Abstract
Background
Acute leukemia with 11q23 aberrations is associated with a poor outcome with therapy. The lack of efficacy of conventional therapy has stimulated interest in developing novel strategies. Recent studies have shown that 11q23-positive acute leukemia cells express the high molecular weight-melanoma associated antigen (HMW-MAA). This tumor antigen represents a useful target to control growth of human melanoma tumors in patients and in severe combined immunodeficient (SCID) mice, utilizing antibody-based immunotherapy. This effect appears to be mediated by inhibition of the HMW-MAA function such as triggering of the focal adhesion kinase/proline-rich tyrosine kinase 2 (Pyk2) pathways. Therefore, in this study we tested whether HMW-MAA-specific monoclonal antibodies (mAb) could inhibit growth of 11q23-positive leukemia cells in SCID mice.
Methods
HMW-MAA-specific mAb were tested for their ability to inhibit the in vitro proliferation of an 11q23-positive acute myeloid leukemia (AML) cell line and blasts from four patients with 11q23 aberrations and their in vivo growth in subcutaneous and disseminated xenograft models.
Results
The HMW-MAA-specific mAb did not affect in vitro proliferation although they down-regulated phosphorylated (P) Pyk2 expression. Furthermore, the mAb enhanced the in vitro anti-proliferative effect of cytarabine. In vivo the mAb inhibited the growth of leukemic cells in a dose-dependent fashion. However, the difference did not reach statistical significance. No effect was detected on P-Pyk2 expression. Furthermore, HMW-MAA-specific mAb in combination with cytarabine did not improve tumor inhibition. Lastly, the combination of two mAb which recognize distinct HMW-MAA determinants had no detectable effect on survival in a disseminated xenograft model.
Conclusions
HMW-MAA-specific mAb down-regulated P-Pyk2 expression and enhanced the anti-proliferative effect of cytarabine in vitro, but had no detectable effect on survival or growth of leukemia cells in vivo. Whether the HMW-MAA-specific mAb can be used as carriers of toxins or chemotherapeutic agents against 11q23-acute leukemia remains to be determined.
Keywords: Acute leukemia, 11q23, HMW-MAA, Immunotherapy
Introduction
Acute leukemia with 11q23 aberrations is associated with a poor outcome to chemotherapy-containing regimens in children [1] and adults [2]. Therefore, other treatment modalities, such as immunotherapy, are sought. The successful application of antibody-based immunotherapy in hematologic malignancies [3] with or without the addition of chemotherapy has prompted us to test this strategy in 11q23-positive acute leukemia. Since no leukemia-specific antigen has been identified in 11q23-positive acute leukemia, we developed an antibody-based immunotherapeutic strategy, taking advantage of the expression of the high molecular weight-melanoma associated antigen [(HMW-MAA), the human homolog of the rat NG2, also known, among others, as chondroitin sulfate proteoglycan, melanoma chondroitin sulfate proteoglycan] on the surface of leukemic blasts [4–10]. The HMW-MAA is a membrane bound proteoglycan that has been targeted in the treatment of malignant melanoma [11–13]. Specifically, induction of HMW-MAA-specific antibodies by HMW-MAA mimics was associated with regression of metastases in a few patients [12] and with survival prolongation [11]. This effect was likely induced by the HMW-MAA-specific antibodies, since administration of HMW-MAA-specific monoclonal antibodies (mAb) inhibits the growth of human melanoma tumors implanted in severe combined immunodeficient (SCID) mice [14]. The anti-tumor effects of HMW-MAA-specific mAb are likely to reflect inhibition of the HMW-MAA function in melanoma cell biology, as mediated by the down-regulation of the focal adhesion kinase (FAK) signaling pathway [15].
We thus tested the in vitro and in vivo effects of HMW-MAA-specific mAb on survival and proliferation of 11q23-positive acute leukemia cells.
Materials and methods
Cells and culture conditions
The acute myeloid leukemia (AML) cell line expressing 11q23, ML-2, [purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany)] and the melanoma cell line expressing HMW-MAA, Colo 38, were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin (100 units/ml) and streptomycin (100 μg/ml) (all from Life Technology, Grand Island, NY). Where indicated, cells were cultured for 72 h at 37°C with tunicamycin (Sigma, St. Louis, MO), at concentrations of 0.25, 0.5 and 1 μg/ml. Higher concentrations were toxic to the ML-2 cells. Tunicamycin was dissolved in dimethyl sulfoxide (5 mg/ml) and diluted with RPMI-1640 medium. Cryopreserved bone marrow samples with more than 80% blasts from four newly diagnosed 11q23-positive acute leukemia [two AML and two acute lymphoblastic leukemia (ALL)] patients were thawed and used immediately for the described experiments.
Lymphocytes, monocytes and granulocytes were isolated from peripheral blood from three normal volunteers. To isolate monocytes, peripheral blood mononuclear cells (PBMC), obtained by density centrifugation, were aspirated and resuspended at 2 × 106 cells/ml of RPMI-1640 medium containing 2% fetal bovine serum and incubated in Petri dishes for 2 h at 37°C. The non-adherent cells were saved to represent lymphocytes and the adherent cells were collected and saved as monocytes. To obtain granulocytes, the cell pellet, following density centrifugation, was collected. Red blood cells were lysed with 1.22% ammonium oxalate, allowing isolation of granulocytes. Serum was collected from normal volunteers and stored at −70°C. Additional controls included CD34 + cells separated by the MACS progenitor cell isolation kit (Milteni Biotec, Auburn, CA) from peripheral stem cell collections from three breast cancer patients without bone marrow involvement as described previously [16].
All of the described studies of patient and normal volunteer samples, as well as the in vivo experiments, including use of primary patient samples in mice, were approved by the Roswell Park Cancer Institute Scientific Review Committee, Institutional Review Board and the Institutional Animal Care and Use Committee.
Antibodies
The HMW-MAA-specific mouse mAb 225.28, 653.25, 763.74, TP61.5 and VT68.2, the control anti-idiotypic mAb MF11-30, MK2-23, and T8-203, elicited with mAb 225.28, 763.74 and CR11-351, respectively, the mAb TP25.99 which recognizes a conformational determinant expressed on all β2m associated HLA-A, B and C heavy chains and a linear determinant expressed on all β2m-free HLA-B heavy chains except HLA-B73, and on β2m free HLA-A1, -A3, -A9, -A11 and -A30 heavy chains, and the HLA-A, B, C, E, F, G-specific mAb W6/32 were developed and characterized as described [17–22]. Phosphorylated (P) extracellular signal regulated kinase (ERK) 1/2-specific, FAK-specific, proline-rich tyrosine kinase 2 (Pyk-2)-specific, and Rous sarcoma virus (Src)-specific rabbit polyclonal antibodies were purchased from Cell Signaling Technology, Inc., Danvers, MA. Nonphosphorylated B cell lymphoma (BCL) 2-associated X protein (Bax)-specific mAb and Bcl-2-specific mAb were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. ERK1/2-specific and FAK-specific rabbit polyclonal antibodies were purchased from Cell Signaling, Pyk2-specific, signal transducer and activator of transcription 3 (STAT3)-specific and Src-specific rabbit polyclonal antibodies were purchased from Upstate Biotechnology, Lake Placid, NY. The CD45-specific mouse phycoerythrin (PE)-conjugated mAb, the human CD45-specific fluorescein isothiocyanate (FITC)-conjugated mAb, the HLA-DR-specific FITC-conjugated mAb and the human CD33-specific FITC-conjugated mAb were purchased from BD Pharmingen, San Diego, CA. The human CD19-specific PE-conjugated mAb and FITC-conjugated mAb, and the PE-conjugated goat anti-mouse immunoglobulin (IgG) antibodies were purchased from Caltag, Burlingame, CA. mAb were biotinylated using NHS-LC-biotin (Pierce, Rockford, IL) according to the manufacturer’s instructions. F(ab′)2 fragments were generated from mouse mAb by digestion with pepsin as described [18].
