Abstract
RhoC is one of the Ras-homologous family genes which has been implicated in tumorigenesis and tumor progression. However, the exact role of RhoC is controversial and yet to be clarified. We have examined the effect of RhoC on prostate tumor cells and found that RhoC had no effect on cell proliferation in vitro or tumor growth in mice. However, RhoC significantly enhanced the metastatic ability of the tumor cells in these animals, suggesting that RhoC affects only metastases but not growth of prostate tumor cells. The results of our immunohistochemical analyses on tumor specimens from 63 prostate cancer patients indicate that RhoC expression had no significant correlation with Gleason grade. However, the expression of RhoC showed significant positive correlation with both lymph-node and distant metastasis, and it was inversely correlated to patient survival. We also found that RhoC significantly augmented the ability of invasion and motility of prostate tumor cells by activating MMP2 and MMP9 in vitro. The results of our antibody array analysis for signal molecules revealed that RhoC significantly activated kinases including MAPK, FAK, Akt and Pyk2. Inhibition of Pyk2 kinase blocked the RhoC-dependent activation of FAK, MAPK and Akt followed by suppression of MMP2 and MMP9. Inhibitors of both MAPK and Akt also significantly blocked activities of these matrix metalloproteinases. Therefore, our results indicate that RhoC promotes tumor metastases in prostate cancer by sequential activation of Pyk2, FAK, MAPK and Akt followed by up-regulation of MMP2 and MMP9, which results in stimulation of invasiveness of tumor cells.
Keywords: metastasis, invasion, Pyk2, Akt, MMP2, MMP9
Introduction
The family of Ras-homologous (Rho) genes that play central roles in cell proliferation and motility has been implicated in tumorigenesis as well as metastatic progression (1). The Rho subfamily includes RhoA, RhoB and RhoC and they share 85% amino acid sequence identity (2). Despite this similarity, each protein has different affinity to various downstream effectors and shows different subcellular localization, suggesting that they have distinct roles in normal cellular function as well as in tumor pathogenesis (3). RhoA appears to be involved in the regulation of actomyosin contractility, and the over-expression of RhoA has been shown to promote invasiveness of tumor cells (2, 4-6). On the other hand, RhoB plays a role in controlling cytokine trafficking as well as in apoptosis induced by DNA damaging agents and has been suggested to act as a suppressor of tumor progression (7, 8).
Recently, RhoC has been shown to be up-regulated in various types of cancer including inflammatory breast cancer (9), hepatocellular carcinoma (10) and non-small cell lung cancer (11). However, the exact role of RhoC in tumorigenesis and tumor progression has remained controversial and needs further clarification. Pille et al. previously found that blocking RhoC expression by siRNA significantly inhibited cell proliferation of breast tumor cells in vitro as well as tumor growth in an animal model (12). More recently, Faried et al. also reported that ectopic expression of RhoC in oesophageal carcinoma cells significantly enhanced the growth of tumor in nude mice. These results suggest that RhoC plays a critical role in cell proliferation and tumor growth both in vitro and in vivo (13). On the contrary, Ikoma et al. reported that ectopic expression of RhoC using retroviral vector in Lewis lung carcinoma cells showed no significant difference in primary tumor growth in mice. However, the rate of lymph-node metastasis was significantly enhanced in these animals (14). In agreement with these results, Hakem et al. recently constructed RhoC knockout mouse and found that loss of RhoC does not affect tumorigenesis but significantly decreased the metastasis in this mouse, suggesting that RhoC is involved only in metastasis but not in tumor cell proliferation (15). These apparent contradictory results by different groups may be due to the difference in the systems or it may be due to the dependency of RhoC on cellular context. Therefore, it is critical to take a more systematic approach of testing the gene both in vitro and in vivo and to validate the outcome results in a clinical setting for each organ or tissue type in order to further clarify the role of RhoC in tumor progression. In this study, we found that RhoC promotes tumor metastasis but not tumor growth by sequential activation of Pyk2, FAK, MAPK and Akt followed by up-regulation of MMP2 and MMP9 in prostate tumor cells and that the expression of RhoC serves as a marker to predict metastatic status and survival of prostate cancer patients.
Materials and Methods
Cell culture and reagents
Human prostate cancer cell line PC3 was obtained from American Type Culture Collection (Manassas, VA), and human prostate cancer cell line PC3MM was kindly provided by Dr. I. J. Fidler (The University of Texas M. D. Anderson Cancer Center, Houston, TX). PC3MM/tet cell line was previously established as a derivative of PC3MM and contains the tetracycline-inducible suppressor. Rat prostate cancer cell line AT2.1 was a gift from Dr. C. W. Rinker-Schaeffer (University of Chicago, Chicago, IL). All cell lines were cultured in RPMI 1640 medium supplemented with 10% FBS (fetal bovine serum), streptomycin (100 μg/ml), penicillin (100 units/ml), and 250 nM dexamethasone, at 37°C in a 5% CO2 atmosphere. The PI3k/Akt inhibitor, Ly294002 and MAPK inhibitor, PD98059, were purchased from Sigma Co and Calbiochem, respectively. FAK inhibitor, TAE226, was previously described and kindly provided by Dr. Honda (16).
Construction of Expression Vectors
To generate a RhoC expression vector, cDNA of the RhoC gene was isolated by PCR amplification from a human cDNA library using a forward primer containing a Flag-tagged Kozak sequence and EcoRI linker and a reverse primer including a XhoI linker. The PCR product was then cloned into the mammalian expression vector pcDNA3 (Invitrogen). To construct a tetracycline-inducible RhoC expression plasmid, the fragment of the RhoC gene in pCDNA3 was subcloned into pcDNA5/TO (Invitrogen) at BamHI /XhoI site. The RhoC expression plasmids or the vector alone were transfected into the AT2.1, PC3MM and PC3MM/tet cells using LipofectAMINE (Invitrogen). To establish stable clones, transfected cells were treated with G418 or Hygromycin and drug-resistant colonies were selected followed by testing RhoC expression by Western blot.
shRNA
Five individual shRNAs against the Pyk2 gene were purchased from Open Biosystem (Huntsville, AL). shRNA with scrambled sequence was purchased from Addgene and used as a negative control (Cambridge, MA). The shRNAs were transfected into the prostate cancer cells using LipofectAMINE (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol, and the culture was further incubated for 48 hours before harvesting the cells for assays.
