Abstract
Background
Fungal (rhino-) sinusitis encompasses a wide spectrum of immune and pathological responses, including invasive, chronic, granulomatous, and allergic disease. However, consensus on terminology, pathogenesis, and optimal management is lacking. The International Society for Human and Animal Mycology convened a working group to attempt consensus on terminology and disease classification.
Discussion
Key conclusions reached were: rhinosinusitis is preferred to sinusitis; acute invasive fungal rhinosinusitis is preferred to fulminant, or necrotizing and should refer to disease of <4 weeks duration in immunocompromised patients; both chronic invasive rhinosinusitis and granulomatous rhinosinusitis were useful terms encompassing locally invasive disease over at least 3 months duration, with differing pathology and clinical settings; fungal ball of the sinus is preferred to either mycetoma or aspergilloma of the sinuses; localized fungal colonization of nasal or paranasal mucosa should be introduced to refer to localized infection visualized endoscopically; eosinophilic mucin is preferred to allergic mucin; and allergic fungal rhinosinusitis (AFRS), eosinophilic fungal rhinosinusitis, and eosinophilic mucin rhinosinusitis (EMRS) are imprecise and require better definition. In particular, to implicate fungi (as in AFRS and EMRS), hyphae must be visualized in eosinophilic mucin, but this is often not processed or examined carefully enough by histologists, reducing the universality of the disease classification. A schema for subclassifying these entities, including aspirin-exacerbated rhinosinusitis, is proposed allowing an overlap in histopathological features, and with granulomatous, chronic invasive, and other forms of rhinosinusitis. Recommendations for future research avenues were also identified.
Keywords: Fungal sinusitis, Aspergillus, zygomycetes, allergy, dematiaceous fungi
INTRODUCTION
Sinusitis, or more accurately rhinosinusitis (RS), is a common disorder affecting approximately 20% of the population.1 Acute rhinosinusitis (ARS) is well categorized. However, controversies surround chronic rhinosinusitis (CRS) and the role of fungi in this condition. CRS accounts for >90% of all cases of rhinosinusitis, and the correct diagnosis of each category of CRS is important for optimum therapy and predicting the course. Recognizing the importance of resolving the many controversies concerning CRS, and to develop a systematic management protocol of fungal rhinosinusitis (FRS), the International Society for Human and Animal Mycology formed a working group on fungal rhinosinusitis. The group participated in a workshop in February 2008 in Chandigarh, India, and deliberated on their clinical experiences of patients with FRS and the research in this area. From this workshop a consensus document was prepared through panel discussions.
The first published attempt to classify FRS came in 1965, when Hora recognized two categories: one was noninvasive, behaving clinically like chronic bacterial sinusitis, and the other invasive, in which the infection results in a mass that behaves like malignant neoplasm, eroding bone and spreading into adjacent tissue.2 The invasive nature of the disease was further confirmed on histopathology.3,4 McGill et al., in 1980, reported a third type of FRS in immunocompromised patients: a fulminant form with rapid and malignant course.5 In 1976, Safirstein noted a combination of nasal polyposis, crust formation, and sinus cultures yielding Aspergillus species, and observed the clinical similarity that this constellation of findings shared with allergic bronchopulmonary aspergillosis (ABPA).6 Similarly, in 1981, Miller et al.,7 and in 1983, Katzenstein et al.,8 independently recognized a pathophysiologic resemblance among a few cases of CRS associated with a mucosal plug in the sinuses of patients with ABPA, which led to a description of a fourth type of FRS, namely allergic Aspergillus sinusitis. Later, it became apparent that melanized fungi are common etiological agents of this allergic type of sinusitis, which led to the renaming of this type of FRS as allergic fungal sinusitis or rhinosinusitis (AFS or AFRS).9–11 In recent years, the definition of AFRS has faced a greater challenge with the demonstration of fungi in eosinophilic mucin independently from type I hypersensitivity in most cases of CRS.12,13 Ponikau et al. proposed a new term for this condition, namely eosinophilic fungal rhinosinusitis (EFRS), to reflect the striking role of eosinophils.12
FRS can be broadly divided into two categories based on histopathological findings: invasive and noninvasive, depending on invasion of the mucosal layer. In the late 1990s, deShazo et al. proposed a new classification for tissue invasive FRS based on the clinical condition, immune status, histopathology, and fungus infection: acute (fulminant) invasive, granulomatous invasive, and chronic invasive types.14 The granulomatous invasive type is mainly described in chronic FRS cases from Sudan, India, and Pakistan, where the patients are immunocompetent, almost exclusively identified with Aspergillus flavus, and present as nonca-seating granuloma with proptosis. These cases have been distinguished from chronic invasive FRS, which has a chronic course, often in subtly immunocompromised patients, such as those with diabetes mellitus and corticosteroid treatment, with dense accumulation of hyphae invading tissue, sometimes in association with the orbital apex syndrome. The described noninvasive FRS disorders are of three types: saprophytic fungal infestation, fungal ball, and fungus-related eosinophilic rhinosinusitis including AFRS. Although a sinus fungal ball is more or less a clear-cut entity, substantial confusion surrounds fungus-related eosinophilic rhinosinusitis and the definition of AFRS. As originally described, the detection of fungi in allergic mucin is considered important in the diagnosis of AFRS, although occasionally hyphae are sparse in the sinus contents. This leads to confusion and potential overlap with eosinophilic mucin rhinosinusitis (EMRS), as described by Ferguson in 2000.15 Ferguson speculated that EMRS is a systemic disease with dysregulation of immunological control where eosinophilic mucin could be present without the presence of fungi.15
Present Classification of Fungal Rhinosinusitis
Although much confusion exists regarding classification, the most commonly accepted system divides fungal rhinosinusitis (FRS) into invasive and noninvasive diseases based on histopathological evidence of tissue invasion by fungi. The invasive diseases include 1) acute invasive (fulminant) FRS, 2) granulomatous invasive FRS, and 3) chronic invasive FRS. The noninvasive diseases include 1) saprophytic fungal infestation, 2) fungal ball, and 3) fungus-related eosinophilic FRS that includes AFRS.
