Abstract
The regulation of metal ion transport within neurons is critical for normal brain function. Of particular importance is the regulation of redox metals such as iron (Fe), where excess levels can contribute to oxidative stress and protein aggregation, leading to neuronal death. The divalent metal transporter 1 (DMT1) plays a central role in the regulation of Fe as well as other metals; hence, failure of DMT1 regulation is linked to human brain pathology. However, it remains unclear how DMT1 is regulated in the brain. Here, we show that DMT1 is regulated by Ndfip1 (Nedd4 family-interacting protein 1), an adaptor protein that recruits E3 ligases to ubiquitinate target proteins. Using human neurons we show the Ndfip1 is upregulated and binds to DMT1 in response to Fe and cobalt (Co) exposure. This interaction results in the ubiquitination and degradation of DMT1, resulting in reduced metal entry. Induction of Ndfip1 expression protects neurons from metal toxicity, and removal of Ndfip1 by shRNAi results in hypersensitivity to metals. We identify Nedd4–2 as an E3 ligase recruited by Ndfip1 for the ubiquitination of DMT1 within human neurons. Comparison of brains from Ndfip1−/− with Ndfip1+/+ mice exposed to Fe reveals that Ndfip1−/− brains accumulate Fe within neurons. Together, this evidence suggests a critical role for Ndfip1 in regulating metal transport in human neurons.
Keywords: cobalt, iron, Nedd4–2, ubiquitin
The brain is a specialized organ that requires metals ions for a number of important cellular processes. As such, the brain contains a relatively high concentration of a number of metals such as Fe, Zn, and Cu (in the order of 0.1–0.5 mM) (1). Importantly, the concentrations of these metals are potentially toxic under stress conditions without the requirement of exogenous uptake through ingestion. It is therefore crucial that the brain has highly efficient homeostatic mechanisms in place to prevent aberrant metal toxicity. Over the last decade, growing evidence suggests that the misregulation of metals within the brain is involved in the neuropathology of a number of disorders, such as Parkinson's and Alzheimer's diseases (2). Metal ions are suggested to have two distinct roles in the pathophysiology of brain disorders. Firstly, redox active metals such as Cu, Fe, Mg, and Co can result in metal-catalyzed protein oxidation that leads to protein damage and denaturation (3). Secondly, metal-protein associations can result in protein aggregation and the formation of insoluble protein bodies (4). In addition to the role metals can play in disease states, it has also now become clear that at times of stress, such as in trauma or stroke, neurons become vulnerable to uncontrolled entry of excess metals (3). It is therefore critical that the brain is able to mount cellular defense mechanisms against sudden surges of metal toxicity.
Normal metal uptake occurs via transferrin-bound Fe that is incorporated into cells by an endocytotic process initiated by transferrin receptor 1 (TfR1) (5). Non-protein-bound metals can also enter cells through a promiscuous metal transporter, divalent metal transporter 1 (DMT1), previously known as DCT1, NRAMP2, or Slc11a2 (6, 7), that is known to actively transport Fe, Zn, Mn, Co, Cd, Cu, Ni, and Pb, via a proton-coupled mechanism (8). DMT1 is predicted to have 12 transmembrane segments and is expressed in neurons, allowing for the incorporation of metals from the extracellular environment and/or the recycling endosomes. A spontaneous mutation at position G185R in DMT1 results in deficits in Fe adsorption in both the mk mouse and the Belgrade rat, giving rise to microcytic, hypochromic anemia (9, 10). These mutant mice also suffer from neurodegeneration in a Parkinsonian model, pointing to an important role for DMT1 in the regulation of metals in the brain (11). Transcriptional control of DMT1 expression through Fe response elements has been described previously (12); however, little is known about the post-translational control mechanisms regulating DMT1 protein levels within the brain. Lessons from yeast studies have demonstrated that the DMT1 homolog, known as Smf1, is degraded by ubiquitination after binding to Bsd2 through an E3 ligase mechanism (13, 14).
In a previous study, we demonstrated that Nedd4 family-interacting protein 1 (Ndfip1) is upregulated in cortical neurons following traumatic brain injury, and this over-expression is protective against neuronal apoptosis in vitro (15). The precise mechanisms of neuroprotection by Ndfip1 remains to be elucidated but given the role of its yeast homologue Bsd2 in metal homeostasis, it is of interest to discover if similar mechanisms apply in neuroprotection, particularly in humans. To test this hypothesis, we used a culture model involving human primary cortical neurons, together with a human neuronal cell line. We show that metal ions upregulate Ndfip1 in human neurons, and induction of Ndfip1 expression is protective against Co or Fe toxicity. Conversely, knock down of the Ndfip1 mRNA results in increased sensitivity of cells to Co and prevents them from downregulating DMT1. We demonstrate that Ndfip1-binding to DMT1 is instrumental for ubiquitination and downregulation of DMT1 and identify Nedd4–2 as a ubiquitin ligase for polyubiquitination of DMT1 under metal-induced stress. Lastly, we show that Ndfip1−/− mice have an increased accumulation of Fe within the brain when exposed to acute metal toxicity.
Results
Ndfip1 in Human Neurons Is Upregulated in Response to Co and Fe Exposure.
