of Gram-negative bacterial exotoxins, referred to as repeats in toxins (RTX). It shares extensive homology with the exotoxins produced by Escherichia coli (16), Actinobacillus pleuropneumoniae (17), and Actinobacillus actinomycetemcomitans (18). However, cytolytic activity of M. haemolytica Lkt is specific for ruminant leukocytes (19, 20). Although all subsets of leukocytes are susceptible to the cytolytic effects of Lkt, polymorphonuclear leukocytes (PMNs) are the most susceptible subset (21). PMN depletion mitigates the lung injury in calves caused by M. haemolytica infection (22). Therefore, Lkt-induced PMN lysis and degranulation are the primary causes of acute inflammation and lung injury characteristic of pneumonic pasteurellosis (22–24).
Previously, we and others identified β2-integrins as the receptor for Lkt on bovine leukocytes (25–28). The β2-integrins are leukocyte-specific integrins that mediate several functions of leukocytes, including homing into areas of inflammation, phagocytosis, antigen presentation, and cytotoxicity. They have a common β-subunit, CD18, which associates with four α-subunits, resulting in four different integrins CD11a/CD18 (LFA-1), CD11b/CD18 (Mac-1), CD11c/CD18 (CR4), and CD11d/CD18 (29, 30). We have previously demonstrated that CD18 is necessary and sufficient to mediate Lkt-induced cytolysis of bovine and ovine leukocytes (31–34) and mapped the Lkt binding site on bovine CD18 to lie between amino acids 1–291 (35). The next logical step was to identify the precise binding site of Lkt on CD18, which formed the objective of this study. Here, we report that the Lkt-binding site is formed by amino acids 5–17 of CD18, which surprisingly, comprise most of the amino acids of the signal peptide that remains intact on mature CD18 molecules on the cell surface. More importantly, a single amino acid substitution in CD18 results in the cleavage of the signal peptide, which abrogates Lkt-induced cytolysis of the target cells.
Results
Peptide Analogs of Bovine CD18 Inhibit Lkt-Induced Cytolysis of Target Cells.
Binding of a ligand to its receptor can be inhibited by synthetic peptides representing the amino acids involved in the binding (36, 37). In this study, we used bovine CD18 peptide analogs to identify the binding site of M. haemolytica Lkt on bovine CD18. Inhibition of Lkt-induced cytolysis of target cells by a nested set of 20-mer peptides spanning amino acids 1–291 of bovine CD18 was used for this purpose. Because the most pronounced difference in the amino acid sequence of CD18 of ruminants and nonruminants was observed in the N-terminal region (amino acids 1–29), the point of origin of the first five peptides was staggered by five amino acids, whereas the rest of the peptides were staggered by 15 amino acids. All of the peptides were tested for their ability to inhibit Lkt-induced cytolysis of target cells by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) dye-reduction cytotoxicity assay. As target cells, we used BL-3 cells (bovine lymphoma cell line) in these experiments, because they are readily available. The findings with BL-3 cells were subsequently confirmed with PMNs of cattle and other ruminants. Two peptides, P1 and P5, strongly inhibited Lkt-induced cytolysis of BL-3 cells (Fig. S1A). Comparison of the concentration of P1 and P5, which causes 50% inhibition of Lkt-induced cytolysis of BL-3 cells, revealed the potency of P5 to be higher than that of P1 (1.7 vs. 17 μg/mL; Fig. S1B). Two other peptides containing the same amino acids as P5, but in a randomly scrambled sequence, failed to inhibit Lkt-induced cytolysis of BL-3 cells, indicating that the inhibition of Lkt-induced cytolysis by P5 was specific (Fig. S1C).
CD18 Peptide Analog Consisting of Amino Acids 5–17 Is the Minimal Peptide That Inhibits Lkt-Induced Cytolysis of Target Cells.
To identify the minimal peptide that would inhibit Lkt-induced cytolysis of target cells as efficiently as P5, peptides with N- and C-terminal truncations were used in the cytotoxicity assay. The percentage inhibition given by peptide P5 was significantly higher than that given by the other peptides with N-terminal truncation, indicating that amino acid 5 is the N-terminal amino acid of the minimal peptide (Fig. S2A). Subsequently, another set of peptides was synthesized with C-terminal truncation by dropping one amino acid at a time while keeping the N terminus constant at amino acid 5. When the amino acids from the C terminus dropped to the 16th, the percentage inhibition decreased, indicating that amino acid 17 is the C-terminal amino acid of the minimal peptide (Fig. S2B). Thus, the peptide composed of amino acids 5–17 is the minimal peptide analog of bovine CD18 that effectively inhibits Lkt-induced cytolysis of BL-3 cells. The peptide P17 (amino acids 5–17) effectively inhibited the Lkt-induced cytolysis of PMNs of cattle, goats, domestic sheep, wild sheep, deer, and bison as well (Fig. S3), confirming the finding with BL-3 cells that amino acids 5–17 is the minimal peptide analog of bovine CD18 that effectively inhibits Lkt-induced cytolysis of target cells. These results indicated that the domain encompassed by amino acids 5–17 of CD18 is the minimal amino acid sequence of CD18 that could serve as the receptor for Lkt on ruminant leukocytes.
