Abstract
Current studies in our laboratory demonstrate a functional link between vesicles, vacuoles and aflatoxin biosynthesis in the filamentous fungus, Aspergillus parasiticus. Under aflatoxin inducing conditions in liquid yeast-extract sucrose medium, A. parasiticus undergoes a shift from vacuole biogenesis to accumulation of an enhanced number of vesicles which exhibit significant heterogeneity in size and density. As a first step in conducting a detailed analysis of the role of these organelles in aflatoxin synthesis, we developed a novel method to purify the vesicle and vacuole fraction using protoplasts prepared from cells harvested during aflatoxin synthesis. The method includes the following steps: 1] preparation of protoplasts from mycelia grown for 36h under aflatoxin inducing conditions; 2] release of vesicles and vacuoles from purified protoplasts in the presence of Triton X-100; and 3] fractionation of the vesicles and vacuoles using a “one-step high density cushion”. The vesicle-vacuole fraction showed a 35 fold enrichment in alpha-mannosidase activity (vacuole marker) and non-detectable succinate dehydrogenase and lactate dehydrogenase activities (mitochondrial and cytoplasmic markers, respectively). Confocal laser scanning microscopy with the vacuole dyes MDY-64 and CMAC demonstrated that the fraction contained pure vesicles and vacuoles and was devoid of membranous debris. Transmission electron microscopy (TEM) confirmed that no mitochondria or unbroken protoplasts contaminated the purified fraction. The purified organelles exhibited significant size heterogeneity with a range of sizes similar to that observed in whole cells and protoplasts.
Keywords: Protoplast, vacuole, vesicle, high density sucrose cushion, Aspergillus parasiticus
1. Introduction
Aspergillus parasiticus is one of a small number of filamentous fungi that synthesize the secondary metabolite, aflatoxin. Aflatoxins are among the most toxic and carcinogenic natural compounds known (Squire, 1981) and, therefore, have been studied extensively. To synthesize aflatoxin, the fungus must orchestrate the regulation, both at the molecular and cellular level, of at least 17 enzyme activities encoded by up to 27 individual genes; these genes are clustered within a 70 kb region in the Aspergillus genome (Yabe & Nakajima, 2004; Yu et al., 2004; Roze et al., 2007). Our previous studies demonstrated the localization of aflatoxin enzymes and aflatoxin to membrane bound organelles. We observed that at 24 to 48 h of growth, OmtA, a late enzyme in the aflatoxin biosynthetic pathway, localized in the cytoplasm together with the early and middle pathway enzymes, Nor1 and Ver1. However, in cells on the substrate surface of the colony observed at this same time period (24-48h), OmtA was predominantly detected in spherical organelles that we proposed were vacuoles (Lee et al., 2004). OrdA, the final enzyme in the pathway, also was thought to localize to vacuoles where it catalyzed the final step of biosynthesis (Lee et al., 2004). Using an alternate approach, we demonstrated that during aflatoxin synthesis, EGFP-tagged Nor-1 and Ver-1 localized to organelles of different sizes; we identified these as vesicles and vacuoles (Hong, 2008).
We recently observed the association of a high vesicle number phenotype with aflatoxin synthesis in A. parasiticus (Chanda et al in preparation). First, we observed a significant rise in the number of vesicles in aflatoxin inducing medium (YES) as compared with aflatoxin non-inducing medium (YEP). Second, treatment with Sortin3, a compound affecting protein trafficking to vacuoles, resulted in an increase in vesicles (fragmented vacuoles morphology) and elevation in aflatoxin enzymes and aflatoxin accumulation. Third, disruption of the gene vb1, a homolog of avaA in A. nidulans, also resulted also in an increase in vesicles, aflatoxin enzymes and aflatoxin accumulation. Based on these observations we hypothesized that vesicles and vacuoles are functionally linked with aflatoxin biosynthesis. As a first step in conducting a detailed analysis of the role of these organelles in aflatoxin biosynthesis, we developed a method to purify vesicles and vacuoles from A. parasiticus during peak levels of aflatoxin biosynthesis (36h of growth in aflatoxin inducing YES medium).
