Abstract
Background
Porphyromonas gingivalis is a gram-negative bacterium that is an important etiologic agent of human adult periodontitis. The goal of the study was to test the hypothesis that two different isoforms, PgLPS1435/1449 and PgLPS1690 exhibit differences in their capacity to stimulate systemic versus local responses compared to E. coli LPS.
Methods
Lipopolysaccharide (LPS) was inoculated into the scalp of mice and the response was measured locally at the site of site of inoculation and systemically in the heart/aorta. VCAM-1 was assessed at the protein level by ELISA and VCAM-1, E-selectin, and ICAM-1 at the RNA level of RNase protection assay. Serum TNF-α levels were also measured.
Results
E. coli LPS and both isoforms of P. gingivalis LPS groups were relatively potent in stimulating expression of inflammatory markers with E. coli LPS being somewhat more potent. In contrast, when the systemic response was measured in the heart/aorta, E. coli but not P. gingivalis LPS significantly induced inflammatory markers. At moderate to low doses (1 and 10 ug per injection) serum TNF–α levels were minimally induced by P. gingivalis LPS compared to E. coli LPS.
Conclusion
The results indicate that both forms of P. gingivalis LPS stimulate an inflammatory response when injected into connective tissue but are minimally stimulatory when a systemic response is measured. In contrast E. coli LPS is a potent stimulus at both the systemic and local level.
Keywords: P. gingivalis, lipopolysaccharide, inflammation, bacteria, periodontal, cytokine
INTRODUCTION
Lipopolysaccharide (LPS) is a major component of the outer membrane of Gram-negative bacteria and a potent modulator by the innate immune system (1). The endotoxin activity of LPS is contained in the lipid region of the molecule, termed lipid A (2). Alterations in lipid A such as changing the number, position, or length of the primary or secondary acyl groups, or subtraction of phosphate or monosaccharide groups results in alteration of the biologic effects (3, 4). It is now understood that bacteria may contain more than one lipid A structural type, and that environmental conditions can regulate the numbers and types of lipid A species found in a single bacterial population (5). Based on this mechanism bacteria might alter host responses to gain advantage for colonization or evading the recognition of host innate immunity. Guo et al reported that a constitutive S. typhimurium mutant could alter LPS-mediated gene expression by regulating structural modifications of lipid A (6). It was also found that environment could alter the lipid A species of Pseudomonas aeruginosa strains isolated from the lungs of individuals with cystic fibrosis, which resulted in more potent stimulation of E-selectin, TNF–α and IL-8 production (7).
Porphyromonas gingivalis is a gram-negative bacterium that is an important etiologic agent of human adult periodontitis, a major cause of tooth loss (8). This bacterium releases copious amounts of outer membrane vesicles containing LPS, which can penetrate periodontal tissue and thus participate in destruction associated with activation of the innate immune response. Compared with enterobacterial LPS, P. gingivalis LPS has variable potency in stimulating biologic activity depending on the cell type stimulated. Several studies demonstrated that the for some cell types such as endothelial cells the biologic activity of P. gingivalis was low compared to that of LPS isolated from enterobacteria (9, 10). However, when injected in vivo, P. gingivalis induces the expression of inflammatory molecules and formation of an inflammatory infiltrate (11, 12). It is possible that some of the variability in response to P. gingivalis or P. gingivalis LPS is due to the predominant forms of LPS produced (13, 14). In this regard, the major isoforms of P. gingivalis LPS have been characterized with lipid A mass ions at m/z 1,435/1,450 and at 1690 (5). It has been predicted that these lipid A modifications will elicit specific alterations in innate host response, with potential pathogenic significance.
The goal of this study was to determine whether P. gingivalis induced both a strong local and systemic inflammatory response and to compare the induction of selected inflammatory markers with that of E. coli LPS. The secondary goal was to compare whether two major isoforms of P. gingivalis LPS exhibited similar inflammatory responses both locally and systemically.