Flow cytometric analysis
Cell surface staining with mAb was performed as described [23]. Briefly, cells were incubated for 15 min on ice with an excess of antibody or an isotype control. Following the addition of the secondary antibody (if needed), cells were incubated with a mouse IgG solution (1 mg/ml) for 10 min on ice to saturate all unbound anti-IgG Fab sites. Cells were then fixed in 300 μl 2% ultrapure formaldehyde (Polyscience Inc., Warington, PA). Apoptosis was analyzed using a BD Pharmingen kit according to the manufacturer’s instructions. Annexin was added first and propidium iodide was added 30 min later. Data were evaluated on a FACS Calibur instrument (BD Bioscience, San Jose, CA) using WinList 6.0 software.
Western blot
Proteins were detected by Western blot analysis as previously described [24]. In brief, whole-cell extracts were separated on 8% polyacrylamide sodium dodecyl sulfate gels and proteins were transferred to nitrocellulose membranes. Membranes were incubated with the primary antibody and then with horseradish peroxidase-labeled anti-mouse or anti-rabbit Ig xenogeneric secondary Ab (Amersham Pharmacia, Piscataway, NJ). To detect the expression of the HMW-MAA in ML-2 cells, cell lysates were incubated at 4°C overnight with HMW-MAA-specific mAb 225.28, then protein A agarose (Upstate Biotechnology) was added and incubation was continued for an additional 2 h at 4°C [24]. After electrophoresis and transfer to nitrocellulose membranes, immunoblotting was performed using HMW-MAA-specific mAb 653.25. Immune complexes were detected using a chemiluminescent detection method (Amersham Pharmacia).
Reverse transcription-polymerase chain reaction (RT-PCR)
The presence of HMW-MAA mRNA was determined by RT-PCR using primers extending from position 2,845 to 3,388 (forward: 5′-GGG ACA CAG AAG ACC AC-3′; reverse: 5′-CCA CAC GGA GGT AGG GTT-3′), 3,364 to 3,872 (forward: 5′-GCC TCG GAA CCC TAC CTC-3′; reverse: 5′-GAC ACC ATC ACC AGG TAG CC-3′), 3,853 to 4,363 (forward: 5′-GGC TAC CTG GTG ATG GTG TC-3′; reverse: 5′-GAT CCA TCT CGG AGG CAT TA-3′), 4,265 to 4,814 (forward: 5′-GGA GAA TGG TGG AAG AGC AG-3′; reverse: 5′-AGG GTC TGA CTG CTG AGT GG-3′) and 4,736 to 5,259 (forward: 5′-AAG TGC TCC TCT CGC TGA AG-3′; reverse: 5′-CTG TGT GAC CTG GAA GAG CA-3′) [25]. Actin was used as an internal standard for mRNA semi quantitation.
Proliferation assays
ML-2 cells were incubated at 37°C in a 5% CO2 atmosphere for up to 48 h with HMW-MAA-specific mAb VT68.2 or 225.28 and cytarabine at varying concentrations. Cell viability was determined by the Trypan blue dye (Invitrogen) exclusion assay.
Interaction assays
All assays were conducted at least in triplicates. The Hill function was fitted to each concentration–response curve for HMW-MAA-specific mAb VT68.2 or 225.28 (at 50 μg/ml) and cytarabine (100 nM–5 μM) [26, 27]. Pharmacodynamic antibody–drug interactions on cell survival (ψ) were evaluated with the following non-competitive equation relating concentrations of both agents to the overall effect [28].
 |
1a |
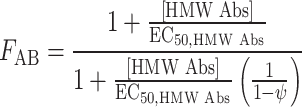 |
1b |
E 0 is the effect baseline when no drug was present, I max was the fraction of the baseline representing the maximum inhibition possible for cytarabine at high concentrations. The value of IC50 was the concentration of cytarabine when given alone to produce 50% of the maximal inhibition. The EC50, HMW Abs value represented the concentration of HMW-MAA-specific mAb that elicited half the maximal change in the IC50 value for cytarabine. The value of ψ simply governed the direction the change in inhibition was in.
Equation 1 was proposed by Ariens [29] for an agent that acts non-competitively to alter a drug’s potency. The HMW-MAA-specific mAb did not change the maximal effect and did not inhibit cell proliferation. This was reflected in the first equation. The only factor in Eq. 1a that contained an influence from the HMW-MAA-specific mAb is F AB. Here F AB acted as a proportionality constant to change the IC50 of cytarabine. The interaction became synergistic and antagonistic when F AB was less than or greater than one, respectively. This could also be reflected in the value of ψ. The interaction taking place was synergistic and antagonistic, when ψ was <1 and when ψ >1, respectively.
Nonlinear regression fitting was performed using the maximum likelihood module in the ADAPT II software [30].
In vivo leukemia models
ML-2 leukemia xenografts
Eight-week-old female SCID/SCID mice (bred at Roswell Park Cancer Institute, Buffalo, NY) were sublethally irradiated with 200 cGy and inoculated with 1 × 107 ML-2 cells subcutaneously (SQ), as described previously, according to an institute-approved animal protocol [31]. When tumor volumes approached 50–100 mm3 (approximately 7–14 days after inoculation), mice were divided into experimental groups of 5–10 mice per group. One group was treated by intraperitoneal (IP) injection of increasing amounts of mAb 225.28 or of the isotype matched control mAb T8-203, twice a week, or of cytarabine at varying concentrations five times per week to determine the maximally tolerated dose (MTD). Following MTD determination, combination regimens of antibodies and chemotherapy used the same dosing regimens and intervals. Mice were routinely assessed for weight loss, anorexia, and other clinical signs. The two largest perpendicular axes of each tumor xenograft (l indicates length; w, width) were measured 2–3 times weekly with calipers, and tumor volume (TV) was calculated using the formula: TV (mm3) = 4/3πr 3, where r = (l + w)/4. Animals were sacrificed when they became moribund from progressive tumor-related signs or when the one-dimensional tumor diameter exceeded 2.0 cm (according to the guidelines of the animal core facility). Mice underwent retro-orbital bleeds during and at completion of treatment for collection of plasma for ELISA analysis. At the end of the experiment, tumors were removed and processed for Western blot analysis and flow cytometry as mentioned above. Results of mAb and chemotherapy treatments are shown as mean tumor volume (mTV) ± standard error (SE) at each time point for each treatment group.
Primary 11q23 leukemia models
Eight-week-old female non-obese diabetic (NOD) SCID mice (Jackson Laboratories, Bar Harbor, ME) were sublethally irradiated with 300 cGy followed by injection of 1 × 107 primary 11q23 positive acute leukemia patient cells into the tail vein, as described previously [32], according to an institute-approved animal protocol. Immediately following inoculation, mice were randomly assigned to one of two groups and treated IP with 0.2 mg of either mAb T8-203 and MF11-30 control antibodies or mAb 225.28 and 763.74. For the first 2 weeks following the injection of primary leukemia cells, mice were treated twice a week. After that initial loading period, treatment was administered once a week for an additional 2 weeks. Mice underwent retro-orbital bleeds during and at completion of treatment for FISH assays to confirm engraftment (see below). Mice were monitored three times per week for signs of clinical deterioration including weight loss >20%, and hind-limb paralysis. At time of moribundity, mice were sacrificed with collection of peripheral blood, bone marrow and spleen samples for additional correlative FISH and flow cytometric studies (see other methods).