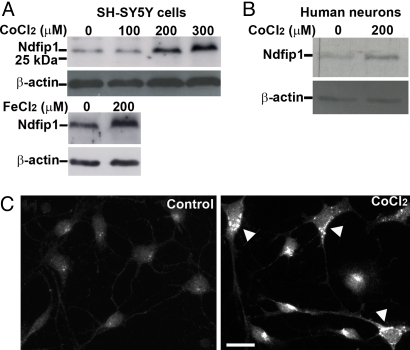
Neuronal exposure to metal ions induces a number of biochemical responses that can result in cellular toxicity. To test the function of Ndfip1 under such conditions, CoCl2 was administered to the human neuroblastoma cell line SH-SY5Y. This cell line has a basal level of Ndfip1 expression (Fig. 1A, control lane). Addition of CoCl2 in increasing molar concentrations caused an upregulation of Ndfip1 in a dose-response manner up to lethal concentrations (Fig. 1A). Similarly cells exposed to 200 μM of FeCl2 also showed an upregulation of Ndfip1 (Fig. 1A). Ndfip1 is also present at basal levels in cultured primary cortical neurons from 18-week-old human embryos (Fig. 1B, control lane). Exposure of these neurons to 200 μM CoCl2 over 18 h showed upregulation of Ndfip1 by approximately two-fold compared to the basal level of the protein (Fig. 1B). We observed that the increased levels of Ndfip1 protein was a result of increased protein expression rather than a change in protein stability (Fig. S1). Immunocytochemistry using polyclonal antibodies showed upregulation of Ndfip1 in cultured human primary neurons following exposure to CoCl2 for 24 h (Fig. 1C).
Fig. 1.
Ndfip1 is upregulated in neurons in response to metal exposure. (A) SH-SY5Y cell lysates after exposure to increasing concentrations of Co for 18 h results in increased Ndfip1 expression as seen in western blots. A similar result was observed when cells were treated with Fe. (B) Treatment of human primary cortical neurons with Co also upregulates Ndfip1 when treated with 200 μM Co for 18 h. Representative blots from at least three independent experiments shown. (C) Immunocytochemistry with anti-Ndfip1 antibodies indicate basal levels of Ndfip1 expression (control) in cultured human primary cortical neurons (11 DIV) but upregulated (arrowheads) when exposed to 200 μM CoCl2. (Scale bar, 10 μm.)
Ndfip1 Promotes the Survival of Neurons from Metal Toxicity.
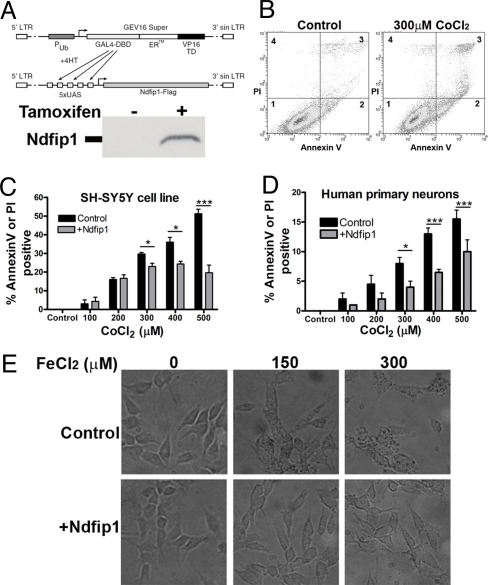
The results from the metal ion treatment suggested that upregulation of Ndfip1 in human neurons may provide a critical defense strategy against metal poisoning. To test this further, an inducible expression system for Ndfip1 was developed wherein the Ndfip1 gene was driven off the yeast Gal4/UAS transcription system (Fig. 2A). As previously described this system relies on the ability of Hsp90 to sequester Gal4 fused to mutant estrogen receptor (ERTM) in the cytoplasm (16); upon addition of 4-hydroxy tamoxifen, Gal4 ERTM VP16 is relocated into the nucleus to initiate transcription of Ndfip1. Following infection of the lentiviral constructs into SH-SY5Y cells, a stable line was isolated. Ndfip1 expression in this cell line was upregulated following induction with 4-hydroxy tamoxifen, but not in the uninduced control (Fig. 2A). To assess the capacity of Ndfip1 to protect against metals, induced Ndfip1 SH-SY5Y cells were exposed to increasing doses of CoCl2 and their viability was determined by flow cytometry staining for the cell death markers annexin V and propidium iodide (17). At the concentrations of CoCl2 used (0–500 μM), there was significant staining with annexin V indicating that CoCl2-induced cell death was occurring in an apoptotic manner and not through necrosis (Fig. 2B). At low levels of Co, both induced and uninduced cells had a similar death response, however at higher concentrations (300–500 μM), a 2- to 3-fold reduction in cell death was observed in cells induced to express Ndfip1 (Fig. 2C). It is likely that at low concentrations of CoCl2, endogenous Ndfip1 in control SH-SY5Y cells is sufficiently protective (Fig. 1A, and see next section). To assess whether primary human cortical neurons are also similarly protected, the inducible Ndfip1 constructs were transiently transfected into dissociated primary cortical neurons from 18-week human cortical tissue. The Amaxa electroporation system was efficient in transducing approximately 60% of the cultured cortical neurons and the entire population was subjected to flow cytometric analysis. Compared to the uninduced cortical neurons, and despite the fact that approximately 40% of sorted neurons did not receive the inducible constructs, the induced human cortical neurons clearly show the benefits of Ndfip1 protection at concentrations above 300 μM CoCl2 (Fig. 2D).