Isolation of a vacuole fraction has been reported for several filamentous fungi, (Forster et al., 1998; Hoppert et al., 2001; Lendenfeld et al., 1993; Martinoia et al., 1979 Vaughn & Davis, 1981), yeast (Rieder & Emr, 2001;; Walworth et al., 1989; Wiemken, 1975), and plants (Bethke et al., 1996; Boudet et al., 1981; Mathieu et al., 1989; Robert et al., 2007; Shimaoka et al., 2004). In general, these vacuole isolation methods involve multistep floatation gradient-, density gradient- or differential centrifugation. However the high degree of size and density heterogeneity in the vesicle and vacuole population in A. parasiticus during aflatoxin production prevented us from directly applying published procedures to obtain a purified vesicle and vacuole fraction.
We report here a novel ‘one-step high density sucrose cushion’ method for purification of the highly heterogeneous vesicle and vacuole fraction from A. parasiticus grown in aflatoxin inducing medium during a transition from exponential to stationary growth; this time frame corresponds to a shift from primary to secondary metabolism (aflatoxin synthesis). The purity of the fraction was confirmed by marker enzyme activity assay, transmission electron microscopy (TEM) and confocal laser scanning microscopy (CLSM). Throughout this work, we defined the size range of vesicles and vacuoles as ≥2.5μm and <2.5μm respectively (Figure 1). The procedure reported here is rapid, easy, and generates a highly pure fraction consisting of vesicles and vacuoles. Functional analysis of this purified fraction has shown the presence of three aflatoxin enzymes (Ver-1, Vbs and OmtA) by Western blot and has functionally linked the vesicle-vacuole fraction with aflatoxin biosynthesis by demonstrating the compartmentalization of the final two steps of the synthesis in this fraction (Chanda et al., in preparation). In follow-up studies, this method will also allow us to conduct detailed ultra-structural, biochemical, and functional analyses of vesicles and vacuoles in aflatoxin producing A. parasiticus.
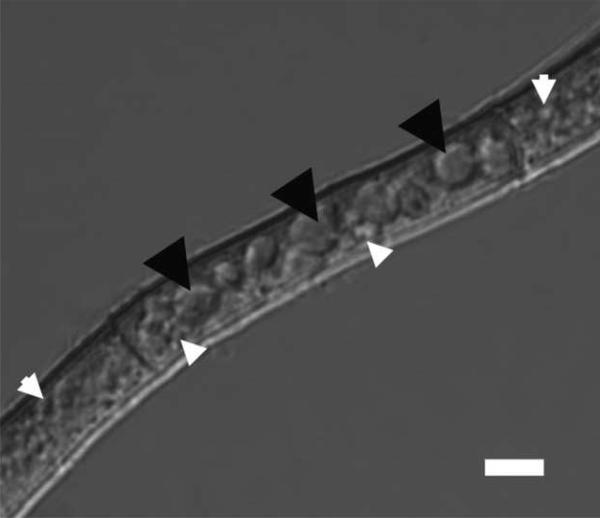
Figure 1. Differential interference contrast image of an A. parasiticus mycelial fragment showing vesicles and vacuoles.
