Abstract
Toll-like receptor-4 (TLR4) recognizes microbial pathogens, such as lipopolysaccharide (LPS), and mediates LPS-induced proinflammatory cytokine secretion, as well as microbial uptake by macrophages. In addition to exogenous pathogens, TLR4 recognizes modified self, such as minimally oxidized low-density lipoprotein (mmLDL). Here we report that mmLDL and its active components, cholesteryl ester (CE) hydroperoxides, induce TLR4-dependent fluid phase uptake typical of macropinocytosis. We show that mmLDL induced recruitment of spleen tyrosine kinase (Syk) to a TLR4 signaling complex, TLR4 phosphorylation, activation of a Vav1-Ras-Raf-MEK-ERK1/2 signaling cascade, phosphorylation of paxillin, and activation of Rac, Cdc42 and Rho. These mmLDL-induced and TLR4- and Syk-dependent signaling events and cytoskeletal rearrangements lead to enhanced uptake of small molecules, dextran and, most importantly, of both native and oxidized LDL, resulting in intracellular lipid accumulation. An intravenous injection of fluorescently labeled mmLDL in wild type mice resulted in its rapid accumulation in circulating monocytes, which was significantly attenuated in TLR4-deficient mice. These data describe a novel mechanism leading to enhanced lipoprotein uptake in macrophages that would contribute to foam cell formation and atherosclerosis. These data also suggest that CE hydroperoxides are an endogenous ligand for TLR4. As TLR4 is highly expressed on the surface of circulating monocytes in patients with chronic inflammatory conditions, and CE hydroperoxides are present in plasma, lipid uptake by monocytes in circulation may contribute to monocytes' pathological roles in chronic inflammatory diseases.
Keywords: macrophage, oxidized lipoprotein, oxidized cholesteryl ester, toll-like receptor-4
INTRODUCTION
Toll-like receptors (TLRs) are pattern recognition receptors (PRRs) that sense the presence of numerous pathogen-associated molecular patterns (PAMPs)1. Activation of PRRs has been widely implicated in signaling mechanisms that contribute to chronic inflammatory diseases, including atherosclerosis. While PRRs were originally postulated to recognize only exogenous pathogens, they are now increasingly documented to respond to endogenous modified self, such as modified and/or oxidized low-density lipoprotein (LDL). We have postulated that such modified LDL could become endogenous PAMPs and initiate low-grade, but sustained, PRR-mediated inflammation and other immune responses2. TLR4 and TLR2 deficiency, as well as a deficiency in MyD88, a signaling adaptor molecule for TLR4 and other TLRs, result in reduced atherosclerosis in apoE-/- or LDLR-/- mice fed a high fat diet3-5. Some human studies, though not all, have shown that inactivating polymorphisms in TLR4 are associated with decreased CVD risk6. Although these data support a general understanding that TLRs regulate inflammation in atherosclerosis, specific mechanisms are poorly understood.
Excessive lipoprotein accumulation by macrophages and the formation of lipid-loaded foam cells is a rate-limiting process in atherogenesis. Foam cell formation involves many pathways, which likely have different quantitative importance at different stages of atherosclerotic lesion progression7,8. For example, at early stages, constitutive macropinocytosis induced during M-CSF-dependent macrophage differentiation in the lesions could contribute to foam cell formation9,10. In advanced lesions, extensively oxidized LDL (OxLDL) becomes a ligand for scavenger receptors (also PRRs), such as CD3611,12. Although CD36-mediated uptake of OxLDL is believed to play a dominant role in the formation of pro-inflammatory lipid-loaded macrophage foam cells in vitro, different laboratories have reported conflicting results about the proatherogenic role of CD36 in hypercholesterolemic murine models7,8,13-15.
Unlike OxLDL, minimally oxidized LDL (mmLDL), as generated in our laboratory, is insufficiently modified to bind to scavenger receptors, but it binds to CD14 and induces TLR4-dependent cytokine expression16,17. In addition to this “classic” TLR4 response, mmLDL induces robust TLR4/MD-2-dependent actin polymerization and spreading in macrophages16. We recently showed that many of the cytoskeletal effects of mmLDL were mediated by oxidized cholesteryl esters (CE) found in the mmLDL. Indeed, the bioactive CE hydroperoxides can be generated by oxidation of CE with 15-lipoxygenase (15LO) and we have also shown that such oxidized CE are found in murine atherosclerotic lesions18. In this study, we report that the extensive ruffling of macrophages induced by mmLDL and by cholesteryl arachidonate oxidized by 15LO (15LO-CE) leads to enhanced fluid phase uptake, mediated by a TLR4-dependent signaling cascade. This TLR4-dependent macropinocytosis results in uptake of both native and modified LDL by differentiated macrophages, as well as by circulating monocytes.
MATERIALS AND METHODS
Detailed information on materials; animals and primary macrophages; cell lines; LDL isolation and modification; oxidation of cholesteryl arachidonate; TLR4 and Syk knockdown; uptake of Lucifer Yellow, fluorescent dextran, and LDL; yeast two hybrid system; GTPase assays; immunoblot and immunoprecipitation analyses; analysis of in vivo uptake of mmLDL; Oil Red O staining; and statistical analysis are described in the expanded Material and Methods section in the online data supplement, available at http://circres.ahajournals.org.