Fig. 2.
Ndfip1 protects human neurons from metal toxicity. (A) Tamoxifen-inducible expression construct of Ndfip1-Flag was created and used in a stable line of SH-SY5Y cells (anti-Flag blot shown). (B) Flow cytometry profile of SH-SY5Y cells treated with Co for 18 h. Different distributions of alive (quadrant 1), apoptotic (quadrant 2), and apoptotic plus necrotic populations (quadrant 3) between control and treated cells are shown. Cells treated with Co are dying through an apoptotic pathway. (C) Flow cytometry analysis of SH-SY5Y cells induced to express exogenous Ndfip1 protein protects against Co toxicity, compared with uninduced controls. (D) Flow cytometry analysis indicates that human embryonic cortical neurons induced to express exogenous Ndfip1 are protected against Co toxicity, compared to uninduced controls. (E) SH-SY5Y cells stained for Fe uptake show that cells induced to express Ndfip1 prevented Fe uptake compared with uninduced controls. Two-way Anova tests (Bonferroni posttests) indicate significant protection by Ndfip1 at higher concentrations of Co in (C) and (D) (P < 0.05* and P < 0.001***, ± SEM). Each flow cytometric analysis represents three independent experiments.
Our results indicate that Ndfip1 is protective against metal poisoning; however, it was unclear whether this was due to a reduction in metal accumulation or the ability of the cells to tolerate higher concentrations of metals. Using inducible Ndfip1 SH-SY5Y cells, we stained for cellular Fe levels after treatment with FeCl2. In the absence of Ndfip1 induction, Fe staining was visible at 150 μM with higher levels observed at 300 μM leading to cell death (Fig. 2E, top row). In cells induced to express Ndfip1, few cells contained any Fe staining at 150 μM concentration. Only at 300 μM was Fe staining observed at levels seen in uninduced cells at 150 μM (Fig. 2E, bottom row). This result suggests that Ndfip1 acts to limit the amount of metals that can enter the cell.
Removal of Ndfip1 Promotes Neuronal Death from Metal Toxicity.
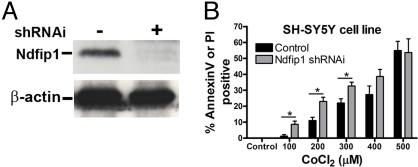
The above experiments with both primary cortical neurons and a neuronal cell line indicate that Ndfip1 is upregulated in response to metals, and is protective against metal-induced apoptosis. To investigate this further, a lentiviral shRNAi construct was used to transiently knock down Ndfip1 in SH-SY5Y cells (Fig. 3A). Control cells were infected with lentiviral particles containing a non-functioning shRNAi. Infected cells (and control infections) were treated with increasing concentrations of CoCl2 (0–500 μM) and compared for cell death using flow cytometry and staining with annexin V and propidium iodide (Fig. 3B). The results reveal effects that are contrary to that of Ndfip1 induction described above (Fig. 2C), and show that cells containing Ndfip1 shRNAi are more susceptible to Co-induced apoptosis at low concentrations of CoCl2 (up to 300 μM). Interestingly, no significant difference was observed between Ndfip1 shRNAi and control shRNAi cells at higher concentrations (400–500 μM) of CoCl2 (Fig. 3B). Nonetheless, the knock-down data mirrors the Ndfip1-induction result, wherein at lower concentrations (0–300 μM) of CoCl2, induction of Ndfip1 has no protective effect on SH-SY5Y cells (Fig. 2C). One possible explanation is that endogenous Ndfip1 levels are protective at low concentrations of CoCl2, therefore removal of Ndfip1 renders these cells susceptible to lower CoCl2 concentrations. At higher concentrations of CoCl2 (500 μM), the endogenous Ndfip1 appears to be as ineffective as the Ndfip1 shRNAi cells in protecting against cell death.
Fig. 3.
Removal of Ndfip1 through shRNAi renders cells more susceptible to metal toxicity. (A) Western blot showing Ndfip1 shRNAi constructs used in (B), showing specific reduction in Ndfip1 protein compared to control with non-functional shRNAi. (B) Flow cytometry analysis of SH-SY5Y cells containing shRNAi directed toward Ndfip1 protein are more susceptible to Co toxicity compared with control cells treated with non-functional shRNAi. Two-way Anova tests (Bonferroni posttests) indicate significant cell death in Ndfip1 shRNAi cells at low concentrations of Co when compared to shRNAi control cells (P < 0.05*, ± SEM).
Ndfip1 Interacts with DMT1 and Facilitates Its Ubiquitination in Neurons.
The data above demonstrate that Ndfip1 can protect neurons (from human fetal brain tissue and a neuronal cell line) from death arising from metal toxicity by limiting the amount of metal entry. A critical issue is to understand the possible mechanism behind this protective function. In yeast, Bsd2 (homolog of Ndfip1) binds and degrades the metal transporter Smf1 (homolog of DMT1) (13, 14). To explore this relationship in human neurons, immunohistochemistry and immunoprecipitation studies were carried out. Tissue sections of the human cortex show co-extensive staining of Ndfip1 and DMT1 (Fig. 4A), implying that these two proteins are compartmentalized in similar locations within the cell. To explore binding between these two proteins, human cortical neurons were used for immunoprecipitation with antibodies to Ndfip1 and DMT1, before and after treatment with CoCl2. The results show that lysates immunoprecipitated with DMT1 antibodies also contain Ndfip1 (Fig. 4B). Conversely, immunoprecipitation with Ndfip1 antibodies brought down DMT1; however, this band is substantially reduced when the cells were pretreated with CoCl2 (Fig. 4C), suggesting that DMT1 levels are reduced in the presence of Co. This data provides evidence that DMT1 is a binding partner for Ndfip1 in human neurons.