Amino Acid Sequence of Bovine CD18 Peptide Analog That Inhibits Lkt-Induced Cytolysis Is from the Signal Sequence of CD18.
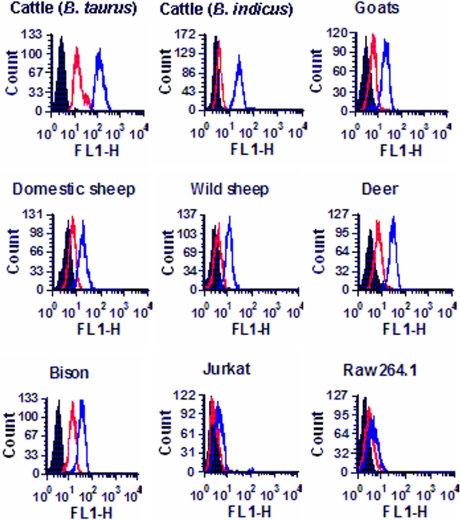
Amino acids 5–17 constitute 13 of the 22 amino acids of the predicted signal sequence of CD18. Therefore, the next logical step was to determine whether the signal peptide remains intact on the mature cell surface CD18 of ruminant leukocytes. Flow cytometric analysis with a chicken antiserum against bovine CD18 signal peptide revealed that the signal peptide indeed remains intact on the CD18 molecules on the PMNs of ruminants (cattle, goats, domestic sheep, wild sheep, deer, and bison) (Fig. 1). The mouse macrophage cell-line RAW264.A and the human T cell leukemia cell line Jurkat were not stained by the antisignal peptide serum, indicating the absence of signal peptide on the CD18 of these cells of nonruminant origin.
Fig. 1.
Antisignal peptide serum binds to membrane CD18 of PMNs of all ruminants tested. PMNs were tested by flow cytometry for binding of a chicken antiserum (1/1,000 dilution) developed against a synthetic peptide spanning amino acids 5–19 of the signal peptide of bovine CD18. The different panels show the binding of the antisignal peptide serum to the PMNs of Bos taurus cattle, Bos indicus cattle, goats, domestic sheep, wild sheep, deer, bison, and the cell lines Jurkat (human T cell leukemia cell line) and RAW264.A (mouse macrophage cell line). Black histogram, cells treated with the secondary Ab only; red histogram, cells treated with preimmune chicken serum; blue histogram, cells treated with antiserum against the signal peptide of bovine CD18. The data (10,000 events) were acquired for forward scatter (FSC) and side scatter (SSC) by using the following settings: FSC, voltage E00; and SSC, voltage 260. Results of one representative experiment of three are shown.
Signal Peptide of Ruminant CD18 Is Not Cleaved from the Mature Protein.
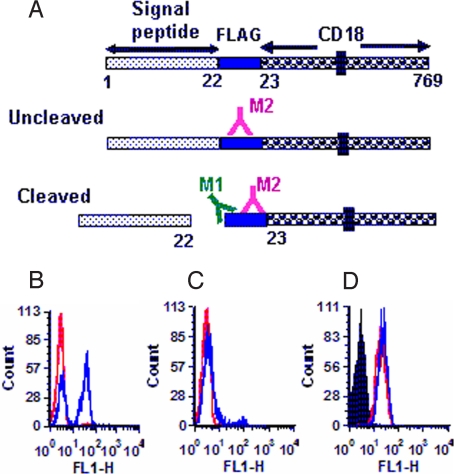
To confirm the fact that the signal peptide of bovine CD18 is not cleaved, the minigene encoding the FLAG epitope (DYKDDDDK) (38) was introduced at the putative signal peptide cleavage site (between amino acids 22 and 23). FLAG-tagged CD18 was transfected into the murine mastocytoma cell line, P815, which is nonsusceptible to M. haemolytica Lkt-induced cytolysis. A transfectant, designated BFL, stably expressing bovine CD18 containing the FLAG epitope between amino acids 22 and 23 was tested with two mAbs, M1 and M2, specific for the FLAG epitope. M1 recognizes the free N-terminal end of FLAG, whereas M2 recognizes FLAG irrespective of its sequence context (Fig. 2A) (38). The mAb M2 bound to the transfectant carrying FLAG-tagged CD18 (BFL), confirming the expression of FLAG epitope on the cell surface (Fig. 2B). The mAb M1 did not bind to the transfectants expressing FLAG-bearing CD18, indicating the lack of cleavage of the signal peptide on the mature membrane CD18 (Fig. 2C). The lack of binding of mAbs M1 and M2 to the transfectant 2B2 (expressing bovine CD18 without the FLAG epitope) (31) indicated the specificity of these two mAbs for the FLAG epitope (Fig. 2 B and C). The level of expression of CD18 on the transfectant BFL was comparable with that of the transfectant 2B2, confirming that the lack of binding of M1 to BFL was not caused by low expression of CD18 on BFL (Fig. 2D).
