Abstract
Local synapses formed by non-dopamine cells within the ventral tegmental area (VTA) are thought to provide an important regulatory influence on the activity patterns of dopamine (DA) neurons. However, ultrastructural confirmation of intra-areal synapses formed by VTA neurons is lacking, and the synaptic targets of these connections have not been examined. We performed discrete injections of the specific anterograde tracer Phaseolus vulgaris leucoagglutinin (PHAL) and used electron microscopy to visualize immunoperoxidase labeling within the local collaterals of VTA cells. The phenotype of target neurons was determined by immunogold-silver labeling for GABA or for tyrosine hydroxylase within DA neurons. Within or immediately adjacent to the VTA injection sites, PHAL was incorporated into the soma and dendrites of both GABA and DA cells. Tracer was also detected within myelinated and unmyelinated axons as well as axon terminals. Some labeled terminals formed identifiable synapses, the majority of which (78%) had symmetric morphology (presumably inhibitory). Both DA and GABA dendrites were contacted by these intrinsic axons. Postembedding immunogold labeling verified that local axon collaterals arose mainly from GABA cells (DA neurons are not known to issue recurrent collaterals). Nevertheless, a few synapses with asymmetric morphology (presumably excitatory) were also noted; whether these derive from local glutamate neurons requires further investigation. Hence, our data provide ultrastructural support for the long standing assumption that GABA VTA neurons synapse locally onto DA cells. The findings also suggest the presence of disinhibitory and possibly excitatory circuitry intrinsic to the VTA.
Keywords: electron microscopy, glutamate, intrinsic connections, ventral midbrain
Introduction
Dopamine (DA) cells in the ventral tegmental area (VTA) are critical modulators of associative learning and preparation for action (Redgrave et al., 2007; Schultz, 2007). Many of these functional studies highlight the importance of inhibition in regulating the behaviorally relevant activity patterns of DA neurons, for example when predicted rewards do not occur (Schultz, 2007) or in response to aversive stimuli (Ungless et al., 2004). GABA synapses onto midbrain DA cells are abundant (Bayer and Pickel, 1991) and in theory arise from both extrinsic and intrinsic sources (Somogyi et al., 1981; Smith and Bolam, 1990; Johnson and North, 1992b; Charara et al., 1996; White, 1996).
GABA neurons in the VTA (Mugniani and Oertel, 1985) have efferent projections that parallel those of DA cells (Van Bockstaele and Pickel, 1995; Carr and Sesack, 2000a; Lee et al., 2001) and mediate similar functions (Laviolette and van der Kooy, 2001; Lee et al., 2001; Steffensen et al., 2001). However, in contrast to midbrain DA neurons which have not been found to issue recurrent collaterals, the axons of GABA cells exhibit extensive local plexuses (Phillipson, 1979; Wassef et al., 1981; Grace and Onn, 1989; Richards et al., 1997; Mailly et al., 2003). This finding suggests that GABA neurons are likely to be involved in local synaptic regulation within the VTA, which is supported by electrophysiological data.
Recordings of DA cells from in vitro preparations indicate the presence of inhibitory postsynaptic potentials of local origin (Johnson and North, 1992b; Johnson and North, 1992a; Sugita et al., 1992; Nugent and Kauer, 2008). Such intrinsic GABA connections in the VTA or adjacent substantia nigra (SN) have also been invoked to explain why activation of inhibitory or excitatory pathways sometimes causes paradoxical excitation or inhibition, respectively, in DA neurons (Grace and Bunney, 1985; Smith and Grace, 1992; Tepper et al., 1995; Tong et al., 1996; White, 1996). On the other hand, the timing of responses evoked in DA and non-dopamine (non-DA) neurons by stimulation of excitatory prefrontal cortical afferents in vivo suggests that non-DA cells may not be a source of inhibition of DA neurons (Overton and Clark, 1997; Tong et al., 1998). Moreover, the relative invariance of DA cell activity across sleep-wake cycles (Miller et al., 1983; Lee et al., 2001) suggests that these neurons may not be so tightly influenced by local GABA cells, which show substantial variation across activity states. Consequently, available electrophysiological evidence does not unequivocally support a local synaptic influence mediated by VTA non-DA neurons. Moreover, there are no ultrastructural studies to date that have directly examined this issue.
We chose to address the synaptic targets of non-DA neurons using discrete injections of the anterograde tracer Phaseolus vulgaris leucoagglutinin (PHAL) within the VTA. In addition to extrinsically directed axons, this tracer labels intra-areal collaterals, which in the VTA have only been reported for non-DA cells. Immunoperoxidase detection of PHAL was combined with preembedding immunogold-silver labeling of GABA or the DA synthetic enzyme tyrosine hydroxylase (TH) in dendrites. Postembedding gold labeling for GABA was also used to detect the presence of this transmitter in local PHAL-labeled axon terminals. Some of this work has been reported previously in abstract form (Omelchenko and Sesack, 2006b).
Materials and Methods
Subjects and surgeries
The procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Seven adult male Sprague-Dawley rats (Hilltop Lab Animals Inc, 330-360 g) were anesthetized with an i.m. injection of 34 mg/kg ketamine, 1 mg/kg acetopromazine, and 7 mg/kg xylazine. After mounting in a stereotaxic apparatus, animals received a unilateral injection of the anterograde tracer PHAL (2.5% in phosphate buffered saline) into the VTA. The solution was delivered by iontophoresis through glass micropipettes (tip diameter 25-60 μm) using 10 sec on/off pulses of 5 μA positive current for 15 min as reported elsewhere (Sesack and Pickel, 1992). Pipettes were lowered to the following coordinates: -5.3 mm posterior to bregma, 0.7 mm lateral to midline, 8.2 mm ventral to the skull surface, according to the atlas of Paxinos and Watson (Paxinos and Watson, 1998).
After a survival period of 8-14 days (39 days in one rat), the animals were anesthetized with pentobarbital (60 mg/kg, i.p., with supplemental doses, if necessary) and treated for 15 minutes with 1 g/kg i.p. of the zinc chelator diethyldithiocarbamic acid (Sigma, St. Louis, MO) in order to prevent silver intensification of endogenous zinc (Veznedaroglu and Milner, 1992). Five animals were then killed by perfusion with heparin saline (Elkins-Sinn, NJ, USA; 1000 U/ml), followed by 50 ml of 3.75% acrolein and 2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (PB), followed by 200-400 ml of 2% paraformaldehyde in 0.1 M PB. The remaining two animals were killed by perfusion with heparin saline, followed by 2% paraformaldehyde and 1% glutaraldehyde in PB. The latter cases were used for postembedding immunolabeling for GABA in axon terminals. Sections from these animals were also labeled by preembedding methods for analysis of the intra-areal dendritic contacts of local axon collaterals. The brains were removed and cut into 4-6 mm coronal blocks containing the VTA, brainstem or forebrain. After 0.5-1 hour of post-fixation in 2% paraformaldehyde, the blocks were sectioned at 50 μm on a vibratome, collected in PB and incubated in 1% sodium borohydride in PB for 30 minutes to improve immunocytochemical labeling.