Fig. 4.
Ndfip1 co-localizes and interacts directly with the divalent metal transporter DMT1 in cultured human neurons. (A) Confocal images of human brain tissue from 18-week embryos demonstrate over-lapping staining for Ndfip1 and DMT1. (B) Immunoprecipitation of DMT1 from human cortical neurons with or without 200 μM Co treatment shows Ndfip1 to be precipitated (IB: immunoblot of Ndfip1). (C) Conversely, immunoprecipitation with Ndfip1 antibodies resulted in DMT1 protein being precipitated (IB: immunoblot of DMT1), although this band is highly reduced in the Co-treated sample (asterisk marks a non-specific band observed in immunoblots with DMT1 antibody). (Scale bar, 10 μm.)
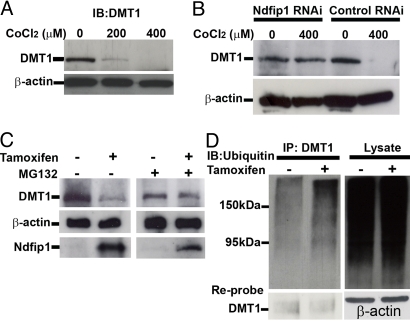
Since Ndfip1 is known to play a pivotal role in targeting proteins for E3 ligase ubiquitination (18), it is of interest to examine whether or not binding of Ndfip1 to DMT1 results in ubiquitination of DMT1. Initially we examined for a direct correlation between DMT1 downregulation and Co treatment, SH-SY5Y cells were subjected to increasing concentrations (0–400 μM) of CoCl2 and western blotted for DMT1. The results indicated that DMT1 was downregulated as CoCl2 concentrations were increased (Fig. 5A). If Ndfip1 is required for the degradation of DMT1, then cells deficient in Ndfip1 should not be able to downregulate DMT1 in the presence of Co. To test this, SH-SY5Y cells containing shRNAi directed toward Ndfip1 were exposed to CoCl2 for 18 h before western blotting for DMT1. Cells with Ndfip1 shRNAi were unable to degrade DMT1 in 400 μM CoCl2 whereas control shRNAi cells were able to efficiently degrade DMT1 (Fig. 5B). Conversely we postulated that upregulation of Ndfip1 would therefore result in the degradation of DMT1. Using the inducible Ndfip1 SH-SY5Y cell line, cells administered 4-hydroxy tamoxifen to induce Ndfip1 expression show a decrease in the levels of DMT1 present (Fig. 5C, lane 2) compared with the non-induced control. Given the function of Ndfip1 in ubiquitination, MG132 was used to determine whether the degradation of DMT1 was proteasome-dependent. With the proteasome blocked by MG132, DMT1 was not downregulated despite Ndfip1 induction (Fig. 5C, lane 4). This suggests Ndfip1-mediated ubiquitination and degradation of DMT1 is proteasome-dependent. To examine DMT1 ubiquitination by Ndfip1, we induced SH-SY5Y cells with 4-hydroxy tamoxifen for 16 h before the cells were harvested. Lysates were immunoprecipitated for DMT1 and blotted for polyubiquitination. Without induction of Ndfip1, immunoprecipitated DMT1 is associated with a basal level of ubiquitination (Fig. 5D, lane 1). Following induction of Ndfip1, the level of ubiquitinated DMT1 is increased (Fig. 5D, lane 2, cell lysate lanes indicate similar loading levels).
Fig. 5.
DMT1 can be ubiquitinated and is degraded in response to Co toxicity. (A) Western blot of SH-SY5Y cell line treated with varying concentrations of Co for 18 h shows decreasing DMT1 levels at higher Co concentrations. (B) Control shRNAi SH-SY5Y cells also decrease the level of DMT1 at 400 μM CoCl2; however Ndfip1 shRNAi cells do not show a significant decrease in DMT1 levels with 400 μM Co. (C) Ndfip1-inducible SH-SY5Y cells when treated with tamoxifen show a decrease in DMT1 protein levels compared to non-tamoxifen treated cells. Addition of the proteasome inhibitor MG132 prevents the decrease in DMT1 levels in the tamoxifen treated cells. (D) Western blot from inducible Ndfip1 SH-SY5Y cells was immunoprecipitated using anti-DMT1 antibodies and probed for polyubiquitin. Induced cells show an increase in DMT1 ubiquitination. Reprobing of the DMT1 IP lanes with anti-DMT1 showed that both lanes contained DMT1 protein.
Ndfip1 Protects Against Fe Toxicity and Its Effect Is Comparable to a Known Anti-Oxidant, Ebselen.