Fig. 2.
The signal peptide of bovine CD18 is not cleaved. (A) Introduction of the FLAG epitope at the signal peptide cleavage site and its detection by two mAbs. The FLAG epitope introduced at the putative signal peptide cleavage site can be detected by two anti-FLAG epitope mAbs M1 and M2. M2 recognizes the FLAG epitope irrespective of its sequence context, whereas M1 recognizes only the free N-terminal end of the FLAG epitope (if cleavage occurs). (B and C) Flow cytometric analysis of expression of the FLAG epitope. The transfectants 2B2 and BFL were tested for the expression of the FLAG epitope with the mAbs M2 (B) and M1 (C). The red and the blue histograms represent 2B2 and BFL, respectively. (D) Flow cytometric analysis of bovine CD18 expression. The untransfected parent cells P815 (black histogram), P815 cells transfected with either bovine CD18 (2B2; red histogram), or bovine CD18 containing the FLAG epitope at the cleavage site (BFL; blue histogram) were tested by flow cytometry for expression of bovine CD18 with an antibovine CD18 mAb. Results of one representative experiment of three are shown.
Signal Peptide of CD18 of Ruminants Contains “Cleavage-Inhibiting” Q at Position −5 Relative to the Cleavage Site.
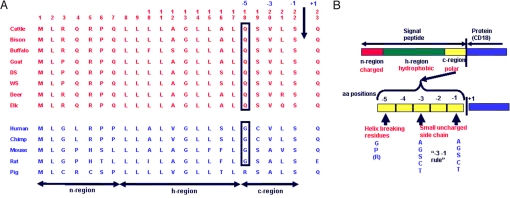
Our finding that the amino acids 5–17 within the signal peptide of ruminant CD18 serve as the receptor for M. haemolytica Lkt, and the fact that the cytolytic activity of Lkt is absolutely specific for ruminant leukocytes, prompted us to examine the signal sequences of CD18 of ruminants (n = 8) and nonruminants (n = 5) (Fig. 3A). The predicted signal sequence of both the ruminant and nonruminant CD18 contains 22 amino acids. The “−3 −1 rule” of Von Hejne (39) for signal peptide cleavage calls for the presence of amino acids with small uncharged side chains at position −1 and −3 relative to the cleavage site (Fig. 3B). Both ruminant and nonruminant CD18 signal peptides conform to this rule. The amino acid residue at position −5 could also determine whether the signal peptide gets cleaved or not (40). Helix-breaking residues G and proline are conducive for signal peptide cleavage (39). Arginine is also conducive to signal peptide cleavage (39, 40). However, Q has been shown to inhibit cleavage of the signal peptide (40). Surprisingly, CD18 of all eight ruminants examined contain cleavage-inhibiting Q, whereas CD18 of all five nonruminants examined contain the “cleavage-conducive” G (humans, mice, rats, and chimpanzees) or arginine (pigs) (Fig. 3A).
Fig. 3.
The signal peptide of CD18 of ruminants contains cleavage-inhibiting Q at amino acid position −5 relative to the cleavage site, whereas that of nonruminants contains cleavage-conducive G. (A) Comparison of signal peptide sequences of ruminants and nonruminants [GenBank accession nos. M81233 (cattle), EU553919 (bison), AY842449 (buffalo), AY452481 (goat), DQ470837 (domestic sheep), DQ104444 (wild sheep), EU623794 (deer), EU553918 (elk), NM0002211 (human), NM001034122 (chimpanzee), X14951 (mouse), NM001037780 (rat), and U13941 (pig)]. DS, domestic sheep; WS, wild sheep; Chimp, chimpanzee. The arrow indicates the signal peptide cleavage site. (B) The −3,−1, rule for cleavage of signal peptides (43).
Mutation of Q to G at Position −5 Relative to the Cleavage Site of CD18 Signal Peptide Abrogates Lkt-Induced Cytolysis.
