Abstract
Although a considerable body of literature indicates that serotoninergic neurons affect diaphragm activity both through direct inputs to phrenic motoneurons and multisynaptic connections involving the brainstem respiratory groups, the locations of the serotoninergic neurons that modulate breathing have not been well defined. The present study identified these neurons in cats by combining the transneuronal retrograde transport of rabies virus from the diaphragm with the immunohistochemical detection of the N-terminal region of tryptophan hydroxylase-2 (TPH2), the brain-specific isoform of the enzyme responsible for the initial and rate-limiting step in serotonin synthesis. TPH2-immunopositive neurons were present in the midline raphe nuclei, formed a column in the ventrolateral medulla near the lateral reticular nucleus, and were spread across the dorsal portion of the pons just below the fourth ventricle. In most animals, only a small fraction of neurons (typically < 20%) labeled for TPH2 in each of the medullary raphe nuclei and the medullary ventrolateral column were infected with rabies virus. However, the percentage of medullary neurons dual-labeled for both rabies and TPH2 was much higher in animals with very advanced infections where virus had spread transneuronally through many synapses. Furthermore, in all cases, TPH2-immunopositive neurons that were infected by rabies virus were significantly less prevalent in the pons than the medulla. These findings suggest that although serotoninergic neurons with direct influences on diaphragm activity are widely scattered in the brainstem, the majority of these neurons are located in the medulla. Many nonserotoninergic neurons in the raphe nuclei were also infected with rabies virus, indicating that midline cells utilizing multiple neurotransmitters participate in the control of breathing.
Keywords: rabies virus, transneuronal tracing, tryptophan hydroxylase-2, raphe nuclei
1. Introduction
A considerable body of literature representing a variety of animal species indicates that neurons located near the medullary midline, including serotoninergic cells, modulate breathing (Bernard et al., 1996; Bernard, 1998; Dias et al., 2007; 2008; Fuller et al., 2000; Hodges et al., 2004; Holtman et al., 1986; Lalley, 1986b, a; 1986b; Lalley et al., 1997; Li et al., 2006b; Lindsey et al., 1998; Messier et al., 2002; Millhorn, 1986; Nattie, 1999; Nattie and Li, 2001; Taylor et al., 2004; 2005; 2006; Verner et al., 2004; 2008). These influences on respiration are mediated both directly through serotoninergic projections to respiratory motoneurons (Holtman et al., 1984; Pilowsky et al., 1990; Tai et al., 1997) and indirectly via serotoninergic inputs to the brainstem respiratory groups (Connelly et al., 1989; Cream et al., 2002; Lalley et al., 1994; 1995; Mulkey et al., 2007; Voss et al., 1990). There is evidence that medullary serotonin-containing neurons serve as central chemoreceptors (Bernard et al., 1996; Li et al., 2006a; Messier et al., 2002; 2004; Nattie, 1999; 2001; Nattie and Li, 2001; Nucci et al., 2008; Penatti et al., 2006; Taylor et al., 2004; 2005; 2006), mediate long-term facilitation of respiratory motor output following episodic hypoxia or electrical stimulation of carotid chemoafferent neurons (Fuller et al., 2000; 2001; Ling et al., 2001; Millhorn et al., 1980; Millhorn, 1986; Mitchell et al., 2001), and are necessary for recovery of respiratory activity following upper cervical spinal injury (Fuller et al., 2005; Tai et al., 1997; Zhou and Goshgarian, 1999; 2000; Zimmer and Goshgarian, 2006). Furthermore, neurons near the medullary midline in felines have been shown to participate in eliciting cough (Baekey et al., 2003; Jakus et al., 1998) and emesis (Miller et al., 1996), although it is unknown whether these cells contain serotonin.
Despite the fact that serotoninergic cells play a role in regulating respiratory muscle discharges, the precise locations of serotonin-containing neurons that influence breathing are unknown. Attempts have been made to pinpoint the cells by examining respiratory responses to electrical or chemical stimulation of midline areas that contain serotoninergic neurons, sometimes in combination with the blockade of serotonin receptors to differentiate the serotonin-dependent and independent components of the responses that were elicited (Alvarenga et al., 2005; Bernard, 1998; Dias et al., 2007; 2008; Holtman et al., 1986; Lalley, 1986b, a; 1986b; Lalley et al., 1997; Li et al., 2006b; Millhorn, 1986; Nattie and Li, 2001; Verner et al., 2004). Other studies have generated focal lesions near the medullary midline to determine which regions participate in regulating respiratory activity (Dias et al., 2007; Hodges et al., 2004; Jakus et al., 1998; Messier et al., 2002; Taylor et al., 2006). However, because the brainstem areas affected by the stimuli or lesions varied widely between studies, and no work has systematically compared the effects of altering the excitability of serotonin-containing neurons in different regions of the brainstem, the precise locations of the serotoninergic cells involved in respiratory control remain unclear. A study in the feline that employed antibodies to 5-hydroxytryptamine (5HT) to localize serotoninergic cells indicated that a large fraction of these neurons were located outside of the raphe nuclei, in regions including the ventrolateral medulla (Jacobs et al., 1984). Although immunohistochemical localization of small molecules such as 5HT can generate false positive results, the experiment suggests that the participation of serotoninergic neurons in breathing control has not been properly assessed. This is because previous studies have not considered whether serotonin-containing cells in regions other than the midline participate in the regulation of respiration.
The use of rabies virus for transneuronal tracing offers a powerful tool to map the polysynaptic pathways regulating the activity of a particular muscle, as the virus is selectively uptaken by motoneurons and moves progressively through neural circuits in a time-dependent retrograde manner (Kelly and Strick, 2000; Ugolini, 1995; Ugolini, 2008). We recently employed the transneuronal transport of rabies virus in the cat to determine the regions of the central nervous system that provide direct and indirect inputs to phrenic motoneurons (Lois et al., 2009). These regions included the medullary and pontine raphe nuclei and adjacent reticular formation as well as additional areas that have been suggested to contain serotoninergic neurons in felines (Jacobs et al., 1984). In the present study, the transneuronal retrograde transport of rabies virus from the diaphragm was coupled with the immunohistochemical identification of serotoninergic cells to establish the relative number of the latter neurons in different brainstem locations that participate in regulating diaphragm contractions. Cats were employed for these experiments, as they utilize the respiratory muscles to produce behaviors such as vomiting and coughing that are not present in rodents. Serotoninergic neurons were identified with the use of an antibody to a unique peptide sequence near the N-terminal region of tryptophan hydroxylase-2 (TPH2), the brain-specific isoform of the enzyme responsible for the initial and rate-limiting step in serotonin synthesis (Kerman et al., 2006). As such, this study also provided a highly-accurate mapping of the locations of serotoninergic neurons in the feline brainstem.