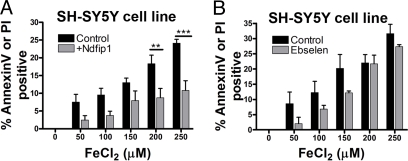
Thus far, we observed that Fe and Co both upregulate Ndfip1 (Fig. 1) and induced expression of Ndfip1 protects neurons from toxic levels of Co (Fig. 2). Given that DMT1 is known to transport a number of metal ions, we postulated that Ndfip1 should protect against other metals. Iron is of particular importance in metabolic processes of neurons and currently implicated in a number of brain pathologies. To test Ndfip1 protection against iron, SH-SY5Y cells were exposed to increasing concentrations of FeCl2, with and without inducible expression of Ndfip1. Flow cytometry analysis of apoptotic cells revealed similar protective effects compared to Co, with two-fold protection against Fe-induced death at concentrations above 200 μM (Fig. 6A). It has been reported that the regulation of Fe transport by DMT1 can be controlled by the drug Ebselen (19) that is currently the subject of a phase III clinical trial in ischemic stroke (20). To compare the efficacy of Ndfip1 relative to Ebselen, the same FeCl2 assay was conducted on SH-SY5Y cells exposed to Ebselen (Fig. 6B). The results showed that Ebselen was equally protective at low concentration of Fe, but at higher concentrations of Fe, Ebselen was less effective compared to Ndfip1 (Fig. 6A and B).
Fig. 6.
Ndfip1 protects against Fe toxicity. (A) Flow cytometry analysis of SH-SY5Y cells induced to express exogenous Ndfip1 protein protects against Fe toxicity, compared with uninduced controls. (B) Flow cytometry analysis of SH-SY5Y cells treated with Ebselen (1 μM) and given toxic levels of Fe. Two-way Anova tests (Bonferroni posttests) indicate significant protection by Ndfip1 at higher concentrations of Fe in (A) (P < 0.01** and P < 0.001***, ± SEM).
The E3 Ligase Nedd4–2 Is Recruited by Ndfip1 To Ubiquitinate DMT1.
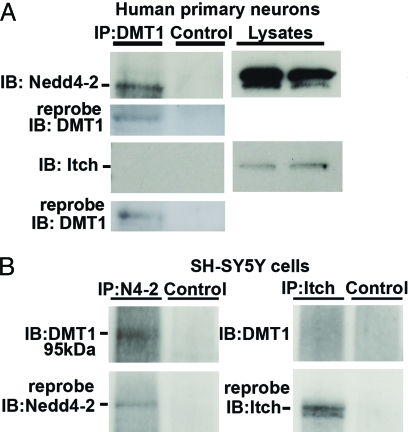
The experiments in the previous sections indicated that Ndfip1 binds to DMT1 under normal and stressed conditions; and that Ndfip1 is not only upregulated by metal ions, but in their presence, DMT1 levels are diminished, most likely by protein ubiquitination. It is therefore of interest to ascertain which E3 ligase mediates ubiquitination since Ndfip1 by itself is unable to catalyze ubiquitin tagging. DMT1 does not contain a PPxY motif that is classically required for binding to members of the Nedd4 family of E3 ligases (21). However, Ndfip1 is known to strongly bind to several Nedd4 family proteins (22). To address this, immunoprecipitation assays were conducted on human cortical neurons using a DMT1 antibody and western blots probed for the Nedd4 E3 ligases, Nedd4–2, and Itch. The results show that Nedd4–2, but not Itch, was immunoprecipitated with DMT1 antibodies (Fig. 7A). Reprobing of the DMT1 immunoprecipitates with anti-Ndfip1 antibody revealed that Ndfip1 was also present in this complex (see Fig. 4B). To further test this result, we immunoprecipitated SH-SY5Y cell lysates with Nedd4–2 and Itch antibodies and probed for DMT1. Only the Nedd4–2 antibody was able to precipitate DMT1 (Fig. 7B). Taken together these results suggest that Ndfip1, DMT1, and Nedd4–2 all form a complex that can result in the polyubiquitination of DMT1.
Fig. 7.
The E3 ligase Nedd4–2 can interact with DMT1. (A) Human primary neurons in culture for 11 days were immunoprecipitated with DMT1 antibodies and probed with antibodies from the E3 ligases Nedd4–2, or Itch. Only Nedd4–2 was observed to interact with DMT1. Cell lysates indicate that both Nedd4–2 and Itch are present in the neurons. (B) Conversely, immunoprecipitation with Nedd4–2 or Itch antibodies using SH-SY5Y cells shows a positive band for DMT1 with Nedd4–2 immunoprecipitation, but not with Itch. Reprobing demonstrated that both Nedd4–2 and Itch have been precipitated in the blots. Control lanes precipitated with protein A beads show no interactions.
Ndfip1−/− Mouse Brains Show an Accumulation of Fe in Neurons.