The observation that the signal peptide of CD18 of ruminants (Lkt-susceptible) contains Q at −5 position, whereas that of nonruminants (Lkt-nonsusceptible) contains G, prompted us to ask whether site-directed mutagenesis of Q to G [Q(−5)G] would result in the abrogation of Lkt-induced cytolysis of transfectants expressing Q(−5)G mutation in the signal peptide of CD18. The amino acid Q at −5 position of bovine CD18 was mutated to G, and the mutated CD18 was transfected into P815 cell line, which is nonsusceptible to Lkt-induced cytolysis. A transfectant stably expressing bovine CD18 carrying the Q(−5)G mutation, designated as BQG, was selected for further analysis. Flow cytometric analysis with an anti-CD18 mAb revealed that the surface expression of bovine CD18 by this transfectant BQG was comparable with that of the transfectant, 2B2, expressing WT CD18 (Fig. 4A). Interestingly, the transfectant 2B2 was effectively lysed by Lkt in a concentration-dependent manner, whereas the transfectant BQG was not lysed at all, indicating that the Q(−5)G mutation in CD18, indeed, abrogates Lkt-induced cytolysis of target cells (Fig. 4B).
Fig. 4.
Mutation of Q to G at position −5 of the signal peptide of bovine CD18 abrogates Lkt-induced cytolysis of transfectants expressing CD18 with Q(−5)G mutation. (A) Transfectants 2B2 and BQG express similar levels of CD18. Transfectants expressing bovine CD18 (2B2; blue histogram), or bovine CD18 containing the Q(−5)G mutation (BQG; red histogram), and the parent cells (P815; green histogram) were tested for expression of bovine CD18 by flow cytometric analysis with an antibovine CD18 mAb. Results of one representative experiment of three are shown. (B) Transfectant 2B2 is lysed by Lkt in a concentration-dependent manner, whereas BQG is not. Transfectants 2B2, BQG, and P815 cells were tested for susceptibility to Lkt-induced cytolysis by the MTT dye-reduction cytotoxicity assay. All data are expressed as mean ± SD (n = 3). (C) CD18 of transfectant BQG exhibits a lower molecular mass than that of 2B2. Cell lysate from the transfectant 2B2 (expressing WT CD18) and BQG [expressing CD18 with Q(−5)G mutation] were subjected to SDS/PAGE (8% gel) followed by Western blot analysis with the anti-CD18 mAb, BAQ30A. Lane 1, lysate from the transfectant BQG. Lane 2, lysate from the transfectant 2B2.
Abrogation of Lkt-Induced Cytolysis of Transfectants Expressing Bovine CD18 Carrying the Q(−5)G Mutation Is Caused by the Cleavage of the Signal Peptide.
The abrogation of cytolysis of the transfectant BQG could be caused by (i) the difference in structural features of Q and G or (ii) the cleavage of the signal peptide. To elucidate the mechanism underlying the abrogation of cytolysis, Western blot analysis of cell lysates of BQG and 2B2 was performed with the anti-CD18 mAb. Single bands representing CD18 were detected with both cell lysates ≈95kDa. However, the CD18 from BQG exhibited a lower molecular mass than the CD18 from 2B2 (Fig. 4C), suggesting that the replacement of cleavage-inhibiting Q by the cleavage-conducive G at −5 position of bovine CD18 resulted in the cleavage of signal peptide.
Discussion
Previously, we have mapped the Lkt-binding site to lie between amino acids 1 and 291 of bovine CD18. In this study, by using synthetic peptides spanning amino acids 1–291 in inhibition of Lkt-induced cytolysis assays, we have shown that the CD18 domain formed by amino acids 5–17 represents the Lkt-binding site on CD18 of bovine PMNs (Fig. S3). Also, inhibition of Lkt-induced cytolysis of PMNs of goats, domestic sheep, wild sheep, deer, and bison by peptide P17 (amino acids 5–17) indicates that the CD18 domain formed by amino acids 5–17 represents the Lkt-binding site on CD18 of other ruminants as well.
Our finding that amino acids 5–17 of bovine CD18 serves as the binding site for Lkt is not in agreement with the finding of Dileepan et al. (41, 42). These authors have previously reported that the Lkt binding site lies within amino acids 500–600 of bovine CD18, more specifically, between amino acids 541 and 581. Our results clearly indicate that this conclusion is incorrect for the following reasons. (i) Two different sets of synthetic peptides spanning amino acids 500–600 failed to inhibit Lkt-induced cytolysis of bovine PMNs (Fig. S4). (ii) Synthetic peptides containing the signal sequence amino acids 5–17 effectively inhibited Lkt-induced cytolysis of PMNs of cattle and other ruminants (Fig. S3). If Lkt binds to CD18 between amino acids 541 and 581, one would expect to see cytolysis of target cells when Lkt is incubated with a synthetic peptide containing amino acids 5–17 only. However, the target cells were not lysed (Figs. S1 and S2). (iii) Our transfectants expressing CD18 containing the Q(−5)G mutation in the signal peptide are not lysed by Lkt (Fig. 4), although amino acids 500–600 are intact in the CD18. The failure of Dileepan et al. (41, 42) to identify amino acids 5–17 in the signal peptide as the Lkt binding site is very likely because their transductants were developed with K562 cells. We note that K562 is a poorly characterized cell line, and the literature (43, 44) contains conflicting reports as to its lineage (erythroleukemia and granulocyte). Our studies indicate that the findings of Dileepan et al. (41, 42) are unique to bovine CD18 transductants developed with K562 cells and do not reflect the molecular events occurring in ruminant leukocytes.