2. Results
2.1. Distribution of TPH2 immunopositive neurons in the cat brainstem
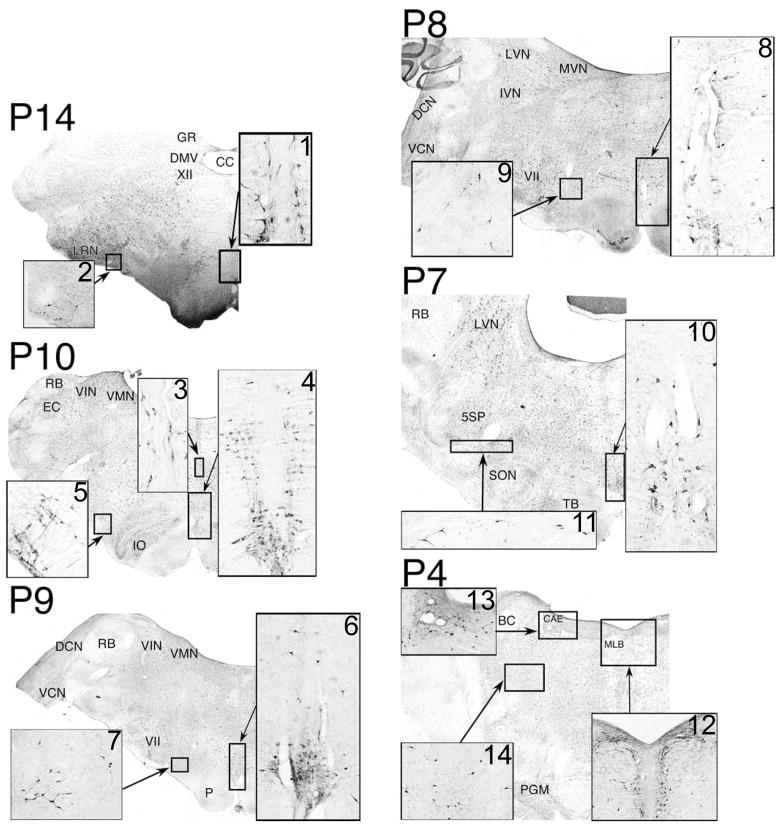
The locations of TPH2-immunopositive neurons were ascertained in two animals from immunoperoxidase-processed brainstem sections separated by 240 μm. The majority of the labeled neurons were localized along the midline in the raphe nuclei. However, a large fraction of cells was also observed elsewhere, as indicated in Fig. 1. A column of TPH2-immunopositive neurons was present in the ventrolateral medulla rostral to the level of the obex. The density of labeled neurons comprising this column was greatest in the caudal medulla, just lateral to the inferior olivary nucleus in the vicinity of the lateral reticular nucleus (see inset panels 2 and 5 of Fig. 1). TPH2-positive cells were also prominent in the medullary subretrofacial nucleus, as previously described in felines (Polson et al., 1992). More rostrally in the brainstem, the column of labeled cells migrated dorsally (Fig. 1, panels 7 and 9) and laterally (Fig. 1, panels 11 and 14), such that it was present in the pontomescencephalic reticular formation. Within the pons, the number and packing density of TPH2-immunopositive neurons comprising the column was very low (Fig. 1, panels 11 and 14). Another high concentration of labeled cells was spread across the dorsal pons, just below the fourth ventricle, as previously described in the guinea pig (Leonard et al., 1995). At its rostral extent, this region was medial to the parabrachial nucleus and in the vicinity of nucleus coeruleus (Fig. 1, panel 13), continuous with the lateral portion of nucleus raphe dorsalis (Fig. 1, panel 12). Only a few scattered labeled neurons were located outside these areas, mainly in the ventral portion of the medullary gigantocellular reticular formation.
Figure 1.
Locations of TPH2-immunopositive neurons in the cat brainstem. The background of each panel is a photomontage generated from micrographs of a section stained with cresyl violet and photographed using a 10X objective. Inset diagrams are micrographs of adjacent sections processed using immunoperoxidase to detect TPH2. Inset panels 1 and 3 show nucleus raphe obscurus, panels 4, 6 and 8 contain raphe pallidus, panel 10 depicts raphe magnus, and panel 12 illustrates raphe dorsalis. Coordinates above each panel indicate the distance in mm posterior to stereotaxic zero (P0). Abbreviations: 5SP, spinal trigeminal nucleus; BC, brachium conjunctivum; CAE, nucleus coeruleus; CC, central canal; DCN, dorsal cochlear nucleus; DMV, dorsal motor nucleus of the vagus; EC, external cuneate nucleus; GR, gracile nucleus; IO, inferior olivary nucleus; LRN, lateral reticular nucleus; LVN, lateral vestibular nucleus; MLB, medial longitudinal bundle; P, pyramid; PGM, pontine gray matter; RB, restiform body; SON, superior olivary nucleus; TB, trapezoid body; VCN, ventral cochlear nucleus; VII, facial nucleus; VIN, inferior vestibular nucleus; VMN, medial vestibular nucleus; XII, hypoglossal nucleus.
To confirm that the TPH2 antibody had specificity for the appropriate antigen, we processed a bin of tissue from two animals with antibody that was preabsorbed to its target peptide. In both cases, no neuronal labeling was evident. For example, panels A–C of Fig. 2 show micrographs of nucleus raphe obscurus and pallidus and the caudal ventrolateral region from a case where the preabsorption control was performed.
Figure 2.