RESULTS
mmLDL induces small molecule uptake by macrophages
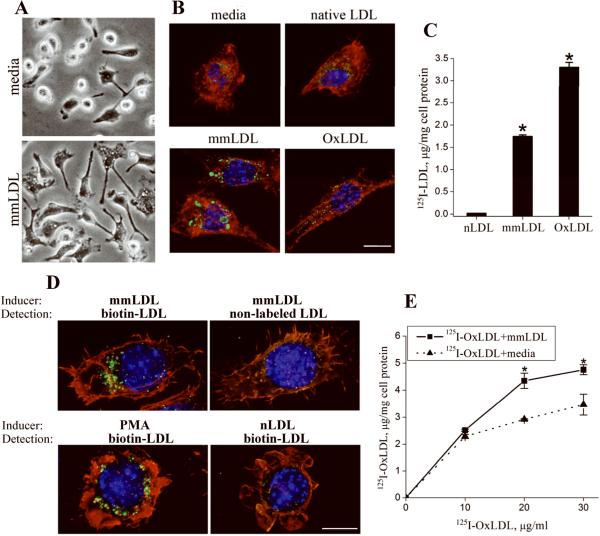
We noticed that the mmLDL-induced ruffling and cytoskeletal rearrangements in macrophages were accompanied by extensive vacuolization, characteristic of macropinocytosis (Figure 1A). Indeed, Lucifer Yellow, a low-molecular weight soluble fluorescent dye, added to the medium, accumulated in heterogeneously sized droplets (0.26-2.97 μm) in macrophages incubated with mmLDL (Figure 1B), consistent with macropinocytosis19. Incubation of macrophages with extensively oxidized LDL (OxLDL) also induced Lucifer Yellow accumulation, but in smaller quantities in uniformly sized vesicles (0.24-0.54 μm). In contrast, Lucifer Yellow did not accumulate in macrophages incubated with native LDL.
Figure 1. mmLDL-induced uptake of small molecules and native and modified LDL.
A, Peritoneal resident macrophages harvested from C57BL6 mice were incubated with media alone or 50 μg/ml mmLDL for 1 hour and imaged with a phase contrast microscope.
B, Peritoneal macrophages harvested from C57BL6 mice were incubated with 50 μg/ml of mmLDL, native LDL or OxLDL for 1 hour, in the presence of 50 μg/ml Lucifer Yellow AC. At the end of incubation, the cells were fixed with formaldehyde and stained for Lucifer Yellow (green), nuclei (blue) and F-actin (red). Scale, 10 μm.
C, Specific cell degradation of iodinated (125I) native LDL,mmLDL and OxLDL by resident peritoneal macrophages was used as a measure of LDL uptake. *, p<0.01 mmLDL vs. native LDL; 3 independent experiments.
D, Uptake of biotinylated native LDL (200 μg/ml) was induced by incubating J774 macrophages for 1 hour with either mmLDL (50 μg/ml) or PMA (1 μg/ml). Biotinylated LDL, green; F-actin cytoskeleton, red; and nuclei, blue. Replacing mmLDL with native LDL, as an inducer of macropinocytosis, resulted in no uptake of biotinylated LDL (lower right panel). Specificity of the biotin-streptavidin staining was controlled by replacing biotinylated LDL with non-labeled LDL (upper right panel). Scale, 10 μm.
E, Total degradation (i.e. specific plus non-specific) of 125I-OxLDL (10, 20 and 30 μg/ml) alone in the media or in the presence of 50 μg/ml unlabeled mmLDL. *, p<0.05 (n=3).
mmLDL accumulates in macrophages and promotes uptake of native LDL and OxLDL
MmLDL itself is insufficiently modified to be taken up via CD36; it does not compete with OxLDL for CD36 binding nor for binding to the oxidized phospholipid-specific monoclonal antibody EO6 (Online Figure I). When iodinated mmLDL was added to primary macrophages, it stimulated its own uptake, at about half the levels of the specific uptake of iodinated OxLDL (Figure 1C). In contrast, there was minimal uptake of iodinated native LDL. In a separate experiment, we observed the comparable uptake of biotinylated native LDL by macrophages stimulated with mmLDL and by macrophages stimulated with PMA, a known activator of macropinocytosis (Figure 1D). Furthermore, addition of unlabelled mmLDL stimulated enhanced uptake of iodinated OxLDL (Figure 1E), suggesting that under these conditions, a certain proportion of OxLDL was internalized independently of saturable scavenger receptor-mediated uptake.
TLR4 mediates mmLDL-induced macropinocytosis
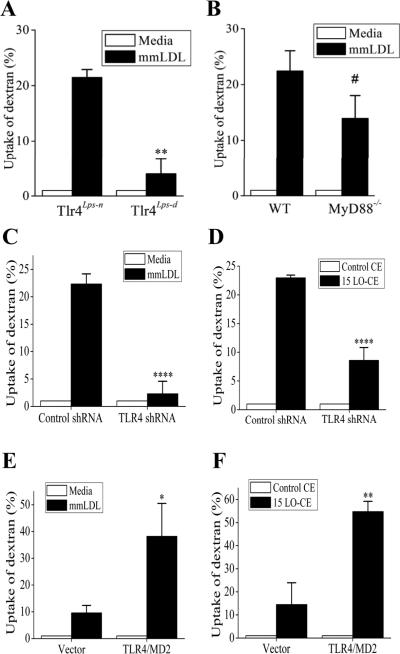
Because mmLDL induces actin polymerization in a TLR4-dependent manner16, we hypothesized that TLR4 also mediates mmLDL-induced macropinocytosis. As a measure of macropinocytosis we used the uptake of fluorescent dextran. Peritoneal macrophages from Tlr4lps-d C3H mice (the lps-d mutation in the TLR4 gene renders it inactive), when stimulated with mmLDL, accumulated 5 times less dextran than wild type Tlr4lps-n macrophages (Figure 2A). In contrast, uptake of dextran by MyD88-/- macrophages was only 38% lower and not significantly different than the uptake by wild type macrophages from C57BL6 mice (Figure 2B), suggesting a major contribution of a MyD88-independent mechanism in the mmLDL-induced macropinocytosis. To further study mechanisms of TLR4-mediated macropinocytosis, we generated a J774 cell line expressing TLR4-specific shRNA. The surface expression of TLR4 in these cells was only 40% of that in J774 cells expressing a scrambled shRNA, and the TLR4-knockdown cells did not spread in response to mmLDL (Online Figure II). The mmLDL-induced uptake of dextran by TLR4-knockdown J774 cells was reduced by 85% compared to control cells (Figure 2C).
Figure 2. TLR4-dependent uptake of dextran.