Our finding that the CD18 domain formed by amino acids 5–17 is the Lkt-binding site on CD18 revealed interesting information about the signal peptide of CD18 of ruminants. Amino acids 5–17 comprise 13 of 22 amino acids of the predicted sequence of bovine CD18 signal peptide, which suggests that the signal peptide of cattle, and other ruminants, may not be cleaved. Paradigm dictates that signal peptides of plasma membrane proteins are cleaved by the signal peptidase in the ER (1–3). However, a few membrane proteins present an exception to this paradigm. Corticotrophin releasing factor receptor type 2a (45), prion protein (46), and sucrase isomaltase (type II) (47) represent the very small minority of proteins with intact signal peptide on the mature molecule.
Flow cytometric analysis with the antibovine CD18 signal peptide serum supported our view that the signal peptide of the CD18 of ruminants remains intact on the mature cell-surface CD18 (Fig. 1). The introduction of the FLAG epitope at the cleavage site and the analysis of transfectants expressing FLAG-tagged CD18 confirmed that the signal peptide of CD18 of cattle is indeed not cleaved from the mature CD18 molecule (Fig. 2). It is very likely that the signal peptide of CD18 of other ruminants is not cleaved as well, because ≥95% amino acid sequence identity exists among them. Reactivity of PMNs from all of the ruminants with the chicken antiserum against bovine signal peptide strongly supports this notion.
The presence of cleavage-inhibiting Q, instead of the cleavage-conducive G, at position −5 relative to the cleavage site of CD18 of all ruminants is very likely responsible for the failure of the ruminant signal peptide to be cleaved (Fig. 4). This view is strongly supported by the fact that the Q(−5)G mutation in the signal peptide of bovine CD18 results in the cleavage of signal peptide as revealed by the Western blot analysis (Fig. 4C) and the resultant abrogation of Lkt-induced cytolysis of the transfectants (Fig. 4B). It is really interesting that a single amino acid substitution in bovine CD18 eliminates the susceptibility of transfectants expressing CD18 carrying the substitution. This finding is of great practical significance. Lkt is the most important virulence factor of M. haemolytica (12, 13, 23, 24). Lkt deletion mutants of M. haemolytica induce milder lung lesions and reduced mortality (12–15). Therefore, cattle and other ruminants that express CD18 without the signal peptide on their leukocytes could be expected to be much less susceptible to M. haemolytica-caused pneumonia. Our finding that the Q(−5)G mutation in the signal peptide of bovine CD18 results in the cleavage of the signal peptide and abrogation of Lkt-induced cytolysis of the transfectants paves the way for the genetic engineering of cattle and other ruminants that are less susceptible to M. haemolytica-caused pneumonia.
In summary, we have demonstrated that amino acids 5–17 within the signal peptide of ruminant CD18 serve as the receptor for M. haemolytica Lkt, and that the failure of the signal peptide to be cleaved from mature CD18 molecules renders the ruminant leukocytes susceptible to Lkt. We propose that engineering cattle and other ruminants to contain this mutation would provide a novel technology to render them less susceptible to pneumonic pasteurellosis and concomitant economic losses.
Methods
Cell Lines and Antibodies.
The cell lines BL-3 (bovine lymphoma), and P815 (murine mastocytoma) were propagated in complete DMEM, supplemented with 10% FBS, 2 mM L-Q, and 20 μg/mL of gentamicin (complete medium). The transfectant 2B2, expressing full-length bovine CD18 on the cell surface, was previously developed in our laboratory by transfecting P815 with cDNA for bovine CD18 (31). The transfectants, BFL and BQG, were selected and propagated in the complete DMEM together with 500 μg/mL of Geneticin (G418; Invitrogen). PMNs were isolated from peripheral blood by density gradient centrifugation using Ficoll-Paque (Amersham Pharmacia Biotech), followed by hypotonic lysis of the erythrocyte pellet, as described (26). Antibovine CD18 mAb, BAQ30A, was obtained from the Washington State University Monoclonal Antibody Center (Pullman, WA). Monoclonal Abs M1 and M2 specific for the FLAG epitope were obtained from Sigma.
Peptides.
A nested set of 20-mer peptides spanning amino acids 1–291 and amino acids 500–600 of bovine CD18 was synthesized at Sigma-Genosys. Once the peptide that inhibits Lkt-induced cytotoxicity was identified, another set of peptides was synthesized with N-terminal truncation by dropping one amino acid at a time while keeping the C-terminal amino acid constant. Once the N-terminal amino acid of the minimal peptide was identified another set of peptides was synthesized with C-terminal truncation by dropping one amino acid at a time while keeping the N-terminal amino acid constant. All of the peptides were resuspended in dimethysulfoxide (ATCC) at a concentration of 10 mg/mL, aliquoted, and stored at −20 °C.