Preembedding immunocytochemistry
The present investigation employed a double-immunolabeling procedure similar to that reported previously by our laboratory (Carr and Sesack, 2000b; Omelchenko and Sesack, 2005). The primary antibodies directed against PHAL, TH and GABA have also been used extensively in our prior publications (Sesack et al., 1989; Sesack and Pickel, 1992; Carr and Sesack, 1998; Omelchenko and Sesack, 2005; Omelchenko and Sesack, 2006a; Omelchenko and Sesack, 2007). The study utilized a mouse monoclonal antibody against TH (Chemicon #MAB318, Temecula, CA) that recognizes an N-terminal 59-61 kDa protein from the TH molecule purified from PC12 cells. It has been shown to specifically recognize TH and not other monoamine synthetic enzymes by western blot analysis. The mouse monoclonal antibody against GABA (Sigma #A-0310) was raised against purified GABA conjugated to bovine serum albumin (BSA) and has been shown to selectively recognize GABA and not several closely related amino acids. The specificity of the rabbit antibody against PHAL (Vector, Burlingame, Calif) is demonstrated by the absence of immunoreactivity from brain sections in which this tracer was neither injected nor transported.
Immunolabeling was conducted on free-floating sections at room temperature with constant shaking. The immunoreagent incubations were always followed by extensive rinses in buffer. Sections were rinsed in 0.1 M tris-buffered saline (TBS; pH 7.6), and then placed for 30 min in a blocking solution containing 1% BSA and 3% normal goat serum in 0.1 M TBS. In order to enhance immunoreagent penetration, the blocking solution contained Triton X-100 (Sigma) at 0.04% for electron microscopy or at 0.2% for light microscopy. Sections were then placed for 12-15 hrs in the blocking solution containing a mixture of primary antibodies: rabbit anti-PHAL (1:1000) and either mouse anti-TH (1:5000) or mouse anti-GABA (1:2000). After that, sections were incubated for 30 min in biotinylated secondary antibody: donkey anti-rabbit (1:400; Jackson ImmunoResearch Laboratories, West Grove, PA) diluted in the blocking solution. Sections were then placed for 30 min in 1:100 avidin-biotin peroxidase complex (Vectastain Elite kit; Vector Laboratories). To visualize the peroxidase reaction, the sections were incubated for 3.5 min in 0.022% diaminobenzidine (Sigma) and 0.003% hydrogen peroxide in TBS.
Following immunoperoxidase labeling, tissue for electron microscopy was further processed by immunogold-silver labeling for TH or GABA. Sections were incubated for 30 min in a washing buffer containing 0.8% BSA, 0.1% fish gelatin, and 3% normal donkey serum in 10 mM PBS. Afterwards, the sections were placed for 12-15 hr in washing buffer to which was added 1:50 donkey anti-mouse IgG conjugated to 1 nm gold particles (Electron Microscopy Sciences, Washington, PA). Following several rinses, the sections were postfixed by 2% glutaraldehyde in PBS for 10 min and placed in 0.2 M sodium citrate buffer, pH 7.4. In order to enhance the size of the gold particles, the sections were incubated in silver solution (Amersham) for 3-6 min.
Tissue preparation for light and electron microscopy
Light microscopic sections were mounted onto glass slides, dried, dehydrated through a series of alcohol solutions with increasing concentrations, defatted in xylene, and coverslipped with DPX. Then sections were studied using bright field microscopy to verify that injection sites were contained within the VTA and that PHAL infusion did not produce spurious retrograde transport to any other brain area that is also afferent to the VTA. In order to match brightness and contrast, digital light microscopic images were adjusted in Adobe Photoshop.
Sections processed for electron microscopy were postfixed for 1 hr in 2% osmium tetroxide in PB, dehydrated via graded alcohol solutions, immersed in propylene oxide, and then left for 12-14 hours in a 1:1 mixture of propylene oxide with either epoxy resin (EMbed 812; Electron Microscopy Sciences) or Durcupan resin (Durcupan ACM; Fluka) in the case of tissue developed for postembedding. After exposure to corresponding straight resin for 2 hr, the sections were embedded between sheets of commercial polymer film. The resin was hardened in an oven for 24-48 hr at 60°C.
The VTA and its subdivisions were identified as previously described (Oades and Halliday, 1987; Bayer and Pickel, 1990), and the exact region within the parabrachial or paranigral subdivisions of the anterior/middle VTA taken for ultrathin sectioning was photodocumented. Ribbons of 3-7 consecutive ultrathin sections were obtained from the surface of the vibratome sections where the tissue interfaced with plastic resin; ultrathin sections were collected on copper or nickel grids. Grids were counterstained with uranyl acetate and lead citrate and examined on an FEI Morgagni transmission electron microscope (Hillsboro, Oregon). Digital micrographs were captured using an AMT XP-60 digital camera (Danvers, MA) and adjusted in Adobe Photoshop.
Postembedding immunocytochemistry
Our extensive experience with preembedding immunogold indicates that this approach is valid for identifying GABAergic dendrites, particularly within the VTA (see also (Van Bockstaele and Pickel, 1995)). However, GABAergic axon terminals labeled by this method are often confined to a narrow band of tissue near the surface interface with plastic embedding. The reasons for this limitation are not known but it hampers efforts to estimate the proportion of PHAL labeled axons that contain GABA. Consequently, we performed postembedding labeling in order to better reveal the phenotype of the axon terminals forming local symmetric synapses.
We utilized a procedure modified from Smith and colleagues (Shink and Smith, 1995; Smith et al., 1996). Although some postembedding protocols employ resin etching to enhance antigenicity, the value of this step for unmasking surface epitopes has not been confirmed by quantitative analysis (Mathiisen et al., 2006) and was not used here. Ultrathin sections from tissue embedded in Durcupan resin were collected on nickel grids from two animals that were perfused with glutaraldehyde. Initially, we used tissue in which PHAL was labeled by preembedding immunoperoxidase. However, this approach appeared to be associated with substantial false-negative outcomes, as also reported by others (Bokor et al., 2005). In order to better estimate the extent of GABA immunoreactivity in PHAL-labeled terminals, an additional set of tissue was developed where PHAL was detected by single-label preembedding immunogold-silver using a 1 nm gold-conjugated goat anti-rabbit IgG. The sections were than incubated in silver solution for 8 min which produced gold-silver particles for PHAL substantially larger than those associated with postembedding gold labeling for GABA.
For postembedding, all steps were performed at room temperature using reagent drops placed on disposable parafilm sheets within a moist Petri dish chamber. All solutions that did not contain antibodies were passed through a 0.22 μm filter before use. Grids were preincubated for 5 min in 0.05 M Tris-buffered saline, pH 7.6, containing 0.1% Triton X-100. Then the grids were incubated overnight with rabbit anti-GABA primary antibody (1:1000, Sigma A-2052) in 0.05 M TBS, pH 7.6, containing 0.01% Triton X-100 and 1% BSA. In dot blot assay, this GABA antibody recognizes native GABA or GABA conjugated to keyhole limpet hemocyanin and does not recognize BSA (manufacture information). In control experiments in which the GABA antiserum was omitted, virtually no postembedding gold particles were detected anywhere in the tissue. In tissue processed by preembedding immunogold-silver for GABA, the same axonal profiles were typically labeled by both preembedding and postembedding for GABA.
Grids were washed for 50 min in several changes of 0.05 M TBS, pH 7.6, containing 0.01% Triton X-100 and 1% BSA and then for 5 min in 0.05 M TBS, pH 8.2, containing 0.1% Triton. Grids were then incubated for 90 min in a 1:25 dilution of 15 nm gold-conjugated goat anti-rabbit IgG (Ted Pella, Redding, CA) in 0.05 M TBS, pH 8.2. The procedure was finished with several washes in the last buffer followed by distilled water. Grids were then counterstained with heavy metal as described earlier.
For postembedding experiments, rabbit primary antibodies were used for both PHAL and GABA. Hence, additional controls were performed to ensure that osmication during tissue preparation for electron microscopy completely destroyed the antigenicity of the rabbit antibody directed against PHAL. To this end, the GABA primary antiserum was omitted, and postembedding application of gold-conjugated anti-rabbit secondary antibody produced no postembedding gold particles over PHAL-containing profiles labeled with either peroxidase or immunogold-silver. This observation is consistent with previous reports regarding the deleterious effects of osmication on antigenicity (Phend et al., 1995).