A and B, Peritoneal resident macrophages from TLR4-competent and TLR4-deficient C3H mice (A) and from MyD88-knockout and wild type C57BL6 mice (B) were incubated with media alone or 50 μg/ml mmLDL for 1 hour in the presence of Alexa Fluor-488 labeled dextran (10,000 Da). The dextran uptake was measured by FACS and presented as percent change in the geometric mean of FACS histograms. **, p<0.01 (n=4); #, not significant (p=0.150, n=6).
C and D, TLR4-knockdown and control J774 macrophages were incubated with media alone or 50 μg/ml mmLDL for 1 hour (C), or 2.5 μg/ml of non-oxidized CE or 15LO-CE for 15 min (D) in the presence of Alexa Fluor-488 labeled dextran (10,000 Da). ****, p<0.0005 (n=5).
E and F, CHO cell lines expressing human TLR4/MD-2 or empty vector were incubated with media alone or 50 μg/ml mmLDL for 1 hour (E), or 2.5 μg/ml of non-oxidized CE or 15LO-CE for 15 min (F) in the presence of Alexa Fluor-488 labeled dextran (10,000 Da). *, p<0.05; **, p<0.01 (n=5).
We have recently identified hydroperoxides of CE, the products of CE oxidation by 15LO, as bioactive components of mmLDL responsible for inducing membrane ruffling and macrophage spreading18. As in the case of the mmLDL-induced changes, 15LO-CE induced spreading in the wild type J774 cells, but not in the TLR4-knockdown cells (Online Figure IIB). Importantly, incubation of macrophages with 15LO-CE also induced the uptake of dextran in a TLR4-dependent manner (Figure 2D), replicating the findings with mmLDL. These data suggest that 15LO-CE constitute a class of endogenous ligands for TLR4. In our earlier experiments, we demonstrated that a CHO cell line stably expressing TLR4/MD-2 responded to mmLDL by enhanced actin polymerization16. In Figure 2E, we show that the TLR4/MD-2 expressing CHO cells, when stimulated with mmLDL, accumulated more fluorescent dextran than the CHO cells expressing an empty vector. The ability of mmLDL to induce macropinocytosis in TLR4/MD-2 CHO cells was fully reproduced by addition of 15LO-CE (Figure 2F).
Signaling of mmLDL-induced, TLR4-dependent macropinocytosis
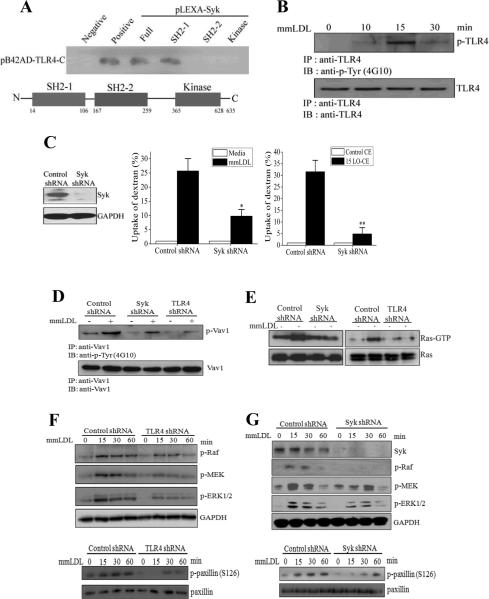
Because the mmLDL-induced, TLR4-dependent endocytic response is characterized by robust cytoskeletal rearrangements, we hypothesized that mmLDL induced a recruitment to TLR4 of a kinase involved in cytoskeleton regulation. Spleen tyrosine kinase (Syk) is known to associate with dectin-1, TLR4 and ITAM receptors and regulate leukocyte cytoskeleton and FcγR-dependent phagocytosis20-24. Yeast two-hybrid experiments, shown in Figure 3A, demonstrated that Syk can directly bind to the C-terminal domain of TLR4 and that this interaction was mediated by the N-terminal SH2 domain in the Syk molecule. We also found that mmLDL stimulated TLR4 phosphorylation (Figure 3B), suggesting that the mmLDL activation leads to an increased Syk SH2 domain binding to phosphorylated tyrosine residues of TLR4. These results agree with our recent findings that mmLDL induced co-immunoprecipitation of TLR4 and Syk in a time-dependent manner as well as TLR4-dependent Syk phosphorylation24. Next, we generated a J774 line expressing Syk shRNA, which had no detectable Syk protein expression. The Syk-knockdown cells accumulated significantly less fluorescent dextran when activated with mmLDL, results fully replicated by the addition of 15LO-CE alone (Figure 3C).
Figure 3. Signaling of mmLDL-induced, TLR4-dependent macropinocytosis.
A, Yeast two-hybrid analysis of binding of the C-terminal domain of TLR4 with the full length Syk, two SH2 domains and the kinase domain of Syk.
B, J774 macrophages were incubated with 50 μg/ml mmLDL for indicated times. Cell lysates were immunoprecipitated with a TLR4 antibody and probed with an antibody against phosphotyrosine (upper panel) or TLR4 (lower panel).
C, Control and Syk-knockdown J774 macrophages (the Syk-knockdown was confirmed in an immunoblot as shown in left-hand panel) were incubated with media alone or 50 μg/ml mmLDL for 1 hour (middle panel), or 2.5 μg/ml of non-oxidized CE or 15LO-CE for 15 min (right-hand panel), in the presence of Alexa Fluor 488-labeled dextran (10,000 Da). The dextran uptake was measured by FACS and presented as an increase in the geometric mean of FACS histograms compared to media or control CE. Mean±standard error from 3 to 5 independent experiments. *, p<0.05; **, p<0.01.
D, Control, Syk-knockdown and TLR4-knockdown J774 macrophages were incubated with 50 μg/ml mmLDL for 30 min. Cell lysates were immunoprecipitated with a Vav1 antibody and probed with an antibody against phospho-tyrosine (upper panel) or Vav1 (lower panel).
E, Control, Syk-knockdown and TLR4-knockdown J774 macrophages were incubated with 50 μg/ml mmLDL for 15 min. Cell lysates were tested for Ras-GTP and total Ras.