Double determinant immunoassay to measure level of mAb 225.28 in mouse serum
Ninety-six-well, flexible, U-bottom Costar 2797 microtiter plates (Costar, Cambridge, MA) were coated with F(ab′)2 fragments of the anti-idiotypic mAb MF11–30 (1 μg/well) in 100 mM NaHCO3, pH 9.6. Following an overnight incubation at 4°C, plates were blocked with 2% bovine serum albumin-phosphate buffered saline (PBS) for 2 h at room temperature and incubated with 100 μl of fivefold serially diluted mouse sera (1:100–1:7,812,500) for an additional 2 h at room temperature. Wells were washed five times with 0.05% Tween-20/PBS and three times with PBS between incubations. Binding of antibody was detected with horseradish peroxidase-conjugated goat anti-mouse IgG (Fc-specific) antibodies. Results were expressed as optical density-absorbance at 450 nm using 3-3′,5,5′-tetramethylbenzidine (TMB) peroxidase as substrate (KPL, Gaithersburg, MD) on an ELISA plate reader (Finstruments RS-232c, Helsinki, Finland). Plates coated with F(ab′)2 fragments of HMW-MAA-specific mAb TP61.5 were used as controls.
Fluorescence in situ hybridization (FISH)
A total of 1 × 104 cells were cytospun onto glass slides and fixed in methanol. Fixed cells were hybridized with commercially available dual-color break apart MLL (mixed leukemia-lymphoma) DNA probe (Vysis/Abbott Molecualr Inc., Des Plaines, IL). Images were captured using the image analysis software (CytoVision Imaging System, Applied Imaging Corp., San Jose, CA). Signals were visualized with a Nikon Eclipse 90i epifluorescence microscope (Nikon Inc.) with appropriate filters, as previously described by us [33].
Immunohistochemistry
Frozen tumor sections were cut on a cryostat and stored on slides at −80°C until processing. Upon processing, slides were warmed up to room temperature and fixed in cold (−20°C) acetone for 10 min. Endogenous peroxidase was quenched with aqueous 1% H2O2 in methanol for 10 min. Biotin detection was accomplished using the ABC reagent (HRP complex; Vector Labs, Burlingame, CA) for 30 min. The chromagen DAB (DAKO, Carpenteria, CA) was then applied and incubation was continued for 5 min (color reaction product, brown). Slides were counterstained with Hematoxylin (Sigma), dehydrated, cleared and coverslipped.
Antibody-dependent-cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) assays
The ADCC and CDC assays were performed as described [34, 35] with minor modifications. Briefly, ML-2 target cells were labeled for 1 h at 37°C with Na2[51Cr]O4, plated in 96-well plates (Costar; Bio-Rad Laboratories, Inc., Hercules, CA) at 1 × 104 cells/well, and incubated for 30 min at room temperature with HMW-MAA-specific mAb 225.28 (3 μg/ml) or isotypic matched control HLA class I antigen-specific mAb W6/32 (3 μg/ml). Cells were then washed with serum-free RPMI-1640 medium and incubated for 4 h at 37°C with human PBMC for ADCC or human serum for CDC. Supernatants were harvested, and the radioactivity was counted in a gamma counter (Packard Cobra II Auto Model 5002; PerkinElmer). Both assays were performed in triplicates, and the percentage of specific cytotoxicity was calculated using the formula: percentage of cytotoxicity = (experimental cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm) × 100. The spontaneous 51Cr release was <25% of maximum 51Cr release.
Statistical analyses
Unpaired Student’s t test was used to compare different groups of mice. The difference in the mean channel fluorescence of signal to background was used to determine the means and ranges for each of the markers investigated. P values <0.05 were considered statistically significant.
Results
Differential expression of HMW-MAA antigenic determinants by 11q23-positive leukemic cells
FACS analysis showed that the four patient samples and the ML-2 cell line were differentially stained by the HMW-MAA-specific mAb tested; the patient samples stained with VT68.2, 225.28 and 763.74 while the cell line stained only with mAb VT68.2 and 225.28 (Fig. 1a). Incubation of ML-2 cells with tunicamycin at increasing concentrations decreased the reactivity with the mAb 225.28 and VT68.2 in a dose-dependent fashion (data not shown). To define the molecular basis of the binding assays, lysates of leukemic cells were tested with HMW-MAA-specific mAb 763.74 in Western blotting. As shown in Fig. 1b, mAb 763.74 reacted with a 450-kDa moiety which corresponds to the large component of the HMW-MAA. Furthermore, mAb 763.74 detected the 280-kDa component in the two ALL samples but not in the two AML samples. To explore whether the lack of mAb 763.74-defined determinant expression was related to deletion of part of the HMW-MAA mRNA, RT-PCR was applied. As shown in Fig. 1c, the size of the HMW-MAA mRNA was similar to that of the mRNA in the melanoma cell line Colo38. Finally, to verify that the intact protein was indeed expressed in ML-2 cells, the HMW-MAA was immunoprecipitated with mAb 225.28 and VT68.2 from ML-2 cells. The 280-kDa protein was detected following immunoblotting with mAb 653.25 (Fig. 1d).
Fig. 1.
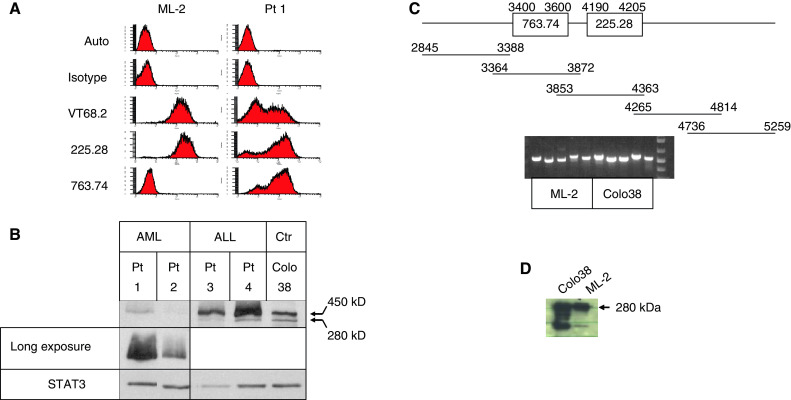
HMW-MAA expression in acute leukemia samples. a FACS analysis of the ML-2 cell line and representative AML patient samples stained with mAb VT68.2, 225.28 and 763.74 which recognize distinct and spatially distant determinants of HMW-MAA. b Western blot analysis of HMW-MAA expression in 11q23-positive acute leukemia samples. The four patient samples were tested with HMW-MAA-specific mAb 763.74. Human melanoma cell line Colo38, which expresses HMW-MAA, served as a positive control. The upper panel represents short exposure and the middle panel represents longer exposure to verify that the 280-kDa component is not expressed in the AML samples. Reaction with anti-STAT3 antibody verifies equal loading. c mRNA expression by RT-PCR of the 763.74 and 225.28-defined determinants. Upper panel the five probes. Lower panel RT-PCR analysis. d SDS-PAGE analysis of HMW-MAA immunoprecipitated from ML-2 cells by mAb 225.28 and 653.25. Of note, the 450-kDa component could not be detected in Colo38 cells using this method most probably due to its degradation, as suggested by the appearance of the lower band
Lack of HMW-MAA expression on normal hematopoietic cells
We analyzed the expression of HMW-MAA by RT-PCR, Western blotting and FACS analysis in CD34 + cells, granulocytes, lymphocytes and monocytes. HMW-MAA expression in these cells was detected neither at the mRNA (data not shown) nor at the protein (data not shown) level.