Acute invasive (fulminant) FRS
The disease is described by a time course of <4 weeks with predominant vascular invasion occurring in patients with immunocompromised status. The histopathology demonstrates hyphal invasion of blood vessels, which may include the carotid arteries and cavernous sinuses, vasculitis with thrombosis, hemorrhage, tissue infarction, and acute neutrophilic infiltrates (Fig. 1).14 The disease has also been termed acute necrotizing FRS, as a necrotizing pathological reaction may be seen in some patients with only minimal inflammation, with plenty of fungi in the necrotic tissue.16 Patients with neutrophil dysfunction or neutropenia, such as hematological malignancies, aplastic anemia, uncontrolled diabetes mellitus, and hemochromatosis, or those undergoing antineoplastic chemotherapy or following transplantation, are especially susceptible to acute invasive FRS,17–19 although this type of infection is reported occasionally in apparently immunocompetent hosts. Aspergillus species, or members of the class zygomycetes are the most frequent etiological agents.16–22
Fig. 1.
(A) Acute invasive fungal sinusitis with bland infarcted area (×400). (B) Necrotizing inflammation with fibrin thrombi (×100). (C) Numerous hyphae of zygomycetes on hematoxylin and eosin stain (×100). (D) Fungal hyphae on Gomori methenamine-silver stain (×200).
Granulomatous invasive FRS
The disease is described by a time course of >12 weeks with an enlarging mass in the cheek, orbit, nose, and paranasal sinuses in immunocompetent hosts. Proptosis is often a prominent feature. Histopathologically, a granulomatous response is seen with considerable fibrosis. Noncaseating granuloma with foreign body or Langhans-type giant cells may be seen, sometimes with vasculitis, vascular proliferation, and perivascular fibrosis (Fig. 2). Hyphae are usually scanty and A. flavus is the primary agent isolated. The presence or absence of precipitating antibodies against antigens from the etiological fungi correlates well with disease progression.23 The disease has primarily been seen in Sudan, India, Pakistan, and Saudi Arabia.14,24,25
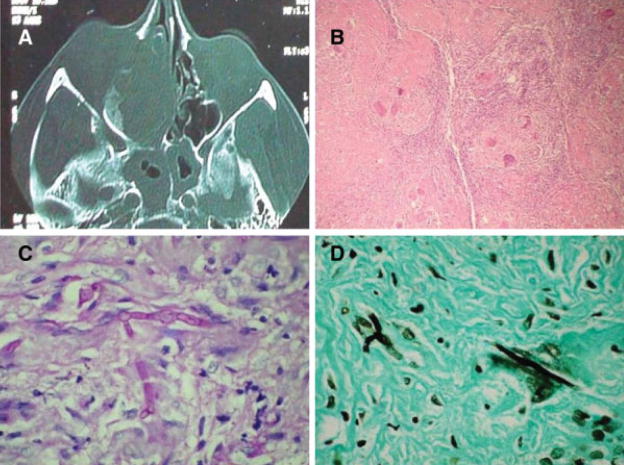
Fig. 2.
(A) Computed tomography scan of patient with chronic granulomatous fungal rhinosinusitis involving the right nasal cavity in a chronic invasive granulomatous fungal rhinosinusitis with bony destruction of paranasal sinuses extending into right orbit. (B) Extensive granulomatous process in a fibrotic background on hematoxylin and eosin stain (×100). (C) Fungal hyphae inside giant cells on periodic acid-Schiff stain (×400). (D) Fungal hyphae inside giant cells on Gomori methenamine-silver stain (×400).
Chronic invasive FRS
Chronic invasive FRS is a slowly destructive process that most commonly affects the ethmoid and sphenoid sinuses, but may involve any paranasal sinus. The disease typically has a time course of >12 weeks. However, in contrast to granulomatous invasive FRS, the entity is characterized as dense accumulation of hyphae, occasional presence of vascular invasion, and sparse inflammatory reaction in association with involvement of local structures (Fig. 3). The entity is usually seen in the context of AIDS, diabetes mellitus, and corticosteroid treatment.14,24,26 Cultures of tissue are positive in >50% of cases and Aspergillus fumigatus is the most common agent isolated.
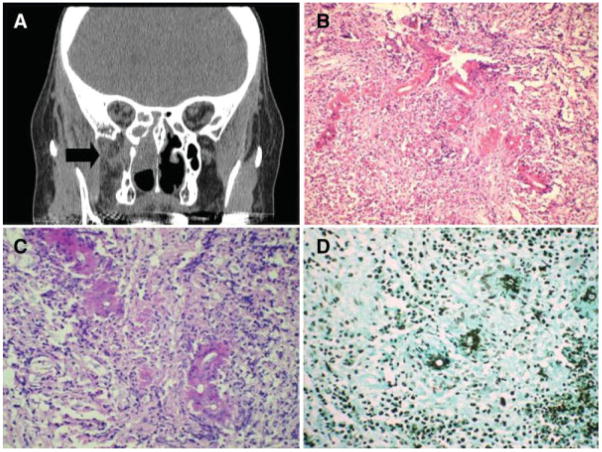
Fig. 3.
(A) Coronal computed tomography scan of immunosuppressed patient with amyloidosis and chronic invasive mucormycosis in chronic invasive fungal rhinosinusitis. Right ethmoid and pterygopalatine space involvement. (B) Nongranulomatous chronic inflammatory infiltrate with transverse section of fungal hyphae eosinophilic Splendore-Hoeppli phenomenon on hematoxylin and eosin stain (×200). (C) Periodic acid-Schiff stain (×400). (D) Gomori methenamine-silver stain (×200).
Saprophytic fungal infestation
Asymptomatic colonization of mucous crusts within the nasal cavity, often in patients who had previous sinus surgery, has been described as saprophytic fungal infestation. The possibility of extension of this growth leading to the formation of fungal ball has been predicted.27
Fungal ball
Fungal ball is described as the presence of noninvasive accumulation of dense conglomeration of fungal hyphae in one sinus cavity, usually the maxillary sinus, although the disease may affect other sinuses or rarely multiple sinuses.28 Various terms, such as mycetoma, aspergilloma, and chronic noninvasive granuloma have been used interchangeably in the literature to designate sinus fungal ball.27 The disease is defined by the following criteria: radiological evidence of sinus opacification with or without radiographic heterogeneity, mucopurulent cheesy or clay-like materials within the sinus, a dense conglomeration of hyphae separate from the sinus mucosa, nonspecific chronic inflammation of the mucosa, no predominance of eosinophils or granuloma or allergic mucin, and no histopathological evidence of fungal invasion of mucosa (Fig. 4).29 Interestingly, the disease has been found more commonly in middle-aged and elderly females, in contrast to all forms of invasive and chronic aspergillosis, which are more common in males.30 Fungi remain non-invasive in the context of a fungal ball, however, and rarely could become invasive after substantial immunosuppression, such as renal transplantation.31 In addition, some patients develop allergic mucin surrounding the fungal balls when corticosteroids are tapered.27,32 Dhong et al. showed that all fungal balls have a characteristic gritty matted gross appearance to the surgeon, whereas the majority, but not all, had computed tomography (CT) characteristics that included radiographic heterogeneity.33 Likewise, such heterogeneity can sometimes be seen in nonfungal sinusitis. In approximately 70% of fungal balls, the diagnosis is made exclusively by histology or microscopy, and cultures are negative. Implication of Aspergillus species as the causative agent may be aided by the use of galactomannan detection in the sinus material.