F and G, Control, TLR4-knockdown and Syk-knockdown J774 macrophages were incubated with 50 μg/ml mmLDL for indicated times. Cell lysates were probed for phosphorylated Raf, MEK1 and ERK1/2, and GAPDH as a loading control, as well as for phosphorylated and total paxillin. The Syk knockdown was confirmed by probing the lysates with a Syk antibody, and the TLR4 knockdown was confirmed in a FACS assay as in Online Figure I.
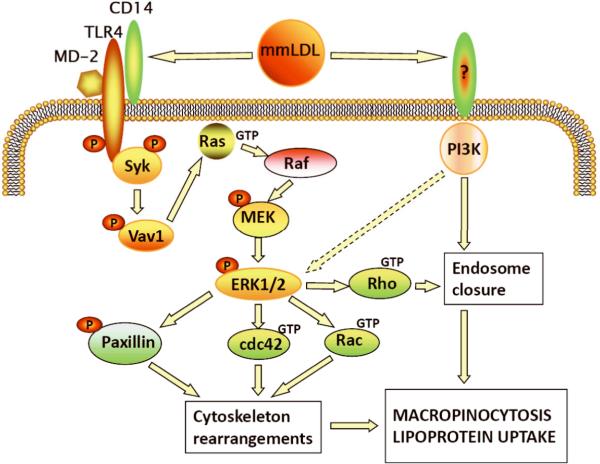
TLR4 and Syk were required for mmLDL activation of guanine nucleotide exchanging factor Vav1, small GTPase Ras, kinases Raf, MEK1 and ERK1/2; in turn, ERK1/2 phosphorylated paxillin at Ser126 and activated small GTPases Cdc42, Rac and Rho (Figure 3 and Online Figure III). We also observed mmLDL-induced, Syk-dependent formation of a N-WASP/Arp2 complex (Online Figure IIID,E), a critical event in actin polymerization. The results with Syk-knockdown cells were confirmed in experiments with Syk and MEK1 pharmacological inhibitors (Online Figures IIIF and IVA-D). Note that the activation of Ras and Vav1 were not inhibited by the MEK1 inhibitor (Online Figure IVA,B), which agrees with their upstream signaling roles. Pharmacological inhibition of ERK1/2 significantly reduced the mmLDL-induced uptake of dextran by macrophages (Online Figure IVD). Importantly, macrophage stimulation with 15LO-CE also induced ERK1/2 and paxillin phosphorylation in a TLR4-dependent manner (Online Figure IVE,F). These results suggest that the TLR4-and Syk-dependent signaling events regulate mmLDL-induced cytoskeletal rearrangements necessary for the process of macropinocytosis. mmLDL also activated Rho (Online Figure IIIC) and PI3K (our earlier reports17,25), two important factors in the closure of a ruffle into an endosome26,27. In addition to Syk, PI3K also contributed to the activation of ERK1/2 (Online Figure IIIG), but not through regulation of Vav1 (Online Figure IVA). A PKC activity may be also involved in mmLDL-induced macropinocytosis, but inhibition of JNK or ROS generation (other targets of mmLDL activation17,24) did not reduce dextran uptake (Online Figure V). The above findings are summarized in a diagram in Figure 7.
TLR4 mediates lipid accumulation in macrophages
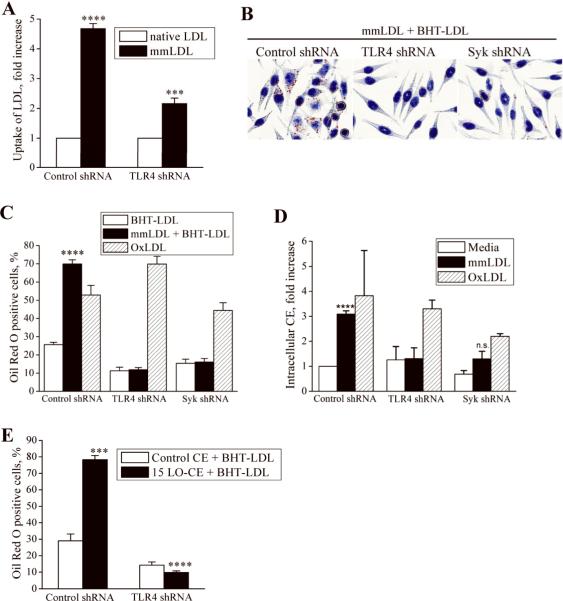
Next, we studied the role of TLR4 in the internalization of mmLDL. In a FACS assay, fluorescently labeled mmLDL accumulated in control J774 macrophages 4.5-fold more than did native LDL. In contrast, the uptake of mmLDL by TLR4-knockdown cells was reduced by 60% (Figure 4A). Furthermore, while mmLDL induced lipid accumulation in control J774 cells, as visualized by Oil Red O staining and intracellular CE accumulation, mmLDL failed to induce lipid uptake by TLR4- or Syk-deficient macrophages (Figure 4B-D). TLR4 activation by LPS also resulted in dextran and native LDL uptake by J774 cells (Online Figure VI). To show that the TLR4 and Syk knockdown did not affect other functions of the macrophage, such as scavenger receptor-mediated uptake of OxLDL, we added OxLDL alone (with no addition of mmLDL) to control, TLR4- and Syk-knockdown macrophages. Importantly, there were no differences in the OxLDL uptake between control and TLR4-knockdown macrophages (Figure 4C,D), suggesting that the scavenger receptor-mediated uptake of OxLDL and the mmLDL-induced, TLR4-mediated macropinocytosis occur via independent mechanisms. There was a trend in the reduction of OxLDL uptake by Syk-knockdown macrophages, which requires further study.
Figure 4. TLR4-dependent uptake of LDL by macrophagesin vitro.