Enhancement by HMW-MAA-specific mAb of the in vitro anti-proliferative effect of cytarabine
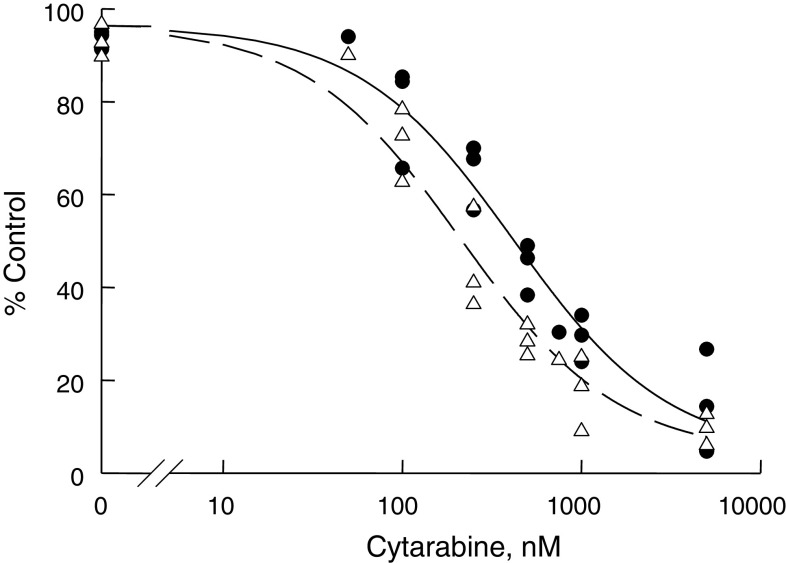
The anti-proliferative effect of the mAb VT68.2 and 225.28 was studied using ML-2 cells. Both mAb had no detectable effect on the growth of ML-2 cells. Furthermore, incubation of ML-2 cells for 48 h at 37°C with mAb VT68.2 (50 μg/ml) and cytarabine (100 nM–5 μM) had no effect when compared to cytarabine alone. However, incubation of these cells for 48 h at 37°C with mAb 225.28 (50 μg/ml) and cytarabine (100 nM–5 μM) reduced the IC50 as compared to cytarabine alone (Fig. 2). The interaction parameter (ψ) was 0.7875.
Fig. 2.
Model fitting of ML-2 cell proliferation inhibition by Cytarabine with or without the HMW-MAA-specific mAb 225.28 (50 μg/ml). Dashed and solid lines are model predictions in the presence and absence of the mAb. Open triangles and solid circles represent observed values in the presence and absence of the mAb, respectively
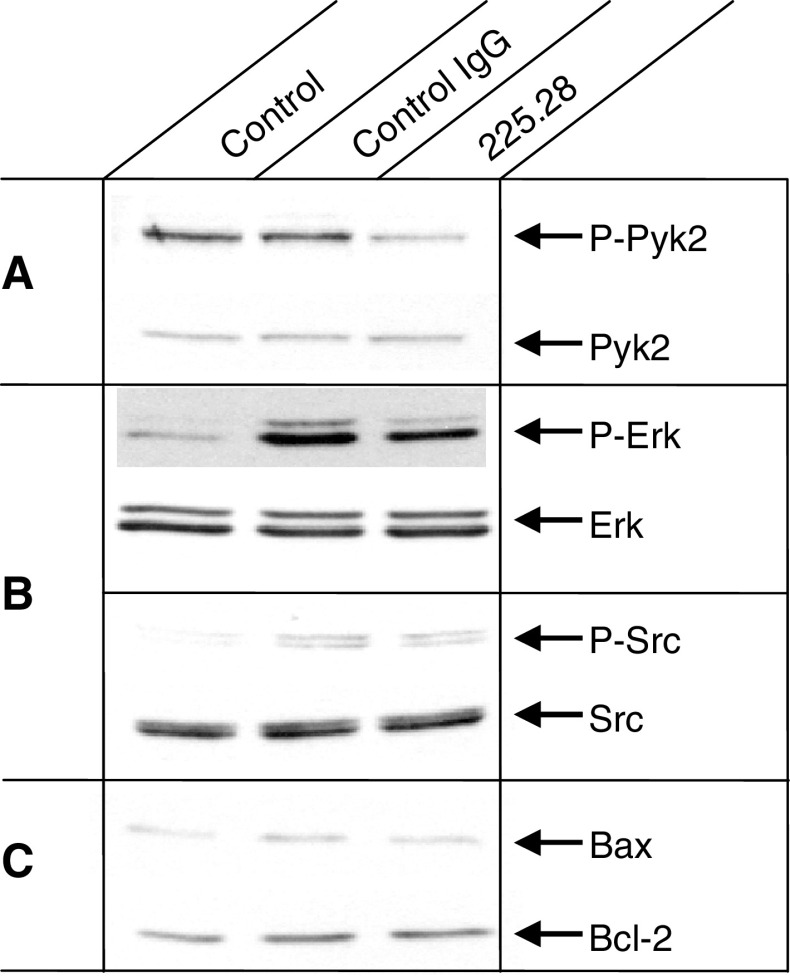
P-Pyk2 down-regulation by HMW-MAA-specific mAb
To determine whether signaling pathways were affected in leukemic cells by treatment with HMW-MAA-specific mAb, we analyzed by Western blots the expression and phosphorylation level of both FAK and Pyk2. While FAK was not detected in ML-2 cells, P-Pyk2 was constitutively expressed in untreated ML-2 cells and mAb 225.28 reduced its phosphorylation (Fig. 3a) at 48 h. This effect is time dependent since no change was detected in the level of P-Pyk2 in cells incubated for 1 or 24 h (data not shown). P-Pyk2 down-regulation was not associated with down-regulation of other signaling pathways such as P-ERK and P-Src (Fig. 3b). Furthermore, reduction of P-Pyk2 was associated with neither upregulation of Bax nor down-regulation of Bcl-2 (Fig. 3c). Lastly, incubation withHMW-MAA-specific mAb for up to 48 h did not increase the percentage of apoptotic/necrotic cells as determined by annexin and propidium iodide staining (data not shown), suggesting lack of effect on the apoptotic pathway.
Fig. 3.
Down-regulation of Pyk2 by HMW-MAA-specific mAb 225.28. ML-2 cells were cultured in the presence of mAb 225.28 (50 μg/ml) of or a control isotype matched mAb T8-203 for 48 h at 37°C. Blots were hybridized with P-Pyk2 and total Pyk2, P-ERK and total ERK, P-Src and total Src, Bax and Bcl-2-specific antibodies
Lack of side effects and favorable pharmacokinetics in mice treated with HMW-MAA-specific mAb
To assess the in vivo relevance of the in vitro data, the mAb 225.28 and VT68.2, which recognize distinct determinants of HMW-MAA, were tested for their ability to inhibit the growth of ML-2 cells transplanted in immunodeficient mice.
In order to determine the optimal time intervals for administration of HMW-MAA-specific mAb to mice, we first examined the levels of mAb 225.28 in the sera of SCID mice following a single injection of increasing doses of mAb 225.28 [14]. High mAb levels were present in sera up to 4 days after injection (Fig. 4a). Mice were therefore treated with mAb twice a week.