MATERIALS and METHODS
Animals
8 week male CD-1 mice were purchased from the Charles River Laboratories in Wilmington, MA and were housed in the Laboratory Animal Science Center (LASC) at Boston University Medical Campus. All animal procedures were approved by the Institutional Animal Care and Use Committee, Boston University Medical Center. For each group there were six animals per data point. Each assay was carried out at least three separate times. Statistical differences between different groups were determined by One-Way ANOVA.
Preparation of PgLPS1435/1449, PgLPS1690, and E. coli LPS
PgLPS1435 was prepared as described in (8)and PgLPS1690 was prepared as described in(15) and both were prepared from P. gingivalis strain 33277. These LPS preparations contained less than 0.1 % protein contamination, the appropriate fatty acid composition, and were highly enriched for the their respective lipid A structures (8, 15). 2. E. coli O111:B4 LPS was obtained from Sigma-Aldrich (St Louis, MO). This preparation was further purified by the method of Manthey and Vogel to remove trace contaminating proteins (16).
LPS treatment and samples collection
PgLPS1435/1449, PgLPS1690, and E. coli LPS were injected subcutaneously in the mid scalp as previously described (9). Following injection mice were euthanized at the indicated time points and the scalp was immediately harvested and frozen in liquid nitrogen. The heart and aorta were obtained by dissection and opened with a scissor so that blood could be removed by rinsing thoroughly with PBS after which they were frozen in liquid nitrogen. To obtain blood, cardiac puncture was performed just prior to sacrifice.
Measurement of serum TNF-α and VCAM-1 in scalp and heart/aorta
Serum TNF-α protein levels were measured by ELISA. Protein was extracted from the heart/aorta or scalp by pulverization of frozen tissue followed by extraction with lysis buffer containing protease inhibitors. Total protein was determined using a BCA Protein Assay kit (Pierce, Rockford, Illinois). VCAM-1 was measured by ELISA of 100ug of protein extract.
RNA analysis
Total RNA from the scalp and heart/aorta were extracted using Trizol Reagent from pulverized frozen tissue following the manufacturer’s instructions. mRNA levels were measured by the RNase protection assay (RPA). P32 labeled probes were incubated with 6 μg total RNA for VCAM-1, ICAM-1 and E-selectin. Samples were subjected to RNase digestion. Following electrophoresis on 6% polyacrylamide gels and the optical density of radiolabeled bands was measured using a PhosphoImager. Each value was then normalized by the value of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the same lane. The mean densitometric values ± SEM from three separate RNase protection assays are shown.