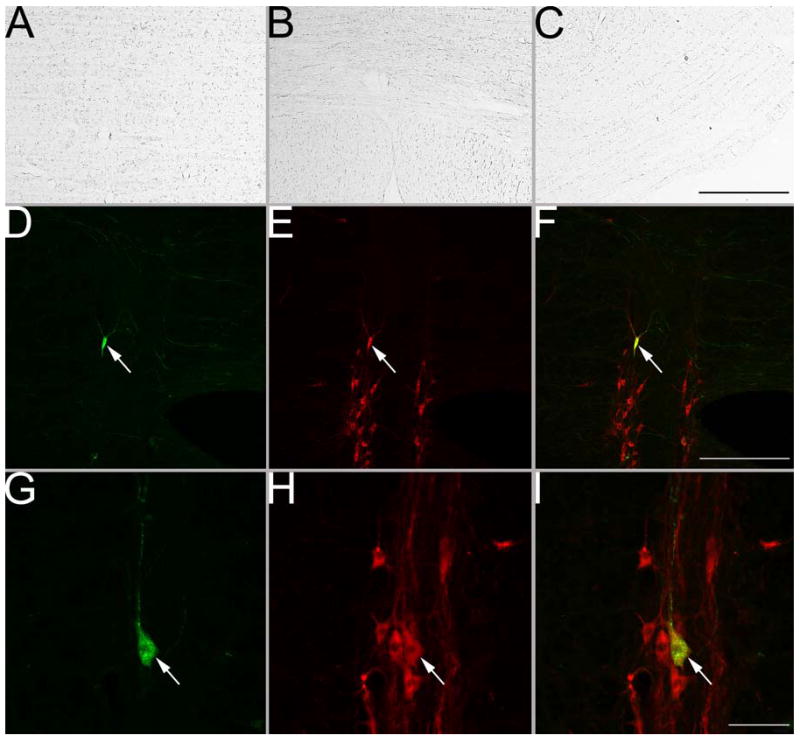
A–C: photomicrographs of raphe obscurus, raphe pallidus, and the caudal ventrolateral medulla near the lateral reticular nucleus, respectively, in sections processed using immunoperoxidase and TPH2 antibody that was preabsorbed with the target peptide. No cellular labeling was evident. D–I: photomicrographs of neurons in raphe obscurus processed using immunofluorescence to dually-localize rabies virus (visualized by the green fluorescence of BODIPY-FL) and TPH2 (denoted by the red fluorescence of CY3). In each row, the first panel (D and G) shows rabies virus coat protein immunopositivity, the middle panel (E and H) shows TPH2 immunopositivity, and the right panel (F and I) shows the combined immunopositivity for both antigens. Arrows in D–I indicate double-labeled neurons. The magnifications for micrographs in each row are the same, and thus only one calibration bar/row is provided. Calibration bars represent 500 μm in C and F and 40 μm in I.
2.2. Colocalization of TPH2 and rabies immunopositive neurons
The regions of the brainstem shown to contain a high density of TPH2-immunopositive neurons in the experiments described above were examined for the colocalization of this protein with rabies virus that had been transneuronally transported from the diaphragm. The distribution of rabies-infected neurons in six of the animals was described in detail in a previous paper (Lois et al., 2009). In two of these six cases (animals C52 and C21), no infected neurons were detected rostral to the pons. Although most of the labeling in these animals was present in the regions known to contain the medullary and pontine respiratory groups, infected cells were also located in areas where TPH2-immunopositive neurons had been identified, particularly the medullary midline and the vicinity of the lateral reticular nucleus. The distribution of rabies immunoreactivity in the brainstem of the additional animal added for these experiments (C95) was similar to that in animals C21 and C52. Rabies infection was much more extensive in the other four cases (Lois et al., 2009). In cat C38, infected cells were present in the midbrain as well as in the brainstem, and a much higher proportion of the labeled medullary neurons was located outside the respiratory groups. Presumably, the virus had spread transneuronally across more synapses in this animal than in the cases C21, C52, or C95. Heavy infection in a variety of regions of the brainstem of cat C39 was prevalent; labeled cells were also present in the diencephalon of this animal, and a limited number of rabies-immunopositive neurons were detected in cerebral cortex. Cat C51 had even more extensive infection than C39, which included an appreciable number of cells in the motor and insular cortices. The infection was the most advanced in C37, which exhibited labeling in multiple areas of the cerebral cortex as well as regions (e.g., substantia nigra) that were uninfected in the other cases.
Panels D–I of Fig. 2 provide examples of neurons that were examined for the colocalization of rabies virus and TPH2. Fig. 3 shows a mapping of the locations of TPH2-immunopositive cells in seven sections from animal C38, as well as the presence of rabies-infected neurons in the same regions. Fig. 3 also indicates the divisions of the raphe nuclei that were considered in quantitative analyses; the divisions were defined based on prior descriptions (Jacobs et al., 1984; Taber et al., 1960). Within the medulla, raphe obscurus was considered to be the dorsal midline region spanning caudally from P8.5; raphe pallidus was designated as the ventral enlarged midline area from P7.5–P14.5; raphe magnus was defined as the dorsal midline area from P6–P7.5 mm. The relatively small divisions of the raphe nuclei in the pons (e.g., raphe pontis and centralis superior) (Taber et al., 1960) were lumped together as the pontine raphe nuclei, and raphe dorsalis was assumed to be the dorsal midline region extending rostrally from P3 (only counts at this single level were included in the present analysis). Fig. 3 also indicates the locations of three serotoninergic cell groups outside the raphe nuclei: the caudal aspect of the lateral column of TPH2-immunopositive neurons (P10.5–P18), which was designated the caudal ventrolateral region; the portion of the lateral serotoninergic column in the rostral medulla (P7–P10), which was defined as the rostral ventrolateral region; and the dorsal pons just below the fourth ventricle, which was termed the central gray. The latter division included cells in the mesopontine tegmentum, and may also have encompassed a portion of the lateral extent of raphe dorsalis.
Figure 3.