Western blot analysis
Cells were collected and dissolved in loading dye solution (125mM Tris-HCL, 4% SDS, 20% Glycerol, 10% β-2- mercaptoethanol, 0.04% Bromophenol Blue), boiled for 5 minutes and subjected to 8-12 % SDS-PAGE. Proteins were transferred to nitrocellulose membranes that were then treated with antibodies against anti-Flag (Sigma-Aldrich, St Louis, Mo), anti-β-tubulin (Upstate Biotechnology, Lake Placid, NY), anti-phospho-Pyk2 (Tyr579/580, Sigma-Aldrich), anti-Pyk2 (Cell Signaling Technology), anti-phospho-Akt (Ser473, Cell Signaling Technology, Danvers, MA), anti-Akt (Cell Signaling Technology), anti-phospho-FAK (Tyr397, Sigma-Aldrich), anti-FAK (Cell Signaling Technology) or anti-phospho-MAPK (Thr183, Sigma-Aldrich) or anti-MAPK (Cell Signaling Technology). The membranes were then incubated with HRP-conjugated secondary antibodies and visualized by the ECL Plus system (Amersham Life Sciences, Piscataway, NJ).
Cell growth assay
Cell lines with or without expressing RhoC gene were cultured in the RPMI1650 medium. At each time point, cells were trypsinized, serially diluted and re-plated in Petri dishes. The resultant colonies were stained with crystal violet and the number of colonies was visually counted. For thymidine uptake assays, cells were treated with or without tetracycline for 24 hours and 3H-thymidine was added to the culture. After 3 hours and 12 hours, cells were collected and acid-insoluble radio-activities were measured by scintillation counter.
Spontaneous metastasis assay
Rat prostate tumor cells AT2.1 (0.5 × 106 cells in 0.2 ml of PBS) were injected s.c. in the dorsal flank of 5-week-old SCID mice (Harlan Sprague-Dawley, Indianapolis, IN). Mice were monitored daily and the tumor volume was measured as an index of the growth rate using the equation: volume = (width + length) / 2 × width × length × 0.5236. The doubling time of tumor during the fastest growing period was calculated by measuring the tumor volume every 4 days. Mice were sacrificed 4 weeks after the inoculation of the cells, and metastatic lesions on the lungs were counted macroscopically.
Immunohistochemical analysis
Formaldehyde-fixed and paraffin embedded tissue specimens from 63 prostate cancer patients were obtained from surgical pathology archives of the Akita Red Cross Hospital (Akita, Japan). Four-micron-thick sections were cut from the paraffin blocks of prostate tumors and mounted on charged glass slides. The sections were deparaffinized and rehydrated, and antigen retrieval was done by heating the slide in 25 mM sodium citrate buffer (pH 9.0) at 80°C for 30 minutes. The slides were incubated overnight at 4°C with anti-RhoC antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-Phospho-Akt (Ser473, Cell Signaling Technology). The sections were then incubated with the HRP-conjugated anti-goat secondary antibody, and 3,3′-diaminobenzidine substrate chromogen solution (Envision Plus kit, DAKO Corp., Carpinteria, CA) was applied followed by counterstaining with hematoxylin. Conditions of immunohistochemical staining with other antibodies (NDRG1, AR, and PTEN) were described previously (17). Results of the immunohistochemistry for RhoC were judged by two independent persons (M.I. and K.W.) based on the intensity of staining combined with percentage of cells with positive staining.
In vitro Motility and Invasion assay
For the motility assay, 1×105 cells were added to the cell culture inserts with microporous membrane without any extracellular matrix coating (Becton Dickinson, Bedford, MA) and RPMI medium containing 20% FBS was added to the bottom chamber. The cells were then incubated for 24 hours at 37°C, and the upper chamber was removed. The cells on the bottom of the upper chambers were stained with tetrazolium dye, and the number of cells was counted under a microscope. For in vitro invasion assay, the working method was similar to that described above, except that the inserts of the chambers to which the cells were seeded were coated with Matrigel (Becton Dickinson).
Wound-healing migration assay
Cells were seeded in a 10cm dish and cultured to confluency. The cell monolayer was then scraped in a form of a cross with a plastic pipette tip. Three “wounded” areas were marked for orientation and photographed by a phase-contrast microscopy before and after 24 hours of incubation.
Real-time reverse transcription-PCR
Forty eight hours after transfection of appropriate plasmid DNA to the cells or 48 hours after induction by tetracycline, total RNA was isolated from the cells and reverse transcribed using random hexamer and MuLV reverse transcriptase (Applied Biosystems, Foster, CA). The cDNA was then amplified with a pair of forward and reverse primers for RhoC (5′-TAAGAAGGACCTGAGGCAAG and 5′-ATCTCAGAGAATGGGACAGC), MMP2 (5′-TGATGGTGTCTGCTGGAAAG and GACACGTGAAAAGTGCCTTG), MMP9 (5′-GGAGACCTGAGAACCAATCTC and 5′ -TCCAATAGGTGATGTTGTGGT), human β -actin (5′-TGAGACCTTCAACACCCCAGCCATG and 5′- GTAGATGGGCACAGTGTGGGTG), Pyk2 (5′-GCTAGACGGCAGATGAAAGT and 5′-AAGCAGACCTTGAGGATACG), Akt1 (5′-ACGTGTACGAGAAGAAGCTC and 5′-ACCCGAGAAATAAAAACCAT) and Akt2 (5′-ACCCAACACCTTTGTCATAC and 5′-AGTCGAAGTCATTCATGGTC). PCRs were done using the Dynamo SYBRGreen qPCR kit (New England Biolabs, Ipswich, MA) and DNA Engine Opticon2 System (MJ Research, Waltham, MA). The thermal cycling conditions composed of an initial denaturation step at 95 °C for 5 minutes followed by 30 cycles of PCR using the following profile: 94°C for 30 seconds, 57°C for 30 seconds, and 72°C for 30 seconds.
Gelatin Zymograph assay
For Zymography assay, cells (2.5 × 105) were seeded in 12-well plates and incubated for 48 hours. Supernatants were collected and mixed with sample buffer followed by electrophoresis on a 10% SDS-polyacrylamide gel containing 5 mg/ml gelatin. The gel was washed with 2.5% Triton-X solution for 2 hours and further incubated in the reaction buffer (50mM Tris-HCl, 5mM CaCl2, 1uM ZnCl2, 1% Triton-X-100) for an additional 18 hours at room temperature. The gel was then stained with 0.5% Coomassie Blue for 9 hours and subsequently immersed with destaining buffer (30% Methanol, 10% Acetic Acid) for 12 hours. The image was photographed and the intensity of each band was digitally quantified.