Membrane-bound organelles <2.5 μm in diameter (white arrowheads) were designated vesicles; organelles ≥2.5μm (black arrowheads) were designated vacuoles. Size bar = 5μm
2. Materials and methods
2.1 Strains, media and growth conditions
A. parasiticus strain SU-1 (ATCC 56775), a wild-type aflatoxin producer, was used in this study. Conidiospores (spores) from a frozen spore stock of A. parasiticus SU-1 were inoculated into YES liquid medium [containing 2% yeast extract and 6% sucrose; pH 5.8] at 104 spores per ml and incubated at 30°C with shaking at 150 rpm for 36 h. Western blot analysis demonstrated accumulation of aflatoxin enzymes beginning at 30 h under these growth conditions (Roze et al., 2007)
2.2 Preparation of protoplasts from mycelia
Protoplasts were prepared by a published method (Birch et al., 2001). Mycelia grown for 36h were harvested by filtration through miracloth [Calbiochem]. The miracloth carrying the mycelia was placed on paper-towels for 30 min to absorb the remaining medium. The mycelia were resuspended [40mg of mycelia per mL] in lysis buffer [10 mM potassium phosphate, pH 5.8] containing 1.2 M MgSO4 as osmotic stabilizer and 50mg/mL of driselase and lysing enzymes [both from Sigma, St.Louis, St. Louis, MO]. The suspension was incubated for 7.5 h at 30°C with shaking at 100 rpm. The protoplasts were separated from debris by filtration through a double layer of cheese cloth and then nylon mesh (30μm mesh, 64μm thickness) [Spectrumlabs, Rancho Dominguez, CA]. 25mL of filtrate was overlaid with 25mL of separation buffer [0.6 M sorbitol, 100 mM Tris-Cl, pH 7.0] in a 50mL conical tube and centrifuged at 1500 × g at room temperature (RT) for 15 min. Protoplasts were collected from the interface in a total volume of 5mL, mixed with an equal volume of protobuffer [1.2 M sorbitol, 10 mM Tris-Cl, pH 7.5], and centrifuged at 1000×g for 10 min (RT) to obtain a protoplast pellet. This pellet was washed with protobuffer and centrifuged at 1000×g for 10 min at 4°C. This final the pellet was resuspended in 0.5mL of protobuffer. The purity of the protoplast fraction was analyzed by bright field microscopy using a Nikon Eclipse E600 microscope [Nikon Inc., Melville, NY].
2.3 Purification of a vesicle-vacuole fraction
The protoplast suspension (0.5mL) was added to 1.5mL of protoplast lysis solution [0.6M sorbitol, 10mM Tris-Cl, 0.025% Triton-X 100 pH 7.5]. Release of vesicles and vacuoles was observed using MDY-64 (Molecular Probes, Invitrogen, Carlsbad, CA) that stains yeast vacuole membranes (green); cells were viewed under a Nikon Eclipse E600 or Labophot fluorescence microscope (Nikon Inc., Melville, NY). After approximately 15 min, when 90-95% of the protoplast were lysed, 1ml of lysis mixture was carefully overlaid on a 1mL high density sucrose cushion [3M sucrose, 1.2M sorbitol, 10mM TrisCl, pH 7.5] and then centrifuged at 3000 × g at RT for 45 min. The vacuole-vesicle fraction was collected from the interface in a volume of 100μL and stored on ice for further analysis.
2.4 Assessment of the vesicle-vacuole fraction purity
Three parallel approaches were utilized to confirm purity of the vesicle-vacuole fraction.
2.4.1 Confocal laser scanning microscopy (CLSM)
MDY-64 and CellTracker Blue CMAC (vacuolar peptidase marker) [Molecular Probes, Invitrogen, Carlsbad, CA] were used to visualize vesicles and vacuoles and to assess their purity. These dyes were utilized according to the manufacturer's protocols with modifications. 2μL of a 10 mM MDY-64 stock solution in DMSO was added to the protoplast lysis mixture (2mL total volume) 5 min before loading on the high density sucrose cushion. 1μL of 10 mM CMAC in DMSO was added to the purified vesicle-vacuole fraction and incubated at RT for 15 min before microscopy. Images were acquired using an Olympus FluoView 1000 confocal laser scanning microscope (CLSM) (Olympus, Center Valley, PA) using a 60x/1.42 oil objective and BP 430-470 emission filter set under excitation with the 405 nm diode laser line for CMAC fluorescence (353 nm excitation/466 nm emission) and BP 505-525 emission filter set under excitation with the 488 nm diode laser line for MDY-64 fluorescence (451 nm excitation/ 497 nm emission).
