Abstract
Typically developing children require years of overt training and practice to learn to read with skill. The relatively recent advent of functional neuroimaging methods amenable to the study of children has provided insight into the neurobiological underpinnings of skilled reading development. In this brief review, we discuss how neuroimaging during reading-related tasks has revealed that, when adult and child skilled readers perform identical reading-related tasks with comparable levels of performance, these groups show similar, but nonidentical patterns of regional brain activity. Children activate some neural regions that adults do not activate (or activate less), and vice versa. The activity patterns in these regions transition to mature levels with increased proficiency and maturity. The dynamic nature of the reading brain as the child matures is thought to be a demonstration of both the inherent flexibility and the increasing efficiency of brain processing over development.
Keywords: development, reading, phonology, children, fMRI
Once you learn to read, you will be forever free.
—Frederick Douglass (1818–1895)
Most people reading this sentence will have some memories of their early reading experiences. With training and practice, the jumble of lines and squiggles eventually resolved into something meaningful and you, as a young reader, accomplished a remarkable task—pairing the abstract symbols of just a few dozen letters with the power of language. Although the human brain has evolved to speak and to understand the speech of others, only very recently in the history of our species has it been necessary for a person to be literate. Thus, reading, per se, is quite unlikely to have been a major target of natural selection. Interpreting language in a graphic form, then, requires the “borrowing” of brain systems that evolved for other purposes (e.g., visual object recognition mechanisms, aural or oral language, social cognition, etc.), and leads to much less “natural” acquisition of reading compared to spoken language (Schlaggar & McCandliss, 2007). Thus, it is all the more impressive that the majority of children who receive adequate instruction learn to read fluently.
However, despite the tremendous importance placed on reading ability in developed countries, the reading disability (e.g., dyslexia) rate remains intolerably high. Disorders of reading development are estimated to occur in 5 to 17% of the American population (Stanovich, 1986). Because reading performance predicts success in school and later life, early recognition of and rational intervention for reading disorders deserve high priority for clinical, social, and economic reasons (Treiman, 1998).
Skilled reading is achieved through the integration of many fundamental and interrelated types of cognitive processes, manifest as phonological awareness (knowledge of or insight into the sound structure of spoken language), orthographic awareness (knowledge of or insight into the composition of the written form of language), and graphotactic awareness (knowledge of or insight into the rules of the written form), as well as others. In a typical population of skilled adult readers, the convergence of this set of cognitive processes supports efficient reading, but this convergence also makes it difficult to dissociate these processes. By studying the neural mechanisms underlying reading skill development in children, there is increased chance of investigating the neurobiological underpinnings of these capacities at different developmental stages, thus potentially separating their effects. Studying skilled reading as it emerges also gives scientists the best chance to directly compare successful and unsuccessful young readers as they are identified and to test strategies to overcome reading difficulties.
Developmental cognitive psychologists and neuroscientists have studied reading using a variety of tools including behavioral methods (reaction times, eye movements, accuracy), electrophysiological methods such as event-related potentials, magnetoencephalography, and functional neuroimaging methods, such as positron emission tomography, and functional magnetic resonance imaging (fMRI). It is important to note that most neuroimaging approaches to reading study components of reading (such as naming letters, matching letter strings, word naming, etc.) or reading-related tasks (such as verb generation or rhyme judgment) rather than the connected text experience that is fundamental to fluent reading. Nonetheless, from these various approaches across laboratories, a number of consistent and interesting observations emerge.
One of the contributions that research in the developmental neuroscience of reading has made to our understanding of reading is the finding that the brain, as it learns to read, is flexible and dynamic (for review, see Schlaggar & McCandliss, 2007). Although many brain regions are called upon uniformly across development, there are some brain regions that have increasing activity over age and others that show decreasing activity over age, even when comparing adults and school-age children performing a particular task at a given level of accuracy and speed. Finding both increases and decreases in activity suggests that no single abiding mechanism (like pruning of developmentally exuberant brain connections, or increasing the speed of neurotransmission via myelination of the brain’s connections) can account for the developmental emergence of skilled reading (Brown et al., 2005).