Ultrastructural analysis
Ultrathin sections were systematically examined at 18,000× magnification and photographed at 22,000×. Neuronal elements were recognized as described by Peters (Peters et al., 1991). For preembedding analysis of the synaptic targets of local collaterals, 2,143,969 μm2 of tissue was examined from TH-labeled sections and 1,945,831 μm2 was examined from GABA-labeled tissue. For postembedding analysis of GABA immunolabeling within local collaterals, 375,100 μm2 was examined from tissue in which PHAL was labeled by preembedding immunogold-silver. The total area examined was estimated by multiplying the number of analyzed grid squares, the area of the each grid square (3,025 μm2), and an estimate of the percentage (25%, 50%, 75% or 100%) of tissue as opposed to plastic resin contained within each square.
In order to minimize false negative results, the analysis of ultrathin sections was performed close to the outermost surface of the tissue where it interfaced with epoxy resin. Specific labeling for PHAL was defined as uniform filling of profiles with immunoperoxidase reaction product. Axon terminals were chosen for quantitative analysis only when they were more heavily labeled than dendrites in the vicinity, consistent with active transport of the tracer. As preembedding immunogold reagents penetrate less deeply into the tissue compared to immunoperoxidase (Sesack et al., 2006), the analysis was restricted to axon terminals in photographic fields (approximately 13.8 μm2) that also contained immunogold-silver labeling within the field. Profiles that contained at least 4 gold-silver particles in a single section were considered to be specifically labeled, although most contained many more than 4 particles. Of the profiles that received synaptic input from PHAL-labeled axons and are represented in Table 1, all contained at least 6 gold particles with the exception of two profiles labeled for TH; these had only 4 particles each but were small diameter distal dendrites, making the number of gold particles per unit area relatively high. Analysis of serial sections was performed in order to establish the morphology of synaptic contacts and the recurrence of sparse immunogold-silver labeling over the same dendrite.
Table 1. Targets of PHA-L Labeled Axons in the Rat VTA.
| Dendrites labeled for: | |||
|---|---|---|---|
| Tissue Labeled for TH | Unlabeled | TH | |
| total axon terminals* | 93 | ||
| total dendritic contacts† | 80 (86%) | 29 (36%) | 51 (64%) |
| total synapses | 30 (32%) | ||
| asymmetric synapses | 8 (27%) | 3 (38%) | 5 (63%) |
| symmetric synapses | 22 (73%) | 12 (55%) | 10 (45%) |
| Dendrites labeled for: | |||
| Tissue Labeled for GABA | Unlabeled | GABA | |
| total axon terminals* | 89 | ||
| total dendritic contacts† | 70 (79%) | 44 (63%) | 26 (37%) |
| total synapses | 25 (36%) | ||
| asymmetric synapses | 4 (16%) | 4 (100%) | 0 (0%) |
| symmetric synapses | 21 (84%) | 10 (48%) | 11 (52%) |
for TH: 11, 38, 6, 30, and 8 axon terminals per animal
for GABA: 24, 20, 22, 11, and 12 axon terminals per animal
dendritic contacts include synapses and appositions
For postembedding immunolabeling, fields were chosen for analysis that contained clearly defined symmetric and asymmetric synapses. Immunoreactivity for GABA was considered specific if the number of gold particles in symmetric synapses was at least three times greater than that found in asymmetric synapses. Moreover, profiles were considered to be immunoreactive for GABA only if they showed a high concentration of gold particles not associated with mitochondria. In our experience, the addition of BSA to the primary antibody substantially reduced background gold particles occurring over dendrites, myelin, and terminals forming asymmetric synapses.
Results
Light microscopy
Typical injection sites consisted of populations of neurons densely immunoreactive for tracer surrounded by a zone of diffusely distributed tracer in the neuropil (Fig. 1A). PHAL injections were localized primarily to the paranigral region (2 cases), parabrachial division (1 case) or both subnuclei (2 cases). All the injections were anterior to the interpeduncular nucleus (i.e. 4.8 to 5.3 mm posterior to Bregma). In one animal, some diffusion of the tracer was also observed along the pipette track into the interstitial nucleus of Cajal, however no labeled neurons were contained within this nucleus, which does not project to the VTA (Geisler and Zahm, 2005). Extrinsically directed fibers containing PHAL were observed in recognized target regions of the VTA (Oades and Halliday, 1987), including the prefrontal cortex and the nucleus accumbens (data not shown). No retrograde transport of PHAL was detected in regions known to innervate the VTA.
Figure 1.
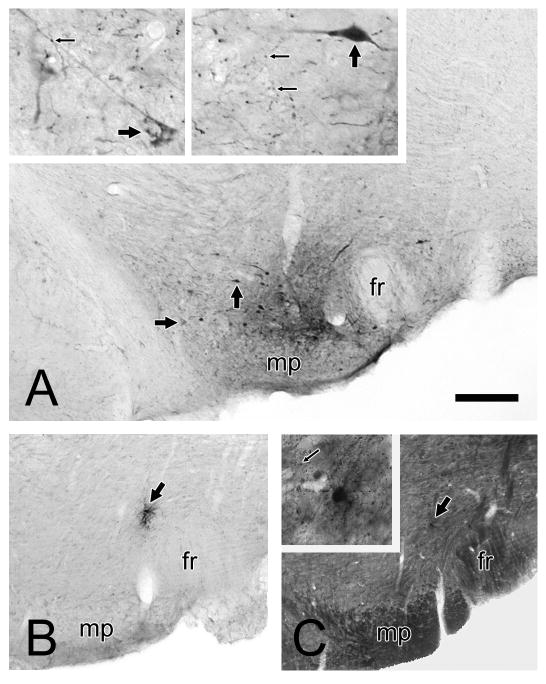
Light microscopic images of coronal brain sections through the rat VTA. Panel A shows a representative injection of PHAL and the labeling of local collaterals. A small injection is centered just above the mammillary peduncle (mp) and lateral to the fasciculus retroflexus (fr). Neurons shown at higher magnification in the inserts are indicated by large arrows. The neuropil surrounding both cells contains numerous puncta that represent probable axon varicosities (small arrows). One of these appears to make contact with a distal dendrite in the left-hand insert. Panels B and C illustrate adjacent coronal sections through the VTA where PHAL was injected but resulted in no evident neuronal uptake. A small deposit of tracer is observed within what appear to be individual cell soma (large arrows). The section in panel C is darker due to osmication and resin embedding in preparation for electron microscopy. The tracer deposit is shown at higher magnification in the insert; a cell body labeled by immunoperoxidase is surrounded by punctate immunogold-silver labeling for GABA (small arrow). Scale bar represents 200 μm in panels A-C and 40 μm in inserts.
Within the VTA, thin PHAL-immunoreactive processes were distributed relatively uniformly and in close proximity to the injection sites (i.e. within approximately 200-400 μm). Some of these exhibited a beaded appearance (Fig. 1A, inserts), consistent with axon varicosities, whereas other thin processes exhibited a smooth morphology characteristic of fibers of passage. The latter were especially observed in the anterior regions that extend into the medial forebrain bundle. A few labeled fibers were noted in the VTA contralateral to the injection site. Otherwise, processes labeled for PHAL and having a beaded appearance were not observed in more distant parts of the VTA.