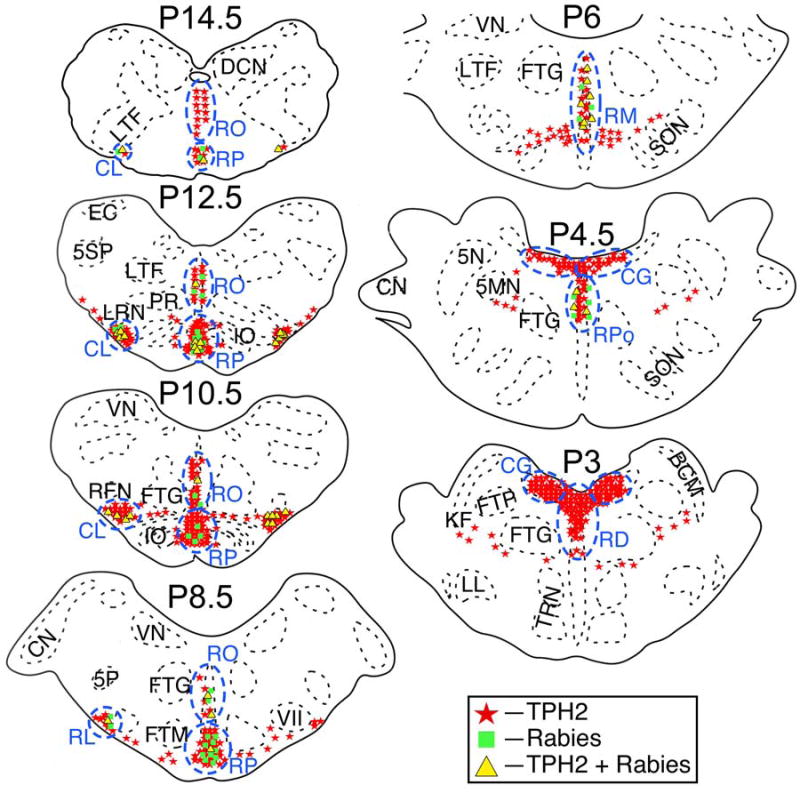
The locations in animal C38 of TPH2-immunopositive neurons, neurons in the same regions that were immunopositive for rabies virus, and neurons that contained both antigens. Areas where a high density of TPH2-immunopositive neurons were observed are designated by blue dashed lines and lettering. Cell locations are shown on standard sections generated through reference to Berman’s cat brainstem atlas (Berman, 1968). The location of each section relative to stereotaxic zero (P0) is indicated. Abbreviations: 5MN, trigeminal motor nucleus; 5N, trigeminal nucleus; 5P, parvocellular spinal trigeminal nucleus; 5SP, spinal trigeminal nucleus; BCM, marginal nucleus of brachium conjunctivum; CG, central gray; CL, caudal ventrolateral serotoninergic region; CN, cochlear nuclei; DCN, dorsal column nuclei; EC, external cuneate nucleus; FTG, gigantocellular tegmental field; FTM, magnocellular tegmental field; FTP, pontine tegmental field; IO, inferior olivary nucleus; KF, Kölliker-Fuse nucleus; LL, nucleus of the lateral lemniscus; LRN, lateral reticular nucleus; LTF, lateral tegmental field; NTS, nucleus tractus solitarius; PR, paramedian reticular nucleus; RD, raphe dorsalis; RFN, retrofacial nucleus; RL, rostral ventrolateral serotoninergic region; RM, raphe magnus; RO, raphe obscurus; RP, raphe pallidus; RPo, pontine raphe nuclei; SON, superior olivary nucleus; TRN, tegmental reticular nucleus; VII, facial nucleus; VN, vestibular nuclei.
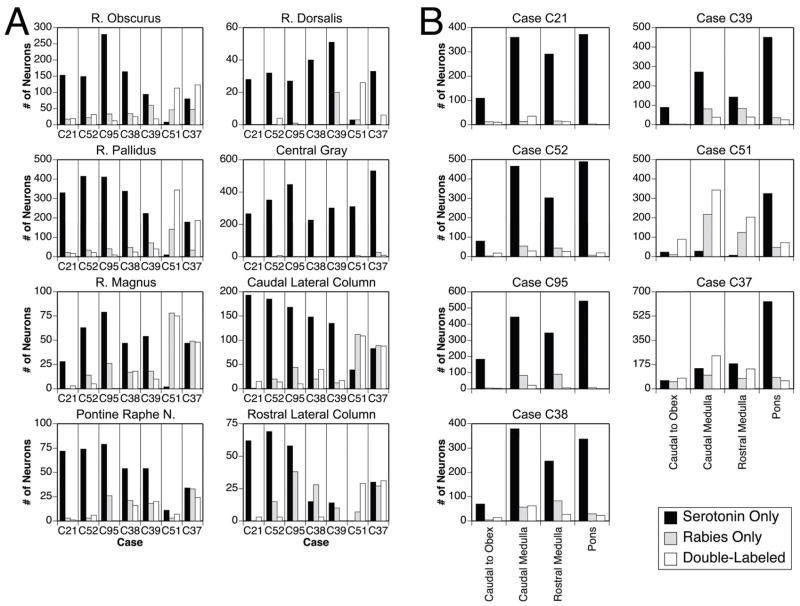
Fig. 4A shows the number of neurons that were immunopositive for TPH2, rabies virus, and both antigens in the areas containing a large number of serotoninergic cells. Neuronal counts were obtained from 18 sections/animal roughly corresponding to the P3–P20 plates (e.g., the levels from 3–20 mm posterior to stereotaxic zero) provided in Berman’s cat brainstem atlas (Berman, 1968). Fig. 4B provides similar data as Fig. 4A, although cell counts in different anterior-posterior divisions of the brainstem are indicated. Those divisions include the area caudal to the obex, the caudal medulla (P10.5–P13.5), the rostral medulla (P7–P10) and the pons (P3–P6). Table 1 indicates the number and percentage of serotoninergic neurons in each of the locations depicted in Fig. 4 that were dual-labeled for the presence of rabies virus.
Figure 4.
Counts from all animals of TPH2-immunopositive neurons, neurons in the same regions that were immunopositive for rabies virus, and neurons that contained both antigens. The values represent the number of labeled cells observed bilaterally. A: Cell counts in different raphe nuclei and regions in the lateral brainstem containing a high concentration of TPH2-immunopositive neurons; the boundaries of these divisions are indicated in Fig. 3. B: Cell counts in the following anterior-posterior divisions of the brainstem: the area caudal to the obex, the caudal medulla (P10.5–P13.5), the rostral medulla (P7–P10) and the pons (P3–P6). Abbreviation: R, raphe.
Table 1.