Antibody Microarray
Antibody microarray was performed using a Panorama Antibody Microarray-Cell Signaling kit (Sigma-Aldrich) according to the manufacturer’s instruction. Briefly, 1.5 × 107 cells were seeded in T-75 flasks and incubated for 48 hours in the medium with or without tetracycline. Cells were collected and protein samples were prepared according to the manufacturer’s protocol. These protein samples were labeled with Cy3 or Cy5 (Amersham Biosciences, UK) and subjected to Antibody Microarray (Sigma-Aldrich) analysis. The array slides were scanned by GenePix Personal 4100A scanner (Molecular Devices, Concord, ON, Canada) and the data was analyzed by GenePix Pro 5.0 (Molecular Devices).
Statistical analysis
For in vitro experiments and animal studies, t-test or one-way ANOVA was used to calculate the P values. The association between RhoC and other clinical markers was calculated by Chi-square test. The Kaplan-Meier method was used to calculate the overall survival rate, and prognostic significance was evaluated by the log-rank test. Univariate and multivariate analyses for the prognostic value of RhoC was performed by the Cox proportional hazard-regression model. For all of the statistical tests, the significance was defined as P < 0.05. SPSS software was used in all cases.
Results
RhoC promotes tumor metastasis, but not cell growth
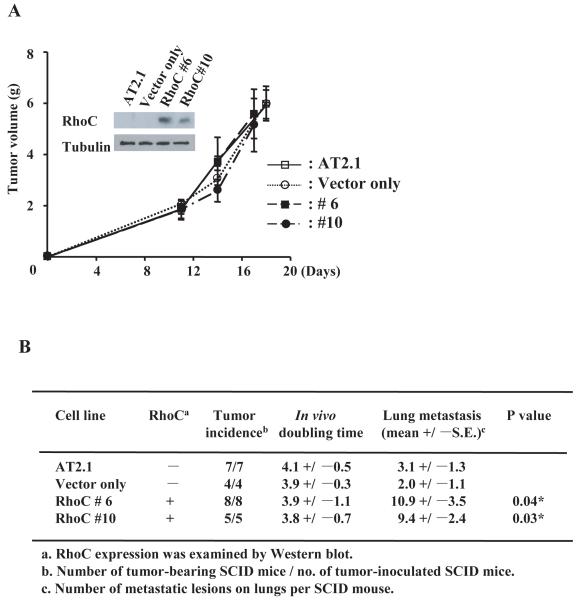
To understand the role of RhoC in prostate cancer, we first established permanent cell lines expressing RhoC using the rat prostate carcinoma cell line AT2.1 which has poor metastatic potential (18). These cell lines expressing RhoC (clone #6, #10) and a clone containing only the vector as well as the parental cell line, AT2.1, were individually injected s.c. into SCID mice. The mice were monitored for the formation and the growth rate of tumors and then sacrificed 3 weeks after the inoculation of the cells. As shown in Fig. 1A, all of the clones and the parental cells formed primary tumors in the animals with similar growth rates during the 3 weeks period, suggesting that RhoC does not have an effect on tumorigenesis or tumor growth. On the other hand, the clones stably expressing RhoC showed a significantly higher incidence of lung metastases compared to the parental cell line and the vector-only clones (Fig. 1B). These results strongly suggest that RhoC can promote the metastatic process of prostate cancer cells without affecting tumorigenicity in vivo. We also examined the effect of RhoC on the growth of these cells in vitro. The results of colorimetric assay for the duration of 72 hours indicate that there was no significant difference in the growth rate between the cells with and without RhoC (Supplementary Fig. S1A). We then examined the rate of DNA synthesis of the cells with and without the expression of RhoC and found that there was no significant difference between these cells (Supplementary Fig. S1B). Furthermore, we established a human prostate cell line, PC3MM/tet/RhoC, which contains the tetracycline-inducible RhoC gene, as well as PC3 cell lines that did or did not ectopically express RhoC. We then examined the rate of cell growth and DNA synthesis of these cells. Again, we found that Rho did not affect the rate of proliferation of the cells (Supplementary Fig. S1A, B), which further supports our notion that RhoC has no apparent role in the growth of prostate cancer cells, while it significantly promotes tumor metastasis.
Figure 1.
RhoC promotes tumor metastasis without affecting the primary tumor growth in vivo. The RhoC expression plasmid was introduced into a low-metastatic rat prostate cell line, AT2.1, and clones (#6 and #10) that constitutively express RhoC were established. As a control, the original vector was also cloned into AT2.1. These clones as well as the parental line were injected s.c. into SCID mice as described previously. The volume of the primary tumor for each clone at the indicated time was measured using the equation, Volume = (Width + Length)/2 × W × L × 0.5236 (A). The inserted photo shows a result of Western blot of RhoC expression for each clone. Mice were sacrificed 4 weeks after the inoculation of the cells, and metastatic lesions on the lungs were counted macroscopically (B). * indicates statistically significant difference (P<0.05).
RhoC expression is significantly increased with advancement of human prostate cancer
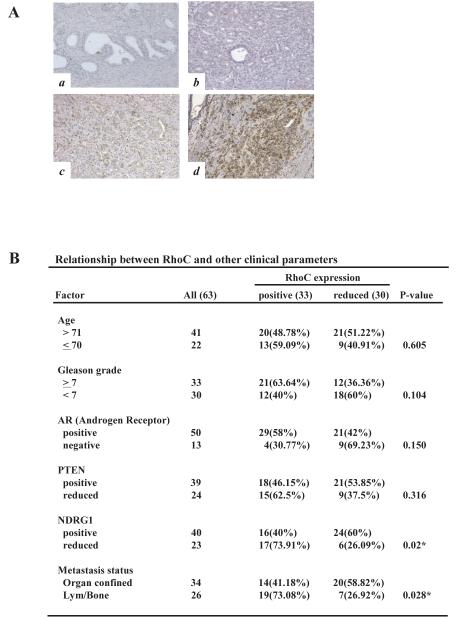
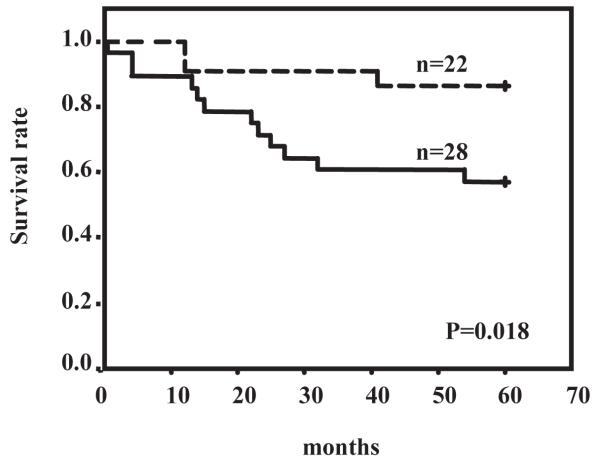
To further corroborate our results in a clinical setting, we examined the status of RhoC expression and its relationship to different clinicopathological factors in prostate cancer by immunohistochemical analysis on 63 prostate tumor specimens. They were randomly selected from surgical pathology archives dating from 1988 to 2001. As shown in Fig. 2A and B, the expression of RhoC was found to be strongly elevated in high-grade tumors, particularly in specimens from patients with metastatic disease, compared to normal prostatic tissue or low-grade tumors. The results of our statistical analyses indicate that RhoC is strongly expressed in tumors with higher Gleason grade although the correlations is not statistically significant (Fig. 2B). Importantly, the RhoC expression showed significant positive correlation to the metastases status of the patients (P=0.028). It is also noted that RhoC expression showed significant inverse correlation to that of NDRG1 (P=0.02) which has been recently shown to be a tumor metastases suppressor in prostate cancer (19). These results suggest that the expression of RhoC is up-regulated at a relatively late stage and