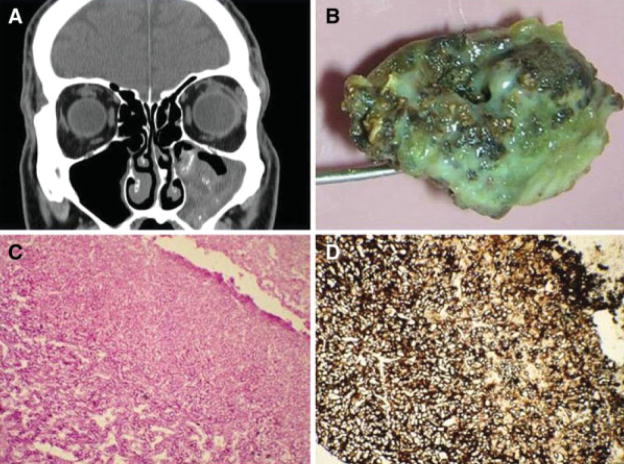
Fig. 4.
(A) Computed tomography scan showing a fungus ball in the left maxillary sinus on coronal view with hyperdense secretions. (B) Gross photo of a fungal ball. (C) Fungal hyphae on periodic acid-Schiff stain (×200). (D) Gomori methenamine-silver stain (×200).
Eosinophil related FRS including AFRS
It is believed that fungal allergens elicit immunoglobulin E (IgE)-mediated allergic and possibly type III (immune complex)-mediated mucosal inflammation in the absence of invasion in an atopic host.34,35 Moreover, when the sensitized individuals are exposed to an environment of high fungal content, symptoms of upper and/or lower airway hyper-responsiveness increase significantly.36 Generalized sinonasal inflammation in combination with viscid allergic mucin effectively obstructs the normal drainage pathway. Fungi persist locally, stimulating locally destructive immune responses. The process then may expand to involve adjacent sinuses and may produce sinus expansion and bony erosion.37,38 Accumulation of eosinophilic mucin in the expanded sinuses leads to elevation of inflammatory mediators, such as major basic protein, eosinophil peroxidase, eosinophil-derived neurotoxin, tumor necrosis factor β, and interleukins (IL)-4, 5, 10, and 13.39,40 Although the presentation of the disease is subtle, occasional dramatic presentations of the disease in the form of acute visual loss, gross facial dysmorphia, or complete nasal obstructions have been observed.10,41,42 Why only some patients behave in this acute fashion is not known.
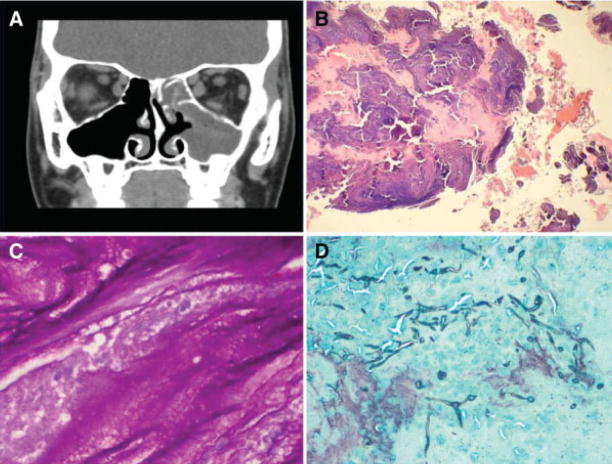
To diagnose AFRS, Bent and Kuhn11 proposed five diagnostic criteria: type I hypersensitivity, nasal polyposis, characteristic findings on CT scan, presence of fungi on direct microscopy or culture, and allergic mucin containing fungal elements without tissue invasion (Fig. 5). These early observations were based on <20 patients capturing the usual clinical findings of AFRS, although there are exceptions to several of the criteria, particularly the presence of nasal polyps. Patients with recurrent AFRS, who have had prior surgery, frequently lack nasal polyps, although their sinuses contain eosinophilic mucin with hyphae. One diagnostic requirement put forth by the guidelines for clinical research in CRS was to consider AFRS a distinct entity, categorized by a type I hypersensitivity to fungi cultured from eosinophilic mucin containing hyphae, harvested from the patient’s nose or sinus cavities, and without evidence of tissue invasion by fungus.42 Although the detection of fungi in allergic mucin is considered important, hyphae may be sparse in sinus content and take considerable time to visualize with the currently used stains. This has led to confusion in categorization of this entity, especially with the description of EMRS.15 However, the use of much more sensitive diagnostic techniques, such as chitin staining43 or polymerase chain reaction amplification,44–46 but not Aspergillus antigen,47 to detect the presence of fungi in the majority cases of chronic rhinosinusitis may reveal that EMRS is predominantly or completely related to a response to one or more fungi. In EMRS cases, disease was uniformly bilateral, combined with a significantly higher frequency of asthma and an increased incidence of aspirin sensitivity, and frequently an immunoglobulin G1 deficiency.15 In a prospective study from India, considerable overlap in findings between AFRS and EMRS were observed, although type I hypersensitivity, Charcot-Leyden crystals, bony erosion, and heterogeneous opacity with sinus expansion on CT scan were found to be significantly associated with AFRS, whereas asthma was significantly associated with EMRS.48 It is possible that EMRS and AFRS are differing manifestations of the same pathological process, with considerable overlap.
Fig. 5.
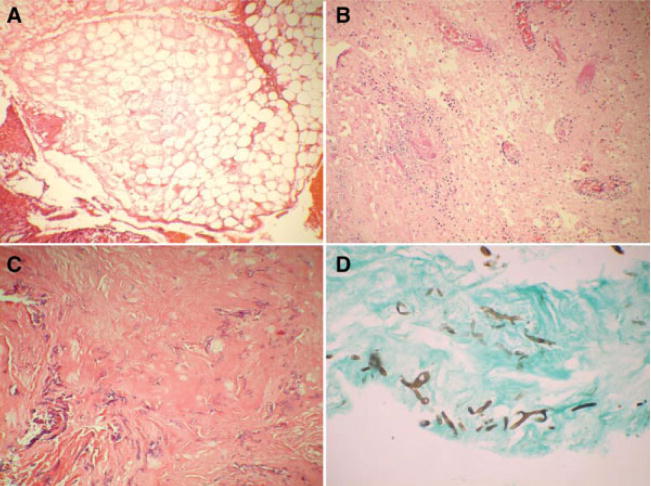
(A) Computed tomography coronal scan showing recurrence of allergic fungal rhinosinusitis following prior surgery. Hyperdensity of mucin within right ethmoid and maxillary sinuses. (B) Allergic fungal sinusitis with allergic mucin (×100). (C) Fungal hyphae inside allergic mucin on periodic acid-Schiff stain (×400). (D) Gomori methenamine-silver stain showing hyphae within the mucin (×100).