RESULTS
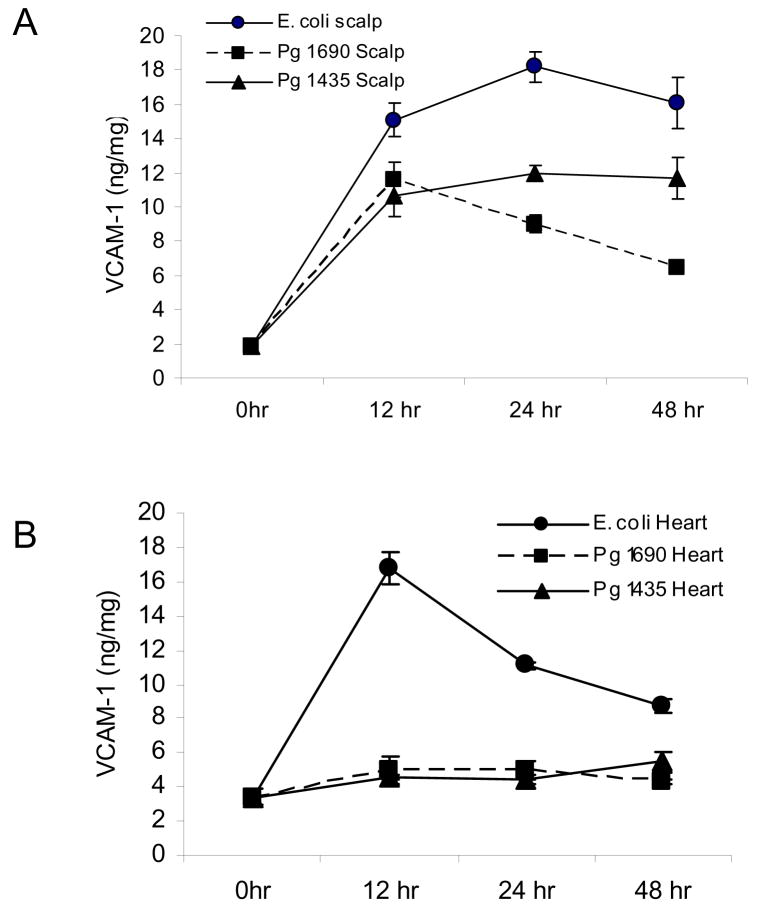
PgLPS1435/1449, PgLPS1690 and E. coli LPS were injected into connective tissue and the response was determined at the local and systemic levels by assessing the expression of adhesion molecules that support inflammation. Vascular-cellular adhesion molecules (VCAM-1), an adhesion molecule that is selectively expressed on vascular cells was measured at the protein level by ELISA. At the site of inoculation all three isoforms of LPS were highly stimulatory inducing a 6 to 8 fold increase at 12 hours and 5 to 10 fold increase at 24 hours compared to baseline (P<0.05) (Fig 1A). Although the difference was relatively small, PgLPS1435/1449 induced levels that were statistically greater than PgLPS1690 at 24 and 48 hours (p< 0.05). The results observed systemically in the heart/aorta differed considerably (Fig 1B). Only E. coli LPS induced VCAM-1 protein levels, which peaked at 12hours when they were five fold higher than baseline (P<0.05). In contrast P. gingivalis LPS had no effect.
Figure 1.
Local and systemic induction of VCAM-1 protein by PgLPS1435/1449, PgLPS1690 LPS and E. coli LPS. 50ug of each LPS was injected adjacent to the periosteum of the murine scalp and animals were euthanized 0, 12, 24 and 48 hours later. (A) Total protein was extracted from scalp. Compared to baseline (0hr) all three isoforms of LPS were significant at each time point (P<0.05). (B) Total protein was extracted from the heart/aorta after thorough rinsing. Only E. coli LPS at 12, 24 and 48 hrs was significant compared to baseline (P<0.05). Each value represents the mean of three separate ELISAs +/− SEM.
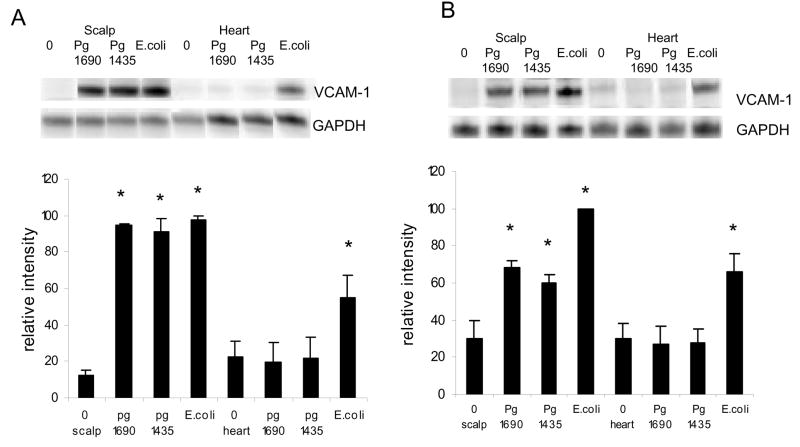
VCAM-1 expression was also assessed at the mRNA level. PgLPS1435/1449, PgLPS1690 and E. coli at a high dose, 50ug, induced a similar expression of VCAM-1 in the scalp, which was approximately 7.5 times higher than baseline (p<0.05) (Fig 2A). In contrast, only E. coli LPS increased mRNA levels of VCAM-1, which were 2.5 fold over baseline in the heart/aorta (p<0.05). At low dose, 1ug, PgLPS1690 and PgLPS1435/1449 stimulated approximately a 2 fold increase while E. coli LPS stimulated a 3 fold increase in VCAM-1 mRNA levels at the site of inoculation. The somewhat higher level of expression stimulated by E. coli LPS compared to P. gingivalis LPS was significant (p<0.05). Only E. coli LPS stimulated VCAM-1 expression in the heart/aorta, which was significantly different from baseline (Fig 2B)(p<0.05).