A, Control and TLR4-knockdown J774 macrophages were incubated with 50 μg/ml of Alexa Fluor 488 labeled native LDL or mmLDL for 1 hour. The cells were fixed, gently scrapped from the plate and analyzed by FACS for the presence of intracellular LDL. Geometric means of the FACS histograms for mmLDL were normalized to that for native LDL. Mean±standard error from 3 independent experiments. ****, p<0.0001 control/nLDL vs. control/mmLDL; ***, p<0.001 control/mmLDL vs. TLR4 KD/mmLDL.
B and C, J774 macrophages, expressing control, TLR4- or Syk-specific shRNA, were incubated for 40 hours with 200 μg/ml native BHT-LDL (LDL protected from oxidation with 10 μM BHT), 50 μg/ml mmLDL plus 200 μg/ml BHT-LDL, or 25 μg/ml OxLDL alone (higher doses of OxLDL cause macrophage apoptosis). At the end of incubation, the cells were stained with Oil Red O. Images in panel B show control, TLR4-knockdown and Syk-knockdown cells incubated with mmLDL+BHT-LDL and correspond to black bars in the graph in panel C. The percentage of cells that displayed positive Oil Red O staining was determined (C). Mean±standard error from 3 independent experiments. ****, p<0.0001 control/mmLDL vs. control/nLDL.
D, J774 macrophages, expressing control, TLR4- or Syk-specific shRNA, were incubated for 40 hours with 50 μg/ml mmLDL or 25 μg/ml OxLDL, and intracellular cholesteryl ester (CE) levels were determined. Mean±standard error from 3 independent experiments. ****, p<0.0001 media vs. mmLDL.
E, J774 macrophages, expressing control or TLR4-specific shRNA, were incubated for 24 hours with 200 μg/ml native BHT-LDL (LDL protected from oxidation with 10 μM BHT) in the presence of 2.5 μg/ml of control CE or 15LO-CE. At the end of incubation, the cells were stained with Oil Red O. The percentage of cells that displayed positive Oil Red O staining was determined. Mean±standard error from 3 independent experiments. ***, p<0.001 non-oxidized CE vs. 15LO-CE in control cells.
Stimulation of J774 macrophages with 15LO-CE also resulted in lipoprotein uptake in a TLR4-dependent manner, while control (non-oxidized) CE did not induce lipoprotein uptake (Figure 4E). Because 15LO-CE, found in mmLDL, were detected in murine atherosclerotic lesions18, these results suggest that a TLR4-dependent endocytosis induced by mmLDL-like lipoproteins may occur in vivo and play a significant role in the formation of lipid-loaded foam cells in atherosclerosis.
In vivo mmLDL uptake by circulating monocytes
It is also conceivable that lipoprotein uptake may begin in the circulation. CD14 and TLR4 are highly expressed on the surface of peripheral blood monocytes, and their levels are significantly increased in patients with cardiovascular disease28,29. Circulating lipoproteins as well as lipoproteins in atherosclerotic lesions contain stable CE hydroperoxides30-32, which may induce TLR4-dependent macropinocytosis in monocytes and macrophages. We found that many circulating monocytes of LDLR-/- mice fed a high-fat diet were positively stained with neutral lipid dyes Oil Red O (chromogenic) or LipidTox Red (fluorescent), indicating increased levels of intracellular neutral lipid (Figure 5A,B). Lipid loaded monocytes were identified as early as 1 week after the start of high-fat feeding, and their numbers tightly correlated with the levels of plasma cholesterol (Figure 5C,D).
Figure 5. Lipid-loaded circulating monocytes.
A, LDLR-/- mice were fed a chow or high fat diet (1.25% cholesterol, 21% milk fat) for 5 weeks. Circulating mononuclear cells were isolated and stained for neutral lipid with Oil Red O.
B, Ex vivo monocytes as in A were stained with LipidTox Red to identify neutral lipid (red), Counterstaining with Alexa Fluor488 (actin cytoskeleton, green) and DAPI (nuclei, blue) demonstrates intracellular localization of the lipid. Scale, 5 μm (lipid, F-actin and merge) and 2 μm (merge-zoom).
C and D, Numbers of lipid-loaded monocytes as a function of total cholesterol and triglycerides levels in plasma.
To test if mmLDL could induce its own accumulation in circulating monocytes, and the role of TLR4 in this process, we intravenously injected two strains of C3H mice, Tlr4lps-n and Tlr4lps-d (the lps-d mutation in the TLR4 gene renders it inactive), with either fluorescently labeled native LDL or mmLDL. After one hour, blood was collected and immediately analyzed by FACS. Labeled mmLDL, but not labeled native LDL, was detected associated with CD11b-positive monocytes. Importantly, monocytes of TLR4-deficient mice had a significantly blunted uptake of mmLDL (Figure 6A). Isolation of the plasma monocytes from these mice and examination by deconvolution microscopy confirmed that mmLDL was indeed internalized by wild type but not TLR4-deficient cells (Figure 6B).
Figure 6. TLR4-dependent uptake of LDL by circulating monocytesin vivo.
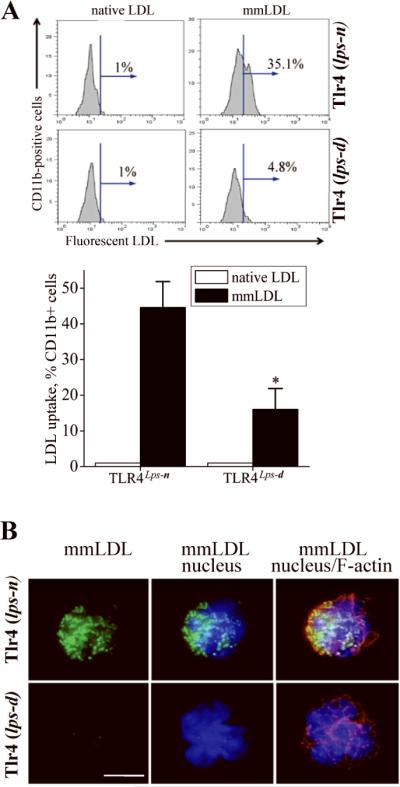
A, Tlr4lps-n and Tlr4lps-d C3H mice were injected with 100 μg of Alexa Fluor 488 labeled native LDL or mmLDL. After 1 hour, blood was collected and the Alexa Fluor 488 fluorescence was detected by FACS in a population of CD11b-positive cells (monocytes). The percentage of Alexa Fluor 488 positive cells was plotted in a bar graph. Mean±standard error from 3 independent experiments. *, p<0.05.