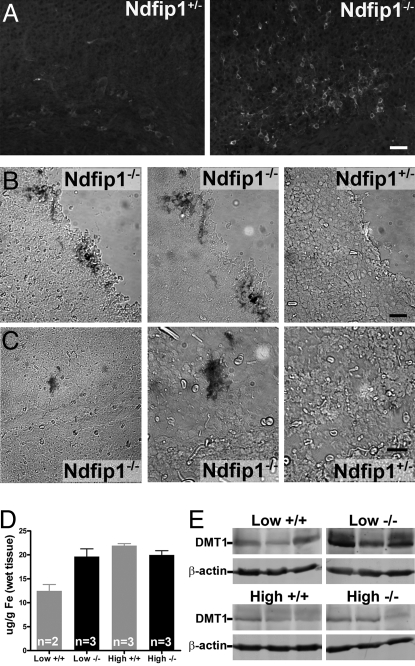
If Ndfip1 can regulate DMT1 levels within neurons, then mice lacking Ndfip1 should accumulate excess metals due to misregulation of DMT1. Ndfip1−/− mice were generated by disruption of the Ndfip1 gene with a gene trap vector, causing truncation of the Ndfip1 transcript encoding only exons 1 and 2 of the mRNA, but with no functional Ndfip1 protein (23). Ndfip1+/− mice are viable but homozygous Ndfip1−/− mice do not survive beyond 14 weeks of age due to severe inflammatory disease. Immunohistochemistry on Ndfip1−/− mice show an increased level of DMT1 protein within the cortex when compared to heterozygous litter mates (Fig. 8A). Brains sections from both Ndfip1−/− and Ndfip1+/− mice at postnatal day 3 (P3) were exposed to 200 μM Fe for 18 h, followed by staining for Fe using Perls Prussian stain. Ndfip1−/− brains showed an accumulation of Fe throughout the cortex compared with their Ndfip1+/− littermates that had no Fe staining (Fig. 8B and C). To further test the uptake of Fe in Ndfip1−/− mice, we used a low and high Fe diet on animals for 3 weeks before analysis of Fe content using inductively coupled plasma mass spectroscopy (ICP-MS). This analysis revealed that Ndfip1−/− animals fed on the low Fe diet had increased levels of Fe within the brain when compared with Ndfip1+/+ littermates (Fig. 8D). Both Ndfip1+/+ and Ndfip1−/− mice had similar level of Fe when fed on the high Fe diet (Fig. 8D). Higher levels of DMT1 protein were observed in Ndfip1−/− mice after the low Fe diet when compared with Ndfip1+/+ littermates (Fig. 8E). However no difference was observed in mice fed the high Fe diet, with the level of DMT1 protein observed to decrease (Fig. 8E). This is most likely due to transcriptional regulation of DMT1 levels as has been previously reported (24). As Ndfip1−/− mice show an increased level of DMT1 protein it is of interest to know whether other metal entry pathways were disrupted due to the misregulation of DMT1. The transferrin receptor is the major Fe entry pathway in most cells. Western blot analysis with TfR antibody showed that in Ndfip1−/− mice there is a decrease in the levels of TfR when compared with wild-type littermates (Fig. S2). Further studies are required to determine whether there is compensation of TfR levels due to the increased DMT1 levels.
Fig. 8.
Loss of Ndfip1 results in increased DMT1 levels and accumulation of Fe in the brain. (A) Ndfip1−/− mice brain sections show increased levels of DMT1 staining when compared with Ndfip1+/− littermates. (B and C) Brains sections from Ndfip1+/− and Ndfip1−/− mice were incubated with FeCl2 for 18 h before staining with Perls Prussian blue for Fe. Ndfip1−/− mice were observed to have significant staining for Fe deposits when compared to Ndfip1+/− littermates at both the cortical surface (B) and within the cortex (C) (left panel, 20× images; two right panels, 40×). (D) ICP-MS analysis of brains of Ndfip1−/− mice fed on a low Fe diet show an accumulation of Fe when compared to wild-type littermates. No difference was observed in the concentration of brain Fe between Ndfip1+/+ and Ndfip1−/− mice when exposed to a high Fe diet. (E) Ndfip1−/− mice when fed a low Fe diet have greater levels of DMT1 when compared to Ndfip1+/+ mice. No difference was observed in the levels of DMT1 in mice fed on a high Fe diet most likely due to transcriptional regulation of the DMT1 gene. (Scale bar, 30 μM.)
Discussion
Transition metals are an essential requirement for brain function. However, the nervous system contains a number of metals at concentrations that if perturbed, acutely or chronically, can lead to cellular toxicity. The effect of excess metals within neurons is well-documented, with reports showing an increase in free radicals or the aggregation of proteins (3, 4). However, there is limited information on the nature of direct molecular and biochemical responses to excess metals in human neurons. The present study has shed light on this process by identifying Ndfip1 as a key protein in the cellular defense mechanism against metal toxicity.
Recently, DMT1 has been implicated in the neuropathology of excitotoxic cell death and also Parkinson's disease (11, 25). During brain trauma or stroke, excitotoxic neuronal death occurs due to the uncontrolled release of glutamate or aspartate. Activation of glutamate-NMDA receptors has been found to stimulate nNOS which results in the activation of Dexras1, this in turn can induce Fe uptake via interaction between PAP7 and DMT1 (25). Increased DMT1 activity leading to excessive Fe uptake was causally linked to cellular toxicity from reactive oxygen species. Excitotoxic neuronal death could be alleviated by using cell-permeable Fe chelators, pointing to a strong link between excess Fe and NMDA-induced neurotoxicity. In Parkinson's patients, it was also found that dopaminergic neurons accumulate DMT1 (11). Additionally, mouse models of Parkinson's disease demonstrate a causal link between Fe accumulation and neuronal death (11). Together, these disease models point to DMT1 misregulation as a focal mechanism for dysregulated metal uptake, leading to neuronal death.