2.4.2. Transmission Electron Microscopy (TEM)
The purity of the vesicle-vacuole fraction also was assessed by TEM. Vacuole-vesicle samples were prepared by first fixing with 1% osmium tetroxide for 1 h at RT. A drop of the solution was then placed on a copper grid and the excess solution was removed by filter paper. The grid was air-dried and stained with 1% uranyl acetate (for negative staining). Images were generated with a JEOL 100CX transmission electron microscope.
2.4.3. Enzyme assays
The activities of specific marker enzymes were examined in the vesicle-vacuole fraction. The α-mannosidase (AMS – a marker tightly associated with vesicles and vacuoles) activity was measured by a method adopted from a published protocol (Boller & Kende, 1979). 10μL of the sample [total protein range from 3μg to 10μg] was added to a reaction mix containing 0.5mL of sodium succinate buffer [50μM Na-succinate, pH 5.0]; then, 3μL of 0.1M p-nitrophenyl substrate [Sigma, St.Louis, MO] was added. The reaction was stopped by addition of 0.8mL of 1M sodium carbonate. The absorbance was determined at 405nm. The specific activity was expressed in nmoles of p-nitrophenol produced per min per μg total protein. Succinate dehydrogenase activity (mitochondrial marker) was assessed according to a standard protocol (Berg, 1995; Bergman, 1990; Reuter, 1995). 10μL of the sample solution [total protein range from 3μg to 10μg] was added to a reaction mixture consisting of 0.3M potassium phosphate, 8.5mM potassium cyanide and 50μg of DCPIP [dichlorophenolindophenol, Na salt]. 0.1mL of sodium succinate was then added to this reaction mixture and the absorbance was recorded at 600nm. Enzyme specific activity was expressed in μmoles DCPIP reduced per minute per μg total protein. Lactate dehydrogenase activity (cytoplasmic marker) was measured according to a standard protocol (Kuznetsov, 2006). 10μL of the sample was added to 1mL of pre-incubated reaction medium (at 30°C) containing 100mM Tris-HCl buffer, 10mM of pyruvate and 0.3mM NADH and the absorbance was measured at 340nm. Enzyme specific activity was expressed in μmoles of lactate formed per min per μg total protein.
3. Results
3.1. Protoplast isolation
Protoplast preparation from mycelia harvested during active aflatoxin production (during a transition from exponential to stationary growth phase) is challenging as aging negatively affects cell wall digestion. Our previous studies showed that aflatoxin gene transcripts and enzymes (Nor-1, Ver-1, VbsA and OmtA) were first detected between 24 and 40 h of growth (Roze et al., 2007). Aflatoxin enzymes and transcripts reached peak levels at 48 h and then declined. We observed that 36 h was an optimum time point because we could detect aflatoxin enzymes and aflatoxin in the mycelia and still digest the cell wall to produce protoplasts. Novozyme 234 [Novozyme Corp], an enzyme routinely used for protoplasting (Bradshaw, 2006; Liang, 1996; Zhou, 1997) is no longer commercially available. We empirically determined that a 1:1 mixture of lysing enzymes and driselase generates protoplasts as efficiently as Novozyme 234 (unpublished data). The protoplasts were isolated using a single step floatation gradient. Upon centrifugation, protoplasts floated to the interface and formed a band which was collected, washed, pelleted, and resuspended in protobuffer. The purity of the protoplast fraction was assessed under bright field microscopy and showed no detectable contamination by mycelial debris (Figure 2). Approximately 106 protoplasts were purified from 4gm of mycelia by this method.