We will address first the implications of developmental increases and next developmental decreases in functional activity during reading-related tasks.
DEVELOPMENTAL INCREASES IN FRONTAL AND PARIETAL CORTEX ACTIVITY LIKELY REFLECT INCREASING TOP-DOWN CONTROL
Regions that have increasing activity over age while performing a particular skill could reflect the mature organization required to perform that skill, an increased ability to perform that skill, or both. Several studies have found regions that show increased activity with development for attention-demanding reading-related tasks (e.g., Bitan et al., 2007; Brown et al., 2005; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003).
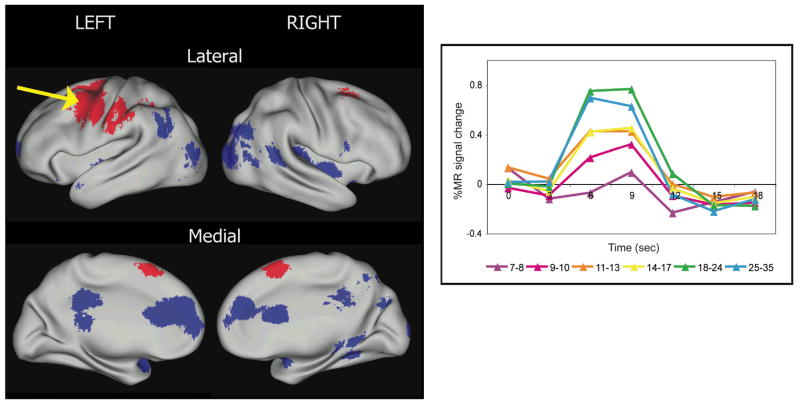
For example, we (Brown et al., 2005) studied lexical tasks such as opposite, rhyme, and verb generation in 95 healthy people ages 7 to 35 years using fMRI. Analyses using fMRI study changes in the blood oxygenation level-dependent (BOLD) signal in different parts of the brain over time, and can compare these changes across groups or tasks. Regions with developmentally increased and decreased activity were identified (Fig. 1). Activity in lateral frontal and parietal cortical regions increased over age, whereas activity in medial frontal, occipital and temporal cortical regions decreased over age. These age-related changes hold even when performance on the task is similar between children and adults, suggesting that the increased activity observed in adults in frontal regions is a maturational feature not due to adults performing more accurately or rapidly than children. The frontal and parietal regions with activity patterns that “grew up” to be largest in adults overlap substantially with regions hypothesized to be important in attention and top-down control over one’s thoughts and actions (e.g., Corbetta & Shulman, 2002; Dosenbach et al., 2006; Posner & Petersen, 1990). Hence, an interesting implication of these findings is that age-related increases in activity in these regions reflects age-related increases in top-down control, affording greater ability to focus on the task at hand and to read efficiently: With efficient reading comes increased opportunity to contemplate the content. These results also support the intuitive idea that with age comes greater access to metalinguistic abilities—that is, awareness of a language’s structure, variations, and cultural context and of one’s own use of that language.
Fig. 1.
Regions that are significantly different over age across reading-related tasks (opposite generation, verb generation, and rhyme generation in auditory and visual modalities; adapted from Brown et al., 2005). Regions where activity increases over age are shown in red, whereas regions where activity decreases over age are shown in blue. The lateral surfaces of the brain images (top row) face outward, such that the posterior aspects of the brain hemispheres are closest together. The medial surfaces of the brain images (bottom row) are rotated from the lateral view, such that the anterior aspects of the brain hemispheres are closest together. The blood-oxygen level-dependent (BOLD) signal can be depicted by graphing percent signal change over time (see graph). At right, the time course for changes in BOLD signal after neural activity typically shows a peak at around 6 to 9 s and resolution at around 20 s. The average BOLD signal time courses in six incremental age bins for 81 people are shown for an example region where activity in reading-related tasks increases over age in frontal cortex (arrow). Brain activations for all figures are displayed using CARET software (Van Essen, 2005).