Figure 2.
Early local and systemic induction of VCAM-1 mRNA by PgLPS1435/1449, PgLPS1690 LPS and E. coli LPS. (A) Low dose (1ug) of each LPS was injected adjacent to the periosteum of the murine scalp. (B) High dose (50ug ) of each LPS was injected. Animals were euthanized 0 or 12 hours later and total RNA was isolated from the scalp and heart/aorta. VCAM-1 was assessed by RNase protection assay. The resulting autoradiograms and densitometric values normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels in the same lane are shown. Each value represents the mean of three separate RPAs +/− SEM. * indicates significantly different from baseline (O hr) (P<0.05).
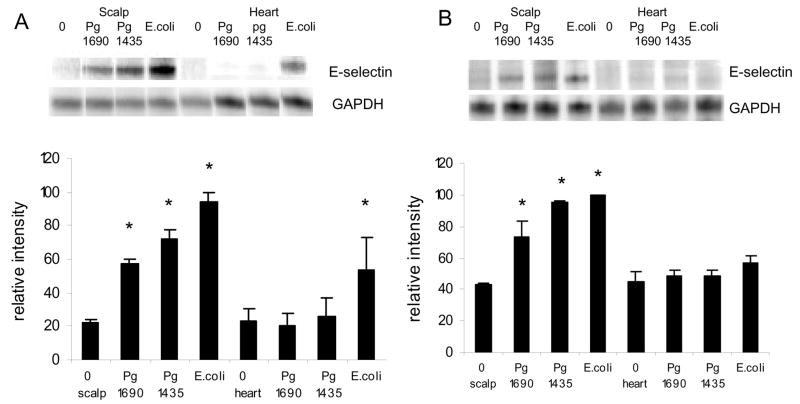
mRNA levels of E-selectin, another pro-inflammatory adhesion molecule was assessed (Fig 3). Ahigh dose of PgLPS1690 and PgLPS1435/1449 at the inoculation site, 50ug, stimulated approximately a 3 fold increase in E-selectin while E. coli LPS stimulated a 4.5 fold increase compared to baseline (p<0.05) (Fig 3A). The differences between E. coli and P. gingivalis LPS was significant (p<0.05), while the difference between PgLPS1690 and PgLPS1435/1449 was not (P>0.05). In the heart/aorta, only E. coli LPS induced E-selectin expression (p<0.05). For the low dose at the inoculation site PgLPS1690 and PgLPS1435/1449 stimulated a 1.7 to 1.8 fold increase and E. coli LPS a 2.1 fold increase (p<0.05) (Fig 3B). The differences among three LPS groups was not significant (p>0.05). In the heart/aorta E-selectin was not induced by any of the LPS groups at the low dose (P>0.05) (Fig 3B).
Figure 3.
Early local and systemic induction of E-selectin mRNA by PgLPS1435/1449or PgLPS1690 LPS and E. coli LPS. (A) Low dose (1ug) of each LPS was injected adjacent to the periosteum of the murine scalp. (B) High dose (50ug ) of each LPS was injected. Animals were euthanized 0 or 12 hours later and total RNA was isolated from the scalp and heart/aorta. E-selectin was assessed by RNase protection assay. The resulting autoradiograms and densitometric values normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels in the same lane are shown. Each value represents the mean of three separate RPAs +/− SEM. * indicates significantly different from baseline (O hr) (P<0.05).