In two animals, PHAL was delivered into the VTA but resulted in no evidence of neuronal uptake, a situation that can occur with this agent for reasons that are not clear. One case produced a small deposit of tracer that was not contained within any evident neuronal or glial structure. This case was not further processed for electron microscopic analysis. In the second case, two adjacent sections, one processed for light microscopy (Fig. 1B) and the other for electron microscopy (Fig. 1C) contained apparent tracer deposit within individual cell bodies. In the latter section, the area around the soma was examined by electron microscopy. Ultrastructural analysis revealed the presence of immunoreaction product for PHA-L in an astrocytic cell body and its processes. No axon terminals containing tracer were detected in the vicinity. These control cases suggest that PHAL must first be taken up into neurons before labeling axon varicosities locally within the VTA. This agrees with prior studies indicating that long-range axonal transport of PHAL is restricted to the neurons at the injection site that took up the tracer (Gerfen and Sawchenko, 1984).
Electron microscopy
Preembedding immunolabeling
Immunoperoxidase labeling for PHAL was observed as a dark flocculent product within neurons and glia. The extreme center of the injection site exhibited compromised morphology and evidence of a small lesion. The area immediately surrounding the center contained tissue with well preserved morphology and readily identifiable profiles that had incorporated PHAL. No unincorporated immunoreaction product was visible in the extracellular space. Tracer was observed in numerous perikarya as well as proximal and distal dendrites (Fig. 2A-C). Peroxidase product was also detected within glial processes (Fig. 2D). Throughout the injection site, immunoreactivity for PHAL was contained within axonal profiles, including thinly myelinated and unmyelinated processes (Fig. 3A,B) as well as axon terminals identified by the presence of small, clear synaptic vesicles (Fig. 3C,D). Almost no labeled axon varicosities contained dense-cored vesicles. Consistent with light microscopic observations, axon terminals were concentrated in close proximity to the injection site.
Figure 2.
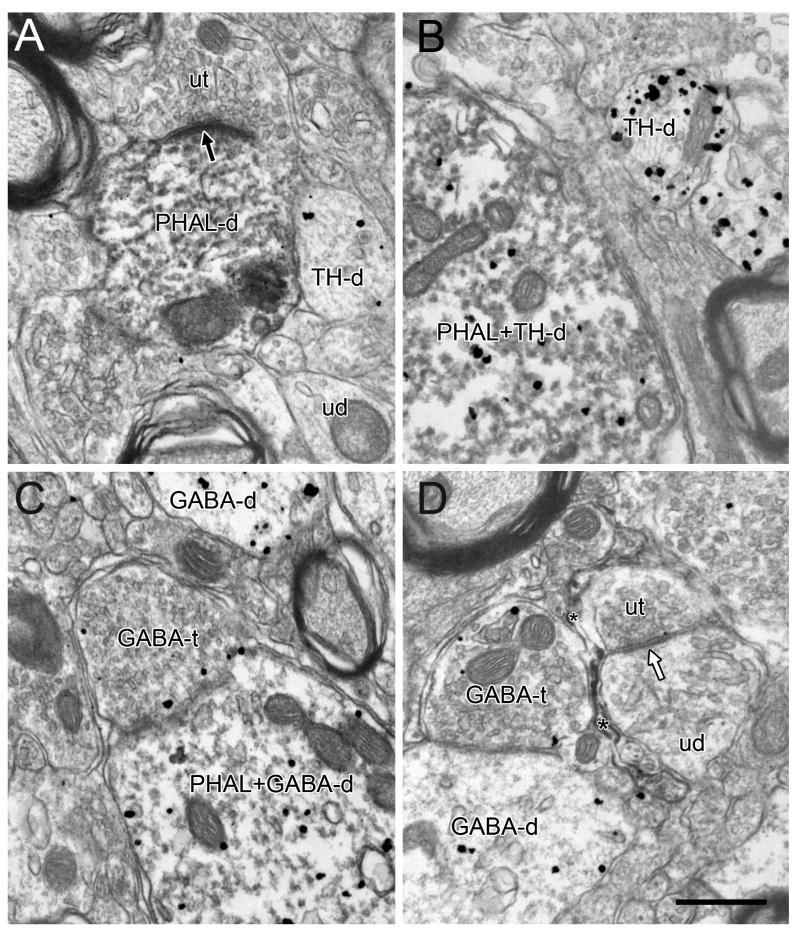
Electron micrographs of the rat VTA showing immunoperoxidase reaction product for PHAL incorporated into dendrites (PHAL-d) and glial processes (asterisks in D). Some PHAL labeled dendrites also contain gold-silver labeling for TH (PHAL+TH-d) or GABA (PHAL+GABA-d). Unlabeled dendrites (ud) are also evident in the adjacent neuropil as are dendrites singly labeled for TH (TH-d) or GABA (GABA-d) and GABA-containing axon terminals (GABA-t). In A, a PHAL-labeled dendrite (PHAL-d) receives an asymmetric synapse (black arrow) from an unlabeled terminal (ut). In D, a glial process (asterisks) containing PHAL surrounds an unlabeled terminal (ut) forming a symmetric synapse (white arrow) onto an unlabeled dendrite (ud). Scale bar, 0.5 μm.
Figure 3.
Electron micrographs showing immunoperoxidase labeling for PHAL in axons. Panels A and B depict thinly myelinated (PHAL-ma) and unmyelinated axons (PHAL-a), the latter within a bundle of unlabeled axons (a). Panels C and D show axon terminals. In C, a PHAL-labeled terminal dually-labeled for GABA (PHAL+GABA-t) apposes a GABA-labeled dendrite (GABA-d). In D, a terminal singly labeled for PHAL (PHAL-t) forms a symmetric synapse (white arrow) onto an unlabeled dendrite (ud). Dendrites singly labeled for TH (TH-d) are observed in the adjacent neuropil. Scale bar, 0.5 μm.
Immunogold-silver labeling for TH or GABA developed on alternative sections was seen as irregularly shaped electron-dense particles. Numerous soma and dendrites, including PHAL-positive profiles, contained gold-silver particles dispersed throughout the structures (Fig. 2). The observation of immunogold-silver labeling in axon terminals was relatively infrequent and typically occurred for GABA (Fig. 2C,D) in profiles located close to the tissue surface. Occasionally, PHAL-positive terminals were observed to contain preembedding immunolabeling for GABA (Fig. 3C) but never for TH.
PHAL-positive terminals often directly contacted dendrites (Fig. 3C,D) and of these points of contact, at least one third exhibited synaptic specializations (Table 1; Fig. 3D). Close attention was paid to the morphology of these synapses, which is correlated with their likely physiological functions (Gray, 1959; Carlin et al., 1980). As confirmed in serial sections, the vast majority (78%; Table 1; Fig. 3D; Fig. 4A,B) had thin or absent postsynaptic densities, associated with a symmetric or Gray's type II morphology. Conversely, 22% of the synapses had thickened postsynaptic densities consistent with an asymmetric (Gray's type I) synaptic type (Fig. 4C,D).