Fig. 4.

Lack of significant leukemic effect of 225.28 mAb with or without cytarabine on the growth of human leukemia ML-2 cells transplanted subcutaneously in SCID mice. a Levels of 225.28 mAb in SCID mouse sera injected with mAb 225.28. b Penetration of 225.28 mAb into subcutaneous tumors. After tumors approached 50–100 mm3, mice were injected with biotinylated mAb 225.28 or T8-203 twice or five times. Twenty-four hours later, mice were sacrificed and tumors were studied by immunohistochemistry. Upper panels mice treated with mAb T8-203 (control), lower panels mice treated with mAb 225.28. Left panels mice treated with two injections, right panels mice treated with five injections (×40). c HMW-MAA expression on ML-2 cells explanted from SCID mice. Cells were stained concomitantly with HLA Class I-specific and HMW-MAA-specific mAb. Results from two representative mice are shown. d Effect of 225.28 mAb with or without cytarabine chemotherapy on tumor volume (5 mice in each group). e Effect of 225.28 mAb with and without cytarabine on mice weight
Next, to determine the optimal tolerated and therapeutic dose of mAb 225.28 in vivo, mice bearing ML-2 xenografts were treated with 0.2, 0.5, and 1.0 mg/kg of mAb. Our results (data not shown) demonstrate a trend towards tumor inhibition with the high (1 mg) but not with the low (0.2 and 0.5 mg) mAb doses. No toxicity due to the mAb therapy was observed in 225.28 mAb versus PBS or T8-203 mAb treated mice (data not shown). Explanted cells from tumor-bearing mice did not show any change in P-Pyk2 expression following in vivo exposure to 225.28 mAb (data not shown).
The penetration of the mAb into the subcutaneous tumors was studied by immunohistochemical staining of tumors explanted from mice at the end of the treatment. As shown in Fig. 4b, 225.28 mAb expression was barely detectable following two injections, but was clearly detected following five injections. There was no significant staining in tumors explanted from mice treated with the isotype control mAb T8-203.
We then tested whether in vivo HMW-MAA expression by ML-2 leukemic cells was affected by the administration of mAb 225.28. To this end, cells were explanted from mice at the end of the treatment and tested with biotinylated mAb 225.28. As shown in Fig. 4c, no changes were detected in the expression of the determinant recognized by mAb 225.28.
We subsequently examined whether HMW-MAA-specific mAb could enhance the anti-leukemic effects of cytarabine chemotherapy in leukemia-bearing mice. Pilot experiments showed that 10–400 mg/kg per day for five days/week were the optimal dose and schedule of cytarabine in ML-2 engrafted SCID mice. We found no statistical difference in tumor inhibition following cytarabine 10 and 100 mg/kg per day with increased morbidity at higher cytarabine doses (data not shown). In contrast to our in vitro data, administration of mAb 225.28 in combination with cytarabine (10 or 100 mg/kg per day for five days/week) enhanced neither the significant anti-proliferative effects (Fig. 4d) nor the systemic toxicities (Fig. 4e) of chemotherapy alone in leukemia-bearing mice.
Because we were unable to find a cell line that expressed the determinant recognized by HMW-MAA-specific mAb 763.74, we developed primary leukemia xenografts in NOD/SCID mice utilizing 11q23 leukemia patient samples which express this determinant. Engraftment of human leukemia cells in peripheral blood, bone marrow, and spleens was shown by flow cytometry (Fig. 5a) and FISH (Fig. 5b). Engraftment took approximately 80 days.
Fig. 5.

Targeting leukemic cells transplanted in NOD/SCID mice with HMW-MAA-specific mAb 763.74 and 225.28. a Flow cytometric confirmation of primary ALL blast engraftment in NOD/SCID mice. Panels represent controls (left panel), dual color staining for human and murine CD45 (middle panel) and dual color staining for HLA-DR and CD19 (right panel). b Representative FISH analysis of 11q23-positive patient-derived ALL cells engrafted in NOD/SCID mice. c Survival of mice treated with 225.28 and 763.74 mAb as compared to control mice (10 mice in each group)
We then tested whether the combination of the HMW-MAA mAb 225.28 and 763.74, which recognize distinct determinants of HMW-MAA, would display greater anti-leukemia effects than one mAb alone in primary leukemia xenograft models established with 11q23 leukemia patient samples, expressing both determinants. Treatment with the two HMW-MAA-specific mAb did not result in prolongation of survival as compared to either antibody alone, or any of the control antibodies (Fig. 5c). Some of the HMW-MAA mAb treated mice experienced weight loss requiring therapy. Four of those mice were sacrificed (hence the drop in the survival curve) and were found to have developed hematomas/localized skin reactions at the injection sites.
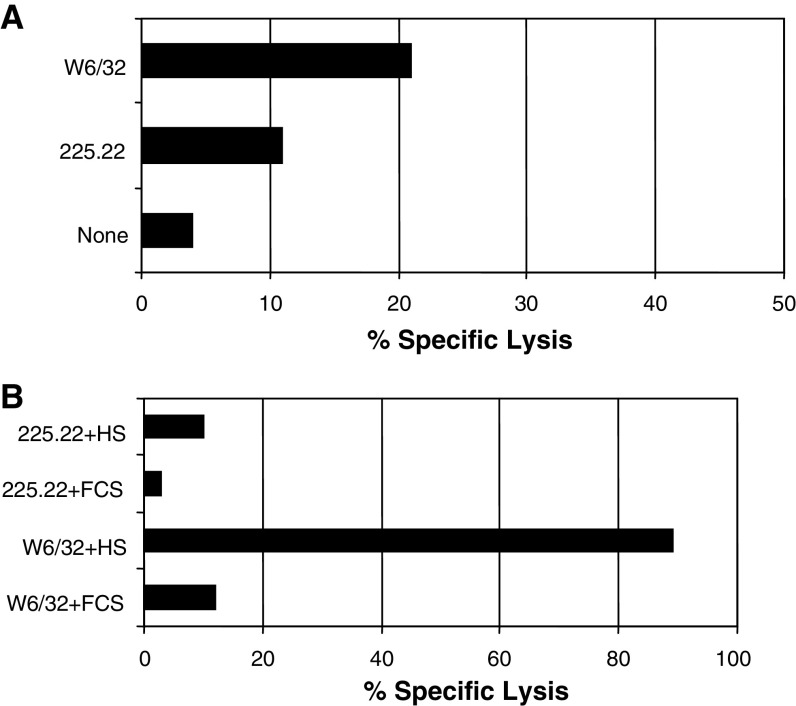
Lack of susceptibility of leukemia cells to complement and cell-dependent lysis mediated by HMW-MAA-specific mAb
To determine whether mAb 225.28 induces immune lysis of HMW-MAA bearing leukemic cells, we tested the susceptibility of ML-2 cells to complement- and cell-dependent lysis mediated by mAb 225.28. No lysis was detected in the two assays (Fig. 6). In contrast, the HLA class I-specific mAb W6/32 efficiently mediated ADCC and CDC.
Fig. 6.