Current understanding of the pathophysiology of AFRS would suggest that the initiation of the inflammatory cascade is a multifunctional event, requiring the simultaneous occurrence of IgE-mediated sensitivity, specific T-cell HLA receptor expression, and exposure to specific fungi.26,34 The fungi causing AFRS are diverse, and in a review of the English literature, Manning and Holman in 1998 reported 168 positive cultures, 87% of the cases due to dematiaceous fungi, and 13% yielded Aspergillus species49 Interestingly, in the Indian scenario A. flavus was isolated in more than 80% of the cases of AFRS.23,48,50–52 A. flavus was also isolated from 50% of patients diagnosed with AFRS in the Middle East.53
Contrary to the prevailing belief that fungi were responsible for CRS in only a selected group of patients with distinct pathophysiology, Ponikau et al. in 1999 demonstrated the presence of fungi in nasal mucus from 96% of patients with CRS and found type I hypersensitivity to be present in <25% of their study group. They detected fungi along with eosinophil and eosinophil degraded products in mucus.12 Often the eosinophils detected in the mucus were in clusters along with a few Charcot-Leyden crystals, but sometimes they found the eosinophils in the form of cellular debris and crystals. They termed this mucin eosinophilic mucin and coined the term eosinophilic fungal rhinosinusitis (EFRS). However, they also cultured a diverse array of airborne fungi from the nose of 100% of healthy volunteers. Later, they further improved the detection technique in eosinophilic mucin by using a fluorescein-labeled chitinase staining technique (Fig. 6).43 From Europe, Braun et al., in 2003, made a similar observation using sensitive techniques to detect fungi.13 Ponikau et al. further progressed their hypothesis by demonstrating high levels of toxic major basic protein (MBP) from eosinophils in the mucus of patients with CRS, and postulated that MBP damages the nasal epithelium from the luminal side, permitting secondary bacterial infection on the damaged epithelium.54 Increasingly the role of bacteria in CRS is questioned. Ponikau proposed that certain fungi could elicit eosinophilic inflammation in the absence of type I hypersensitivity reactions in patients with CRS.12,54 This concept of nonatopic eosinophilia from fungi is supported by studies that demonstrate that peripheral blood mononuclear cells (PBMCs) from patients with CRS show exaggerated humoral and cellular responses, both Th1 and Th2 types, after exposure to common airborne fungi, particularly of the Alternaria species, which are absent in PBMCs from healthy control subjects. The authors claimed that the anomalous immune and inflammatory responses to ubiquitous fungi might explain the chronic eosinophilic inflammation of CRS.55 These findings raise several questions: 1) Is AFRS a separate distinct entity under CRS that requires not only the presence of eosinophilic mucin with hyphae, but also the presence of atopy? 2) Is EFRS a nonallergic fungal eosinophilic inflammation that exists as a separate CRS entity? 3) Do secondary bacterial infections exist in a large group of patients with CRS? 4) If secondary bacterial infection is present, is this promoted by damage to the nasal and sinus epithelium from fungal induced eosinophilia in many of the patients with CRS? Alternatively, do AFRS and EFRS represent the limits of a range of fungal eosinophilic inflammation?
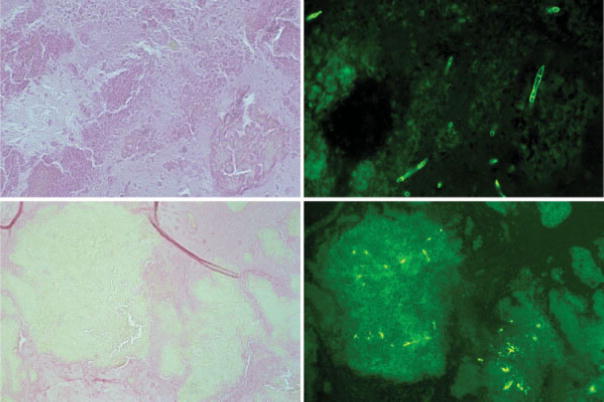
Fig. 6.
Histological images of eosinophilic mucin taken from two patients (top and bottom). The left panels show the mucin stained with Gomori methenamine-silver (GMS), and no hyphae or fungal elements are visible in these sections. The right panels are serial sections and show alternative ways of visualizing fungi. At the top a chitinase stain, which detects chitin present in all fungal cell walls, but not bacteria (note the septate hyphae) (×400). The bottom right shows a serial section of the right side GMS stain, but instead stained with an anti-Alternaria polyclonal antibody. Note again the lack of fungal visualization on GMS, which is in contrast to anti-Alternaria staining, and demonstrates not only the presence of intact fungi but also its remnants and fungal antigens. These images question the sensitivity of GMS staining to rule the presence of fungal matter, including hyphae (×200).
With the confusion in discrete definitions of AFRS, EFRS and EMRS, another possibility is that fungi may be bystanders or one of several contributors to the whole process. In the analysis of pathophysiology of eosinophil related FRS, it has been suggested that fungal elements trapped in the mucus in sinuses are the source of antigenic material that stimulates IgE, IgG, and IgA production.8,56 Numerous stimuli, other than fungi, or in addition to fungi, may be responsible for the pathophysiology of this disorder, including the putative role of allergens, bacteria, and bacterial-derived superantigens.57 A role has been proposed for Staphylococcal-derived superantigens in the pathogenesis of CRS associated with nasal polyps.58 All of these studies indicate that until there is definite evidence of T cell activity within the sinuses that responds to fungal antigens, together with further demonstration that removal of fungal antigens ameliorates the disease, the case against fungal involvement remains only circumstantial. The confusion in this area was further complicated with the well-documented but rare reports of histologic tissue invasion in possible cases of AFRS.59 It may be due to two separate pathogenic processes, allergic disease and infection by the fungus in the same host. Extending the hypersensitivity process in the causation of AFRS, some researchers claimed the consistent presence of AFRS and ABPA in the same patients, and termed the process as sino-bronchial allergic mycosis syndrome.60
All these controversies in the definition of the categories of FRS have emphasized the need for collaborative work and exchange of findings among doctors and scientists interested in this field.