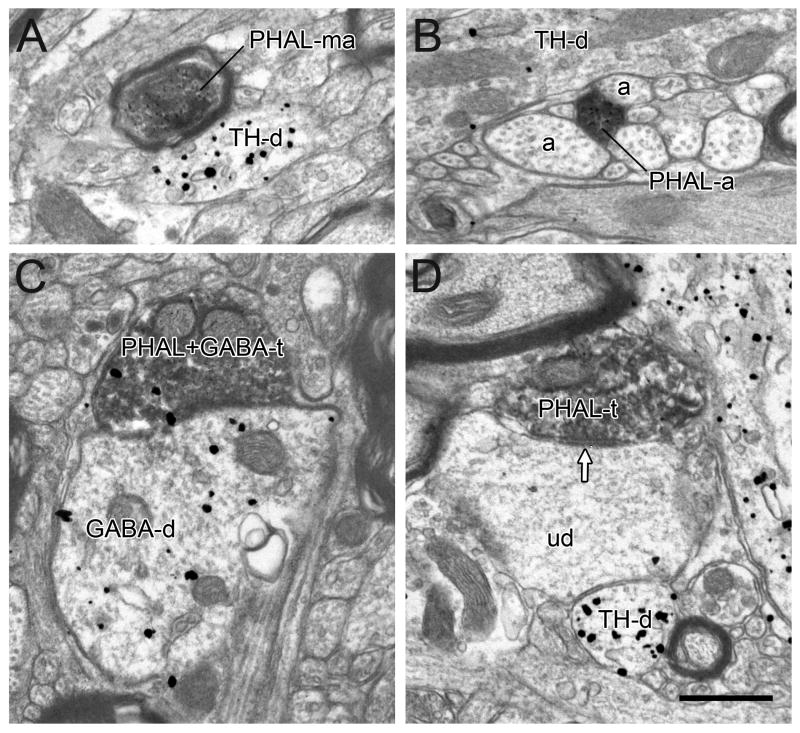
Figure 4.
Electron micrographs showing PHAL-labeled terminals forming symmetric (white arrows) or asymmetric synapses (black arrows) onto unlabeled dendrites (ud) or dendrites labeled for TH (TH-d) or GABA (GABA-d). Commonly, the same dendrites receive additional synaptic inputs from unlabeled terminals (ut) and/or GABA-labeled (GABA-t) terminals. Scale bar, 0.5 μm.
In tissue sections immunolabeled for TH, many of the PHAL-labeled symmetric synapses contacted TH-labeled dendrites (45%, Table 1, Fig. 4A). The remaining terminals synapsed onto dendrites that did not contain gold-silver labeling. In tissue labeled for GABA, PHAL-labeled axons forming symmetric synapses often contacted GABA-immunoreactive dendrites (52%; Table 1; Fig. 4B); the rest contacted unlabeled dendrites.
Although less frequent, PHAL-labeled terminals forming asymmetric synapses were also observed in both TH and GABA labeled tissue sets. In tissue sections immunolabeled for TH, some of these PHAL-positive axons formed asymmetric synapses onto TH-labeled dendrites (63%; Table 1; Fig. 4C); the rest synapsed onto dendrites that did not contain gold-silver labeling. In tissue labeled for GABA, all of the observed PHAL-labeled asymmetric synapses were onto dendrites immunonegative for GABA (Table 1; Fig. 4D).
Postembedding immunolabeling
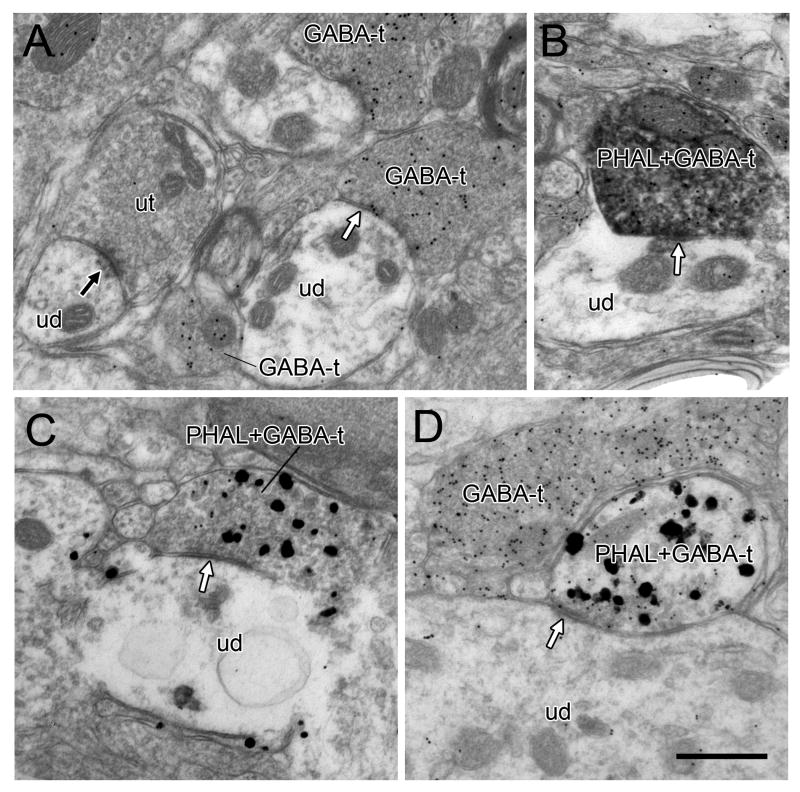
Tissue from animals perfused with glutaraldehyde-containing fixative was embedding in Durcupan resin and used for GABA postembedding immunostaining. The gold postembedding labeling was seen as uniform small round electron-dense particles concentrated in some axonal profiles and light to absent in others (Fig. 5A). Few gold particles were observed overlaying dendrites or soma, consistent with prior reports of the relative insensitivity of the postembedding method for detecting dendritic GABA (Shink and Smith, 1995; Jia et al., 2003). All of the axon terminals that were observed to contain abundant gold particles for GABA and to form clear synapses made symmetric specializations (Fig. 5). Conversely, adjacent axons forming asymmetric synapses exhibited few if any gold particles for GABA (Fig. 5A). This precise pattern of labeling provides additional evidence of specificity for the rabbit polyclonal GABA antibody used for postembedding.
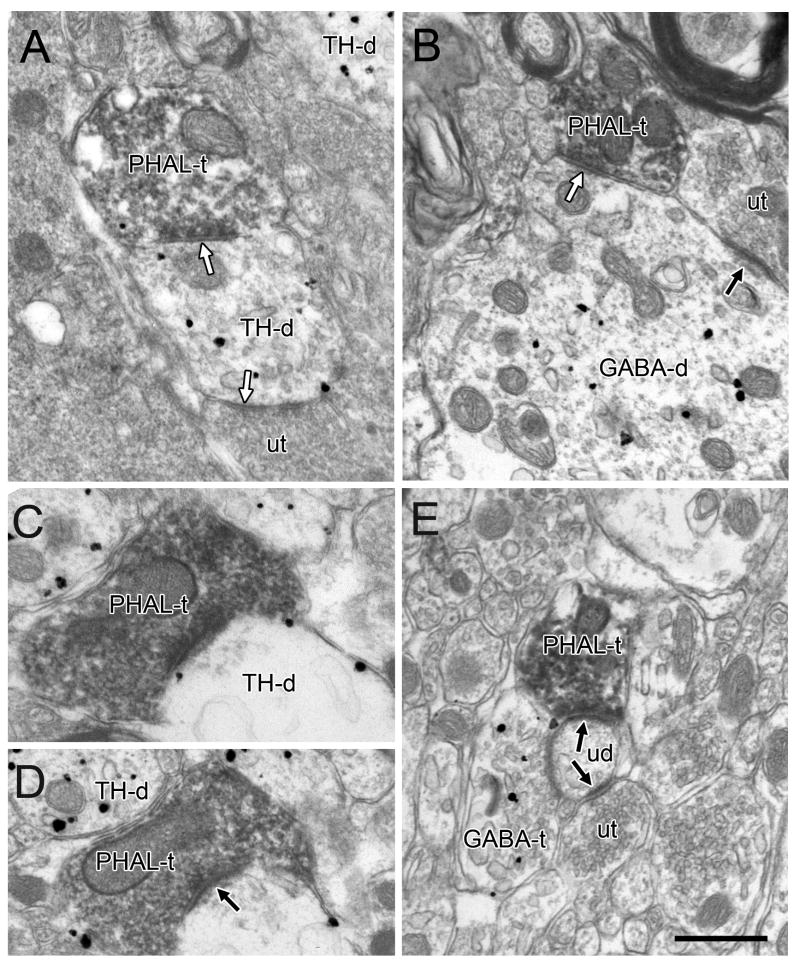
Figure 5.