DEVELOPMENTAL DECREASES IN TEMPORAL-PARIETAL CORTEX ACTIVITY LIKELY REFLECT DECREASING RELIANCE ON PHONOLOGICAL MECHANISMS
As mentioned previously, although studies of the functional neuroanatomy of reading-related tasks show that activity in some brain regions increases over development, largely independent of performance, there are also many neural regions, particularly in the posterior of the brain, that demonstrate decreased activity over age. One helpful hypothesis about why these decreases might occur has been proposed by Pugh and colleagues (Pugh et al., 2001). These authors suggest that, when children learn to read, they rely first and foremost on phonological processing mechanisms in the left temporal-parietal cortex, and these mechanisms serve to train up orthographic processing mechanisms in the left ventral occipital-temporal cortex. After the ventral occipital-temporal system is trained, processing there becomes more efficient and reliance on phonological mechanisms declines for frequently encountered words, and more direct access to lexical (word) or semantic (meaning) processing may increase.
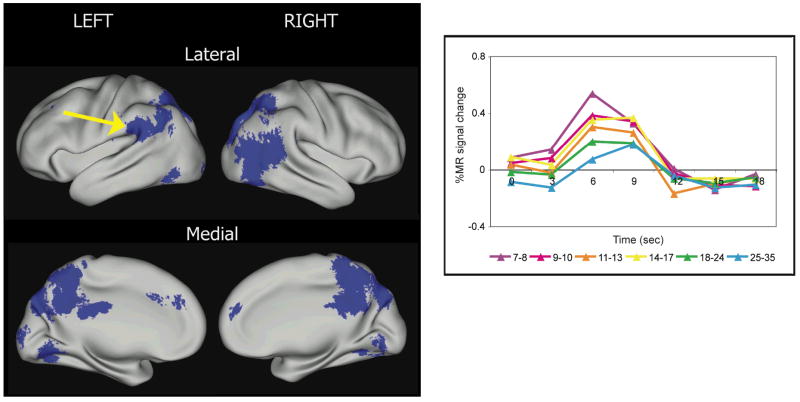
In a related fMRI study, we (Church, Coalson, Lugar, Petersen, & Schlaggar, 2008) compared 25 children (age range = 7–10 years) and 25 adults (age range = 18–32 years), who performed similarly when asked to read single high-frequency words aloud and to repeat aurally presented high-frequency words. In support of the hypothesis of Pugh et al. (2001), we found that children show significantly greater activity in the temporal-parietal regions than adults during reading (Fig. 2), suggesting that children rely more on phonological processing mechanisms than do adults.
Fig. 2.
Regions that are significantly different over age for high-frequency single word reading and auditory repeating tasks (adapted from Church, Coalson, Lugar, Petersen, & Schlaggar, 2008). All regions decreased in activity over age and are shown in blue. The brain images are organized in the same manner as described in Figure 1. At right, the time courses of blood-oxygen level-dependent signal change for the reading task in six incremental age bins for 81 people are shown on a graph for a left parietal region where activity decreases over age (arrow). This region is in a location hypothesized to be important in phonological processing, and the higher level of activity in children suggests that adults have a relatively decreased reliance on phonological processing when reading aloud high-frequency words.
Interestingly, in the same study, we also observed significant activation in bilateral occipital-temporal cortex in children for the repeat task (“say aloud the word you hear”) in addition to the reading task. In adults, by contrast, we observed no activation in these regions for the repeating task. From this result, it appears that, over development, the bilateral occipital-temporal cortex transitions from being generally utilized for both auditory and visual lexical processing tasks in children to being more specialized for visual than for auditory processing in adults. This transition is in keeping with the notion that ventral occipital-temporal cortex becomes gradually specialized for reading.