directly involved in metastatic progression of prostate cancer, which is in good agreement with our in vivo data. Furthermore, our results of survival analyses on 50 prostate cancer patients over a period of 5 years indicate that patients with positive expression of RhoC had significantly worse overall survival rate than the patients with reduced expression of the gene (P=0.018, log-rank test) (Fig. 3). The results of univariate Cox regression analysis revealed that the death risk of patients with increased RhoC expression was 4.8 times higher than the risk of patients with RhoC negativity. However, when we performed a multivariate analysis for RhoC, Gleason score and metastasis, only the metastasis status gave a significant value (P=0.015) and other two factors were excluded. The fact that multivariate analyses of these three factors excluded RhoC status indicate that the profiles of the RhoC expression and metastasis status of patients significantly overlaps and that each factor has enough “power” of predicting patient outcome. In fact, when we did multivariate analysis for a combination of RhoC status and Gleason score, which is the most widely used pathological marker for prostate cancer, RhoC status turned out to be a better predicting marker than Gleason score (P=0.037 and P=0.237 for RhoC and Gleason score status, respectively). Although Rho C expression did not significantly and independently predict survival compared to metastasis, increased Rho C correlates with aggressive disease which could account for increased metastatic disease.
Figure 2.
Immunohistochemical analysis of RhoC in human prostate cancer. Immunohistochemical staining was performed on paraffin-embedded human prostate tissue sections using anti-RhoC antibody and the results were compared to other clinical parameters. Representative field with immunostaining for RhoC were shown in (A): normal prostate tissue (a), low grade carcinoma (b), high grade localized carcinoma (c), and high grade-metastatic carcinoma tissue (d). (B) Association of RhoC with other clinical parameters was analyzed by standard chi-square test using SPSS software. * indicates statistically significant difference (P<0.05).
Figure 3.
Prognostic value of RhoC expression. Overall survival rate over a period of 5 years was calculated in 50 patients with prostate cancer in relation to the expression of the RhoC genes by Kaplan-Meier method. The P value (=0.018) was determined by a log rank test. Solid line and dotted line indicate RhoC positive patients and patients with reduced expression of RhoC, respectively.
RhoC promotes invasiveness and motility of prostate cancer cells in vitro
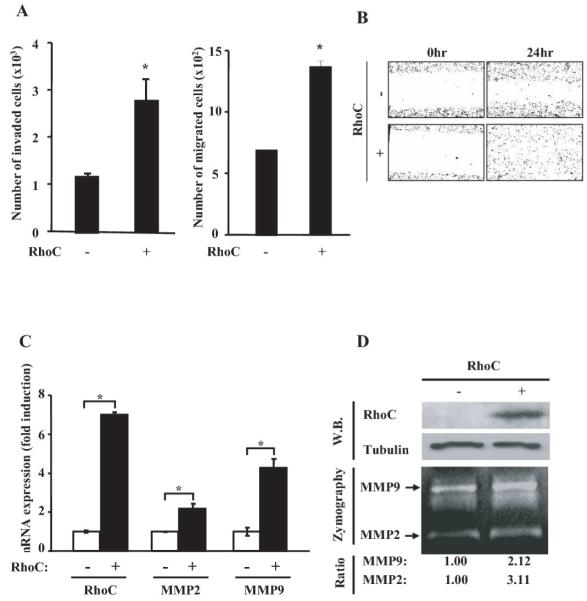
To understand how RhoC contributes to the progression of prostate cancer, we ectopically expressed the RhoC gene in the human prostate cancer cell line, PC3, followed by examining the invasiveness and migration of the cells in vitro. We found that the expression of RhoC significantly enhanced both cell invasiveness and migration (P=0.03 and 0.004, respectively) (Fig. 4A), which is in good agreement with the previous results of Yao et al. (20). The effect of RhoC on cell motility was also examined by the “wound healing” assay. As shown in Fig. 4B, cells with ectopically expressing RhoC showed much higher rate of motility compared to the cells with an empty-vector transfectant. These results strongly suggest that RhoC promotes metastasis by enhancing invasiveness and/or motility of tumor cells. Because the invasive ability of tumor cells is known to be often correlated with their production of secretory proteases (21), we examined the expression of MMP2 and MMP9 in the cells that over-expressed RhoC. As shown in Fig. 4C, quantitative RT-PCR analysis for the cell over-expressing RhoC significantly augmented the level of the expression of the MMP2 and MMP9 genes (P= 0.049 and 0.02, respectively). These results were further validated by Gelatin Zymography and Western blot analyses as shown in Fig. 4D. Therefore, our results indicate that the invasiveness of tumor cells induced by RhoC is, at least in part, due to over-expression of MMP2 and MMP9.
Figure 4.
RhoC promotes invasiveness and motility of prostate cancer cells in vitro. (A) The RhoC expression plasmid (pcDNA3/RhoC) or the vector alone was transfected into the PC3 cell line. After 24hours, cells were collected and subjected to invasion (left panel) and migration (right panel) assays. * indicates statistically significant difference (P<0.05). (B) For the motility assay, the PC3 cells stably transfected with the RhoC expression plasmid or an empty vector were cultured to confluency. The monolayer was scratched by drawing lines and photographed under a microscope. After 24 hours of incubation, they were photographed again. (C) To test the effect of RhoC on MMP2 and MMP9, PC3 cells that have been stably transfected with the RhoC expression plasmid or an empty vector were cultured in 12-well plates. Cells were then collected and their total RNA was treated with DNase. The RNA was then subjected to qRT-PCR using specific primers for the RhoC, MMP2 and MMP9 genes. Results were presented as ratios of the expression level of each gene in RhoC-positive and RhoC-negative cells. * indicates statistically significant difference (P<0.05). (D) MMP2 and MMP9 activities in the conditioned medium from the PC3 cells with or without the RhoC expression plasmid as described in (C) were assayed by Gelatin Zymography. The image was photographed and the intensity of each band was digitally quantified. The expression of Flag-RhoC was confirmed by Western blot (upper panels).