Number and (percentage, in parentheses) of TPH2-immunopositive neurons in different nuclei (top) and different anterior-posterior locations (bottom) that were infected by rabies virus. Data from each animal are shown in a separate column. Values were generated from bilateral counts of labeled cells.
| # and (%) of TPH2-Immunopositive Neurons Infected in Different Nuclei | |||||||
|---|---|---|---|---|---|---|---|
| Animal | C21 | C52 | C95 | C38 | C39 | C51 | C37 |
| Survival Time (hrs) | 96 | 87 | 99 | 96 | 96 | 107 | 101 |
| Raphe Obscurus | 19 (11) | 31 (17) | 12 (4) | 25 (13) | 18 (16) | 113 (93) | 123 (61) |
| Raphe Pallidus | 17 (5) | 21 (5) | 9 (2) | 24 (7) | 40 (15) | 344 (97) | 187 (51) |
| Raphe Magnus | 3 (10) | 5 (7) | 0 (0) | 18 (28) | 10 (16) | 75 (97) | 48 (51) |
| Pontine Raphe Nuclei | 1 (1) | 6 (8) | 0 (0) | 16 (23) | 20 (20) | 7 (39) | 24 (41) |
| Raphe Dorsalis | 0 (0) | 4 (11) | 0 (0) | 0 (0) | 0 (0) | 26 (90) | 6 (15) |
| Central Gray | 0 (0) | 8 (2) | 0 (0) | 0 (0) | 0 (0) | 2 (1) | 9 (2) |
| Caudal Lateral Column | 15 (7) | 14 (7) | 10 (6) | 40 (21) | 17 (11) | 109 (74) | 88 (52) |
| Rostral Lateral Column | 3 (5) | 3 (4) | 0 (0) | 3 (17) | 0 (0) | 29 (100) | 31 (51) |
| # and (%) of TPH2-Immunopositive Neurons Infected at Different Anterior-Posterior Locations | |||||||
| Caudal to Obex | 10 (8) | 18 (18) | 3 (2) | 14 (17) | 3 (3) | 89 (79) | 77 (56) |
| Caudal Medulla (P10.5–P13.5) | 35 (9) | 29 (6) | 22 (5) | 62 (14) | 38 (12) | 343 (92) | 238 (62) |
| Rostral Medulla (P7–P10) | 12 (4) | 26 (8) | 6 (2) | 27 (10) | 39 (22) | 203 (97) | 143 (44) |
| Pons (P3–P6) | 1 (0) | 19 (4) | 0 (0) | 23 (6) | 25 (5) | 70 (18) | 58 (8) |
The total number of TPH2-immunopositive neurons observed in each animal was relatively consistent: 1190 in C21, 1088 in C51, 1199 in C38, 1547 in C95, 1057 in C39, 1430 in C52, and 1533 in C37. In all of the cases except the two with the most advanced infection (C51 and C37), neurons that were dual-labeled for both rabies and TPH2 were diffusely scattered throughout the medullary and pontine raphe nuclei and bilaterally in the lateral medullary serotoninergic column. Although there was some variability between animals, only a low fraction of serotoninergic neurons in each location was infected with rabies virus (see Table 1). In contrast, >50% of medullary TPH2-immunopositive neurons were infected by rabies virus in cases C37 and C51. Furthermore, in all animals, the fraction of TPH2 immunopositive cells infected by rabies virus was higher in the medulla (P7–P18) than in the pons (P3–P6) (see Fig. 4 and Table 1), as confirmed by the use of a Wilcoxon signed rank test (p<0.05). All of the regions where TPH2-immunopositive neurons were located also contained a large number of cells that were selectively labeled for the presence of rabies virus (see Fig. 4). The fraction of infected neurons in these regions that was not serotoninergic varied from animal to animal, but was always considerable. For example, in all animals except those with the most advanced infections (C37 and C51), more cells in medullary raphe nuclei were selectively immunopositive for rabies virus than were double-labeled for both rabies and TPH2.
3. Discussion
The locations of TPH2-immunopositive neurons in the present study largely overlapped those of cells labeled for the presence of 5HT in the cat (Jacobs et al., 1984). This provides validation for the prior work, and confirms that a large number of serotoninergic neurons in felines are located outside of the classical raphe nuclei, particularly in a ventrolateral column in the medulla and the dorsal portion of the pons. The ventrolateral column is much less prominent in rodents (Kerman et al., 2006). The present study additionally showed that the serotoninergic neurons with the most direct connections with phrenic motoneurons are widely dispersed in the medullary and pontine raphe nuclei and the lateral medullary serotoninergic cell column, but are more prevalent in the medulla than the pons. In most animals, ~20% or less of TPH2-immunopositive cells in each medullary raphe nucleus and the lateral medullary column were dual-labeled for the presence of rabies, including in animals (e.g., C38 and C39) where a substantial number of neurons were infected in regions such as the deep cerebellar nuclei that are only indirectly connected to phrenic motoneurons (see (Lois et al., 2009) for a full description of the distribution of rabies labeling in these cases). Thus, neurons providing synaptic inputs to the brainstem respiratory group neurons should have been infected in these cases as well as cells with direct projections to the phrenic motor pool. A large fraction of TPH2-immunopositive neurons was infected only in animals with the most extensive rabies labeling, particularly C51 and C37 (Lois et al., 2009), where the virus had spread transneuronally through a large number of synapses to infect many cells. As such, many of the serotoninergic neurons that were infected by rabies virus in these advanced cases likely provided very indirect influences on diaphragm activity.
A number of caveats must be weighed when interpreting the present data. First, it must be considered whether the infection of only a small fraction of serotoninergic neurons by rabies was related to a limited transport of the virus by motor pathways regulating diaphragm activity. To address this concern, we dispersed a large amount of virus at many sites in the crural diaphragm on one side, which resulted in the infection of a large fraction of cells in the regions containing the brainstem respiratory groups (see (Lois et al., 2009) for a thorough description of this labeling). As such, it seems likely that the pathways controlling diaphragm function where heavily infected in this study. A converse issue is whether rabies virus infected neurons other than those regulating diaphragm activity. As discussed in our prior manuscript (Lois et al., 2009), numerous observations argue against this point, including the fact that there was no apparent infection of sympathetic and parasympathetic circuitry innervating visceral organs adjacent to the diaphragm and there was no rabies immunopositivity in motoneurons other than those in the known location of the phrenic motor pool. A third potential concern is that the infection of neurons by rabies suppressed the expression of TPH2, and thus the occurrence of dual labeling. This also seems unlikely, since the number of serotoninergic cells observed was similar from animal to animal, and in fact the colocalization of TPH2 and rabies immunoreactivity was highest in the cases where the infection was most advanced.