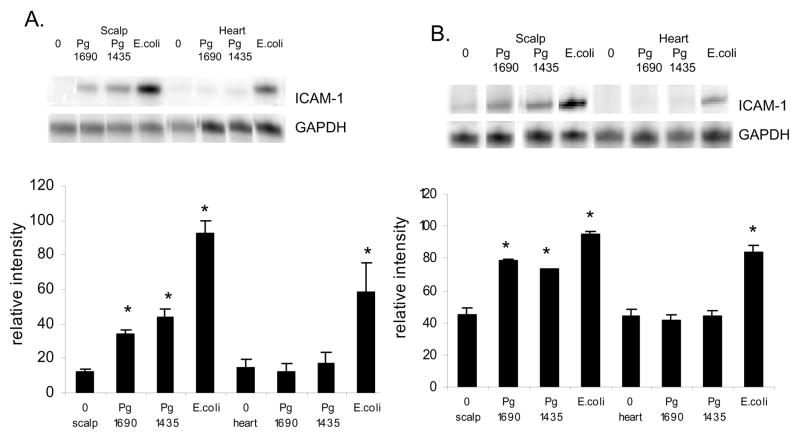
PgLPS1690 and PgLPS1435/1449 high dose, 50ug, induced an approximately 3 fold increase in mRNA levels of ICAM-1, while E. coli LPS induced a 7.5 fold enhancement (p<0.05) (Fig 4A). The differences between E. coli and P. gingivalis LPS were significant (p<0.05). In the heart/aorta only E. coli LPS induced ICAM-1 expression (p<0.05). mRNA levels of ICAM-1 induced by a low dose of each LPS isoform stimulated a 1.8 to 2.1 fold increase (P<0.05)(Fig 4B). The difference between E. coli and P. gingivalis LPS was not significant (P>0.05). In the heart/aorta only E. coli LPS was stimulatory (P<0.05).
Figure 4.
Early local and systemic induction of ICAM-1 mRNA by PgLPS1435/1449or PgLPS1690 and E. coli LPS. (A) Low dose (1ug) of each LPS was injected adjacent to the periosteum of the murine scalp. (B) High dose (50ug ) of each LPS was injected. Animals were euthanized 0 or 12 hours later and total RNA was isolated from the scalp and heart/aorta. ICAM-1 was assessed by RNase protection assay. The resulting autoradiograms and densitometric values normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels in the same lane are shown. Each value represents the mean of three separate RPAs +/− SEM. * indicates significantly different from baseline (O hr) (P<0.05).
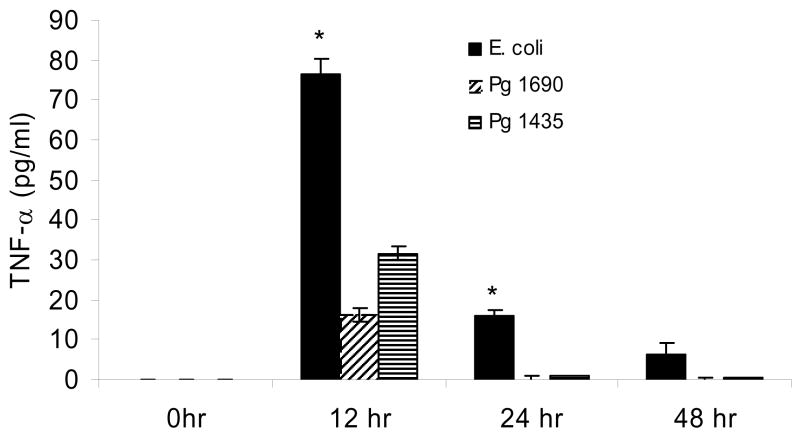
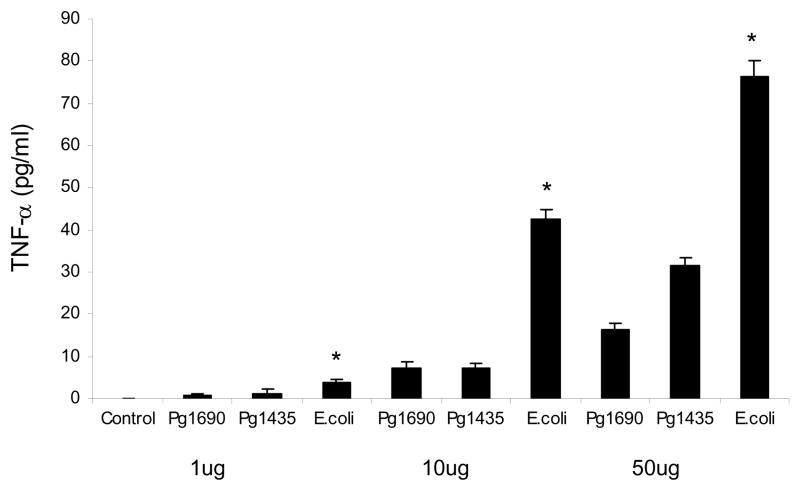
The systemic response to the different forms of LPS was also assessed by measuring TNF–α protein levels in serum at different time points (Fig 5). There was no TNF–α detected at baseline. At 12 hours TNF–α levels induced by E. coli LPS were approximately 140% higher than PgLPS435/1449 and 370% greater than that stimulated by PgLPS1690. The difference between each was significant (P<0.05). At 24hours both P. gingivalis LPS groups returned to baseline while TNFα was still elevated in mice injected with E. coli LPS (P<0.05). At 48 hours all groups were at or close to baseline. When a dose response was measured E. coli LPS induced significantly higher level of TNF-α compared to both isoforms of P. gingivalis LPS at all doses (Fig 6) (p<0.05). A difference between PgLPS435/1449 andPgLPS1690 was found only at the highest dose, 50ug.
Figure 5.