RhoC activates MMP through the Pyk2 signal pathway
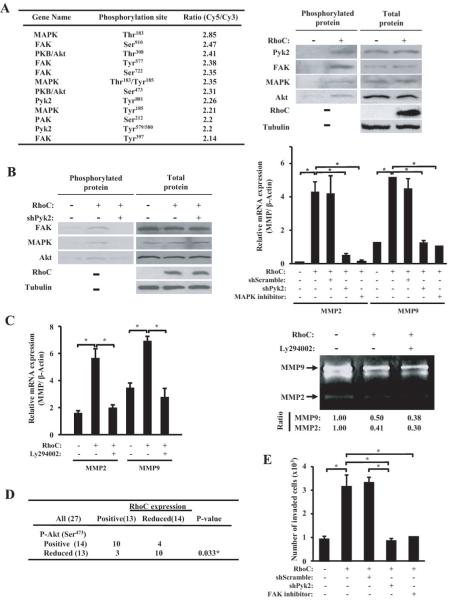
To further gain insight into signaling pathways by which RhoC promotes invasive phenotype, we prepared cell lysates from PC3MM/tet/RhoC with or without induction of the RhoC gene by tetracycline. The lysates were labeled with Cy3 and Cy5 and analyzed on an antibody microarray which contained 224 antibodies for various key molecules of cell signaling and cell cycle, and the results of ratios were rank-ordered. As shown in Fig. 5A (left panel), ectopic expression of RhoC significantly phosphorylated a series of protein kinases including MAPK, FAK, Akt, and Pyk2. The result of the array analysis was also confirmed by Western blot using the antibodies specific to phosphorylated proteins as well as the antibodies to the total proteins for each signal molecules (Fig. 5A right panel; Supplementary Fig. S2A). These results suggest that RhoC can directly activate a cascade of signal pathways involving these key signal molecules that are closely related to cell motility and tumor progression.
Figure 5.
RhoC activates MMPs through the Pyk2/FAK pathway. (A) For antibody array analysis, cell lysates were prepared from the PC3MM/tet cells containing the tetracycline-inducible RhoC gene with or without induction of RhoC. The proteins were labeled with Cy3 or Cy5 and subjected to Antibody Microarray (Sigma-Aldrich, MO) analysis. The scanned data was analyzed by GenePix Pro 5.0 (Axon Instrument). The result of the antibody array data was confirmed by Western Blot using phospho-specific antibodies to Pyk2, FAK, MAPK and Akt as well as using antibodies to total protein of each corresponding gene. (B) PC3 cells stably transfected with the RhoC-expression plasmid or an empty vector were transfected with the expression plasmid of shRNA for Pyk2 or a scrambled sequence. After 48 hours, cells were collected and subjected to Western blot analysis using phospho-specific antibodies (left panel). To examine the effect of Pyk2 and MAPK on the MMP expression, the same set of cells were treated with or without the MAPK inhibitor, PD98059 (100 μM) for 48 hours. RNA was extracted from each sample (triplicate) and subjected to qRT-PCR using specific primers for MMP2 and MMP9 (right panel). (C) Effect of Akt phosphorylation on MMP expression was examined. Cells with or without expression of RhoC were treated with or without PI3K/Akt inhibitor, Ly294002 (100nM), for 48 hours. The cells were then collected and RNA was extracted followed by qRT-PCR analysis for MMP2 and MMP9 expression (left panel). The conditioned culture mediums of the same set of samples were subjected to Zymography assay for MMP2 and MMP9 (right panel). The image was photographed and the intensity of each band was digitally quantified. (D) To examine the clinical status of RhoC and p-Akt expression, 27 samples from prostate cancer patients were analyzed by immunohistochemistry using antibodies to RhoC and p-Akt. The result was analyzed by Chi-square test. (E) PC3 cells with or without expressing RhoC was treated with shPyk2 or the FAK-specific inhibitor, TAE226, for 48 hrs. The cells were then assayed for their invasiveness by using Matrigel invasion assay as described in Materials and Methods.
Pyk2 is a tyrosine kinase and belongs to a member of the focal adhesion kinase (FAK) subfamily which plays a critical role in cell migration and motility of various cell types (22, 23). Pyk2 is also known to be able to phosphorylate Akt (23). Therefore, we sought a possibility that Pyk2 is the immediate effecter of the RhoC signal and controls the downstream pathways. PC3/RhoC cells were transfected with the expression vector of shRNA targeted to Pyk2. After 48 hours of incubation, cell lysates were prepared and subjected to Western blot analysis using antibodies to RhoC, p-FAK, p-MAPK and p-Akt. As shown in Fig. 5B (left panel) and Supplementary Fig. S2B, induction of RhoC strongly phosphorylated FAK, MAPK and Akt, and this RhoC-dependent phosphorylation of these molecules was strongly blocked by addition of shRNA to the Pyk2 gene, suggesting that RhoC first activates Pyk2 which then phosphorylates FAK, MAPK and Akt. We then examined whether MMP2 and MMP9 is indeed activated by Pyk2 and MAPK in a RhoC-dependent manner. RNA was prepared from PC3/RhoC cells that were cultured in the presence or absence of shRNA for Pyk2 and the MAPK inhibitor, PD98059. RNAs were then examined for the expression of MMP2 and MMP9 by qRT-PCR. As shown in Fig. 5B (right panel) and Supplementary Fig. S2C (left panel), RhoC-dependent activation of both MMP2 and MMP9 was significantly abrogated in the presence of shRNA for Pyk2 or the MAPK inhibitor, suggesting that the activation of MMP2 and MMP9 by RhoC is at least partly due to phosphorylation of Pyk2 followed by the activation of MAPK. Because our results indicate that Akt is also phosphorylated at Ser473 by RhoC in a Pyk2-dependent fashion, we examined whether Akt is also involved in the activation of MMP2 and MMP9 in the RhoC signal pathway. As shown in Fig. 5C (left panel) and Supplementary Fig. S2C (right panel), we found that the RhoC-dependent induction of MMP2 and MMP9 was indeed significantly blocked by PI3K/Akt inhibitor, Ly294002. This result was further confirmed by Gelatin Zymography analysis as shown in Fig. 5C (right panel). To further corroborate the in vitro result, we examined 27 clinical specimens from prostate cancer patients by conducting immunohistochemistry using anti-RhoC and anti-phosphoAkt (Ser473) antibodies. As shown in Fig. 5D (left panel), we found that RhoC expression was significantly correlated to the expression of phospho-Akt in these tumor tissues. Therefore, these clinical data as well as the in vitro results strongly suggest that Akt is a part of the down-stream effector of RhoC signal and plays an important role in RhoC-dependent activation of MMP2 and MMP9. To further validate the role of Pyk2 and FAK in the RhoC-induced signal, we treated the PC3 cells that do or do not express RhoC with shPyk2 or the FAK-specific inhibitor, TAE226, followed by measuring invasiveness of these cells by the Matrigel invasion chamber assay. As shown in Fig. 5E, we found that inhibition of Pky2 and FAK indeed significantly blocked the RhoC-induced invasiveness of the prostate tumor cells, which strongly suggests the functional involvement of Pyk2 and FAK in the RhoC signaling pathway.