Preparation of Lkt.
Production of Lkt from M. haemolytica A1 has been described (48). Detailed description is given in SI Text.
Detection of Inhibition of Lkt-Induced Cytolysis of Target Cells by the Peptide Analogs of CD18.
The MTT (Sigma) dye reduction cytotoxicity assay for detection of Lkt-induced cytolysis of target cells has been described (48). Detailed description is given in SI Text.
Cloning and Expression of Bovine CD18 Carrying the FLAG Epitope at the Cleavage Site.
The GeneTailor site-directed mutagenesis system (Invitrogen) was used to insert the FLAG epitope (DYKDDDDK) into the vector pMD1 carrying bovine CD18 cDNA, at the signal peptide cleavage site (between amino acids 22 and 23). The insertion was carried out in two steps (12 bp at a time). The insertion of FLAG epitope into CD18 was confirmed by DNA sequencing. The vector carrying the FLAG-tagged CD18 was transfected into P815 cells with Lipofectamine 2000 according to the manufacturer's protocol.
Cloning and Expression of Bovine CD18 Carrying the Q(−5)G Mutation.
The bovine cDNA for CD18 was previously subcloned into the mammalian expression vector pCI-neo to yield pMD1 (31). To produce Q(−5)G mutation in bovine CD18, site-directed mutagenesis was performed by using the GeneTailor site-directed mutagenesis system (Invitrogen). The CD18 sequence after the point mutation was checked by DNA sequencing. Transfection of P815 cells with Lipofectamine 2000 (Invitrogen) was carried out according to the manufacturer's recommendations.
Flow Cytometric Analysis.
This analysis was performed as described (31). Cell surface expression of bovine CD18 by transfectants 2B2, BFL, and BQG was detected with the anti-CD18 mAb BAQ30A. The presence of intact signal peptide of CD18 on PMNs of ruminants, Jurkat cells, and RAW264.1 cells was detected with the chicken antiserum developed against the synthetic peptide spanning amino acids 5–19 of bovine CD18 signal peptide. Cell surface expression of FLAG epitope on the transfectants 2B2 and BFL was detected with mAbs M1 and M2. Detailed description is given in SI Text.
Western Blot Analysis.
Bovine CD18 in the cell lysates of 2B2 and BQG was detected by Western blot analysis as described in SI Text.
Statistical Analysis.
One-way ANOVA was used to determine whether the differences in percentage inhibition caused by the different peptides are statistically significant.
Acknowledgments.
We thank Dr. R. A. Goldsby (Amherst College, Amherst, MA) and Dr. B. A. Osborne (University of Massachusetts, Amherst, MA) for reading the manuscript and providing comments and criticisms; Dr. S. K. Maheswaran (University of Minnesota, Minneapolis) for providing the K562 transductants; and Dr. Chad Chase (SubTropical Agricultural Research Station of U.S. Department of Agriculture in Brooksville, FL) for suppling Bos indicus blood. This work was supported by funds from the Department of Veterinary Microbiology and Pathology at Washington State University and the Foundation for North American Wild Sheep.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906775106/DCSupplemental.
References
- 1.Blobel G, Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and nonprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975;67:835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Heijne G. The signal peptide. J Membr Biol. 1990;115:195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]
- 3.Tuteja R. Type I signal peptidase: An overview. Arch Biochem Biophys. 2005;441:107–111. doi: 10.1016/j.abb.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Mosier DA. Bacterial pneumonia. Vet Clin N Am Food Anim Pract. 1997;13:483–493. doi: 10.1016/s0749-0720(15)30310-8. [DOI] [PubMed] [Google Scholar]
- 5.Ackermann MR, Brogden KA. Response of the ruminant respiratory tract to Mannheimia (Pasteurella) haemolytica. Microbes Infect. 2000;2:1079–1088. doi: 10.1016/s1286-4579(00)01262-4. [DOI] [PubMed] [Google Scholar]
- 6.Miller MW. Pasteurellosis. In: Williams ES, Barker IK, editors. Infectious Diseases of Wild Mammals. Ames: Iowa State Univ Press; 2001. pp. 330–339. [Google Scholar]
- 7.Odugbo MO, Odama LE, Umoh JE, Lombin LH. The comparative pathogenecity of strains of eight serovas and untypable strains of Mannheimia haemolytica in experimental pneumonia of sheep. Vet Res. 2004;35:661–669. doi: 10.1051/vetres:2004044. [DOI] [PubMed] [Google Scholar]
- 8.Bowland SL, Shewn PE. Bovine respiratory disease: Commercial vaccines currently available in Canada. Can Vet J. 2000;41:33–48. [PMC free article] [PubMed] [Google Scholar]