Electron micrographs showing postembedding labeling for GABA in the VTA. In A, a GABA-labeled terminal (GABA-t) forms a symmetric synapse (white arrow) onto an unlabeled dendrite (ud). Other GABA-ts are evident nearby and a second unlabeled dendrite receives an asymmetric synapse (black arrow) from an unlabeled terminal (ut). In B, an axon terminal contains both immunoperoxidase for PHAL and postembedding gold labeling for GABA (GABA+PHAL-t). A slight specialization (white arrow) is evident where the terminal contacts an unlabeled dendrite. In C and D, axon terminals exhibit both preembedding gold-silver labeling for PHAL and postembedding gold particles for GABA and form symmetric synapses (white arrows) onto unlabeled dendrites. The terminal in C contains sparse gold particles that nevertheless exceed background levels and were verified in serial sections. In D, the postembedding gold particles in the dually-labeled axon are noticeably less dense than a nearby terminal singly labeled for GABA (GABA-t). Scale bar, 0.5 μm.
Some examples of GABA postembedding labeling in axons labeled by immunoperoxidase for PHAL were found (Fig. 5B). However, postembedding immunogold labeling for GABA was difficult to detect in many axons that contained heavy peroxidase product. In order to better characterize the extent of GABA labeling in terminals containing PHAL, we developed an additional tissue set for two animals in which PHAL was labeled by preembedding immunogold-silver. This approach was facilitated by the larger size and irregular shape of preembedding gold-silver particles that were clearly distinguished from the uniform smaller gold particles associated with postembedding (Fig. 5C,D). In this tissue, 16 PHAL-positive terminals were identified and 10 of them (63%) contained postembedding gold labeling for GABA. Of these 10 dually-labeled axons, 6 formed synapses and all were of the symmetric type; 4 dually-labeled axons did not form synapses. Of the remaining 6 PHAL-labeled axons, two formed asymmetric synapses and did not exhibit postembedding labeling for GABA.
Discussion
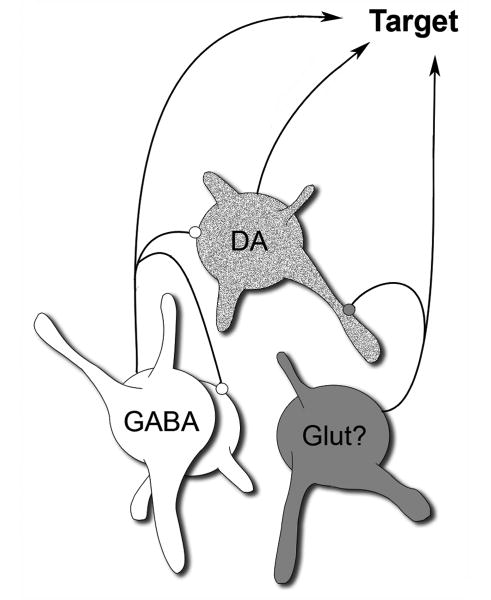
This study provides the first direct anatomical evidence that non-DA VTA neurons synapse locally onto DA cells, in agreement with physiological evidence for local regulation of DA neurons. This study also demonstrates that GABA cells receive local synaptic innervation, suggesting a complex disinhibitory circuitry intrinsic to the VTA that was not fully appreciated in prior studies. The fact that many of the local axon terminals formed symmetric synapses and were immunoreactive for GABA indicates their likely origin from VTA GABA cells (Mugniani and Oertel, 1985; Van Bockstaele and Pickel, 1995; Steffensen et al., 1998; Carr and Sesack, 2000a). The observation of local synapses with asymmetric, presumably excitatory physiology is consistent with the recently established presence of glutamate neurons in the VTA (Hur and Zaborszky, 2005; Kawano et al., 2006; Yamaguchi et al., 2007). If the latter are verified to be the source, then our findings suggest that these glutamate cells also participate in the local synaptic regulation of VTA cell activity. Figure 6 illustrates the main observations of the present study.
Figure 6.
Schematic drawing of the VTA showing the most prominent local connections of GABA cells, which appear to target both DA and GABA neurons. Local axon synapses may also derive from VTA glutamate cells, although this remains to be verified.
Methodological considerations
Local synaptic connectivity within the VTA was addressed using discrete injections of PHAL, a specific anterograde tracer. Following uptake by soma and dendrites, PHAL is transported into both extrinsically directed axons and their intra-areal collaterals (Gerfen and Sawchenko, 1984).
Several observations suggest that the axon terminals labeled with PHAL in the current study originated from VTA neurons that were filled with the tracer and not by retrograde transport or uptake into fibers of passage. PHAL reportedly undergoes retrograde transport in at least some systems (Gerfen and Sawchenko, 1984; Shu and Peterson, 1988), and so special attention was given to ensure the absence of retrogradely labeled cells in areas known to be afferent to the VTA.
PHAL is not transported effectively by fibers of passage following iontophoretic administration (Gerfen and Sawchenko, 1984). When injected into major fiber bundles (e.g. ventral hippocampal commisure or fimbria), PHAL has occasionally been observed in sparse axons near the injection site but not in known targets of these pathways (Gerfen and Sawchenko, 1984). The intensity of this labeling was noted to be considerably weaker than when neuronal processes actively transported PHAL. Following injection into gray matter structures as in the present study, it would be difficult to rule out any such local diffusion of PHAL into occasional short range axons. Hence, as an added precaution we ensured that the terminals included in the sample were more heavily labeled than dendrites in the vicinity, consistent with active transport of the tracer. It is also important to note that in cases of injections where PHAL was absent from neurons or present only in glia, tracer incorporation into axon terminals was not detected. Hence, we feel that spurious diffusion of PHAL into local axons in the VTA did not contribute substantially to the present findings. Nevertheless, it needs to be acknowledged that such non-specific uptake of tracer cannot be definitively ruled out in every case.
The goals of this study were facilitated by the rapid sequestration of tracer following intra-parenchymal injection so that electron microscopic examination of the neuropil showed no residual tracer within the extracellular space. The same results have also been reported for biotin dextran amine used to identify local axon collaterals in the monkey cortex (Melchitzky et al., 1998). There are no reports of specific neuronal phenotypes that fail to take up and transport PHAL, and indeed the present study found ample uptake into both DA and GABA neurons.
A significant potential limitation of the present study is the frequent occurrence of false negative outcomes in electron microscopic examination of tissue prepared with minimal detergent concentrations and associated with restricted antibody penetration. In our experience, this problem is most evident when examining dual immunolabeling of axon terminals by preembedding methods (Omelchenko and Sesack, 2005; Omelchenko and Sesack, 2006a; Sesack et al., 2006). Indeed the results here indicate that only occasional axons containing tracer were dually-labeled for GABA using this approach. Consequently, we performed postembedding labeling in order to better reveal the phenotype of the axons forming local symmetric synapses. Although we did observe some postembedding gold labeling for GABA in axons labeled by immunoperoxidase for PHAL, the rate of false negatives also appeared to be substantial using this approach. This limitation has been reported by other laboratories (Bokor et al., 2005). Fortunately, satisfactory results were obtained using a combination of preembedding immunogold-silver for PHAL and postembedding gold for GABA in which the sizes and shapes of gold particles were clearly distinct. In this tissue, the observation of specific GABA immunoreactivity in all tracer-labeled terminals forming symmetric synapses suggests that the majority of these synapses derive from local GABA neurons.