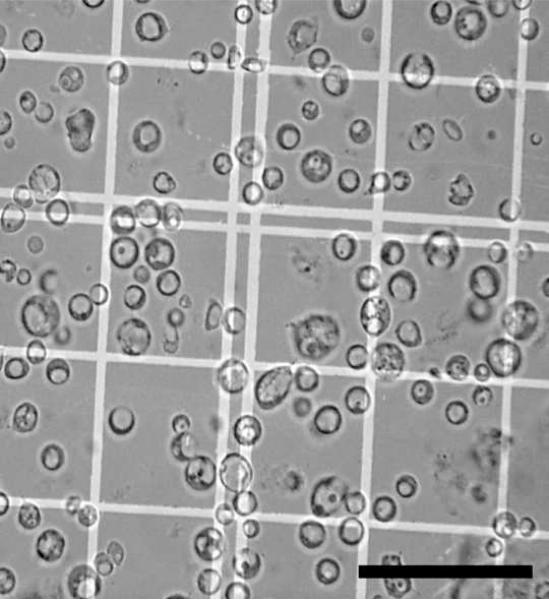
Figure 2. Bright field image of a purified protoplast fraction.
A. parasiticus was grown for 36 h under aflatoxin inducing conditions and protoplasts prepared and purified as described in Methods. 10μL of the pure protoplast fraction were dispensed into a hemacytometer and observed by bright field microscopy to estimate purity and total number. Size bar = 50μm.
3.2. Purification of the vesicle-vacuole fraction
Vesicles and vacuoles were released from protoplasts soon after addition of Triton X-100 to the protoplast suspension (Movie 1). We monitored release during the first 30 minutes after Triton X-100 addition in the presence of MDY-64 [data not shown]. Within 15 min, most protoplasts (> 90%) released vesicles and vacuoles. Storing the reaction for longer periods of time negatively affected the stability of the vesicles and vacuoles; after 30 min few vesicles and vacuoles could be observed
To fractionate vesicles and vacuoles, we layered the lysis mixture on top of a high density (3M) sucrose cushion. Centrifugation (3000×g, 45 min) caused the vacuole-vesicle population to migrate down to the sucrose cushion forming a thin band at the interface; the remaining cell debris banded either within the cushion (small protoplast fragments) or pelleted at the bottom of the cushion (large protoplast fragments). Experimental conditions were optimized empirically. This procedure (Figure 3) routinely yielded sufficient vesicles and vacuoles to generate 30 ug of protein from 4 grams of initial mycelia (wet weight).
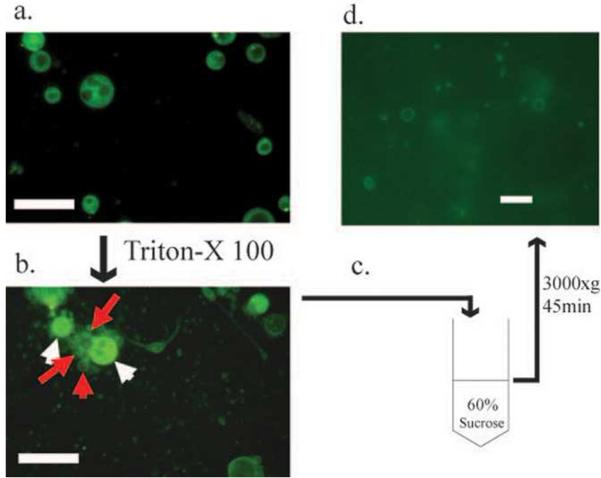
Figure 3. Purification of a vesicle-vacuole fraction.
a. Pure protoplasts treated with the vacuole membrane stain MDY-64. b. Release of vesicles and vacuoles (red arrows) after treatment of protoplasts (white arrows) with Triton X-100. c. High density sucrose cushion for separation of vesicle-vacuole fraction. d. Pure vesicle-vacuole fraction after isolation from sucrose cushion illustrated in panel C. Bars in a) and b), 25μm; bar in d), 10μm.
3.4. Fraction purity
Three methods were employed to test the purity of the vesicle-vacuole fraction. Alpha-mannosidase has been used frequently as a vacuole marker enzyme in plants (Boller & Kende, 1979), filamentous fungi (Hoppert et al., 2001; Lendenfeld et al., 1993) and yeast (Yoshihisa et al., 1988) and also has been reported to co-purify with vesicles (Campbell & Rome, 1983). In Aspergillus oryzae, alpha mannosidase localized to intracellular vesicles (Akao et al., 2006) and in yeast, the “cytoplasm-to-vacuole targeting pathway (CVT)” is reported to transport the enzyme to vacuole membranes (Hutchins & Klionsky, 2001). We therefore measured α-mannosidase specific activity in the vesicle-vacuole fraction and compared it with specific activity values in protoplasts and in whole-cells as one measure of purity.