B, The monocytes that were analyzed by FACS in panel B, were cytospun on a glass slide and stained with Hoechst 33258 (blue) for nuclei and TRITC-phalloidin (red) for F-actin. The green fluorescence shows intracellular accumulation of Alexa Fluor 488 labeled mmLDL. Scale, 5 μm.
DISCUSSION
The formation of lipid-laden foam cells is an early and critical step in the initiation and progression of atherosclerosis33. Although the mechanisms leading to such foam cell formation are complex, involving several independent pathways7,8,34, modified LDL is likely to be involved in both the regulation of lipoprotein uptake by macrophages, as well as directly contributing lipid. Oxidized lipids and modified proteins in extensively oxidized LDL, i.e. “OxLDL”, directly mediate its internalization via scavenger receptors7. In addition, Jones et al.35 and Kruth and colleagues9,10 have suggested that foam cell formation can occur due to increased uptake of native LDL mediated by scavenger receptor-independent macropinocytosis. Recent studies suggest that enzymatically modified LDL bound to cell surface proteoglycans has enhanced uptake by a macropinocytosis pathway as well36.
In this study, we demonstrate a novel mechanism that stimulates fluid phase uptake in macrophages leading to enhanced uptake of both native and modified LDL. This process was characterized by enhanced uptake of small molecules (Lucifer Yellow) and dextran, typical of macropinocytosis. Furthermore, we show that the mmLDL-induced stimulation of macropinocytosis was TLR4-dependent and resulted in lipid accumulation in macrophages (Figures 1, 2, 4). We also show that cholesteryl ester hydroperoxides, such as those formed by oxygenation with 15LO, are oxidized lipid moieties in mmLDL responsible for the TLR4-dependent uptake (Figures 2 and 4). The intracellular signaling pathway that regulates the TLR4-dependent uptake involves the recruitment of Syk to the intracellular domain of TLR4 and consequent Syk-dependent downstream signaling leading to cytoskeletal rearrangements (Figure 3). Further, we demonstrate the in vivo relevance of these observations by showing that mmLDL injected intravenously induces its own uptake by circulating monocytes in a TLR4-dependent manner (Figure 6). These results agree with our recent study in which TLR4-competent or TLR4-deficient murine macrophages were transplanted into hypercholesterolemic zebrafish and the macrophage uptake of endogenous (dietary) lipid was monitored in live animals37. Because strong lipoprotein oxidation occurs in hypercholesterolemic zebrafish, an in vivo generated zebrafish analog of mmLDL likely induced TLR4-dependent lipid uptake.
We demonstrate that mmLDL significantly enhances macropinocytic accumulation of small molecules, dextran and LDL, even in highly differentiated peritoneal resident macrophages and macrophage-like cell lines (Figures 1-2). Although there is no specificity in regard to the material that is being internalized via mmLDL-induced macropinocytosis, the process itself is highly regulated. The stimulation of macropinocytosis by mmLDL and by its active component 15LO-CE requires the expression of functional TLR4 (Figures 2 and 4). These results imply that CE hydroperoxides constitute a class of endogenous agonists of TLR4 and could be important in sustained activation of immune cells. Although hydroperoxides of free fatty acids and phospholipids are not stable and rapidly break down into aldehydes and other advanced oxidation products, hydroperoxides of CE are much more stable and present in plasma and in atherosclerotic lesions30-32. Such CE hydroperoxides are likely produced in vivo by oxidation of CE in LDL mediated by cellular 15LO, as found in macrophages and endothelial cells. 15LO, an intracellular enzyme, can nonetheless oxidize CE in extracellular LDL38. Presumably, this occurs following binding of LDL to LRP-1, leading to translocation of 15LO to the plasma membrane, where it oxidizes the CE of LDL. The 15LO-oxidized CE is then transferred back to the LDL via an LRP1-dependent mechanism39. Indeed, LDLR-/- and apoE-/- mice lacking 12/15LO (the mouse homolog of human 15LO) develop less LDL oxidation and less atherosclerosis compared to the LDLR-/- and apoE-/- controls40-42. Furthermore, enhanced 12/15LO expression and activity have been reported in macrophages and endothelial cells in atherosclerotic mouse models43,44.
Our data have also revealed a novel downstream signaling pathway by which mmLDL activation of TLR4 leads to enhanced macropinocytosis. mmLDL induces Syk recruitment to TLR4 via the Syk N-terminal domain binding to the TLR4 C-terminal domain. In turn, this leads to activation of a Syk-dependent Vav1-Ras-Raf-MEK1-ERK1/2 signaling cascade, with subsequent activation of paxillin and Cdc42 and Rac GTPases, and the formation of a N-WASP/Arp2 complex (Figure 3 and Online Figures III-IV). These signaling events lead to actin polymerization, a necessary step in membrane ruffling. However, the fact that a nearly complete Syk knockdown resulted in incomplete inhibition of dextran uptake (Figure 3C) suggests Syk-independent pathways may also contribute to TLR4-dependent uptake. Our results agree with the reports of Syk-dependent ERK1/2 activation in phagocytosis45, ERK1/2 activation in TLR4-dependent antigen uptake by dendritic cells46, and the role of paxillin and small GTPases in actin reorganization and pinocytic vesicle formation9,26,27,47. Subsequent ruffle closing into an endosome is regulated by activated Rho and PI3K. Indeed, in this study we show that mmLDL activates Rho (Online Figure IIIC) and earlier we have demonstrated robust PI3K activation induced by mmLDL25. The signaling mechanism leading from mmLDL activation to macropinocytosis and lipoprotein uptake is schematically presented in Figure 7. We have recently demonstrated that mmLDL also induces activation of Nox2 and ROS generation in a TLR4-and Syk-dependent manner, with subsequent activation of PLCγ and PKC24. These data and the results of this study suggest an important pro-inflammatory role for TLR4-dependent mmLDL activation of macrophages.