In the workshop the following consensus opinions and definitions were made through panel discussion:
IS THE OPTIMAL TERMINOLOGY FUNGAL RHINOSINUSITIS OR FUNGAL SINUSITIS?
It is essential to clarify the terminology because there are other sinuses in the body besides the paranasal sinuses. As most cases of nasal fungal sinusitis have a proceeding or concomitant involvement of the nasal cavity, except with isolated fungal ball lesions, fungal rhinosinusitis was the term considered most appropriate. However, the terms rhinitis and rhinosinusitis are two different entities and should not be confused.
IS THE OPTIMAL TERMINOLOGY ACUTE INVASIVE, FULMINANT, OR NECROTIZING FUNGAL RHINOSINUSITIS?
The key characteristics of this life-threatening category of fungal rhinosinusitis in immunosuppressed patients are invasion of tissue and duration of illness <4 weeks. In some patients, a necrotizing reaction may be seen histopathologically with minimal inflammation, but necrotizing lesions are not seen in all patients in this group. The extent and nature of lesions also depends on the degree of immunosuppression. The term fulminant conveys the rapid destruction and often fatal outcome that occurs in patients with severe immunosuppression or when left untreated. However, illness usually takes a more protracted course when the treatment is started early or with reversal of immunosuppression. Therefore, the consensus was to use the term acute invasive fungal rhinosinusitis, with the etiological agent substituted, if known (i.e., acute invasive Mucorales rhinosinusitis).
DISTINCTION BETWEEN ACUTE AND CHRONIC FRS
Acute disease is when the duration of illness is <1 month, and that of chronic disease is >3 months, although other factors, such as host immune status and vascular invasion, may also distinguish the two forms of disease. In the acute variety, a neutrophilic tissue reaction, and in the chronic course an eosinophilic reaction, is usually seen. Questions were raised as to whether it is required to include a subacute category to cover the transition time between acute and chronic form, and whether introduction of the new term subacute would change the management strategies. The introduction of a new term, or the change of the term, may affect the clinicians’ considerations of seriousness of the disease. After deliberation, it was unanimously decided that the new term subacute might be used in the rare situation when the duration of illness is within 1 to 3 months and the pathology is of mixed cellular reaction (both neutrophilic and eosinophilic).
ARE GRANULOMATOUS AND CHRONIC FRS SEPARATE ENTITIES?
This was one of the unresolved issues. In the granulomatous type, a histopathologic granulomatous response with considerable fibrosis, typified by nonca-seating granuloma with foreign body or Langhans-type giant cells, occasional vasculitis, vascular proliferation, and perivascular fibrosis, hyphae are usually sparse and A. flavus is consistently isolated. It may be a geographically or ethnicity-related entity, as it is most commonly seen in Sudan, India, Pakistan, and Saudi Arabia. In contrast, the chronic invasive type is characterized by dense accumulation of hyphae, sometimes with vascular invasion, chronic or sparse inflammatory reaction, and isolation of A. fumigatus with direct destruction of adjacent tissues. It is not clear whether fibrosis is present consistently in the chronic invasive form. The clinicopathological distinction between these two types is not sharp. Both have a chronic course and frequently prominent orbital involvement. Moreover, no difference in prognosis or therapy is yet apparent based on this distinction. However, in the panel discussion, the participants agreed that chronic invasive and granulomatous FRS should be differentiated, primarily on pathological grounds, until more data are forthcoming.
IS THE OPTIMAL TERMINOLOGY FUNGAL BALL, MYCETOMA, OR ASPERGILLOMA?
These terms have been used interchangeably in the literature to designate the sinus fungal ball. The disease is defined as the presence of noninvasive dense accumulation of fungi in sinus cavities. The use of the term mycetoma is technically not correct, as mycetoma is a chronic local invasion of subcutaneous tissue by bacteria or fungi with the formation of sinus tract, swelling, and granule. The term aspergilloma is also not appropriate, because the disease is not always due to Aspergillus species. Therefore, the sinus fungal ball seems to be the most appropriate term.
Because the presence of a fungal ball in the sphenoid sinus is potentially much more serious than that in the maxillary sinus due to the proximity to the brain, the consensus view was that it is desirable to describe which sinus is involved. The treatment of sinus fungal ball requires removal of the mass but fatal invasive aspergillosis has been reported following surgery of sphenoid sinus fungal balls. Thus it was decided to re-examine the management of fungal balls to find out whether mucosal biopsy to document lack of invasion is required when the preoperative scan and operative findings suggest a sinus fungal ball. Regarding the description of each condition the consensus was to describe it as localization + fungal ball ± causative fungus (e.g., maxillary sinus fungal ball due to A. flavus).
IS THERE AN ENTITY SAPROPHYTIC FUNGAL INFESTATION OF NASAL MUCOSA?
Simple colonization of nasal or paranasal sinuses without any symptoms has been described as saprophytic fungal infestation.27 The colonization occurs often over mucous crusts in patients who had a history of previous sinus surgery, and is detected upon endoscopic examination. It remains silent until it is detected or presents with foul odor. Further extension of the growth may lead to fungal ball formation. After deliberation, the consensus was to describe the condition as localized fungal colonization of nasal or paranasal mucosa.
WHAT IS THE DISTINCTION AMONG AFRS/EFRS/EMRS?
Much discussion focused on finding commonality among the different forms of eosinophilic diseases of rhinosinusitis. In AFRS, there is so called allergic mucin with many eosinophils and the presence of noninvasive fungi with raised fungal specific IgE. The EFRS and EMRS (EFRS-like) cases do not have specific IgE and they differ in the presence (EFRS) or absence of fungus visualized microscopically (EMRS). One specific problem was that the presence or absence of fungal hyphae depends on the thoroughness of the histopathological examination of the mucous. Sometimes the mucous is not provided to pathology, especially with the use of microdebriders, which suction away removed tissue and mucus. The possibility of another AFRS-like group was, therefore, evoked in which the presence of fungus is not demonstrated, although there is a positive fungal-specific IgE response demonstrable.
The first consensus reached was to call the mucus in such conditions as eosinophilic mucin rather than allergic mucin, irrespective of presence or absence of atopy, as eosinophilic mucin describes the presence of eosinophil or eosinophil degraded products in mucus, which is present in all described conditions. The diseases with eosinophilic mucin may be broadly divided into nonfungal and fungal categories. The nonfungal side includes the AFRS-like group with fungal specific IgE, the EMRS (EFRS-like) category, and aspirin-exacerbated RS (previously known as aspirin-sensitive RS). The fungal side includes AFRS, EFRS, and a limited number of cases of aspirin-exacerbated RS, which also have fungal hyphae demonstrable (Fig. 7).