Electron microscopic analysis of protoplasts frequently demonstrates the presence of multiple mitochondria. We reasoned that analysis of succinate dehydrogenase activity, a mitochondrial marker enzyme (Pycock & Nahorski, 1971) would provide an independent measure of this contaminant in the vesicle-vacuole fraction.
We also measured activity of lactate dehydrogenase, a cytosolic marker (Kobayashi et al., 2006); like succinate dehydrogenase, one would expect reduction or elimination of this activity during the purification process.
The data demonstrated a significant increase (30 fold) in α-mannosidase specific activity at each purification step starting from whole cells to protoplasts to vesicles and vacuoles accompanied by elimination of detectable activities of succinate dehydrogenase and lactate dehydrogenase (Figure 4 a-c).
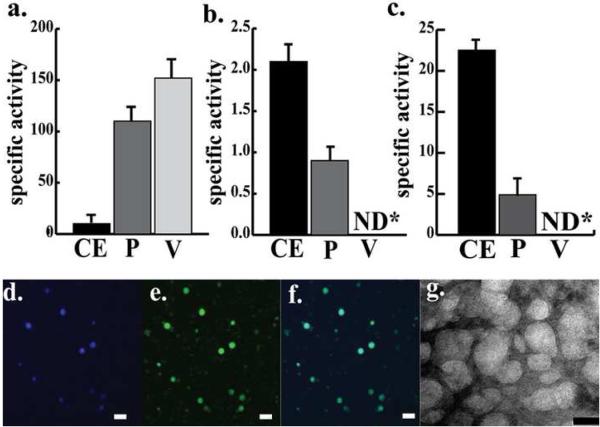
Figure 4. Analysis of purity of the vesicle-vacuole fraction.
a-c: Measurement of marker enzyme specific activities during purification of vesicles and vacuoles with protein extracts from whole cells, protoplasts and the vesicle-vacuole fraction. Protein extracts were prepared from whole cells (CE), pure protoplasts (P), and the pure vesicle-vacuole fraction (V) as described in Methods. The specific activities of alpha mannosidase (A) (vacuole marker; specific activity is expressed as nmoles of p-nitrophenol produced per min per μg of total protein), succinate dehydrogenase (B)(mitochondrial marker; specific activity is expressed as μmoles of DCPIP reduced per minute per μg of total protein and lactate dehydrogenase (C) (cytoplasmic marker; specific activity is expressed as μmoles of lactate formed in the reaction per min per μg of total protein) were measured. Data summarize three independent experiments. Specific activities are reported as the mean ± standard error. ND = not detectable. d-g: Microscopic analysis of a vesicle-vacuole fraction. The vesicle-vacuole fraction was purified and prepared for microscopic analyses as described in Methods. d-f: CLSM images of a vesicle-vacuole fraction. d: Vesicle-vacuole fraction stained with CMAC. e: Vesicle-vacuole fraction stained with MDY-64 stain. f: digital overlay of d and e. g: TEM images of the vesicle-vacuole fraction. (Bars in panels d-f = 5μm and bar in panel g = 50nm)
The second method employed to assess purity as well as to characterize the vesicle-vacuole fraction was CLSM. The vacuole membrane stain MDY-64 and the vacuole peptidase stain CMAC demonstrated great affinity for all organelles in the vesicle-vacuole preparation; these organelles were spherical in shape and were highly diverse in size (range approximately 20 to 2500 nm) (Figure 4d-f). We observed ten different fields under CLSM with at least 50 vesicles and vacuoles in each field and found no protoplast debris or unbroken protoplasts associated with that fraction.