Each region of the raphe nuclei receives distinct inputs and mediates different behavioral responses (Aghajanian and Liu, 2009; Azmitia, 1999; Hornung, 2003). The finding that a diffuse network of serotoninergic neurons, and not a concentrated population of cells, participates in regulating diaphragm activity likely reflects the multifunctional nature of the muscle. In cats, the diaphragm is involved in a number of different roles in addition to breathing; the muscle participates in generating coughing and vomiting, and its activity is adjusted during postural alterations, locomotion, exercise, sleep, and emotional responses (Miller et al., 1997). Considering the diversity of diaphragm functions, it is not surprising that phrenic motoneurons receive multisynaptic serotoninergic inputs from neurons in a number of locations. However, this observation confounds the ability to study serotoninergic influences on phrenic motoneuron activity, since the serotoninergic neurons are distributed so widely. Nonetheless, the results do provide some insights for new experiments. For example, previous studies have suggested that neurons located in the medulla near the lateral reticular nucleus are involved in producing cardiorespiratory adjustments during exercise (Waldrop et al., 1996). The current findings indicate that a column of serotoninergic neurons, some of which participate in regulating diaphragm activity, is positioned in the same region. As such, future work is warranted to explore whether serotoninergic neurons in the ventrolateral medulla participate in exercise-related alterations in breathing.
In summary, the present findings show that serotoninergic neurons with the most direct influences on diaphragm function are diffusely distributed throughout the medullary and pontine raphe nuclei, as well as in the ventrolateral medulla. Since each raphe nucleus receives different inputs, this widespread network of cells is likely capable of altering phrenic motoneuron activity during a variety of different behaviors. Many non-serotoninergic neurons within the medullary raphe nuclei also provide inputs to phrenic motoneurons, indicating that multiple neurotransmitters mediate the effects of stimulation of midline brainstem areas on breathing (Alvarenga et al., 2005; Bernard, 1998; Dias et al., 2007; 2008; Holtman et al., 1986; Lalley, 1986b, a; 1986b; Lalley et al., 1997; Li et al., 2006b; Millhorn, 1986; Nattie and Li, 2001; Verner et al., 2004).
4. Experimental Procedures
All of the procedures used in this study conformed to the “Guide for the Care and Use of Laboratory Animals” (Council, 1996) and were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee. Brainstem tissue obtained from 11 adult cats (Liberty Research, Waverly, NY) was employed in the present study. Tissue acquired from two animals was utilized for an immunoperoxidase analysis that documented the locations of serotoninergic neurons in the brainstem. Tissue from two other animals was utilized in a control experiment verifying the selectivity of the antibody employed in the analysis for recognizing TPH2. The other seven animals received injections of the N2C strain of rabies virus at a titer of 1 × 108 plaque forming units/ml into the left diaphragm and brainstem sections from these cases were processed for dual localization of rabies and TPH2 positive neurons. The distribution of rabies-infected neurons in the brainstem of six of these animals (cases C52, C21, C37, C38, C39, and C51) was described previously (Lois et al., 2009). These animals received an injection of 200 μl of rabies virus spread across eight sites in the crural diaphragm (an exception is C37, which received 300 μl of rabies distributed at 12 sites in the left diaphragm). Rabies inoculations were performed on one additional cat (C95) to increase the number of cases available for the present analysis. This extra animal received an injection of 250 μl of rabies virus across 10 sites in the crural diaphragm; the surgical procedures and biosafety practices employed in this experiment were identical to those utilized previously (Lois et al., 2009). At the end of the survival period (see Table 1 for a listing of these timepoints), animals were anesthetized and perfused using paraformaldehyde-lysine-periodate fixative as described in our prior publication (Lois et al., 2009). Transverse brainstem sections were cut at a thickness of 40 μm using a freezing microtome, collected in six wells of either phosphate-Tris-azide (PTA) buffer or cryoprotectant (Watson et al., 1986), and stored at either 4°C (tissue placed in PTA) or −20°C (tissue placed in cryoprotectant).
One well of tissue from two animals was processed using avidin-biotin immunoperoxidase techniques (Hsu et al., 1981) to detect TPH2-containing neurons; a 1:1000 concentration of rabbit anti-TPH2 antibody (Kerman et al., 2006) was employed in the analysis. The laboratory of Dr. Stanley Watson at the University of Michigan provided this antibody. For each case, an adjacent bin of sections was stained with cresyl violet so the relative location of TPH2 immunopositive neurons to brainstem nuclei could be ascertained. One well of tissue from two other animals was immunoprocessed similarly, except that the antibody was preabsorbed with TPH2 peptide at 50 μM concentration. In these control experiments no staining of neurons was noted in areas that were robustly labeled in the primary experiments, confirming that the antibody has the same specificity in the cat as previously demonstrated in the rat (Kerman et al., 2006).
One bin of tissue from the other seven animals was processed using immunofluorescence techniques to dual-localize rabies and TPH2. Sections were incubated for 2 days at 4°C in a combination of rabbit anti-TPH2 (1:200) and mouse monoclonal antibody directed against the rabies virus phosphoprotein that resides in the infective nucleocapsid core (M957, 1:30)(Kelly and Strick, 2000). Dr. Peter Strick provided the latter antibody, and its specificity for detecting rabies virus has previously been described (Lois et al., 2009; Nadin-Davis et al., 2000). Subsequently, after rinsing in phosphate-buffered saline, the sections were incubated in a combination of goat secondary antibody conjugated to CY3 (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA) and goat secondary antibody conjugated to BODIPY-FL (1:300, Molecular Probes, Eugene, OR). In 4 cases, anti-rabbit BODIPY-FL and anti-mouse CY3 were employed; for two others, anti-mouse BODIPY-FL and anti-rabbit CY3 were utilized. Two bins were processed for the seventh animal, using both combinations of secondary antibody. Analogous results were obtained despite the secondary antibody employed to visualize each antigen. On completion of the immunohistochemical processing, the tissue was mounted on gelatin-coated slides, dehydrated, cleared, and coverslipped using Cytoseal 60 (VWR Scientific, West Chester, PA).
Immunoperoxidase sections were inspected to determine all the regions of the medulla and pons that contain TPH2-immunopositive neurons. Subsequently, the entirety of selected immunoperoxidase and cresyl violet stained sections were photographed using a 10X objective, and the micrographs were assembled into a montage using Stereo Investigator 7 software (MicroBrightField, Williston, VT). Selected regions of these montages were imported into Photoshop CS3 software (Adobe Systems, San Jose, CA) and gathered into plates for presentation.