Lack of significant activity of HMW-MAA-specific mAb in cell-dependent and complement-dependent cytotoxicity assays targeting ML2 cells. a Freshly isolated PBMC were cultured with 51Cr labeled ML-2 cells at the effector: target ratio of 50:1 in the presence or absence of the released HMW-MAA-specific mAb 225.28 (3 μg/ml). Following a 4-h incubation, supernatants were harvested and the 51Cr released radioactivity was measured. The HLA class I antigen-specific mAb W6/32 was used as a positive control. b ML-2 cells were mixed with the HMW-MAA-specific mAb 225.28 in the presence or absence of human serum. Supernatants were harvested following a 4-h incubation and the released radioactivity was measured. The HLA class I antigen-specific mAb W6/32 was used as a positive control
Discussion
Our results have demonstrated that HMW-MAA-specific mAb, have no anti-proliferative effect on HMW-MAA bearing leukemic cells, but are effective in down-regulating P-Pyk2 and enhanced the anti-proliferative activity of cytarabine in vitro. The mAb are well tolerated and have favorable pharmacokinetics in vivo, suggesting that future studies of targeted HMW-MAA immunotherapy with radiolabeled or toxin-conjugated mAb for 11q23-positive leukemia are warranted.
Not all the HMW-MAA determinants tested were detected on the primary 11q23 acute leukemia samples analyzed. The results of our analysis of the HMW-MAA mRNA by RT-PCR and of our SDS-PAGE analysis of the antigen immunoprecipitated from leukemic samples argue against the possibility that our findings are caused by large deletions at the mRNA level and/or by protein truncation. On the other hand, we cannot exclude that the lack of reactivity of some of the HMW-MAA-specific mAb with leukemic samples is caused by loss of the corresponding determinant(s) because of point mutations or by inaccessibility of the corresponding determinant(s) because of differential glycosylation of the protein moiety of the HMW-MAA [36].
Differential glycosylation of proteins has been demonstrated not only for HMW-MAA but also for other tumor antigens, e.g., the mucin-1 antigen [37]. Over-glycosylation prevents the immune system from accessing the peptide core and hides epitopes, which are usually exposed in under-glycosylated cells. Whatever the mechanism(s), it is noteworthy that the differential expression of HMW-MAA antigenic determinants is not unique to leukemic cells, since it has been previously shown in melanoma cells [38].
When designing immunotherapy, one has to be sure that the treatment will target the malignant clone and will spare normal hematopoietic cells. Our results showing no expression of HMW-MAA on normal hematopoietic cells are in concordance with the literature using another HMW-MAA-specific mAb [5] and suggest that HMW-MAA is a useful target to apply immunotherapy for the treatment of 11q23-positive acute leukemia. Further, the expression of HMW-MAA on the surface of pericytes, the mesenchymal cells associated with the walls of small blood vessels [39], and the recent increased interest of targeting angiogenesis in acute leukemia [40, 41], suggest an additional role for HMW-MAA-specific mAb immunotherapy in this disease.
Our in vitro results demonstrating synergism between HMW-MAA-specific mAb and cytarabine were expected, in view of the synergism between mAb and chemotherapy agents [42]. However, such a synergy has not been shown before for HMW-MAA-specific mAb. We selected cytarabine as the chemotherapeutic agent because it is the mainstay of both AML and ALL therapies and since it is not exported by multi-drug resistance mechanisms. We examined the combined effects of HMW-MAA-specific mAb with cytarabine on cell killing using the Ariens non-competitive functional interaction model with an interaction parameter (ψ). Interaction parameters may be useful in various mechanism-based models to account for the synergism or antagonism not predicted by the mechanistic expectations of the modeling scheme. The estimated value of this parameter indicates the intensity of the drug–drug interaction when compared to the no-interaction value (i.e., the value that does not influence the underlying mechanistic model, based on single drug effect alone). This interaction model is not limited to the level of mass-balance drug-receptor binding equations, but assumes that each drug contributes to the interaction after binding to their respective targets. Effect is assumed to be a function of bound drug-target and the Hill equation relates single drug concentrations to effect.
Our in vitro results demonstrated a non-immunological mechanism of action for HMW-MAA-specific mAb in our leukemia model, as was previously demonstrated for their action in melanoma [43]. A similar non-immunological mechanism was demonstrated for integrins that can mediate signaling from the extracellular space into the cell through adaptor molecules such as FAK [44]. Interestingly, HMW-MAA has been demonstrated to bind α4β1 integrin in melanoma cells and has been suggested to amplify integrin-mediated signal transduction [45]. Though FAK was known to be expressed in all cell types, its homologue, Pyk2, was shown to be expressed at higher levels in hematopoietic cells [46]. Both were constitutively expressed in AML blasts though only Pyk2 was detected in ML-2 cells. In our model, in vitro exposure of ML-2 cells to HMW-MAA-specific mAb triggered neither ADCC nor CDC but down-regulated constitutive Pyk2 activity, allowing us to test the effect of the HMW-MAA-specific mAb in NOD/SCID mice.
The in vivo studies presented here demonstrate that HMW-MAA-specific mAb alone had minimal anti-leukemia effect, but no anti-Pyk2 effect, at high doses in mice with rapidly growing leukemia disease. We then asked whether the HMW-MAA-specific mAb can be added to cytarabine without worsening the toxicities associated with the cytotoxic agent. Cytarabine alone generated the expected anti-leukemia effect in vivo. The addition of HMW-MAA-specific mAb to cytarabine resulted in no additional toxicity but no significant anti-leukemia effect, beyond that of cytarabine, was detected. Finally, combining two HMW-MAA-specific mAb did not result in any significant anti-leukemia activity. Failure of antibody-based immunotherapy is unfortunately common in the setting of active leukemia. For example, the addition of lintuzumab, the naive anti-CD33 mAb, to salvage induction chemotherapy failed to improve the response rate of the chemotherapy regimen alone [47]. Conjugating anti-CD33 mAb with chemotherapy (calicheamicin) resulted in significant benefit that was augmented by the addition of cytarabine [48]. Interestingly, using the CD33-specific mAb in patients with minimal residual acute promyelocytic leukemia resulted in conversion to RT-PCR negative state [49] highlighting a possible role for the mAb-based immunotherapy.
One mechanism by which tumor cells can escape immune recognition and destruction is represented by the down-regulation or loss of the targeted tumor antigens, especially following immunotherapy [reviewed in 50]. We therefore asked whether the expression of the HMA-MAA determinants recognized by the mAb used was lost in mice treated with HMW-MAA-specific mAb. Our results demonstrate persistence of the HMW-MAA determinants on explanted cells, suggesting that the expression of HMW-MAA was not subject to immuno-editing. These results support further development of HMW-MAA-specific mAb to deliver radioisotopes or toxins to 11q23-positive acute leukemia cells, known to be resistant to conventional chemotherapeutic agents.
Acknowledgments
Supported partially by grants from the National Cancer Institute Grant CA16056 (SF, ESW, MW) and CA 105500 (SF), the National Institute of Health Grant GM57980 (JCE), the Szefel Foundation, Roswell Park Cancer Institute (ESW) and the Heidi Leukemia Research Fund, Buffalo, NY (MW).