Fig. 7.
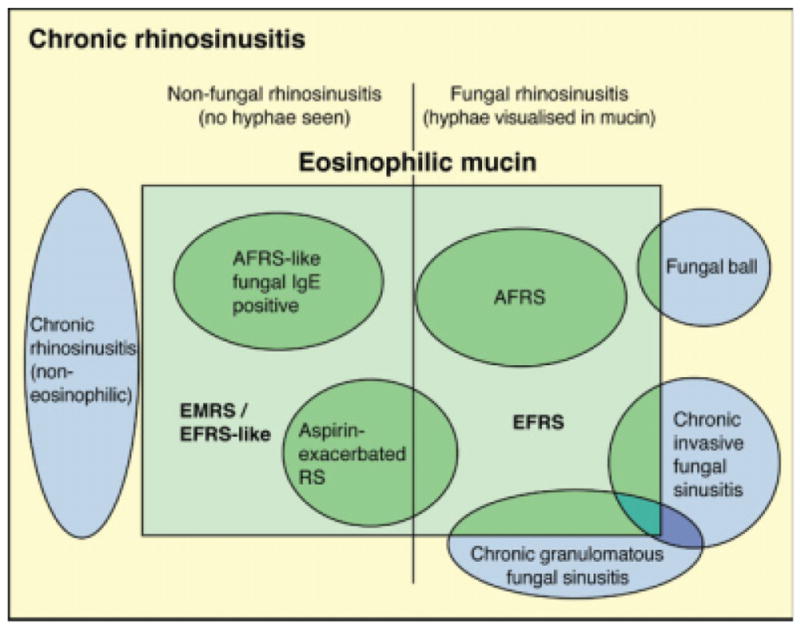
The inter-relationships of various forms of chronic rhinosinusitis derived by consensus discussion. AFRS = allergic fungal rhinosinusitis; EMRS = eosinophilic mucin rhinosinusitis; EFRS = eosinophilic fungal rhinosinusitis; RS = rhinosinusitis.
There was a vigorous discussion by the panel on whether it is essential to demonstrate the presence of fungal hyphae in eosinophilic mucin, or is it enough to demonstrate the presence of fungal antigen or nucleic acid in the mucus to differentiate the two broad groups. There was agreement that there is no evidence whether antifungal treatment is useful for eosinophilic diseases with fungus demonstrable, and further, that there is no clear information as to whether any fungus that is seen is the cause of eosinophilic infiltration or contributing to the disease process. There was also discussion on the definition of eosinophilic mucin/nasal exudates and the percentage of occurrence of nasal polyps. In most cases, there are clusters of eosinophils and eosinophil degraded products present in mucus with or without Charcot Leyden crystals. Sometimes there is sensitivity to one or more specific fungi (as detectable IgE in AFRS), and sometimes there is presence of IgG antibodies to specific fungi. It is also not known whether unilateral disease represents a precursor stage to bilateral disease in any of the eosinophilic diseases.
The role of fungi in AFRS, EFRS, and EMRS remains unclear. Fungal and nonfungal entities with associated eosinophilia are shown in the consensus diagram of Figure 7, whereas outside the eosinophilic histopathology box lie invasive fungal entities and fungal balls with little overlap with eosinophilic patterns.
The consensus developed during the panel discussion is summarized and tabulated in Table I.
TABLE I.
Consensus Developed During Panel Discussion About the Controversies.
| Sl No. | Controversy | Consensus |
|---|---|---|
| 1 | Fungal rhinosinusitis or fungal sinusitis? | Fungal rhinosinusitis |
| 2 | Acute invasive, fulminant, or necrotizing fungal rhinosinusitis? | Acute invasive fungal rhinosinusitis (when etiological agent is known, e.g., acute invasive Aspergillus rhinosinusitis) |
| 3 | Distinction between acute and chronic FRS | Acute when duration is <1 month Chronic when duration is >3 months Subacute when duration 1–3 months |
| 4 | Are granulomatous and chronic FRS separate entities? | Keep the entities separate until more data clarify the facts |
| 5 | Fungal ball, mycetoma, or aspergilloma? | Fungal ball with the description localization + fungal ball ± causative fungus (e.g., maxillary sinus fungal ball due to Aspergillus flavus) |
| 6 | Saprophytic fungal infestation of nasal mucosa? | Localized fungal colonization of nasal or paranasal sinus mucosa |
| 7 | Allergic mucin or eosinophilic mucin? | Eosinophilic mucin |
| 8 | Distinction between AFRS/EFRS/EMRS? | See Fig. 7 |
FRS = fungal rhinosinusitis; AFRS = allergic fungal rhinosinusitis; EFRS = eosinophilic fungal rhinosinusitis; EMRS = eosinophilic mucin rhinosinusitis.
Footnotes
This work is a summary of the panel discussion during the Workshop on Fungal Sinusitis, Chandigarh, India, February 9–11, 2008. The major sponsors of the workshop were the International Society for Human and Animal Mycology, Lifecare Innovations, India, Pfizer Inc., and Merek Sharp & Dohme.
Additional Supporting Information may be found in the online version of this article.