As indicated above, the Pugh et al. (2001) model has had a strong influence on the reading development field, providing a framework for understanding how reading impairments could occur. Reading difficulties may be the result of initial inadequacies in phonological processing mechanisms. Inappropriately low activity in phonological processing regions in children at risk for dyslexia may ultimately lead to an orthographic processing system inadequately tuned for reading resulting in a dyslexic phenotype. Further support for this model comes from a large-scale longitudinal fMRI study of children with reading disabilities, which showed that these children do not show appropriate levels of activation of either the phonological or the orthographic processing systems, compared to their age-matched peers (Shaywitz et al., 2002; Shaywitz, Mody, & Shaywitz, 2006), but that this activation profile “improves” with phonologically based remediation (Shaywitz et al., 2004).
IMPLICATIONS: DEVELOPMENT AS A TOOL
One potentially powerful application of information derived from the developmental neuroscience of reading is for the investigation of the neuroscience of adult reading. Developmental phenomena observed in a particular set of regions, for example, can help to frame specific questions about those brain regions in adults. As a case in point, recall the earlier-mentioned finding that there is decreased activity observed in adults compared to children in a left temporal-parietal region for reading aloud high-frequency words (Fig. 2). This result was interpreted to indicate that this decrease in activity comes about because of the decreasing reliance on phonological mechanisms for high-frequency words that comes with reading skill and age. If this interpretation is correct, then, in principle, the “child-like” level of activity should be able to be resurrected in adults when they are presented with more phonologically challenging stimuli in the same task structure. Two factors that have been shown to increase the phonological demand of visually presented words are length (i.e., serial length of words measured as number of letters) and familiarity (i.e., a measure of usage).
Therefore, in an fMRI experiment, we (Church, Petersen, & Schlaggar, 2006) examined two levels of familiarity (low-frequency words and pronounceable nonwords) and two levels of length (one-syllable, four- to six-letter stimuli, and three-syllable, seven- to nine-letter stimuli) in 24 healthy adults (age range = 21–30 years). Low-frequency words and nonwords produced stronger activation in the left temporal-parietal region than did high-frequency words. Most importantly, the length of low-frequency and nonwords modulated the activity increase with longer strings leading to greater activity. In other words, manipulation of stimulus features produced a child-like level of activity in a region previously shown to be developmentally transient in its involvement in reading aloud of single words (Church et al., 2006). This preliminary result lends further support to the idea that this particular region in the left temporal-parietal cortex region is functioning as a phonological processor.
More generally, the results of this manipulation suggest that regions that have the appearance of developmental transience for a particular reading-related function are not necessarily uninvolved in these processes in adults; the brain remains flexible, calling on apparently more developmentally significant regions in order to deal with more challenging stimuli.
FUTURE DIRECTIONS
Investigations of reading-remediation approaches that use neuroimaging as an evaluation tool are starting to be conducted (reviewed in Schlaggar & McCandliss, 2007). As discussed earlier, it is clear that neuroimaging methods can help to test specific hypotheses (e.g., phonological deficit) regarding the origins and development of dyslexic or impaired reading (Shaywitz et al., 2004, 2006). This pioneering work by Shaywitz, Shaywitz, and their colleagues demonstrates how characterizing the typical developmental context for brain regions involved in reading can contribute substantially to understanding the neurobiology underpinning improvements in response to remediation.
Recent work comparing alphabetic languages (like English) to nonalphabetic languages (like Chinese) has been very instructive regarding culturally determined neural manifestations of dyslexia (Siok, Niu, Jin, Perfetti, & Tan, 2008). For example, children with impaired reading in Chinese, a logographic-based language, have both structural and functional brain differences in the left middle frontal gyrus. Importantly, they do not appear to have the fMRI differences noted in the posterior brain regions in impaired readers of alphabetic languages. One of the most intriguing observations from this work is that, in Chinese readers, orthographic awareness appears to be much more salient than phonological awareness for the acquisition of skilled reading (Tan, Spinks, Eden, Perfetti, & Siok, 2005). Recognizing and exploring the consequences of cultural differences in reading impairment across languages (or across writing systems) creates opportunities for broader considerations of approaches to treatment.