Serum TNF-α protein levels induced by local injection of PgLPS1435/1449, PgLPS1690 and E. coli LPS. 50ug of each LPS was injected adjacent to the periosteum of the murine scalp and animals were euthanized 0, 12, 24 and 48 hours later. Serum TNF-α levels were measured by ELISA. Each value represents the mean of three separate experiments +/− SEM. * indicates E. coli LPS was significantly greater than P. gingivalis LPS under the same conditions (P<0.05).
Figure 6.
Dose-dependent induction of serum TNF–α protein levels by local injection PgLPS1435/1449, PgLPS1690 and E. coli LPS. Low (1ug) medium (10ug) and high dose (50ug) of each LPS was injected and animals were euthanized 0 or 12 hours later. Serum TNF-α levels were measured by ELISA. Each value represents the mean of three separate experiments +/− SEM. * indicates E. coli LPS was significantly greater than P. gingivalis LPS under the same conditions (P<0.05).
DISCUSSION
In vitro experiments indicate that the response to P. gingivalis LPS varies considerably (8, 17). This may be dependent upon the cell type examined or the type of LPS produced. In order to investigate this issue we examined two major isoforms of P. gingivalis LPS in a connective tissue setting where complex cell to cell interactions would be maintained. The scalp model was chosen for this purpose as we and others have previously described (11, 18). P. gingivalis LPS and E. coli LPS were potent inducers of VCAM-1 measured at the protein level at the site of inoculation. E. coli LPS had a small but statistically significant increase compared to P. gingivalis LPS, with PgLPS1435/1449 being slightly more stimulatory than PgLPS1690. In contrast only E. coli LPS was a potent inducer when measuring VCAM-1 protein at a distant site, in the heart/aorta. This general pattern was supported by expression of the mRNA levels of VCAM-1, E-selectin and ICAM-1. Both isoforms of P. gingivalis LPS were potent stimulators of inflammation although somewhat less than that of E. coli at the local site of inoculation. In the heart/aorta only E. coli LPS was stimulatory. The systemic response was also assessed by measuring TNF–α in the serum. At a high dose both PgLPS1435/1449 and PgLPS1690 were stimulatory but less so compared to E. coli LPS with PgLPS1435/1449 somewhat more potent that PgLPS1690. At a moderate dose, 10μg per injection, only E. coli LPS induced high levels of serum TNF-α Thus, these studies indicate that the local response to P. gingivalis LPS is relatively strong whereas the systemic response is relatively weak. In contrast E. coli LPS is a strong stimulus of both a local and systemic inflammatory response.