- 9.Frank GH. Pasteurellosis of cattle. In: Adlam C, Rutter JM, editors. Pasturella and Pasteurellosis. New York: Academic; 1989. pp. 197–222. [Google Scholar]
- 10.Rehmtulla AJ, Thomson RG. A review of the lesions in shipping fever of cattle. Can Vet J. 1981;22:1–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Confer AW, Panciera RJ, Clinkenbeard KD, Moiser DA. Molecular aspects of virulence of Pasteurella haemolytica. Can J Vet Res. 1990;54:S48–S52. [PubMed] [Google Scholar]
- 12.Petras SF, et al. Antigenic and virulence properties of Pasteurella haemolytica leukotoxin mutants. Infect Immun. 1995;63:1033–1039. doi: 10.1128/iai.63.3.1033-1039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatum FM, et al. Construction of an siogenic leukotoxin deletion mutant of Pasteurella haemolytica serotype 1: Characterization and virulence. Microb Pathog. 1998;24:37–46. doi: 10.1006/mpat.1997.0181. [DOI] [PubMed] [Google Scholar]
- 14.Highlander SK, et al. Inactivation of Pasteurella (Mannheimia) haemolytica leukotoxin causes partial attenuation of virulence in a calf challenge model. Infect Immun. 2000;68:3916–3922. doi: 10.1128/iai.68.7.3916-3922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dassanayake RP, et al. Mannheimia haemolytica serotype A1 exhibits differential pathogenicity in two related species Ovis Canadensis and Ovis aries. Vet Microbiol. 2009;133:366–371. doi: 10.1016/j.vetmic.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Strathdee CA, Li RY. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989;171:916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devenish J, Rosendal S, Johnson R, Hubler S. Immunoserological comparison of 104-kilodalton proteins associated with hemolysis and cytolysis in Actinobacillus pleuropneumoniae, Actinobacillus suis, Pasteurella haemolytica, and Escherichia coli. Infect Immun. 1989;57:3210–3213. doi: 10.1128/iai.57.10.3210-3213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolodrubetz D, Dailey T, Ebersole J, Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect Immun. 1989;57:1465–1469. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaehler KL, Markham RJ, Muscoplat CC, Johnson DW. Evidence of species specificity in the cytocidal effects of Pasteurella haemolytica. Infect Immun. 1980;30:615–616. doi: 10.1128/iai.30.2.615-616.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YF, Renshaw HW, Martens RJ, Livingston CW., Jr Pasteurella haemolytica leukotoxin: Chemiluminescent responses of peripheral blood leukocytes from several different mammalian species to leukotoxin- and opsonin-treated living and killed Pasteurella haemolytica and Staphylococcus aureus. Am J Vet Res. 1986;47:67–74. [PubMed] [Google Scholar]
- 21.Shewen PE, Wilkie BN. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982;35:91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slocombe RJ, Marark J, Ingersoll R, Derksen FJ, Robinson NE. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am J Vet Res. 1985;46:2253, 2258. [PubMed] [Google Scholar]
- 23.Highlander SK. Molecular genetic analysis of virulence in Mannheimia (Pasteurella) haemolytica. Front Biosci. 2001;1:D1128–D1150. doi: 10.2741/highland. [DOI] [PubMed] [Google Scholar]
- 24.Jayaseelan S, Sreevatsan S, Maheswaran SK. Role of Mannheimia haemolytica leukotoxin in the pathogenesis of bovine pneumonic pasteurellosis. Anim Health Res Rev. 2002;3:69–82. doi: 10.1079/ahrr200242. [DOI] [PubMed] [Google Scholar]
- 25.Wang JF, et al. Molecular and biochemical mechanisms of Pasteurella haemolytica leukotoxin-induced cell death. Microb Pathog. 1998;25:317–331. doi: 10.1006/mpat.1998.0236. [DOI] [PubMed] [Google Scholar]
- 26.Ambagala T, Ambagala APN, Srikumaran S. The leukotoxin of Pasteurella haemolytica binds to β2-integrins on bovine leukocytes. FEMS Microbiol Lett. 1999;179:161–167. doi: 10.1111/j.1574-6968.1999.tb08722.x. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Clinkenbeard KD, Ritchey JW. Bovine CD18 identified as a species specific receptor for Pasteurella haemolytica leukotoxin. Vet Microbiol. 1999;67:91–97. doi: 10.1016/s0378-1135(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 28.Jayaseelan S, et al. Lymphocyte function-associated antigen 1 is a receptor for Pasteurella haemolytica leukotocin in bovine leukocytes. Infect Immun. 2000;68:72–79. doi: 10.1128/iai.68.1.72-79.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gahmberg CG, et al. Leukocyte integrins and inflammation. Cell Mol Life Sci. 1998;54:549–555. doi: 10.1007/s000180050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noti JD, Johnson AK, Dillon JD. Structural and functional characterization of the leukocyte integrin gene CD11d. Essential role of Sp1 and Sp3. J Biol Chem. 2000;275:8959–8969. doi: 10.1074/jbc.275.12.8959. [DOI] [PubMed] [Google Scholar]