References
- 1.Pui CH, Gaynon PS, Boyett JM, Chessells JM, Baruchel A, Kamps W, Silverman LB, Biondi A, Harms DO, Vilmer E, Schrappe M, Camitta B. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359:1909–1915. doi: 10.1016/S0140-6736(02)08782-2. [DOI] [PubMed] [Google Scholar]
- 2.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS, Koduru PR, Moore JO, Stone RM, Mayer RJ, Feldman EJ, Davey FR, Schiffer CA, Larson RA, Bloomfield CD. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 3.Gokbuget N, Hoelzer D. Treatment with monoclonal antibodies in acute lymphoblastic leukemia: current knowledge and future prospects. Ann Hematol. 2004;83:201–205. doi: 10.1007/s00277-003-0752-8. [DOI] [PubMed] [Google Scholar]
- 4.Behm FG, Smith FO, Raimondi SC, Pui CH, Bernstein ID. Human homologue of the rat chondroitin sulfate proteoglycan, NG2, detected by monoclonal antibody 7.1, identifies childhood acute lymphoblastic leukemias with t(4;11)(q21;q23) or t(11;19)(q23;p13) and MLL gene rearrangements. Blood. 1996;87:1134–1139. [PubMed] [Google Scholar]
- 5.Smith FO, Rauch C, Williams DE, March CJ, Arthur D, Hilden J, Lampkin BC, Buckley JD, Buckley CV, Woods WG, Dinndorf PA, Sorensen P, Kersey J, Hammond D, Bernstein ID. The human homologue of rat NG2, a chondroitin sulfate proteoglycan, is not expressed on the cell surface of normal hematopoietic cells but is expressed by acute myeloid leukemia blasts from poor-prognosis patients with abnormalities of chromosome band 11q23. Blood. 1996;87:1123–1133. [PubMed] [Google Scholar]
- 6.Hilden JM, Smith FO, Frestedt JL, McGlennen R, Howells WB, Sorensen PH, Arthur DC, Woods WG, Buckley J, Bernstein ID, Kersey JH. MLL gene rearrangement, cytogenetic 11q23 abnormalities, and expression of the NG2 molecule in infant acute myeloid leukemia. Blood. 1997;89:3801–3805. [PubMed] [Google Scholar]
- 7.Mauvieux L, Delabesse E, Bourquelot P, Radford-Weiss I, Bennaceur A, Flandrin G, Valensi F, MacIntyre EA. NG2 expression in MLL rearranged acute myeloid leukaemia is restricted to monoblastic cases. Br J Haematol. 1999;107:674–676. doi: 10.1046/j.1365-2141.1999.01730.x. [DOI] [PubMed] [Google Scholar]
- 8.Wuchter C, Harbott J, Schoch C, Schnittger S, Borkhardt A, Karawajew L, Ratei R, Ruppert V, Haferlach T, Creutzig U, Dorken B, Ludwig WD. Detection of acute leukemia cells with mixed lineage leukemia (MLL) gene rearrangements by flow cytometry using monoclonal antibody 7.1. Leukemia. 2000;14:1232–1238. doi: 10.1038/sj.leu.2401840. [DOI] [PubMed] [Google Scholar]
- 9.Borkhardt A, Wuchter C, Viehmann S, Pils S, Teigler-Schlegel A, Stanulla M, Zimmermann M, Ludwig WD, Janka-Schaub G, Schrappe M, Harbott J. Infant acute lymphoblastic leukemia—combined cytogenetic, immunophenotypical and molecular analysis of 77 cases. Leukemia. 2002;16:1685–1690. doi: 10.1038/sj.leu.2402595. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz S, Rieder H, Schlager B, Burmeister T, Fischer L, Thiel E. Expression of the human homologue of rat NG2 in adult acute lymphoblastic leukemia: close association with MLL rearrangement and a CD10(−)/CD24(−)/CD65 s(+)/CD15(+) B-cell phenotype. Leukemia. 2003;17:1589–1595. doi: 10.1038/sj.leu.2402989. [DOI] [PubMed] [Google Scholar]
- 11.Mittelman A, Chen ZJ, Yang H, Wong GY, Ferrone S. Human high molecular weight melanoma-associated antigen (HMW-MAA) mimicry by mouse anti-idiotypic monoclonal antibody MK2–23: induction of humoral anti-HMW-MAA immunity and prolongation of survival in patients with stage IV melanoma. Proc Natl Acad Sci USA. 1992;89:466–470. doi: 10.1073/pnas.89.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittelman A, Chen ZJ, Liu CC, Hirai S, Ferrone S. Kinetics of the immune response and regression of metastatic lesions following development of humoral anti-high molecular weight-melanoma associated antigen immunity in three patients with advanced malignant melanoma immunized with mouse antiidiotypic monoclonal antibody MK2-23. Cancer Res. 1994;54:415–421. [PubMed] [Google Scholar]
- 13.Bender H, Grapow M, Schomburg A, Reinhold U, Biersack HJ. Effects of diagnostic application of monoclonal antibody on survival in melanoma patients. Hybridoma. 1997;16:65–68. doi: 10.1089/hyb.1997.16.65. [DOI] [PubMed] [Google Scholar]
- 14.Hafner C, Breiteneder H, Ferrone S, Thallinger C, Wagner S, Schmidt WM, Jasinska J, Kundi M, Wolff K, Zielinski CC, Scheiner O, Wiedermann U, Pehamberger H. Suppression of human melanoma tumor growth in SCID mice by a human high molecular weight-melanoma associated antigen (HMW-MAA) specific monoclonal antibody. Int J Cancer. 2005;114:426–432. doi: 10.1002/ijc.20769. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Price MA, Neudauer CL, Wilson C, Ferrone S, Xia H, Iida J, Simpson MA, McCarthy JB. Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms. J Cell Biol. 2004;165:881–891. doi: 10.1083/jcb.200403174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia Z, Sait SN, Baer MR, Barcos M, Donohue KA, Lawrence D, Ford LA, Block AM, Baumann H, Wetzler M. Truncated STAT proteins are prevalent at relapse of acute myeloid leukemia. Leuk Res. 2001;25:473–482. doi: 10.1016/S0145-2126(00)00158-2. [DOI] [PubMed] [Google Scholar]
- 17.Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens—new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 18.Wilson BS, Imai K, Natali PG, Ferrone S. Distribution and molecular characterization of a cell-surface and a cytoplasmic antigen detectable in human melanoma cells with monoclonal antibodies. Int J Cancer. 1981;28:293–300. doi: 10.1002/ijc.2910280307. [DOI] [PubMed] [Google Scholar]
- 19.Imai K, Pellegrino MA, Wilson BS, Ferrone S. Higher cytolytic efficiency of an IgG2α than of an IgG1 monoclonal antibody reacting with the same (or spatially close) determinant on a human high-molecular-weight melanoma-associated antigen. Cell Immunol. 1982;72:239–247. doi: 10.1016/0008-8749(82)90472-5. [DOI] [PubMed] [Google Scholar]
- 20.Tsujisaki M, Sakaguchi K, Igarashi M, Richiardi P, Perosa F, Ferrone S. Fine specificity and idiotype diversity of the murine anti-HLA-A2, A28 monoclonal antibodies CR11-351 and KS1. Transplantation. 1988;45:632–639. doi: 10.1097/00007890-198803000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Kusama M, Kageshita T, Chen ZJ, Ferrone S. Characterization of syngeneic antiidiotypic monoclonal antibodies to murine anti-human high molecular weight melanoma-associated antigen monoclonal antibodies. J Immunol. 1989;143:3844–3852. [PubMed] [Google Scholar]