Figure 7. Signaling pathways of mmLDL-induced, TLR4-dependent macropinocytosis and lipoprotein uptake.
It is generally believed that macrophage lipid accumulation occurs exclusively in the vessel wall. This hypothesis is indeed well founded. There are numerous mechanisms that differentiated tissue macrophages use to internalize OxLDL, mmLDL, enzymatically modified LDL and LDL retained by its binding to the extracellular matrix (reviewed in7,8,34). Foam cells have previously been observed in the circulation of non-human primates after months of hypercholesterolemia, and were thought to represent lipid-laden macrophages that entered the circulation from the intima48. In this study, however, we provide new data suggesting that under hyperlipidemic conditions, in addition to excessive accumulation of lipid by tissue macrophages, peripheral blood monocytes can also accumulate lipoproteins (Figures 5 and 6). Peripheral blood monocytes are a source of tissue macrophages, which in large part determine the initiation, progression and outcomes of atherosclerosis. Monocytes already enriched in neutral lipids even before immigration into the artery intima may be more susceptible to further lipid accumulation, as well as having altered metabolic properties.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING This study was supported by the NIH grants HL081862 (Y.I.M.), GM069338 (R.H., J.L.W. and Y.I.M.), HL088093 (J.L.W. and Y.I.M.), grant 0530159N from the American Heart Association (Y.I.M.), as well as a grant from the Leducq Fondation (J.L.W.) and the Korean National Research Laboratory Program grant ROA-2007-000-20004-0 (Y.S.B).
Footnotes
DISCLOSURES: None
References
- (1).Krieger M. The other side of scavenger receptors: pattern recognition for host defense. Curr Opin Lipidol. 1997;8:275–280. doi: 10.1097/00041433-199710000-00006. [DOI] [PubMed] [Google Scholar]
- (2).Miller YI, Chang MK, Binder CJ, Shaw PX, Witztum JL. Oxidized low density lipoprotein and innate immune receptors. Curr Opin Lipidol. 2003;14:437–445. doi: 10.1097/00041433-200310000-00004. [DOI] [PubMed] [Google Scholar]
- (3).Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. PNAS. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- (5).Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bjorkbacka H. Multiple roles of Toll-like receptor signaling in atherosclerosis. Curr Opin Lipidol. 2006;17:527–533. doi: 10.1097/01.mol.0000245258.25387.ec. [DOI] [PubMed] [Google Scholar]
- (7).Witztum JL. You are right too! J Clin Invest. 2005;115:2072–2075. doi: 10.1172/JCI26130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Webb NR, Moore KJ. Macrophage-derived foam cells in atherosclerosis: lessons from murine models and implications for therapy. Curr Drug Targets. 2007;8:1249–1263. doi: 10.2174/138945007783220597. [DOI] [PubMed] [Google Scholar]
- (9).Kruth HS, Jones NL, Huang W, Zhao B, Ishii I, Chang J, Combs CA, Malide D, Zhang WY. Macropinocytosis Is the Endocytic Pathway That Mediates Macrophage Foam Cell Formation with Native Low Density Lipoprotein. J Biol Chem. 2005;280:2352–2360. doi: 10.1074/jbc.M407167200. [DOI] [PubMed] [Google Scholar]
- (10).Zhao B, Li Y, Buono C, Waldo SW, Jones NL, Mori M, Kruth HS. Constitutive receptor-independent low density lipoprotein uptake and cholesterol accumulation by macrophages differentiated from human monocytes with macrophage-colony-stimulating factor (MCSF) J Biol Chem. 2006;281:15757–15762. doi: 10.1074/jbc.M510714200. [DOI] [PubMed] [Google Scholar]
- (11).Boullier A, Bird DA, Chang MK, Dennis EA, Friedman P, Gillotre-Taylor K, Hörkkö S, Palinski W, Quehenberger O, Shaw P, Steinberg D, Terpstra V, Witztum JL. Scavenger receptors, oxidized LDL, and atherosclerosis. Ann N Y Acad Sci. 2001;947:214–222. doi: 10.1111/j.1749-6632.2001.tb03943.x. [DOI] [PubMed] [Google Scholar]
- (12).Hazen SL. Oxidized Phospholipids as Endogenous Pattern Recognition Ligands in Innate Immunity. J Biol Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally Modified LDL Binds to CD14, Induces Macrophage Spreading via TLR4/MD-2, and Inhibits Phagocytosis of Apoptotic Cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- (17).Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- (18).Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized LDL. J Biol Chem. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Swanson JA, Watts C. Macropinocytosis. Trends in Cell Biology. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- (20).Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends in Immunology. 2005;26:208–214. doi: 10.1016/j.it.2005.02.002. [DOI] [PubMed] [Google Scholar]
- (21).Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- (22).Arndt PG, Suzuki N, Avdi NJ, Malcolm KC, Worthen GS. Lipopolysaccharide-induced c-Jun NH2-terminal Kinase Activation in Human Neutrophils: ROLE OF PHOSPHATIDYLINOSITOL 3-KINASE AND Syk-MEDIATED PATHWAYS. J Biol Chem. 2004;279:10883–10891. doi: 10.1074/jbc.M309901200. [DOI] [PubMed] [Google Scholar]
- (23).Chaudhary A, Fresquez TM, Naranjo MJ. Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol Cell Biol. 2007;85:249–256. doi: 10.1038/sj.icb7100030. [DOI] [PubMed] [Google Scholar]