BIBLIOGRAPHY
- 1.International Rhinosinusitis Advisory Board. Infectious rhinosinusitis in adults: classification, etiology and management. Ear Nose Throat J. 1997;76:5–22. [PubMed] [Google Scholar]
- 2.Hora JF. Primary aspergillosis of the paranasal sinuses and associated areas. Laryngoscope. 1965;75:768–773. doi: 10.1288/00005537-196505000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Jahrsdoerfer RA, Ejercito VS, Johns MME, Contrell RW, Sydnor JB. Aspergillosis of the nose and paranasal sinuses. Am J Otol. 1979;1:6–14. doi: 10.1016/s0196-0709(79)80003-4. [DOI] [PubMed] [Google Scholar]
- 4.Lowe J, Bradley J. Cerebral and orbital Aspergillus infection due to invasive aspergillosis of ethmoid sinus. J Clin Pathol. 1986;39:774–778. doi: 10.1136/jcp.39.7.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGill TJ, Simpson G, Healy GB. Fulminant aspergillosis of the nose and paranasal sinuses: a new clinical entity. Laryngoscope. 1980;90:748–754. [PubMed] [Google Scholar]
- 6.Safirstein B. Allergic broncho-pulmonary aspergillosis with obstruction of the upper respiratory tract. Chest. 1976;70:788–790. doi: 10.1378/chest.70.6.788. [DOI] [PubMed] [Google Scholar]
- 7.Millar JN, Johnston A, Lamb D. Allergic aspergillosis of the maxillary sinuses. Thorax. 1981;36:710. [Google Scholar]
- 8.Katzenstein AA, Sole SR, Greenberger PA. Allergic Aspergillus sinusitis: a newly recognized form of sinusitis. J Allergy Clin Immunol. 1983;72:82–93. doi: 10.1016/0091-6749(83)90057-x. [DOI] [PubMed] [Google Scholar]
- 9.Allphin AL, Strauss M, Abdul Karim FW. Allergic fungal sinusitis: problems in diagnosis and treatment. Laryngoscope. 1991;101:815–820. doi: 10.1288/00005537-199108000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Manning SC, Schaefer SD, Close LG, Vuitch F. Culture positive allergic fungal sinusitis. Arch Otolaryngol. 1991;117:174–178. doi: 10.1001/archotol.1991.01870140062007. [DOI] [PubMed] [Google Scholar]
- 11.Bent JP, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111:580–588. doi: 10.1177/019459989411100508. [DOI] [PubMed] [Google Scholar]
- 12.Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74:877–884. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 13.Braun H, Buzina W, Freudenschuss K, Beham A, Stammberger H. “Eosinophilic fungal rhinosinusitis”: a common disorder in Europe? Laryngoscope. 2003;113:264–269. doi: 10.1097/00005537-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 14.deShazo RD, Chapin K, Swain R. Fungal sinusitis. N Eng J Med. 1997;337:254–259. doi: 10.1056/NEJM199707243370407. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson BJ. Eosinophilic mucin rhinosinusitis: a distinct clinicopathological entity. Laryngoscope. 2000;110:799–813. doi: 10.1097/00005537-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Gowing NFC, Hamlin IME. Tissue reaction to Aspergillus in cases of Hodgkin’s disease and leukemia. J Clin Pathol. 1960;13:396–413. doi: 10.1136/jcp.13.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.deShazo RD. Fungal sinusitis. Am J Med Sci. 1998;316:39–45. doi: 10.1097/00000441-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson BJ. Mucormycosis of the nose and paranasal sinuses. Otolaryngol Clin North Am. 2000;33:349–365. doi: 10.1016/s0030-6665(00)80010-9. [DOI] [PubMed] [Google Scholar]
- 19.Adelson RT, Marple BF. Fungal rhinosinusitis: state-of-art diagnosis and treatment. J Otolaryngol. 2005;34(suppl 1):S18–S23. [PubMed] [Google Scholar]
- 20.Zapico ADV, Suarez AR, Encinas PM, Angulo CM, Pozuelo EC. Mucormycosis of the sphenoid sinus in an otherwise healthy patient. Case report and literature review. J Layngol Otol. 1996;110:471–473. doi: 10.1017/s0022215100134012. [DOI] [PubMed] [Google Scholar]
- 21.Sridhara SR, Paragache G, Panda NK, Chakrabarti A. Mucormycosis in immunocompetent individuals: an increasing trend. J Otolaryngol. 2005;34:402–406. doi: 10.2310/7070.2005.34607. [DOI] [PubMed] [Google Scholar]
- 22.Chakrabarti A, Das A, Mandal J, et al. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med Mycol. 2006;44:335–342. doi: 10.1080/13693780500464930. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti A, Sharma SC, Chander J. Epidemiology and pathogenesis of paranasal sinus mycoses. Otolaryngol Head Neck Surg. 1992;107:745–750. doi: 10.1177/019459988910700606.1. [DOI] [PubMed] [Google Scholar]
- 24.Veress B, Malik OA, el-Tayeb AA, el-Daoud S, Mahgoub ES, el-Hassan AM. Further observations on primary paranasal Aspergillus granuloma in the Sudan. A morphological study of 46 cases. Am J Trop Med Hyg. 1973;2:765–772. doi: 10.4269/ajtmh.1973.22.765. [DOI] [PubMed] [Google Scholar]
- 25.deShazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Gardner L, Swain R. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1997;123:1181–1188. doi: 10.1001/archotol.1997.01900110031005. [DOI] [PubMed] [Google Scholar]
- 26.Milroy CM, Blanshard JD, Lucas S, Michaels L. Aspergillosis of the nose and paranasal sinuses. J Clin Pathol. 1989;42:123–127. doi: 10.1136/jcp.42.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferguson BJ. Definitions of fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33:227–235. doi: 10.1016/s0030-6665(00)80002-x. [DOI] [PubMed] [Google Scholar]
- 28.Grosjean P, Weber R. Fungus balls of the paranasal sinuses: a review. Eur Arch Otorhinolaryngol. 2007;264:461–470. doi: 10.1007/s00405-007-0281-5. [DOI] [PubMed] [Google Scholar]
- 29.deShazo RD, O’Brien M, Chapin K, et al. Criteria for the diagnosis of sinus mycetoma. J Allergy Clin Immunol. 1997;99:475–485. doi: 10.1016/s0091-6749(97)70073-3. [DOI] [PubMed] [Google Scholar]
- 30.Dufour X, Kauffmann-Lacroix C, Ferrie JC, Goujon JM, Rodier MH, Klossek JM. Paranasal sinus fungal ball epidemiology, clinical features and diagnosis. A retrospective analysis of 173 cases from a single center in France, 1989–2002. Med Mycol. 2006;44:61–67. doi: 10.1080/13693780500235728. [DOI] [PubMed] [Google Scholar]
- 31.Gungor A, Adusumilli V. Fungal sinusitis: progression of diseases in immunosuppression—a case report. Ear Nose Throat J. 1998;77:207–215. [PubMed] [Google Scholar]
- 32.Graham SM, Ballas ZK. Postoperative steroids confuse the diagnosis of allergic fungal sinusitis. J Allergy Clin Immunol. 1998;101:139–140. doi: 10.1016/S0091-6749(98)70211-8. [DOI] [PubMed] [Google Scholar]