Immunofluorescent sections were inspected using an Olympus BX51TRF photomicroscope equipped with a Hamamatsu camera (Hamamatsu Photonics, Hamamatsu, Japan) and a Simple-32 PCI image analysis system (Compix, Lake Oswego, OR) or a Nikon Eclipse E600N photomicroscope equipped with a Spot RT monochrome digital camera (Diagnostic Instruments, Sterling Heights, MI) and MetaMorph imaging software (Universal, Downingtown, PA). Approximately 18 sections from each case, corresponding to the P3 – P18 levels illustrated in Berman’s atlas of the cat brainstem (Berman, 1968), were selected for photography and analysis. All of the brainstem regions containing TPH2 immunopositive neurons were photographed at both low and high magnification using epifluorescence in combination with filters that selectively excited CY3 or BODIPY-FL. In the dual-labeling immunofluorescence analysis, each field was photographed in single exposures that recorded the location of cells harboring each antigen and in double exposures that revealed the cellular localization of both TPH2 and rabies. Great care was taken to ascertain that yellow fluorescence reflected the colocalization of both the BODIPY-FL and CY3 fluorophors and was not attributable to the presence of overlapping cells that each contained one of the fluorophors. Some selected sections were also photographed with an Olympus FluoView 1000 laser-scanning confocal microscope; a 10X objective was employed and 7 optical sections per field were acquired. For each image, the z-stack was compressed with the use of ImageJ software (National Institutes of Health, Bethesda, MD). Final preparation of figures for presentation, including the pseudocoloring of monochrome images, was performed using Adobe Photoshop CS3 software.
Acknowledgments
The authors thank Lucy Cotter, Derek Reighard, Bailey Commander, Allison Waggoner, and Sarah Weber for technical assistance in the completion of these experiments. We are also grateful to Mr. James Beals for his expert help and advice regarding microscopy and image processing. We additionally thank Dr. Peter Strick of the University of Pittsburgh for providing rabies virus and anti-rabies antibodies and Dr. Stanley Watson of the University of Michigan for supplying anti-TPH2 antibody. This work was supported by National Institutes of Health (NIH) grants R01-DC-03732 (to Bill Yates) and K99-MH-081927 (to Ilan Kerman), a NARSAD Young Investigator Award (to Ilan Kerman), as well as by grant P40-RR-018604 from NIH’s National Center for Research Resources.
Abbreviations
- 5HT
5-hydroxytryptamine
- TPH2
tryptophan hydroxylase-2
References
- Aghajanian G, Liu RJ. Serotonin (5-hydroxytryptamine; 5-HT): CNS pathways and neurophysiology. In: Squire LR, editor. Encyclopedia of Neuroscience. Academic Press; Oxford: 2009. pp. 725–732. [Google Scholar]
- Alvarenga RM, Pires JG, Futuro Neto HA. Functional mapping of the cardiorespiratory effects of dorsal and median raphe nuclei in the rat. Braz J Med Biol Res. 2005;38:1719–1727. doi: 10.1590/s0100-879x2005001100022. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacol. 1999;21:33S–45S. doi: 10.1016/S0893-133X(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R. Medullary raphe neuron activity is altered during fictive cough in the decerebrate cat. J Appl Physiol. 2003;94:93–100. doi: 10.1152/japplphysiol.00341.2002. [DOI] [PubMed] [Google Scholar]
- Berman AI. The Brain Stem of the Cat. University of Wisconsin Press; Madison: 1968. [Google Scholar]
- Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphe. J Appl Physiol. 1996;80:108–115. doi: 10.1152/jappl.1996.80.1.108. [DOI] [PubMed] [Google Scholar]
- Bernard DG. Cardiorespiratory responses to glutamate microinjected into the medullary raphe. Resp Physiol. 1998;113:11–21. doi: 10.1016/s0034-5687(98)00050-4. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett. 1989;105:34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Cream C, Li A, Nattie E. The retrotrapezoid nucleus (RTN): local cytoarchitecture and afferent connections. Respir Physiol Neurobiol. 2002;130:121–137. doi: 10.1016/s0034-5687(01)00338-3. [DOI] [PubMed] [Google Scholar]
- Dias MB, Nucci TB, Margatho LO, Antunes-Rodrigues J, Gargaglioni LH, Branco LG. Raphe magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J Appl Physiol. 2007;103:1780–1788. doi: 10.1152/japplphysiol.00424.2007. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie E. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol. 2008;105:83–90. doi: 10.1152/japplphysiol.00120.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Opansky C, Qian B, Davis S, Bonis J, Bastasic J, Leekley T, Pan LG, Forster HV. Transient attenuation of CO2 sensitivity after neurotoxic lesions in the medullary raphe area of awake goats. J Appl Physiol. 2004;97:2236–2247. doi: 10.1152/japplphysiol.00584.2004. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Norman WP, Gillis RA. Projections from the raphe nuclei to the phrenic motor nucleus in the cat. Neurosci Lett. 1984;44:105–111. doi: 10.1016/0304-3940(84)90229-5. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Dick TE, Berger AJ. Involvement of serotonin in the excitation of phrenic motoneurons evoked by stimulation of the raphe obscurus. J Neurosci. 1986;6:1185–1193. doi: 10.1523/JNEUROSCI.06-04-01185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C: 1996. [Google Scholar]
- Jacobs BL, Gannon PJ, Azmitia EC. Atlas of serotonergic cell bodies in the cat brainstem: an immunocytochemical analysis. Brain Res Bul. 1984;13:1–31. doi: 10.1016/0361-9230(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Jakus J, Stransky A, Poliacek I, Barani H, Bosel’ova L. Effects of medullary midline lesions on cough and other airway reflexes in anaesthetized cats. Physiol Res. 1998;47:203–213. [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Meth. 2000;103:63–71. doi: 10.1016/s0165-0270(00)00296-x. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Shabrang C, Taylor L, Akil H, Watson SJ. Relationship of presympathetic-premotor neurons to the serotonergic transmitter system in the rat brainstem. J Comp Neurol. 2006;499:882–896. doi: 10.1002/cne.21129. [DOI] [PubMed] [Google Scholar]