- 22.Temponi M, Gold AM, Ferrone S. Binding parameters and idiotypic profile of the whole immunoglobulin and Fab′ fragments of murine monoclonal antibody to distinct determinants of the human high molecular weight-melanoma associated antigen. Cancer Res. 1992;52:2497–2503. [PubMed] [Google Scholar]
- 23.Wetzler M, Baer MR, Stewart SJ, Donohue K, Ford L, Stewart CC, Repasky EA, Ferrone S. HLA class I antigen cell surface expression is preserved on acute myeloid leukemia blasts at diagnosis and at relapse. Leukemia. 2001;15:128–133. doi: 10.1038/sj.leu.2401982. [DOI] [PubMed] [Google Scholar]
- 24.Wetzler M, Brady MT, Tracy E, Li ZR, Donohue KA, O’Loughlin KL, Cheng Y, Mortazavi A, McDonald AA, Kunapuli P, Wallace PK, Baer MR, Cowell JK, Baumann H. Arsenic trioxide affects signal transducer and activator of transcription proteins through alteration of protein tyrosine kinase phosphorylation. Clin Cancer Res. 2006;12:6817–6825. doi: 10.1158/1078-0432.CCR-06-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24:267–296. doi: 10.1615/CritRevImmunol.v24.i4.40. [DOI] [PubMed] [Google Scholar]
- 26.Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol. 1910;40:iv–vii. [Google Scholar]
- 27.Levasseur LM, Faessel H, Slocum HK, Greco WR. Implications for clinical pharmacodynamic studies of the statistical characterization of an in vitro antiproliferation assay. J Pharmacokinet Biopharm. 1998;26:717–733. doi: 10.1023/A:1020755124451. [DOI] [PubMed] [Google Scholar]
- 28.Ariens EJ, Van Rossum JM, Simonis AM. Affinity, intrinsic activity and drug interactions. Pharmacol Rev. 1957;9:218–236. [PubMed] [Google Scholar]
- 29.Ariens EJ. Affinity and intrinsic activity in the theory of competitive inhibition. I. Problems and theory. Arch Int Pharmacodyn Ther. 1954;99:32–49. [PubMed] [Google Scholar]
- 30.D’Argenio DZ, Schumitzky A (1997) ADAPT II users guide: pharmacokinetc/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles
- 31.Wang ES, Teruya-Feldstein J, Wu Y, Zhu Z, Hicklin DJ, Moore MA. Targeting autocrine and paracrine VEGF receptor pathways inhibits human lymphoma xenografts in vivo. Blood. 2004;104:2893–2902. doi: 10.1182/blood-2004-01-0226. [DOI] [PubMed] [Google Scholar]
- 32.Lumkul R, Gorin NC, Malehorn MT, Hoehn GT, Zheng R, Baldwin B, Small D, Gore S, Smith D, Meltzer PS, Civin CI. Human AML cells in NOD/SCID mice: engraftment potential and gene expression. Leukemia. 2002;16:1818–1826. doi: 10.1038/sj.leu.2402632. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Sait SN, Wetzler M. Characterization of dendritic-like cells derived from t(9;22) acute lymphoblastic leukemia blasts. Int Immunol. 2004;16:1377–1389. doi: 10.1093/intimm/dxh139. [DOI] [PubMed] [Google Scholar]
- 34.Ragupathi G, Liu NX, Musselli C, Powell S, Lloyd K, Livingston PO. Antibodies against tumor cell glycolipids and proteins, but not mucins, mediate complement-dependent cytotoxicity. J Immunol. 2005;174:5706–5712. doi: 10.4049/jimmunol.174.9.5706. [DOI] [PubMed] [Google Scholar]
- 35.Wagner S, Hafner C, Allwardt D, Jasinska J, Ferrone S, Zielinski CC, Scheiner O, Wiedermann U, Pehamberger H, Breiteneder H. Vaccination with a human high molecular weight melanoma-associated antigen mimotope induces a humoral response inhibiting melanoma cell growth in vitro. J Immunol. 2005;174:976–982. doi: 10.4049/jimmunol.174.2.976. [DOI] [PubMed] [Google Scholar]
- 36.Desai SA, Wang X, Noronha EJ, Kageshita T, Ferrone S. Characterization of human anti-high molecular weight-melanoma-associated antigen single-chain Fv fragments isolated from a phage display antibody library. Cancer Res. 1998;58:2417–2425. [PubMed] [Google Scholar]
- 37.Moore A, Medarova Z, Potthast A, Dai G. In vivo targeting of underglycosylated MUC-1 tumor antigen using a multimodal imaging probe. Cancer Res. 2004;64:1821–1827. doi: 10.1158/0008-5472.CAN-03-3230. [DOI] [PubMed] [Google Scholar]
- 38.Kageshita T, Nakamura T, Yamada M, Kuriya N, Arao T, Ferrone S. Differential expression of melanoma associated antigens in acral lentiginous melanoma and in nodular melanoma lesions. Cancer Res. 1991;51:1726–1732. [PubMed] [Google Scholar]
- 39.Ruiter DJ, Schlingemann RO, Westphal JR, Denijn M, Rietveld FJ, De Waal RM. Angiogenesis in wound healing and tumor metastasis. Behring Inst Mitt. 1993;92:258–272. [PubMed] [Google Scholar]
- 40.Perez-Atayde A, Sallan S, Tedrow U, Connors S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol. 1997;150:815–821. [PMC free article] [PubMed] [Google Scholar]
- 41.Hatfield KJ, Olsnes AM, Gjertsen BT, Bruserud O. Antiangiogenic therapy in acute myelogenous leukemia: targeting of vascular endothelial growth factor and interleukin 8 as possible antileukemic strategies. Curr Cancer Drug Targets. 2005;5:229–248. doi: 10.2174/1568009054064651. [DOI] [PubMed] [Google Scholar]
- 42.Giles F, Estey E, O’Brien S. Gemtuzumab ozogamicin in the treatment of acute myeloid leukemia. Cancer. 2003;98:2095–2104. doi: 10.1002/cncr.11791. [DOI] [PubMed] [Google Scholar]
- 43.Chang CC, Campoli M, Luo W, Zhao W, Zaenker KS, Ferrone S. Immunotherapy of melanoma targeting human high molecular weight melanoma-associated antigen: potential role of nonimmunological mechanisms. Ann NY Acad Sci. 2004;1028:340–350. doi: 10.1196/annals.1322.040. [DOI] [PubMed] [Google Scholar]
- 44.Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775:163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Iida J, Meijne AM, Oegema TR, Jr, Yednock TA, Kovach NL, Furcht LT, McCarthy JB. A role of chondroitin sulfate glycosaminoglycan binding site in alpha4beta1 integrin-mediated melanoma cell adhesion. J Biol Chem. 1998;273:5955–5962. doi: 10.1074/jbc.273.10.5955. [DOI] [PubMed] [Google Scholar]
- 46.Gelman IH. Pyk 2 FAKs, any two FAKs. Cell Biol Int. 2003;27:507–510. doi: 10.1016/S1065-6995(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 47.Feldman EJ, Brandwein J, Stone R, Kalaycio M, Moore J, O’Connor J, Wedel N, Roboz GJ, Miller C, Chopra R, Jurcic JC, Brown R, Ehmann WC, Schulman P, Frankel SR, De Angelo D, Scheinberg D. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23:4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 48.Piccaluga PP, Martinelli G, Rondoni M, Malagola M, Gaitani S, Visani G, Baccarani M. First experience with gemtuzumab ozogamicin plus cytarabine as continuous infusion for elderly acute myeloid leukaemia patients. Leuk Res. 2004;28:987–990. doi: 10.1016/j.leukres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Jurcic JG, DeBlasio T, Dumont L, Yao T-J, Scheinberg DA. Molecular remission induction with retinoic acid and anti-CD33 monoclonal antibody HuM195 in acute promyelocytic leukemia. Clin Cancer Res. 2000;6:372–380. [PubMed] [Google Scholar]
- 50.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]