Discussion
RhoC has been shown to be involved in various types of tumors (9-11). However, the exact role of RhoC in tumor progression and its underlying mechanism are unclear, and the previous results from different groups have presented an apparently contradictory picture of the function of this gene (12-15). In this study, we have integrated multiple approaches, both in vitro and in vivo to clarify the functional role of RhoC in prostate cancer progression. Our results of animal experiment clearly indicate that RhoC plays a critical role in metastatic progression of prostate tumor but it is not essential for tumor cell growth. The results of immunohistochemical analysis of human prostate cancer specimens also indicate that RhoC expression is significantly correlated with the metastatic status of the patients but not with Gleason grade, which strongly support our notion that RhoC is implicated mainly in metastatic process but not in tumorigenesis. Importantly, the RhoC expression is inversely correlated with patient survival, suggesting that RhoC can serve as a prognostic marker as well as a potential therapeutic target for prostate cancer.
The molecular mechanism by which RhoC promotes tumor progression is an intriguing question. We have constructed a RhoC inducible cell line and examined its protein expression profile using an antibody array to clarify the signal pathway. The results of the array analysis revealed that Pyk2, FAK, MAPK and Akt were all phosphorylated upon induction of the RhoC expression, and the knockdown of Pyk2 resulted in significant reduction in phosphorylation of FAK, MAPK and Akt, suggesting that Pyk2 is the up-stream effector and plays a central role in the RhoC signal pathway. Pyk2 belongs to the subfamily of focal adhesion protein tyrosine kinases and it has been shown to be involved in cell migration, invasion and proliferation (24-28). It was reported that in the in vitro model of TGF beta-induced EMT, Pyk2 was strongly phosphorylated at Tyr881 and that Tyr580 during migration (22). It should be noted that both of these sites were found to be phosphorylated by our antibody array analysis (Fig. 5A). Pyk2 is capable of transducing signal via several known pathways, and one of the effectors is FAK which has been shown to be phosphorylated at Tyr397, Tyr576/577 and Tyr925 by Pyk2 (29). The results of our antibody array data also revealed that both of these sites were indeed phosphorylated upon induction of RhoC. These results suggest that RhoC activates FAK via phosphorylation of Pyk2. FAK is a focal-adhesion kinase and plays a critical role in cell migration and motility (30-32). The enhanced expression of FAK has been documented in a number of different types of human cancers (33-41). The phosphorylation of FAK is known to be linked to the activation of several downstream signaling including ERK and JNK/MAPK as well as PI3K/Akt (42, 43). Furthermore, it was previously shown that invasive ability of RhoC was significantly attenuated by a MAPK inhibitor in vitro (44). Notably, our results of knockdown experiment using Pyk2-specific shRNA has shown that the RhoC-dependent phosphorylation of both ERK/MAPK and Akt was significantly blocked by knockdown of Pyk2, suggesting that MAPK and Akt are activated by RhoC via phosphorylation of Pyk2 and FAK.
We have shown that RhoC promotes metastasis by augmenting motility and invasion of tumor cells (Fig. 4, 5) via activation of MMP2 and MMP9, two key proteases for invasion of tumor cells. It should be noted that the expression of both MMP2 and MMP9 were previously shown to be modulated by activation of Akt and MAPK (45-47). We indeed have shown that inhibitors of both molecules significantly blocked the RhoC-dependent activation of MMP2 and MMP9. In this context, it should be noted that Ruth et al. have recently shown that RhoC promoted invasion of human melanoma cells in a PI3K/Akt dependent manner (48). Our results also indicate that Akt was significantly phosphorylated at Ser473 by RhoC, and the phosphorylation of this serine residue has previously been found to be involved in motility and invasiveness of tumor cells (45, 46, 49). The activation of Akt has also been shown to be clinically associated with aggressiveness and earlier recurrence of prostate cancer (50). Collectively, our results indicate that RhoC enhances invasiveness and metastatic ability of tumor cells by activating the Pyk2/FAK pathway followed by phosphorylation of Akt and MAPK that in turn activate MMP2 and MMP9. RhoC is considered to serve as an independent prognostic marker to predict patient outcome, and an intervention of the RhoC signal may be an effective therapeutic strategy for prostate cancer.
Supplementary Material
Supplemental Figure 1. RhoC does not affect the cell proliferation in vitro. (A) AT2.1, PC3 and PC3mm/tet cells were transfected with the RhoC expression plasmid, and clones were established for each cell line. These cells were then cultured, trypsinized and serially diluted followed by plating in Petri dishes. The resultant colonies after 10 days were stained and their numbers were visually counted. The expression of RhoC and the induction of this protein by tetracycline were confirmed by Western blot (inserted photos). (B) The rate of DNA synthesis for each cell line with or without expression of RhoC was measured by thymidine incorporation assay.
Supplemental Figure 2. RhoC activates MMPs through the Pyk2/FAK pathway. (A) The result of antibody array data was confirmed by Western Blot using phospho-specific antibodies as well as antibodies to the total protein of each genes. Left panels show the result of the cell line of PC3MM/tetRhoC with or without expression of RhoC. The right panels are the result of AT2.1 with or without RhoC expression. (B) PC3MM/tet cells containing the tetracycline-inducible RhoC gene was tranfected with the expression plasmid of shRNA for Pyk2 or for scrambled sequence. After 6 hours, tetracycline was added to the medium and cells were further incubated for 72 hours. Cells were then collected and subjected to Western blot analysis using phospho-specific antibodies as well as antibodies to total protein for each gene. (C) To examine the effect of Pyk2 and MAPK on the MMP expression, the same set of cells were treated with or without shPyk2 or the MAPK inhibitor, PD98059 (100 M) for 48 hours. RNA was extracted from each sample (triplicate) and subjected to qRT-PCR using specific primers for MMP2 and MMP9 (left panel). To examine the effect of Akt on the RhoC signal, cells with or without induction of RhoC were treated with or without PI3K/Akt inhibitor, Ly294002 (100nM), for 48 hours. The cells were then collected and RNA was extracted followed by qRT-PCR analysis for MMP2 and MMP9 expression (right panel).