The above suggests that post-translational control of DMT1 is a critical regulatory system in the prevention of metal accumulation and neuronal apoptosis. Our central hypothesis was framed around Ndfip1 for two reasons. First, previous work from this laboratory had identified Ndfip1 to be a critical protein for improving neuronal survival during stress in vitro and associated with neuronal survival in vivo following traumatic brain injury (15). Ndfip1 is endogenously present in cortical neurons at low levels, most likely residing in the Golgi complex. Upon activation by injury or stress, Ndfip1 is dramatically upregulated in neurons extending to the cytoplasm and neuronal processes. Importantly, upregulation of Ndfip1 is consistently and robustly associated with neuronal survival, and neurons undergoing apoptosis do not show Ndfip1 upregulation. Second, the yeast homolog of Ndfip1 has been previously shown to be critical for defending against toxicity by transition metals (13). Given the evolutionary divergence between yeast and man, it is remarkable that Ndfip1 and its yeast homolog Bsd2 share similar mechanistic traits in protecting cells against metal poisoning. Importantly, both yeast and humans require an E3 ligase to catalyze degradation of the metal transporter by protein ubiquitination. In the case of humans we have found the E3 ligase is Nedd4–2, a mammalian relative of yeast Rsp5. As in yeast, the mammalian metal transporter DMT1 lacks a PPxY motif that is required for binding to Nedd4 ligases; this is circumvented by binding of DMT1 to Ndfip1 whose PPxY motifs are capable of binding to WW domains of Nedd4–2 (18). It appears that multiple members of the Nedd4 family are capable of binding to Ndfip1 for E3 ligase activity; in non-neuronal cells, Ndfip1 modulates Fe entry by interacting with the E3 ligase, WWP2, for DMT1 regulation (26). Hence, protection against toxicity by metals through the ubiquitination of metal transporters is an ancient and highly conserved mechanism.
Ubiquitination of proteins is a potent mechanism used for regulating protein stability, protein activity, and subcellular localization (27). The nervous system uses this versatile process in the development and migration of neurons, elaboration of synapses, and maintenance of neuronal circuits (28). Another aspect of the present study is the demonstration that protein ubiquitination by Ndfip1 via Nedd4–2 is instrumental for improving the survival of neurons under stress from metal toxicity, and as previously reported, for traumatic brain injury (15). In particular, the involvement of Nedd4-family of E3 ligases is significant, given that this family of genes was originally cloned in the developing nervous system (29), and its function in the nervous system has recently gained prominence. For example, in Drosophila, Nedd4 binding and ubiquitination of Commissureless has important effects in regulating axon guidance by the Commissureless target, Roundabout (30). In the rat dorsal ganglion, Nedd4–2 binding and ubiquitination of TrkA receptor selectively influences the survival of NGF-but not BDNF-dependent neurons (31). The present study adds a further dimension to this important mechanism in the nervous system by emphasizing the role of Nedd4 adaptors such as Ndfip1. So while many proteins may not be recognized by Nedd4 ligases due to the lack of the recognition motif PPxY, these proteins may bind to Ndfip1 and be vicariously presented for ubiquitination by Nedd4 proteins. The list of known Ndfip1 targets remains small; apart from DMT1, Ndfip1 is linked to the ubiquitination of JunB (23). The use of adaptors such as Ndfip1 can endow greater versatility to the system; by adding a higher level of selectivity and access to targets that may otherwise be inaccessible to Nedd4. Since Ndfip1 can also be ubiquitinated by Nedd4 (22), the process may be rapidly turned off by degradation of Ndfip1.
In conclusion, we have shown that human neurons in culture are susceptible to metal toxicity in cellular assays involving increasing concentrations of environmental Fe and Co. This serves as an in vitro paradigm of brain injury paralleling stroke and brain trauma where transient levels of metals are known to flood the injury site. We have identified DMT1 as the key player in the entry of metal ions in the brain, and this process may be reversed by DMT1 degradation. Regulation of DMT1 levels is achieved by ubiquitination, via binding of DMT1 to the Nedd4-adaptor, Ndfip1. We demonstrate that increasing Ndfip1 in neurons provides effective defense against high concentrations of metal. In addition to these acute assays, we also show that Ndfip1 is critical for the longer-term regulation of metal in the brain. In mice lacking Ndfip1, Fe is abnormally concentrated in cortical neurons suggesting a housekeeping role for Ndfip1 in metal homeostasis.
Materials and Methods
Human Embryonic Cortical Neuron Cultures.
Human cortices from 18-week-old embryos were obtained from NSW Fetal Tissue Consortium. Brain tissue was collected in HEPES buffered MEM and all procedures were conducted under sterile conditions. Tissue was dissociated and plated in neurobasal media supplemented with 1 × B27 (Invitrogen) and 0.5 mM L-glutamate. Neurons were seeded at 4 × 106 cells per plate, and cultured with 5% CO2 at 37 °C. Transfection of neurons was performed using Amaxa according to the manufactures protocol. Inducible Ndfip1 plasmids (2 μg total) were added and cells electroporated using the mouse hippocampal neuron protocol.
Cell Culture and Lentiviral Infection.
Metal Toxicity and Apoptosis Quantification by Flow Cytometry.
Cobalt Uptake and Staining of SH-SY5Y Cells.
Western Blots and Immunoprecipitation Assays.
Immunohistochemistry.
Histology and Brain Fe Measurements.
Supplementary Material
Acknowledgments.