It has previously been shown that in endothelial cells P. gingivalis LPS is a weak inducer of adhesion molecule expression (1). However, in vivo under conditions where multiple cell types are present, studies shown here demonstrate that highly purified P. gingivalis LPS stimulates expression of adhesion molecules associated with endothelial cells, VCAM-1 and E-selectin. Thus it is likely that other cells, which respond to P. gingivalis LPS, induce a cascade leading to indirect activation of endothelial cells. For example, isolated microphages are highly reactive to P. gingivalis LPS (17) and may mediate P. gingivalis induction of endothelial cells. Alternatively, it is possible that minor contaminants of LPS at high concentrations had some activity affecting the local inflammatory response. However, it should be noted that even at high concentrations P. gingivalis LPS was weak at inducing a systemic inflammatory response.
Porphyromonas gingivalis has been shown to cause septic shock-like symptoms and even animal death (19). However, P. gingivalis LPS is considerably less potent in inducing septic shock when applied intravenously compared to LPS derived from enteric bacteria (13). Results presented here are consistent with the latter findings and suggest that when endothelial cell responses predominate, as reflected in serum TNF-α levels and the short term response of cardiovascular tissue, P. gingivalis LPS is much less potent than LPS from E. coli.. This may reflect A TLR2 response to PgLPS1435/1449 and PgLPS1690 that is specifically associated with endothelial cells (7, 15, 20). Furthermore the results given insight into how P. gingivalis may contribute to a systemic disease such as atherosclerosis. It has been reported that invasion of P. gingivalis is required for accelerated progression of atherogenesis in an animal model. This may be necessary since P. gingivalis does not elicit a strong systemic inflammatory response and may require local invasion to promote atherogenesis (21). However, when the cellular environment is more complex and multiple cellular interactions occur, the response to P. gingivalis LPS is more pro-inflammatory and more similar to that of E. coli LPS.
Acknowledgments
We would like to thank Dr. Graves’ Administrative Assistant Ms. Alicia Ruff for help in preparing this manuscript. This work was supported by NIH/NIDCR grants DE017732 and DE012768. All authors attest that there are no conflict of interest.
Footnotes
- R&D Systems, Minneapolis, MN (Elisa)
- R&D System, Minneapolis, MN (Lysis buffer)
- Pierce, Rockford, Illinois (BCA Protein Assay Kit)
- Life Technologies Inc., Rockville, MD (Trizol Reagent)
- BD Bioscience, Franklin Lakes, NJ
- Biorad Laboratories, Hercules, CA (PhosphoImager)
References
- 1.Darveau R, Cunningham M, Bailey T, et al. Ability of bacteria associated with chronic inflammatory disease to stimulate E-selectin expression and promote neutrophil adhesion. Infect Immun. 1995;63:1311–1317. doi: 10.1128/iai.63.4.1311-1317.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rietschel ET, Brade L, Brandenburg K, et al. Chemical structure and biologic activity of bacterial and synthetic lipid A. Rev Infect Dis. 1987;9 Suppl 5:S527–36. doi: 10.1093/clinids/9.supplement_5.s527. [DOI] [PubMed] [Google Scholar]
- 3.Schromm A, Brandenburg K, Loppnow H, et al. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur J Biochem. 2000;267:2008–13. doi: 10.1046/j.1432-1327.2000.01204.x. [DOI] [PubMed] [Google Scholar]
- 4.Rietschel ET, Brade H, Holst O, et al. Bacterial endotoxin: Chemical constitution, biological recognition, host response, and immunological detoxification. Curr Top Microbiol Immunol. 1996;216:39–81. doi: 10.1007/978-3-642-80186-0_3. [DOI] [PubMed] [Google Scholar]