In addition, research is starting to move beyond the regional level of analysis to looking at reading networks at a more brain-wide level. These techniques, generally called functional or effective connectivity, should prove to be a robust method for revealing how the functional relationships between regions change over typical development and in the presence of impaired reading development. Functional connectivity, in all its forms, has been applied to studies of typical and atypical reading for close to a decade. Critical insights have come from these studies. For example, Horwitz, Rumsey, and Donohue (1998) found that correlations in blood flow between the angular gyrus (part of the putative phonological processing system) and other brain regions were decreased in dyslexics. More recently, Hampson et al. (2006) demonstrated that an individual’s reading ability could be correlated to the strength of the functional connectivity between Broca’s area and the left angular gyrus during reading. These studies demonstrate the precedent for using functional connectivity to investigate the neural systems for reading.
The resting-state version of functional connectivity MRI examines regional correlations in low-frequency BOLD signal while a participant is resting quietly, not performing any specific task. This technique has the potential benefit of being taken into younger, preliterate children who are typically difficult to scan, or other instances where task performance is complicated with confounding factors. Observation of the correlations between reading-related brain regions over the whole reading skill progression (preliterate, early literacy, and skilled reading), over the ages that typically span this progression in literate cultures, or both will be a powerful way to explore the dynamic nature of the brain’s involvement in reading.
Recommended Readings
Pugh, K.R. et al. (2001). (See References). A functional neuroanatomy-based model for understanding the development of reading.
Wolf, M. (2007). Proust and the Squid: The story and science of the reading brain. New York, NY: HarperCollins. A clearly written, user-friendly book summarizing what is currently known about reading and the brain.
Brown, T.T. et al. (2005). (See References). An fMRI study of lexical processing tasks from ages 7 to 32 years, examining similarities and differences over age and performance.
Schlaggar, B.L., & McCandliss, B.D. (2007). (See References). A detailed, comprehensive review of many aspects of reading development and the challenges faced in that research field.
Acknowledgments
This work was supported in part by a National Institutes of Health (NIH) Neurological Sciences Academic Development Award (to B.L.S.), NIH Grant NS053425 (to B.L.S.), The McDonnell Center for Higher Brain Function (B.L.S.), The Olin Fellowship Program (J.A.C.), and The Markey Pathway in Human Pathobiology (J.A.C.). We thank Steve Petersen and Alecia Vogel for helpful comments.
References
- Bitan T, Cheon J, Lu D, Burman DD, Gitelman DR, Mesulam MM, Booth JR. Developmental changes in activation and effective connectivity in phonological processing. NeuroImage. 2007;38:564–575. doi: 10.1016/j.neuroimage.2007.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cerebral Cortex. 2008;18:2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Petersen SE, Schlaggar BL. Regions showing developmental effects in reading studies show length and lexicality effects in adults. Poster presented at the annual meeting of the Society for Neuroscience; Atlanta, GA. 2006. Nov, [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. NeuroImage. 2006;31:513–519. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy of Sciences, USA. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annual Review of Neuroscience. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, et al. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biological Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Mody M, Shaywitz BA. Neural mechanisms in dyslexia. Current Directions in Psychological Science. 2006;15:278–281. [Google Scholar]
- Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH. A structural-functional basis for dyslexia in the cortex of Chinese readers. Proceedings of the National Academy of Sciences, USA. 2008;105(14):5561–5566. doi: 10.1073/pnas.0801750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanovich KE. Matthew effects in reading: Some consequences of individual differences in the acquisition of literacy. Reading Research Quarterly. 1986;21:360–406. [Google Scholar]
- Tan LH, Spinks JA, Eden GF, Perfetti CA, Siok WT. Reading depends on writing, in Chinese. Proceedings of the National Academy Sciences, USA. 2005;102:8781–8785. doi: 10.1073/pnas.0503523102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiman R. Why spelling? The benefits of incorporating spelling into beginning reading instruction. In: Metsala JL, Ehri LC, editors. Word recognition in beginning literacy. Mahwah, NJ: Erlbaum; 1998. pp. 289–313. [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]