This work was supported by a grant from the Victorian Neurotrauma Initiative, a joint venture between the Transport Accident Commission of Victoria with the Department of Innovation, Industry and Regional Development, and the National Health and Medical Research Council (NHMRC). J.H. is supported by a Howard Florey Centenary Fellowship awarded by the NHMRC. This work was carried out under the NHMRC National Statement on Ethical Conduct in research involving humans and approved by Melbourne Health Human Research Ethics committee.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.A.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904880106/DCSupplemental.
References
- 1.Bush AI. Metals and neuroscience. Curr Opin Chem Biol. 2000;4:184–191. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 2.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing, and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 4.Valko M, Morris H, Cronin MT. Metals, toxicity, and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 5.Taylor EM, Crowe A, Morgan EH. Transferrin and iron uptake by the brain: Effects of altered iron status. J Neurochem. 1991;57:1584–1592. doi: 10.1111/j.1471-4159.1991.tb06355.x. [DOI] [PubMed] [Google Scholar]
- 6.Picard V, Govoni G, Jabado N, Gros P. Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J Biol Chem. 2000;275:35738–35745. doi: 10.1074/jbc.M005387200. [DOI] [PubMed] [Google Scholar]
- 7.Forbes JR, Gros P. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood. 2003;102:1884–1892. doi: 10.1182/blood-2003-02-0425. [DOI] [PubMed] [Google Scholar]
- 8.Gunshin H, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 9.Fleming MD, et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 10.Fleming MD, et al. Nramp2 is mutated in the anemic Belgrade (b) rat: Evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar J, et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proc Natl Acad Sci USA. 2008;105:18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunez MT, et al. Iron-activated iron uptake: A positive feedback loop mediated by iron regulatory protein 1. Biometals. 2003;16:83–90. doi: 10.1023/a:1020743405347. [DOI] [PubMed] [Google Scholar]
- 13.Liu XF, Supek F, Nelson N, Culotta VC. Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J Biol Chem. 1997;272:11763–11769. doi: 10.1074/jbc.272.18.11763. [DOI] [PubMed] [Google Scholar]
- 14.Liu XF, Culotta VC. Post-translation control of Nramp metal transport in yeast. Role of metal ions and the BSD2 gene. J Biol Chem. 1999;274:4863–4868. doi: 10.1074/jbc.274.8.4863. [DOI] [PubMed] [Google Scholar]
- 15.Sang Q, et al. Nedd4-WW domain-binding protein 5 (Ndfip1) is associated with neuronal survival after acute cortical brain injury. J Neurosci. 2006;26:7234–7244. doi: 10.1523/JNEUROSCI.1398-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vince JE, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 17.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 18.Shearwin-Whyatt L, Dalton HE, Foot N, Kumar S. Regulation of functional diversity within the Nedd4 family by accessory and adaptor proteins. Bioessays. 2006;28:617–628. doi: 10.1002/bies.20422. [DOI] [PubMed] [Google Scholar]
- 19.Wetli HA, Buckett PD, Wessling-Resnick M. Small-molecule screening identifies the selanazal drug ebselen as a potent inhibitor of DMT1-mediated iron uptake. Chem Biol. 2006;13:965–972. doi: 10.1016/j.chembiol.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi T, et al. Ebselen in acute ischemic stroke: A placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 21.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 22.Harvey KF, Shearwin-Whyatt LM, Fotia A, Parton RG, Kumar S. N4WBP5, a potential target for ubiquitination by the Nedd4 family of proteins, is a novel Golgi-associated protein. J Biol Chem. 2002;277:9307–9317. doi: 10.1074/jbc.M110443200. [DOI] [PubMed] [Google Scholar]
- 23.Oliver PM, et al. Ndfip1 protein promotes the function of itch ubiquitin ligase to prevent T cell activation and T helper 2 cell-mediated inflammation. Immunity. 2006;25:929–940. doi: 10.1016/j.immuni.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupic F, et al. Duodenal mRNA expression of iron related genes in response to iron loading and iron deficiency in four strains of mice. Gut. 2002;51:648–653. doi: 10.1136/gut.51.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheah JH, et al. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron. 2006;51:431–440. doi: 10.1016/j.neuron.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foot NJ, et al. Regulation of the divalent metal ion transporter DMT1 and iron homeostasis by a ubiquitin-dependent mechanism involving Ndfips and WWP2. Blood. 2008;112:4268–4275. doi: 10.1182/blood-2008-04-150953. [DOI] [PubMed] [Google Scholar]
- 27.Ciechanover A. Proteolysis: From the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 28.DiAntonio A, Hicke L. Ubiquitin-dependent regulation of the synapse. Annu Rev Neurosci. 2004;27:223–246. doi: 10.1146/annurev.neuro.27.070203.144317. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, et al. cDNA cloning, expression analysis, and mapping of the mouse Nedd4 gene. Genomics. 1997;40:435–443. doi: 10.1006/geno.1996.4582. [DOI] [PubMed] [Google Scholar]
- 30.Myat A, et al. Drosophila Nedd4, a ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the roundabout receptor. Neuron. 2002;35:447–459. doi: 10.1016/s0896-6273(02)00795-x. [DOI] [PubMed] [Google Scholar]
- 31.Arevalo JC, et al. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50:549–559. doi: 10.1016/j.neuron.2006.03.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.