- (24).Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages Generate Reactive Oxygen Species in Response to Minimally Oxidized Low-Density Lipoprotein: Toll-Like Receptor 4- and Spleen Tyrosine Kinase-Dependent Activation of NADPH Oxidase 2. Circ Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Miller YI, Worrall DS, Funk CD, Feramisco JR, Witztum JL. Actin polymerization in macrophages in response to oxidized LDL and apoptotic cells: role of 12/15-lipoxygenase and phosphoinositide 3-kinase. Mol Biol Cell. 2003;14:4196–4206. doi: 10.1091/mbc.E03-02-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- (27).Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- (28).Methe H, Kim JO, Kofler S, Weis M, Nabauer M, Koglin J. Expansion of circulating toll-like receptor 4-positive monocytes in patients with acute coronary syndrome. Circulation. 2005;111:2654–2661. doi: 10.1161/CIRCULATIONAHA.104.498865. [DOI] [PubMed] [Google Scholar]
- (29).Satoh M, Shimoda Y, Maesawa C, Akatsu T, Ishikawa Y, Minami Y, Hiramori K, Nakamura M. Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. International Journal of Cardiology. 2006;109:226–234. doi: 10.1016/j.ijcard.2005.06.023. [DOI] [PubMed] [Google Scholar]
- (30).Letters JM, Witting PK, Christison JK, Eriksson AW, Pettersson K, Stocker R. Time-dependent changes to lipids and antioxidants in plasma and aortas of apolipoprotein E knockout mice. J Lipid Res. 1999;40:1104–1112. [PubMed] [Google Scholar]
- (31).Christison J, Karjalainen A, Brauman J, Bygrave F, Stocker R. Rapid reduction and removal of HDL- but not LDL-associated cholesteryl ester hydroperoxides by rat liver perfused in situ. Biochem J. 1996;314(Pt 3):739–742. doi: 10.1042/bj3140739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Yamamoto Y. Fate of lipid hydroperoxides in blood plasma. Free Radic Res. 2000;33:795–800. doi: 10.1080/10715760000301311. [DOI] [PubMed] [Google Scholar]
- (33).Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- (34).Tabas I, Williams KJ, Boren J. Subendothelial Lipoprotein Retention as the Initiating Process in Atherosclerosis: Update and Therapeutic Implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- (35).Jones NL, Reagan JW, Willingham MC. The Pathogenesis of Foam Cell Formation: Modified LDL Stimulates Uptake of Co-Incubated LDL Via Macropinocytosis. Arterioscler Thromb Vasc Biol. 2000;20:773–781. doi: 10.1161/01.atv.20.3.773. [DOI] [PubMed] [Google Scholar]
- (36).Boyanovsky BB, Shridas P, Simons M, van der Westhuyzen DR, Webb NR. Syndecan-4 mediates macrophage uptake of group V secretory phospholipase A2-modified low density lipoprotein. J Lipid Res. 2008;50:641–650. doi: 10.1194/jlr.M800450-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Stoletov K, Fang L, Choi SH, Hartvigsen K, Hansen LF, Hall C, Pattison J, Juliano J, Miller ER, Almazan F, Crosier P, Witztum JL, Klemke RL, Miller YI. Vascular Lipid Accumulation, Lipoprotein Oxidation, and Macrophage Lipid Uptake in Hypercholesterolemic Zebrafish. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.108.189803. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Ezaki M, Witztum JL, Steinberg D. Lipoperoxides in LDL incubated with fibroblasts that overexpress 15-lipoxygenase. J Lipid Res. 1995;36:1996–2004. [PubMed] [Google Scholar]
- (39).Zhu H, Takahashi Y, Xu W, Kawajiri H, Murakami T, Yamamoto M, Iseki S, Iwasaki T, Hattori H, Yoshimoto T. Low density lipoprotein receptor-related protein-mediated membrane translocation of 12/15-lipoxygenase is required for oxidation of low density lipoprotein by macrophages. J Biol Chem. 2003;278:13350–13355. doi: 10.1074/jbc.M212104200. [DOI] [PubMed] [Google Scholar]
- (40).George J, Afek A, Shaish A, Levkovitz H, Bloom N, Cyrus T, Zhao L, Funk CD, Sigal E, Harats D. 12/15-Lipoxygenase gene disruption attenuates atherogenesis in LDL receptor-deficient mice. Circulation. 2001;104:1646–1650. doi: 10.1161/hc3901.095772. [DOI] [PubMed] [Google Scholar]
- (41).Cyrus T, Pratico D, Zhao L, Witztum JL, Rader DJ, Rokach J, FitzGerald GA, Funk CD. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein e-deficient mice. Circulation. 2001;103:2277–2282. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- (42).Huo Y, Zhao L, Hyman MC, Shashkin P, Harry BL, Burcin T, Forlow SB, Stark MA, Smith DF, Clarke S, Srinivasan S, Hedrick CC, Pratico D, Witztum JL, Nadler JL, Funk CD, Ley K. Critical Role of Macrophage 12/15-Lipoxygenase for Atherosclerosis in Apolipoprotein E-Deficient Mice. Circulation. 2004;110:2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- (43).Ylä-Herttuala S, Rosenfeld ME, Parthasarathy S, Sigal E, Sarkioja T, Witztum JL, Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991;87:1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Bolick DT, Srinivasan S, Whetzel A, Fuller LC, Hedrick CC. 12/15 Lipoxygenase Mediates Monocyte Adhesion to Aortic Endothelium in Apolipoprotein E-Deficient Mice Through Activation of RhoA and NF-{kappa}B. Arterioscler Thromb Vasc Biol. 2006;26:1260–1266. doi: 10.1161/01.ATV.0000217909.09198.d6. [DOI] [PubMed] [Google Scholar]
- (45).Parsa KVL, Butchar JP, Rajaram MVS, Cremer TJ, Tridandapani S. The tyrosine kinase Syk promotes phagocytosis of Francisella through the activation of Erk. Molecular Immunology. 2008;45:3012–3021. doi: 10.1016/j.molimm.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Zaru R, Ronkina N, Gaestel M, Arthur JS, Watts C. The MAPK-activated kinase Rsk controls an acute Toll-like receptor signaling response in dendritic cells and is activated through two distinct pathways. Nat Immunol. 2007;8:1227–1235. doi: 10.1038/ni1517. [DOI] [PubMed] [Google Scholar]
- (47).Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- (48).Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984;4:323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.