- Lalley PM. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol. 1986a;380:373–385. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. Responses of phrenic motoneurones of the cat to stimulation of medullary raphe nuclei. J Physiol. 1986b;380:349–371. doi: 10.1113/jphysiol.1986.sp016290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J Physiol. 1994;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Schwarzacher SW, Richter DW. 5-HT2 receptor-controlled modulation of medullary respiratory neurones in the cat. J Physiol. 1995;487:653–661. doi: 10.1113/jphysiol.1995.sp020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM, Benacka R, Bischoff AM, Richter DW. Nucleus raphe obscurus evokes 5-HT-1A receptor-mediated modulation of respiratory neurons. Brain Res. 1997;747:156–159. doi: 10.1016/s0006-8993(96)01233-4. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Kerman I, Blaha G, Taveras E, Taylor B. Interdigitation of nitric oxide synthase-, tyrosine hydroxylase-, and serotonin-containing neurons in and around the laterodorsal and pedunculopontine tegmental nuclei of the guinea pig. J Comp Neurol. 1995;362:411–432. doi: 10.1002/cne.903620309. [DOI] [PubMed] [Google Scholar]
- Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol. 2006a;577:307–318. doi: 10.1113/jphysiol.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Song G, Cao Y, Wang H, Wang G, Yu S, Zhang H. Modulation of the Hering-Breuer reflex by raphe pallidus in rabbits. Neurosci Lett. 2006b;397:259–262. doi: 10.1016/j.neulet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Arata A, Morris KF, Hernandez YM, Shannon R. Medullary raphe neurones and baroreceptor modulation of the respiratory motor pattern in the cat. J Physiol. 1998;512:863–882. doi: 10.1111/j.1469-7793.1998.863bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol. 2009;106:138–152. doi: 10.1152/japplphysiol.91125.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier ML, Li A, Nattie EE. Muscimol inhibition of medullary raphe neurons decreases the CO2 response and alters sleep in newborn piglets. Respir Physiol Neurobiol. 2002;133:197–214. doi: 10.1016/s1569-9048(02)00168-4. [DOI] [PubMed] [Google Scholar]
- Messier ML, Li A, Nattie EE. Inhibition of medullary raphe serotonergic neurons has age-dependent effects on the CO2 response in newborn piglets. J Appl Physiol. 2004;96:1909–1919. doi: 10.1152/japplphysiol.00805.2003. [DOI] [PubMed] [Google Scholar]
- Miller AD, Nonaka S, Jakus J, Yates BJ. Modulation of vomiting by the medullary midline. Brain Res. 1996;737:51–58. doi: 10.1016/0006-8993(96)00663-4. [DOI] [PubMed] [Google Scholar]
- Miller AD, Bianchi AL, Bishop BP, editors. Neural Control of the Respiratory Muscles. CRC Press; Boca Raton, FL: 1997. [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Resp Physiol. 1980;42:171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Millhorn DE. Stimulation of raphe (obscurus) nucleus causes long-term potentiation of phrenic nerve activity in cat. J Physiol. 1986;381:169–179. doi: 10.1113/jphysiol.1986.sp016320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadin-Davis SA, Sheen M, Abdel-Malik M, Elmgren L, Armstrong J, Wandeler AI. A panel of monoclonal antibodies targeting the rabies virus phosphoprotein identifies a highly variable epitope of value for sensitive strain discrimination. J Clin Microbiol. 2000;38:1397–1403. doi: 10.1128/jcm.38.4.1397-1403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nucci TB, Branco LG, Gargaglioni LH. 5-HT1A, but not 5-HT2 and 5-HT7, receptors in the nucleus raphe magnus modulate hypoxia-induced hyperpnoea. Acta Physiol. 2008;193:403–414. doi: 10.1111/j.1748-1716.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J Appl Physiol. 2006;101:1177–1188. doi: 10.1152/japplphysiol.00376.2006. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, de Castro D, Llewellyn-Smith I, Lipski J, Voss MD. Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci. 1990;10:1091–1098. doi: 10.1523/JNEUROSCI.10-04-01091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson JW, Halliday GM, McAllen RM, Coleman MJ, Dampney RA. Rostrocaudal differences in morphology and neurotransmitter content of cells in the subretrofacial vasomotor nucleus. J Auton Nerv Syst. 1992;38:117–137. doi: 10.1016/0165-1838(92)90232-6. [DOI] [PubMed] [Google Scholar]
- Taber E, Brodal A, Walberg F. The raphe nuclei of the brain stem in the cat. I Normal topography and cytoarchitecture and general discussion. J Comp Neurol. 1960;114:161–187. doi: 10.1002/cne.901140205. [DOI] [PubMed] [Google Scholar]
- Tai Q, Palazzolo KL, Goshgarian HG. Synaptic plasticity of 5-hydroxytryptamine-immunoreactive terminals in the phrenic nucleus following spinal cord injury: A quantitative electron microscopic analysis. J Comp Neurol. 1997;386:613–624. [PubMed] [Google Scholar]
- Taylor NC, Li A, Green A, Kinney HC, Nattie EE. Chronic fluoxetine microdialysis into the medullary raphe nuclei of the rat, but not systemic administration, increases the ventilatory response to CO2. J Appl Physiol. 2004;97:1763–1773. doi: 10.1152/japplphysiol.00496.2004. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Ventilatory effects of muscimol microdialysis into the rostral medullary raphe region of conscious rats. Respir Physiol Neurobiol. 2006;153:203–216. doi: 10.1016/j.resp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ugolini G. Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J Comp Neurol. 1995;356:457–480. doi: 10.1002/cne.903560312. [DOI] [PubMed] [Google Scholar]
- Ugolini G. Use of rabies virus as a transneuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesis. Dev Biol (Basel) 2008;131:493–506. [PubMed] [Google Scholar]
- Verner TA, Goodchild AK, Pilowsky PM. A mapping study of cardiorespiratory responses to chemical stimulation of the midline medulla oblongata in ventilated and freely breathing rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R411–421. doi: 10.1152/ajpregu.00019.2004. [DOI] [PubMed] [Google Scholar]
- Verner TA, Pilowsky PM, Goodchild AK. Retrograde projections to a discrete apneic site in the midline medulla oblongata of the rat. Brain Res. 2008;1208:128–136. doi: 10.1016/j.brainres.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Voss MD, De Castro D, Lipski J, Pilowsky PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol. 1990;295:208–218. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]
- Waldrop TG, Eldridge FL, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, Section 12, Exercise: Regulation and Integration of Multiple Systems. Oxford University Press; New York: 1996. [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. Effects of serotonin on crossed phrenic nerve activity in cervical spinal cord hemisected rats. Exp Neurol. 1999;160:446–453. doi: 10.1006/exnr.1999.7213. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol. 2000;89:1528–1536. doi: 10.1152/jappl.2000.89.4.1528. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J Spinal Cord Med. 2006;29:147–155. doi: 10.1080/10790268.2006.11753868. [DOI] [PMC free article] [PubMed] [Google Scholar]