Morphological features and likely sources of local axon collaterals within the VTA
Our finding of a moderate density of intrinsic synaptic connections is the first direct anatomical evidence to support previous physiological reports predicting the existence of a local inhibitory innervation within the VTA (Johnson and North, 1992b; Johnson and North, 1992a; Sugita et al., 1992; White, 1996; Nugent and Kauer, 2008) or the adjacent SN (Grace and Bunney, 1985; Smith and Grace, 1992; Tepper et al., 1995). The observations that the majority of local synapses had a symmetric, presumably inhibitory morphology and that these terminals were immunoreactive for GABA indicate that VTA GABA neurons are the main source of these intrinsic connections. DA neurons apparently do not contribute to local synaptic connectivity (Phillipson, 1979; Wassef et al., 1981; Grace and Onn, 1989; Richards et al., 1997) and tracer-labeled terminals immunoreactive for TH were not observed in the current study. Moreover, the relative absence of dense-cored vesicles in tracer-labeled terminals suggests that local connections are unlikely to release neuropeptides (Thureson-Klein et al., 1986).
GABA-containing collaterals may derive from local circuit interneurons whose axons are entirely intrinsic to the VTA or from the recurrent branches of GABA projection cells whose major axon is directed toward extrinsic targets. Definitive evidence for true interneurons in the VTA and adjacent SN has yet to be provided, whereas physiological studies of midbrain GABA neurons consistently report antidromic activation of these cells from distant sites (Steffensen et al., 1998; Tepper and Lee, 2007). Nevertheless, the current findings cannot distinguish between these two possible sources of local GABA axons.
GABA is unlikely to be the only neurotransmitter involved in local VTA regulation based on the observation of axons containing tracer and forming asymmetric synapses. This morphology is suggestive of an excitatory neurotransmitter in these terminals, although the association between synapse structure and physiology is only correlative (Gray, 1959; Carlin et al., 1980). The relatively low number of asymmetric synapses and lack of specific antibodies suitable for postembedding labeling of transmitter glutamate precluded us from obtaining definitive evidence regarding the phenotype of these axons. Nevertheless, these local collaterals may derive from the recently described population of cells in the VTA that express the vesicular glutamate transporter type 2 (VGlut2) (Hur and Zaborszky, 2005; Kawano et al., 2006; Yamaguchi et al., 2007). A preliminary report from another laboratory indicates that these glutamate cells do form local axon connections (Dobi and Morales, 2007).
Synaptic targets of local axon collaterals within the VTA
The finding that VTA DA neurons received presumed inhibitory synaptic innervation from intrinsic cells is consistent with in vitro physiological recordings describing inhibitory postsynaptic potentials of local origin in principal neurons (Johnson and North, 1992b; Johnson and North, 1992a; Sugita et al., 1992; Nugent and Kauer, 2008). These same studies were less clear regarding the occurrence of local inhibitory events in secondary GABA cells. Hence, the observation here that VTA GABA neurons receive intrinsic symmetric synapses was unexpected. Nevertheless, such connections are commonly reported for SN zona reticulata neurons (Comoli et al., 2003; Mailly et al., 2003). Such intrinsic connections between midbrain GABA neurons provide the circuitry for potential disinhibitory processes to increase the excitability of DA cells.
Functional significance
The GABA innervation to VTA neurons comes from both intrinsic (present study) and extrinsic sources (Somogyi et al., 1981; Oades and Halliday, 1987; Smith and Bolam, 1990; Charara et al., 1996; White, 1996). The observation that local collaterals synapse mainly onto dendritic shafts suggests that these intrinsic connections are likely to be physiologically weaker than axosomatic inhibitory inputs. Synapses onto soma originate primarily from extrinsic GABAergic sources, including the nucleus accumbens, ventral pallidum, and mesopontine tegmentum (Somogyi et al., 1981; Smith and Bolam, 1990; Charara et al., 1996; Omelchenko and Sesack, 2005). This synaptic organization suggests that local connectivity provides an important means for coordinating the activity of neighboring cells within the VTA but with a limited capacity to override more proximal extrinsic inhibition.
The influence of local GABA collaterals is also spatially limited, in that the majority of synapses formed by intrinsic axons are located in close proximity to the injection site. This innervation pattern is consistent with a role for these inputs in regulating adjacent cells versus distant subregions of the VTA (Ferreira et al., 2008). Such focalization of regulatory control may help to synchronize discharge patterns (Wang and Buzsaki, 1996) and at the same time limit the spread of influence to neighboring cells that project to common or immediately adjacent regions of the forebrain (Haber et al., 2000). This idea is further supported by observations from the nearby SN zona reticulata where the projection fields of recurrent axons remain mostly confined to the dendritic fields of the parent neurons (Mailly et al., 2003).
An important question for future investigation is why the influence of local GABA synapses is sometimes not evident in DA neurons. For example, timing differences in the onset of prefrontal cortex-evoked responses in DA and non-DA neurons suggests that local GABA connections may not be the source of inhibition in some DA cells (Tong et al., 1998). We have shown that prefrontal cortical inputs to the VTA demonstrate specificity in their cellular targets (Carr and Sesack, 2000b), synapsing onto some populations of DA and GABA neurons but not others. Hence, the combined evidence from physiological and anatomical studies raises the possibility that distinct groups of VTA cells are more or less regulated by local GABA collaterals. However, this hypothesis still does not explain the relative absence of activity changes in midbrain DA neurons across the sleep/wake cycle despite evidence for state changes in the firing rate of VTA GABA neurons (Lee et al., 2001). This finding appears to argue against a tonic local influence of GABA cells, making it an important area for future investigation.
Varied physiological actions and diverse cellular targets of intrinsic synaptic connections suggest that local axon collaterals play a complex role in VTA cell regulation based on a synaptic organization that may include direct inhibition and excitation as well as indirect disinhibition. Additional studies are required to more precisely characterize the physiological actions of local VTA collaterals on specific neuronal populations across multiple behavioral states.