- 5.Kumada H, Haishima Y, Umemoto T, Tanamoto K. Structural study on the free lipid A isolated from lipopolysaccharide of Porphyromonas gingivalis. J Bacteriol. 1995;177(8):2098–106. doi: 10.1128/jb.177.8.2098-2106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo L, Lim KB, Gunn JS, et al. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276(5310):250–3. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 7.Ernst RK, Yi E, Guo L, et al. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science. 1999;286:1561–5. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 8.Darveau RP, Pham TT, Lemley K, et al. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect Immun. 2004;72(9):5041–51. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa T, Asai Y, Yamamoto H, et al. Immunobiological activities of a chemically synthesized lipid A of Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2000;28(4):273–81. doi: 10.1111/j.1574-695X.2000.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa T, Suda Y, Kashihara W, et al. Immunobiological activities of chemically defined lipid A from Helicobacter pylori LPS in comparison with Porphyromonas gingivalis lipid A and Escherichia coli-type synthetic lipid A (compound 506) Vaccine. 1997;15(15):1598–605. doi: 10.1016/s0264-410x(97)00102-3. [DOI] [PubMed] [Google Scholar]
- 11.Naguib G, Al-Mashat H, Desta T, Graves D. Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J Invest Dermatol. 2004;123:87–92. doi: 10.1111/j.0022-202X.2004.22711.x. [DOI] [PubMed] [Google Scholar]
- 12.Graves D, Naguib G, Lu H, Desta T, Amar S. Porphyromonas gingivalis fimbriae are pro-inflammatory but do not play a prominent role in the innate immune response to P. gingivalis. J Endotoxin Res. 2005;11:13–8. doi: 10.1179/096805105225006722. [DOI] [PubMed] [Google Scholar]
- 13.Nair BC, Mayberry WR, Dziak R, Chen PB, Levine MJ, Hausmann E. Biological effects of a purified lipopolysaccharide from Bacteroides gingivalis. J Periodontal Res. 1983;18(1):40–9. doi: 10.1111/j.1600-0765.1983.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 14.Tanamoto K, Azumi S, Haishima Y, Kumada H, Umemoto T. Endotoxic properties of free lipid A from Porphyromonas gingivalis. Microbiology. 1997;143 ( Pt 1):63–71. doi: 10.1099/00221287-143-1-63. [DOI] [PubMed] [Google Scholar]
- 15.Reife RA, Coats SR, Al-Qutub M, et al. Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity: differential activities of tetra- and penta-acylated lipid A structures on E-selectin expression and TLR4 recognition. Cell Microbiol. 2006;8(5):857–68. doi: 10.1111/j.1462-5822.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 16.Manthey CL, Vogel SN. Interactions of lipopolysaccharide with macrophages. Immunol Ser. 1994;60:63–81. [PubMed] [Google Scholar]
- 17.Zhou Q, Desta T, Fenton M, Graves DT, Amar S. Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its FimA protein. Infect Immun. 2005;73(2):935–43. doi: 10.1128/IAI.73.2.935-943.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zubery Y, Dunstan C, Story B, Kesavalu L, Ebersole J, Holt S, et al. Bone resorption caused by three periodontal pathogens in vivo in mice is mediated in part by prostaglandin. Infect Immun. 1998;66:4158–4162. doi: 10.1128/iai.66.9.4158-4162.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Lin Y, Lai Y, Hu S. Lethal outcome caused by Porphyromonas gingivalis A7436 in a mouse chamber model is associated with elevated titers of host serum interferon-gamma. Oral Microbil Immunol. 2006;21:100–6. doi: 10.1111/j.1399-302X.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- 20.Martin M, Katz J, Vogel SN, Michalek SM. Differential induction of endotoxin tolerance by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. J Immunol. 2001;167(9):5278–85. doi: 10.4049/jimmunol.167.9.5278. [DOI] [PubMed] [Google Scholar]
- 21.Gibson FC, 3rd, Hong C, Chou HH, et al. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109(22):2801–6. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]