Acknowledgements
Grant support: NIH (1R01CA124650, 1R01CA129000 to KW), Department of Defense (PC031038, PC061256, BC044370 to KW), Illinois Department of Public Health, Penny Severns Breast, Cervical and Ovarian Cancer Research Fund, William McElroy Charitable Foundation, and American Lung Association, Illinois.
References
- 1.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301:43–9. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 4.Fiordalisi JJ, Keller PJ, Cox AD. PRL tyrosine phosphatases regulate rho family GTPases to promote invasion and motility. Cancer Res. 2006;66:3153–61. doi: 10.1158/0008-5472.CAN-05-3116. [DOI] [PubMed] [Google Scholar]
- 5.Yoshioka K, Matsumura F, Akedo H, Itoh K. Small GTP-binding protein Rho stimulates the actomyosin system, leading to invasion of tumor cells. J Biol Chem. 1998;273:5146–54. doi: 10.1074/jbc.273.9.5146. [DOI] [PubMed] [Google Scholar]
- 6.Yoshioka K, Nakamori S, Itoh K. Overexpression of small GTP-binding protein RhoA promotes invasion of tumor cells. Cancer Res. 1999;59:2004–10. [PubMed] [Google Scholar]
- 7.Prendergast GC. Actin’ up: RhoB in cancer and apoptosis. Nat Rev Cancer. 2001;1:162–8. doi: 10.1038/35101096. [DOI] [PubMed] [Google Scholar]
- 8.Mazieres J, Antonia T, Daste G, et al. Loss of RhoB expression in human lung cancer progression. Clin Cancer Res. 2004;10:2742–50. doi: 10.1158/1078-0432.ccr-03-0149. [DOI] [PubMed] [Google Scholar]
- 9.Kleer CG, Griffith KA, Sabel MS, et al. RhoC-GTPase is a novel tissue biomarker associated with biologically aggressive carcinomas of the breast. Breast Cancer Res Treat. 2005;93:101–10. doi: 10.1007/s10549-005-4170-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Yang LY, Huang GW, et al. Genomic analysis reveals RhoC as a potential marker in hepatocellular carcinoma with poor prognosis. Br J Cancer. 2004;90:2349–55. doi: 10.1038/sj.bjc.6601749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shikada Y, Yoshino I, Okamoto T, Fukuyama S, Kameyama T, Maehara Y. Higher expression of RhoC is related to invasiveness in non-small cell lung carcinoma. Clin Cancer Res. 2003;9:5282–6. [PubMed] [Google Scholar]
- 12.Pillé JY, Denoyelle C, Varet J, et al. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol Ther. 2005;11:267–74. doi: 10.1016/j.ymthe.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Faried A, Faried LS, Kimura H, et al. RhoA and RhoC proteins promote both cell proliferation and cell invasion of human oesophageal squamous cell carcinoma cell lines in vitro and in vivo. Eur J Cancer. 2006;42:1455–65. doi: 10.1016/j.ejca.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Ikoma T, Takahashi T, Nagano S, et al. A definitive role of RhoC in metastasis of orthotopic lung cancer in mice. Clin Cancer Res. 2004;10:1192–200. doi: 10.1158/1078-0432.ccr-03-0275. [DOI] [PubMed] [Google Scholar]
- 15.Hakem A, Sanchez-Sweatman O, You-Ten A, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–9. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halder J, Lin YG, Merritt WM, et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67:10976–83. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- 17.Bandyopadhyay S, Pai SK, Hirota S, et al. PTEN up-regulates the tumor metastasis suppressor gene Drg-1 in prostate and breast cancer. Cancer Res. 2004;64:7655–60. doi: 10.1158/0008-5472.CAN-04-1623. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs JT, Isaacs WB, Feitz WF, Scheres J. Establishment and characterization of seven Dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate. 1986;9:261–81. doi: 10.1002/pros.2990090306. [DOI] [PubMed] [Google Scholar]
- 19.Bandyopadhyay S, Pai SK, Gross SC, et al. The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res. 2003;63:1731–6. [PubMed] [Google Scholar]
- 20.Yao H, Dashner EJ, van Golen CM, van Golen KL. RhoC GTPase is required for PC-3 prostate cancer cell invasion but not motility. Oncogene. 2006;25:2285–96. doi: 10.1038/sj.onc.1209260. [DOI] [PubMed] [Google Scholar]
- 21.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Yano H, Schaefer E, Sabe H. Different modes and qualities of tyrosine phosphorylation of Fak and Pyk2 during epithelial-mesenchymal transdifferentiation and cell migration: analysis of specific phosphorylation events using site-directed antibodies. Oncogene. 2001;20:2626–35. doi: 10.1038/sj.onc.1204359. [DOI] [PubMed] [Google Scholar]
- 23.Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25:6889–98. doi: 10.1128/MCB.25.16.6889-6898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwabara K, Nakaoka T, Sato K, Nishishita T, Sasaki T, Yamashita N. Differential regulation of cell migration and proliferation through proline-rich tyrosine kinase 2 in endothelial cells. Endocrinology. 2004;145:3324–30. doi: 10.1210/en.2003-1433. [DOI] [PubMed] [Google Scholar]
- 25.Lipinski CA, Tran NL, Menashi E, et al. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005 May;7(5):435–45. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang X, Jacamo R, Zhukova E, Sinnett-Smith J, Rozengurt E. RNA interference reveals a differential role of FAK and Pyk2 in cell migration, leading edge formation and increase in focal adhesions induced by LPA in intestinal epithelial cells. J Cell Physiol. 2006;207:816–28. doi: 10.1002/jcp.20629. [DOI] [PubMed] [Google Scholar]
- 27.Fernandis AZ, Prasad A, Band H, Klösel R, Ganju RK. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23:157–67. doi: 10.1038/sj.onc.1206910. [DOI] [PubMed] [Google Scholar]
- 28.Zrihan-Licht S, Fu Y, Settleman J, et al. RAFTK/Pyk2 tyrosine kinase mediates the association of p190 RhoGAP with RasGAP and is involved in breast cancer cell invasion. Oncogene. 2000;19:1318–28. doi: 10.1038/sj.onc.1203422. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Dy RC, Cance WG, Graves LM, Earp HS. Interactions between two cytoskeleton-associated tyrosine kinases: calcium-dependent tyrosine kinase and focal adhesion tyrosine kinase. J Biol Chem. 1999;274:8917–24. doi: 10.1074/jbc.274.13.8917. [DOI] [PubMed] [Google Scholar]
- 30.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–15. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 31.Sieg DJ, Hauck CR, Ilic D, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–56. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 32.van Nimwegen MJ, Verkoeijen S, van Buren L, Burg D, van de Water B. Requirement for focal adhesion kinase in the early phase of mammary adenocarcinoma lung metastasis formation. Cancer Res. 2005;65:4698–706. doi: 10.1158/0008-5472.CAN-04-4126. [DOI] [PubMed] [Google Scholar]