- 31.Deshpande MS, Ambagala TC, Ambagala APN, Kehrili ME, Jr, Srikumaran S. Bovine CD18 is necessary and sufficient to mediate Mannheimia (Pasteurella) haemolytica leukotoxin-induced cytolysis. Infect Immun. 2002;70:5058–5064. doi: 10.1128/IAI.70.9.5058-5068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W, et al. Mannheimia (Pasteurella) heamolytica leukotoxin utilizes CD18 as its receptor on bighorn sheep leukocytes. J Wildl Dis. 2007;43:75–81. doi: 10.7589/0090-3558-43.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Dassanayake RP, Maheswaran SK, Srikumaran S. Monomeric expression of bovine β2-integrin subunits reveals their role in Mannheimia haemolytica leukotoxin-induced biological effects. Infect Immun. 2007;75:5004–5010. doi: 10.1128/IAI.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dassanayake RP, Shanthalingam S, Davis WC, Srikumaran S. Mannheimia haemolytica leukotoxin-induced cytolysis of ovine (Ovis aries) leukocytes is mediated by CD18, the β-subunit of β2-integrins. Microb Pathog. 2007;42:167–173. doi: 10.1016/j.micpath.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Gopinath RS, Ambagala TC, Deshpande MS, Donis RO, Srikumaran S. Mannheimia (Pasteurella) heamolytica leukotoxin binding domain lies within amino acids 1–291 of bovine CD18. Infect Immun. 2005;73:6179–6182. doi: 10.1128/IAI.73.9.6179-6182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tibbetts SA, et al. Peptides derived from ICAM-1 and LFA-1 modulates T cell adhesion and immune function in a mixed lymphocyte culture. Transplantation. 1999;68:685–692. doi: 10.1097/00007890-199909150-00015. [DOI] [PubMed] [Google Scholar]
- 37.Tibbetts SA, Seetharama JD, Siahaan TJ, Benedict SH, Chan MA. Linear and cyclic LFA-1 and ICAM-1 peptides inhibit T cell adhesion and function. Peptides. 2000;21:1161–1167. doi: 10.1016/s0196-9781(00)00255-2. [DOI] [PubMed] [Google Scholar]
- 38.Stewart RS, Drisaldi B, Harris DA. A transmembrane form of the prion protein contains an uncleaved signal peptide and is retained in the endoplasmic reticulum. Mol Biol Cell. 2001;12:881–889. doi: 10.1091/mbc.12.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983;133:17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- 40.Rutz C, et al. The corticotropin-releasing factor receptor type 2a contains an N-terminal pseudo signal peptide. J Biol Chem. 2006;281:24910–24921. doi: 10.1074/jbc.M601554200. [DOI] [PubMed] [Google Scholar]
- 41.Dileepan T, Thumbikat P, Walcheck B, Kannan MS, Maheswaran SK. Recombinant expression of bovine LFA-1 and characterization of its role as a receptor for Mannheimia haemolytica leukotoxin. Microb Pathog. 2005;38:249–257. doi: 10.1016/j.micpath.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Dileepan T, Kannan MS, Walcheck B, Maheswaran SK. Integrin-EGF-3 domain of bovine CD18 is critical for Mannheimia haemolytica leukotoxin species-specific susceptibility. FEMS Microbiol Lett. 2007;274:67–72. doi: 10.1111/j.1574-6968.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 43.Klein E, et al. Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int J Cancer. 1976;18:421–431. doi: 10.1002/ijc.2910180405. [DOI] [PubMed] [Google Scholar]
- 44.Anderson LC, Nilsson K, Gahmberg CG. K562, a human erythroleukemic cell line. Int J Cancer. 1979;23:143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- 45.Rutz C, et al. The corticotrophin-releasing factor receptor type 2a contains an N-terminal pseudo signal peptide. J Biol Chem. 2006;281:24910–24921. doi: 10.1074/jbc.M601554200. [DOI] [PubMed] [Google Scholar]
- 46.Stewart RS, Drisaldi B, Harris DA. A transmembrane form of the prion protein contains an uncleaved signal peptide and is retained in the endoplasmic reticulum. Mol Biol Cell. 2001;12:881–889. doi: 10.1091/mbc.12.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hegner M. Single amino acid substitutions can convert the uncleaved signal anchor of sucrose-isomaltase to a cleaved signal sequence. J Biol Chem. 1992;267:16928–16933. [PubMed] [Google Scholar]
- 48.Gentry MJ, Srikumaran S. Neutralizing monoclonal antibodies to Pasteurella haemolytica leukotoxin affinity-purify the toxin from crude culture supernatants. Microb Pathog. 1991;10:411–417. doi: 10.1016/0882-4010(91)90086-p. [DOI] [PubMed] [Google Scholar]