Acknowledgments
Grant support: USPHS grant NIMH 067937
Literature Cited
- Bayer VE, Pickel VM. Ultrastructural localization of tyrosine hydroxylase in the rat ventral tegmental area: relationship between immunolabeling density and neuronal associations. J Neurosci. 1990;10:2996–3013. doi: 10.1523/JNEUROSCI.10-09-02996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase-immunoreactivity in rat ventral tegmental area. Brain Res. 1991;559:44–55. doi: 10.1016/0006-8993(91)90285-4. [DOI] [PubMed] [Google Scholar]
- Bokor H, Frere SG, Eyre MD, Slezia A, Ulbert I, Luthi A, Acsady L. Selective GABAergic control of higher-order thalamic relays. Neuron. 2005;45:929–940. doi: 10.1016/j.neuron.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–843. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Callosal Terminals in the rat prefrontal cortex: synaptic targets and association with GABA-immunoreactive structures. Synapse. 1998;29:193–205. doi: 10.1002/(SICI)1098-2396(199807)29:3<193::AID-SYN1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000a;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000b;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp Neurol. 1996;364:254–266. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci. 2003;6:974–980. doi: 10.1038/nn1113. [DOI] [PubMed] [Google Scholar]
- Dobi A, Morales M. Dopaminergic neurons in the rat ventral tegmental area (VTA) receive glutamatergic inputs from local glutamatergic neurons. Soc Neurosci Abstr. 2007:916. [Google Scholar]
- Ferreira JG, Del-Fava F, Hasue RH, Shammah-Lagnado SJ. Organization of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neuroscience. 2008;153:196–213. doi: 10.1016/j.neuroscience.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anteriograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: Immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris Leucoagglutinin (PHA-L) Brain Res. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A, Bunney B. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 1985;333:271–284. doi: 10.1016/0006-8993(85)91581-1. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn S. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization. J Comp Neurol. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Jia HG, Yamuy J, Sampogna S, Morales FR, Chase MH. Colocalization of gamma-aminobutyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopic study. Brain Res. 2003;992:205–219. doi: 10.1016/j.brainres.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992a;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992b;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. GABAA receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems. Eur J Neurosci. 2001;13:1009–1015. doi: 10.1046/j.1460-9568.2001.01458.x. [DOI] [PubMed] [Google Scholar]
- Lee RS, Steffensen SC, Henriksen SJ. Discharge profiles of ventral tegmental area GABA neurons during movement, anesthesia, and the sleep-wake cycle. J Neurosci. 2001;21:1757–1766. doi: 10.1523/JNEUROSCI.21-05-01757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailly P, Charpier S, Menetrey A, Deniau JM. Three-dimensional organization of the recurrent axon collateral network of the substantia nigra pars reticulata neurons in the rat. J Neurosci. 2003;23:5247–5257. doi: 10.1523/JNEUROSCI.23-12-05247.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen T, Nagelshus E, Jouleh B, Torp R, Feydennlund D, Mylonakou MN, Amiry-Moghaddam M, Covolan L, Utvik J, Riber B, Gujord K, Knutset J, Skare O, Laake P, Davanger S, Haug FM, Rinvik E, Ottersen O. Postembedding immunogold cytochemistry of membrane molecules and amino acid transmitters in the central nervous system. In: Zaborszky L, Wouterlood J, Lanciego F, editors. Neuroanatomical Tract-Tracing 3: Molecules, Neurons, Systems. NY: Springer, New York; 2006. pp. 72–108. [Google Scholar]
- Melchitzky DS, Sesack SR, Pucak ML, Lewis DA. Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J Comp Neurol. 1998;390:211–224. doi: 10.1002/(sici)1096-9861(19980112)390:2<211::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Miller JD, Farber J, Gatz P, Roffwarg H, German DC. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 1983;273:133–141. doi: 10.1016/0006-8993(83)91101-0. [DOI] [PubMed] [Google Scholar]
- Mugniani E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy Vol 4: GABA and Neuropeptides in the CNS, Part I. New York: Elsevier; 1985. pp. 436–608. [Google Scholar]
- Nugent FS, Kauer JA. LTP of GABAergic synapses in the ventral tegmental area and beyond. J Physiol. 2008;586:1487–1493. doi: 10.1113/jphysiol.2007.148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oades R, Halliday G. Ventral tegmental (A10) system: Neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol. 2006a;494:863–875. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural evidence that non-dopamine cells in the rat ventral tegmental area synapse locally onto dopamine and GABA neurons. Soc Neurosci Abstr. 2006b:722–12. [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton P, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Peters A, Palay SL, Webster H. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. New York: Oxford; 1991. [Google Scholar]
- Phend KD, Rustioni A, Weinberg RJ. An osmium-free method of epon embedment that preserves both ultrastructure and antigenicity for post-embedding immunocytochemistry. J Histochem Cytochem. 1995;43:283–292. doi: 10.1177/43.3.7532656. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. A Golgi study of the ventral tegmental area of Tsai and interfascicular nucleus in the rat. J Comp Neurol. 1979;187:99–115. doi: 10.1002/cne.901870107. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.10.007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Tr Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Miner LAH, Omelchenko N. Pre-embedding immunoelectron microscopy: applications for studies of the nervous system. In: Zaborszky L, Wouterlood F, Lanciego J, editors. Neuroanatomical Tract-Tracing 3: Molecules, Neurons, Systems. NY: Springer New York; 2006. p. 6. [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Shink E, Smith Y. Differential synaptic innervation of neurons in the internal and external segments of the globus pallidus by the GABA- and glutamate-containing terminals in the squirrel monkey. J Comp Neurol. 1995;358:119–141. doi: 10.1002/cne.903580108. [DOI] [PubMed] [Google Scholar]
- Shu SY, Peterson GM. Anterograde and retrograde axonal transport of Phaseolus vulgaris leucoagglutinin (PHA-L) from the globus pallidus to the striatum of the rat. J Neurosci Methods. 1988;25:175–180. doi: 10.1016/0165-0270(88)90156-2. [DOI] [PubMed] [Google Scholar]
- Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse. 1992;12:287–303. doi: 10.1002/syn.890120406. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. The output neurons and the dopaminergic neurons of the substantia nigra receive a GABA-containing input from the globus pallidus in the rat. J Comp Neurol. 1990;296:47–64. doi: 10.1002/cne.902960105. [DOI] [PubMed] [Google Scholar]
- Smith Y, Charara A, Parent A. Synaptic innervation of midbrain dopaminergic neurons by glutamate-enriched terminals in the squirrel monkey. J Comp Neurol. 1996;364:231–253. doi: 10.1002/(SICI)1096-9861(19960108)364:2<231::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Bolam JP, Totterdell S, Smith AD. Monosynaptic input from the nucleus accumbens--ventral striatum region to retrogradely labelled nigrostriatal neurones. Brain Res. 1981;217:245–263. doi: 10.1016/0006-8993(81)90002-0. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Lee RS, Stobbs SH, Henriksen SJ. Responses of ventral tegmental area GABA neurons to brain stimulation reward. Brain Res. 2001;906:190–197. doi: 10.1016/s0006-8993(01)02581-1. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Johnson SW, North RA. Synaptic inputs to GABAA and GABAB receptors originate from discrete afferent neurons. Neurosci Lett. 1992;134:207–211. doi: 10.1016/0304-3940(92)90518-c. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Lee CR. GABAergic control of substantia nigra dopaminergic neurons. Prog Brain Res. 2007;160:189–208. doi: 10.1016/S0079-6123(06)60011-3. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15:3092–3103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thureson-Klein A, Klein RL, Zhu PC. Exocytosis from large dense cored vesicles as a mechanism for neuropeptide release in the peripheral and central nervous system. Scan Electron Microsc. 1986:179–187. [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse. 1996;22:195–208. doi: 10.1002/(SICI)1098-2396(199603)22:3<195::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Martinezcue C, Clark D. Do non-dopaminergic neurons in the ventral tegmental area play a role in the responses elicited in A10 dopaminergic neurons by electrical stimulation of the prefrontal cortex. Exp Brain Res. 1998;118:466–476. doi: 10.1007/s002210050303. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995;682:215–221. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- Veznedaroglu E, Milner TA. Elimination of artifactual labeling of hippocampal mossy fibers seen following pre-embedding immunogold-silver technique by pretreatment with zinc chelator. Microsc Res Tech. 1992;23:100–101. doi: 10.1002/jemt.1070230110. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef M, Berod A, Sotelo C. Dopaminergic dendrites in the pars reticulata of the rat substantia nigra and their striatal input. Combined immunocytochemical localization of tyrosine hydroxylase and anterograde degeneration. Neuroscience. 1981;6:2125–2139. doi: 10.1016/0306-4522(81)90003-8. [DOI] [PubMed] [Google Scholar]
- White FJ. Synaptic regulation of mesocorticolimbic dopamine neurons. Annu Rev Neurosci. 1996;19:405–436. doi: 10.1146/annurev.ne.19.030196.002201. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]