- 33.Owens LV, Xu L, Dent GA, et al. Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann Surg Oncol. 1996;3:100–5. doi: 10.1007/BF02409059. [DOI] [PubMed] [Google Scholar]
- 34.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer. 1996;68:164–71. doi: 10.1002/(sici)1097-0215(19961009)68:2<169::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 35.McCormack SJ, Brazinski SE, Moore JL, Jr, Werness BA, Goldstein DJ. Activation of the focal adhesion kinase signal transduction pathway in cervical carcinoma cell lines and human genital epithelial cells immortalized with human papillomavirus type 18. Oncogene. 1997;15:265–74. doi: 10.1038/sj.onc.1201186. [DOI] [PubMed] [Google Scholar]
- 36.Kornberg LJ. Focal adhesion kinase expression in oral cancers. Head Neck. 1998;20:634–9. doi: 10.1002/(sici)1097-0347(199810)20:7<634::aid-hed10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 37.Kornberg LJ. Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck. 1998;20:745–52. doi: 10.1002/(sici)1097-0347(199812)20:8<745::aid-hed14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 38.Judson PL, He X, Cance WG, Van Le L. Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer. 1999;86:1551–6. doi: 10.1002/(sici)1097-0142(19991015)86:6<1551::aid-cncr23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 39.Cance WG, Harris JE, Iacocca MV, et al. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res. 2000;6:2417–23. [PubMed] [Google Scholar]
- 40.Lark AL, Livasy CA, Calvo B, et al. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: immunohistochemistry and real-time PCR analyses. Clin Cancer Res. 2003;9:215–22. [PubMed] [Google Scholar]
- 41.Gabriel B, Mildenberger S, Weisser CW, et al. Focal adhesion kinase interacts with the transcriptional coactivator FHL2 and both are overexpressed in epithelial ovarian cancer. Anticancer Res. 2004;24:921–7. [PubMed] [Google Scholar]
- 42.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–78. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 43.Besson A, Robbins SM, Yong VW. PTEN/MMAC1/TEP1 in signal transduction and tumorigenesis. Eur J Biochem. 1999;263:605–11. doi: 10.1046/j.1432-1327.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 44.van Golen KL, Bao LW, Pan Q, Miller FR, Wu ZF, Merajver SD. Mitogen activated protein kinase pathway is involved in RhoC GTPase induced motility, invasion and angiogenesis in inflammatory breast cancer. Clin Exp Metastasis. 2002;19:301–11. doi: 10.1023/a:1015518114931. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki A, Lu J, Kusakai G, Kishimoto A, Ogura T, Esumi H. ARK5 is a tumor invasion-associated factor downstream of Akt signaling. Mol Cell Biol. 2004;24:3526–35. doi: 10.1128/MCB.24.8.3526-3535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zi X, Guo Y, Simoneau AR, et al. Expression of Frzb/secreted Frizzled-related protein 3, a secreted Wnt antagonist, in human androgen-independent prostate cancer PC-3 cells suppresses tumor growth and cellular invasiveness. Cancer Res. 2005;65:9762–70. doi: 10.1158/0008-5472.CAN-05-0103. [DOI] [PubMed] [Google Scholar]
- 47.Thant AA, Nawa A, Kikkawa F, et al. Fibronectin activates matrix metalloproteinase-9 secretion via the MEK1-MAPK and the PI3K-Akt pathways in ovarian cancer cells. Clin Exp Metastasis. 2000;18:423–8. doi: 10.1023/a:1010921730952. [DOI] [PubMed] [Google Scholar]
- 48.Ruth MC, Xu Y, Maxwell IH, Ahn NG, Norris DA, Shellman YG. RhoC promotes human melanoma invasion in a PI3K/Akt-dependent pathway. J Invest Dermatol. 2006;126:862–8. doi: 10.1038/sj.jid.5700211. [DOI] [PubMed] [Google Scholar]
- 49.Guan Z, Wang XR, Zhu XF, et al. Aurora-A, a negative prognostic marker, increases migration and decreases radiosensitivity in cancer cells. Cancer Res. 2007;67:10436–44. doi: 10.1158/0008-5472.CAN-07-1379. [DOI] [PubMed] [Google Scholar]
- 50.Ayala G, Thompson T, Yang G, et al. High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clin Cancer Res. 2004;10:6572–8. doi: 10.1158/1078-0432.CCR-04-0477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. RhoC does not affect the cell proliferation in vitro. (A) AT2.1, PC3 and PC3mm/tet cells were transfected with the RhoC expression plasmid, and clones were established for each cell line. These cells were then cultured, trypsinized and serially diluted followed by plating in Petri dishes. The resultant colonies after 10 days were stained and their numbers were visually counted. The expression of RhoC and the induction of this protein by tetracycline were confirmed by Western blot (inserted photos). (B) The rate of DNA synthesis for each cell line with or without expression of RhoC was measured by thymidine incorporation assay.
Supplemental Figure 2. RhoC activates MMPs through the Pyk2/FAK pathway. (A) The result of antibody array data was confirmed by Western Blot using phospho-specific antibodies as well as antibodies to the total protein of each genes. Left panels show the result of the cell line of PC3MM/tetRhoC with or without expression of RhoC. The right panels are the result of AT2.1 with or without RhoC expression. (B) PC3MM/tet cells containing the tetracycline-inducible RhoC gene was tranfected with the expression plasmid of shRNA for Pyk2 or for scrambled sequence. After 6 hours, tetracycline was added to the medium and cells were further incubated for 72 hours. Cells were then collected and subjected to Western blot analysis using phospho-specific antibodies as well as antibodies to total protein for each gene. (C) To examine the effect of Pyk2 and MAPK on the MMP expression, the same set of cells were treated with or without shPyk2 or the MAPK inhibitor, PD98059 (100 M) for 48 hours. RNA was extracted from each sample (triplicate) and subjected to qRT-PCR using specific primers for MMP2 and MMP9 (left panel). To examine the effect of Akt on the RhoC signal, cells with or without induction of RhoC were treated with or without PI3K/Akt inhibitor, Ly294002 (100nM), for 48 hours. The cells were then collected and RNA was extracted followed by qRT-PCR analysis for MMP2 and MMP9 expression (right panel).