- 33.Dhong HJ, Jung JY, Park JH. Diagnostic accuracy in sinus fungus balls: CT scan and operative findings. Am J Rhinol. 2000;14:227–231. doi: 10.2500/105065800779954446. [DOI] [PubMed] [Google Scholar]
- 34.Horst M, Hejjaoni A, Horst V, Michel FB, Bonsquent J. Double-blind, placebo controlled rush immunotherapy with a standardized Alternaria extract. J Allergy Clin Immunol. 1990;85:460–472. doi: 10.1016/0091-6749(90)90156-x. [DOI] [PubMed] [Google Scholar]
- 35.Marple BF. Allergic fungal rhinosinusitis: current theories and management strategies. Laryngoscope. 2001;111:1006–1019. doi: 10.1097/00005537-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Downs S, Mitkakis T, Marks G, et al. Clinical importance of Alternaria exposure in children. Am J Respir Crit Care Med. 2001;164:455–459. doi: 10.1164/ajrccm.164.3.2008042. [DOI] [PubMed] [Google Scholar]
- 37.Honser SM, Corey JP. Allergic fungal rhinosinusitis: pathophysiology, epidemiology, and diagnosis. Otolaryngol Clin North Am. 2000;33:399–408. doi: 10.1016/s0030-6665(00)80014-6. [DOI] [PubMed] [Google Scholar]
- 38.Ghegan MD, Lee FS, Schlosser RJ. Incidence of skull base and orbital erosion in allergic fungal rhinosinusitis (AFRS) and non-AFRS. Otolaryngol Head Neck Surg. 2006;134:592–595. doi: 10.1016/j.otohns.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 39.Nussenbaum B, Marple BF, Schwade ND. Characteristics of bony erosion in allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg. 2001;124:150–154. doi: 10.1067/mhn.2001.112573. [DOI] [PubMed] [Google Scholar]
- 40.Kuhn FA, Swan R. Allergic fungal sinusitis: diagnosis and treatment. Curr Opin Otolarynogol Head Neck Surg. 2003;11:1–5. doi: 10.1097/00020840-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Marple BF, Gibbs SR, Newcomer MT, Mabry RL. Allergic fungal sinusitis-induced visual loss. Am J Rhinol. 1999;13:191–195. doi: 10.2500/105065899781389740. [DOI] [PubMed] [Google Scholar]
- 42.Meltzer E, Hamilos D, Hadley J, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114(suppl):S155–S212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor MJ, Ponikau JU, Sherris DA, et al. Detection of fungal organisms in eosinophilic mucin using a fluorescein-labeled chitin-specific binding protein. Otolaryngol Head Neck Surg. 2002;127:377–383. doi: 10.1067/mhn.2002.128896. [DOI] [PubMed] [Google Scholar]
- 44.Rao AK, Mathers PH, Ramadan HH. Detection of fungi in the sinus mucosa using polymerase chain reaction. Otolaryngol Head Neck Surg. 2006;134:581–585. doi: 10.1016/j.otohns.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 45.Kim ST, Choi JH, Jeon HG, Cha HE, Hwang YJ, Chung YS. Comparison between polymerase chain reaction and fungal culture for the detection of fungi in patients with chronic sinusitis and normal controls. Acta Otolaryngol. 2005;125:72–75. doi: 10.1080/00016480410018133. [DOI] [PubMed] [Google Scholar]
- 46.Polzehl D, Weschta M, Podbielski A, Riechelmann H, Rimek D. Fungus culture and PCR in nasal lavage samples of patients with chronic rhinosinusitis. J Med Microbiol. 2005;54:31–37. doi: 10.1099/jmm.0.45881-0. [DOI] [PubMed] [Google Scholar]
- 47.Kostamo K, Richardson M, Eerola E, et al. Negative impact of Aspergillus galactomannan and DNA detection in the diagnosis of fungal rhinosinusitis. J Med Microbiol. 2007;56:1322–1327. doi: 10.1099/jmm.0.47101-0. [DOI] [PubMed] [Google Scholar]
- 48.Saravanan K, Panda NK, Chakrabarti A, Bapuraj RJ. Allergic fungal rhinosinusitis: an attempt to resolve the diagnostic dilemma. Arch Otolaryngol Head Neck Surg. 2006;132:173–178. doi: 10.1001/archotol.132.2.173. [DOI] [PubMed] [Google Scholar]
- 49.Manning SC, Holman M. Further evidence for allergic pathophysiology in allergic fungal sinusitis. Laryngoscope. 1998;108:1485–1496. doi: 10.1097/00005537-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Chhabra A, Handa KK, Chakrabarti A, Mann SBS, Panda N. Allergic fungal sinusitis: clinicopathological characteristics. Mycoses. 1996;39:437–441. doi: 10.1111/j.1439-0507.1996.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 51.Panda NK, Sharma SC, Chakrabarti A, Mann SBS. Paranasal sinus mycoses in north India. Mycoses. 1998;41:281–286. doi: 10.1111/j.1439-0507.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 52.Dhiwakar M, Thakar A, Bahadur S, et al. Pre-operative diagnosis of allergic fungal sinusitis. Laryngoscope. 2003;113:688–694. doi: 10.1097/00005537-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 53.Taj-Aldeen SJ, Hilal AA, Schell WA. Allergic fungal rhinosinusitis: a report of 8 cases. Am J Otolaryngol. 2004;25:213–218. doi: 10.1016/j.amjoto.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Ponikau JU, Sherris DA, Kephart GM, et al. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005;116:362–369. doi: 10.1016/j.jaci.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 55.Shin SH, Ponikau JU, Sherris DA, et al. Chronic rhinosinusitis: an advanced immune response to ubiquitous airborne fungi. J Allergy Clin Immunol. 2004;114:1369–1375. doi: 10.1016/j.jaci.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Manning SC, Vuitch F, Weinberg AG, Brown OE. Allergic aspergillosis: a newly recognized form of sinusitis in the pediatric population. Laryngoscope. 1989;99:681–685. doi: 10.1288/00005537-198907000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Borish L, Rosenwasser L, Steinke JW. Fungi in chronic hyperplastic eosinophilic sinusitis. Clin Rev Allergy Immunol. 2006;30:1–9. doi: 10.1385/CRIAI:30:3:195. [DOI] [PubMed] [Google Scholar]
- 58.Bachert C, Gevaert P, Holtappels G, Johansson SG, Van Cauwenberge P. Total and specific IgE in the nasal polyps in related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–614. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 59.Thakar A, Sarkar C, Dhiwakar M, Bahadur S, Dahiya S. Allergic fungal sinusitis: expanding the clinicopathological spectrum. Otolaryngol Head Neck Surg. 2004;130:209–216. doi: 10.1016/j.otohns.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Venarske DL, deShazo RD. Sinobronchial allergic mycosis: the SAM Syndrome. Chest. 2002;121:1670–1676. doi: 10.1378/chest.121.5.1670. [DOI] [PubMed] [Google Scholar]