In the third method to measure purity, we prepared the vesicle-vacuole fraction for transmission electron microscopy (TEM) (Figure 4g). The resulting images supported the CLSM data strongly suggesting that the fraction was not contaminated with mitochondria or other organelles.
4. Discussion
Initial attempts to directly apply vacuole isolation methods used in yeast [ficoll gradient] (Hutchins & Klionsky, 2001; Wiemken, 1975) and Neurospora [ficoll-sucrose-sorbitol gradient] (Martinoia et al., 1979) did not enable us to collect the vesicle- vacuole fraction in a single band. Vesicles and vacuoles with a wide range of sizes were observed along the entire length of density gradients (data not shown); these data suggested significant heterogeneity in density. The differential centrifugation method developed for Penicillium (Lendenfeld et al., 1993) also was not effective; forces below 500 g did not pellet the vesicle-vacuole fraction while forces above 500 g resulted in a loss of 70-80% of vesicles and vacuoles presumably due to breakage (data not shown). Because of the high degree of heterogeneity in density of the vesicles and vacuoles, we reasoned (correctly) that a high density sucrose (3M) cushion would effectively collect the vesicle-vacuole fraction in a single band at the interface without breaking the organelles.
In generating protoplasts for transformation, the time for cell wall digestion time is significantly less [3-5h] than the time required to achieve sufficient numbers of protoplasts for vesicle-vacuole fraction purification [7.5 h]. In transformation protocols, the cells are harvested at 16-18 h at which time the cell wall is thinner than the wall in mycelia harvested at 36 h. A significant number of empirical experiments were required to optimize the mass of mycelium, the digestion time, and the enzyme concentration. The fact that protoplasts exhibited lactate dehydrogenase, succinate dehydrogenase, and alpha-mannosidase activity and that vacuole peptidases in protoplasts acted on the CMAC stain to render blue fluorescence suggested that the protoplasts retained most if not all metabolic activity even after the long digestion period. During the protoplasting procedure, (digestion with driselase and lysing enzyme) mycelia lose their cell walls and the cytoplasmic membrane evaginates to form protoplasts. The efficacy of this method is highly dependent on fungal age, culture conditions, pH of incubation, enzymes used and osmotic stabilizers (van den Broek et al., 1979; Zigel & Kobzeva, 1980).
Microscopy and measurement of alpha-mannosidase activity suggest that our procedure enriched a fraction of protoplasts in which the majority of the volume was occupied by vesicles and vacuoles. We propose that the high volume of vesicles and vacuoles enhanced the ability of the protoplasts to migrate to the interface of the lysis mixture and to the high density sucrose cushion.
We made one interesting observation regarding the membrane morphology of vesicles and vacuoles (stained with OsO4) in protoplasts using TEM (Supplementary figure 1). Approximately 60% of the organelles of all sizes showed a lipid bilayer along the organelle margin reminiscent of the plasma membrane. In contrast, approximately 40% of the organelles of all sizes showed irregular boundaries that were not stained with OsO4 suggesting the absence of a membrane. Future studies will analyze whether the apparent absence of a membrane is a result of cellular aging (natural causes) or is an artifact of either the protoplasting or thin section preparation procedures.
We were successful in preparation of thin sections of vesicles and vacuoles in whole cells (Lee et al., 2004) and protoplasts (Supplementary Figure 1) for TEM analysis. However this method was not successful for preparation of purified vesicles and vacuoles, presumably because these organelles are quite fragile. So, we adopted a simple method for TEM analysis which involved fixation of the vesicle-vacuole fraction with OsO4, drying the sample directly on the copper grid, and viewing under TEM. TEM images presented here thus represent the view of a very concentrated suspension of vesicles and vacuoles as seen from the top and not a fraction that has been embedded and sectioned. This method did not allow us to conduct a detailed ultrastructural analysis of the vesicle-vacuole fraction, but did allow us to judge the purity and size heterogeneity in this pure fraction.
Conclusions
Direct comparison of the size range of vesicles and vacuoles in whole cells (Figure 1), protoplasts (Supplementary figure 1) and in the purified vesicle-vacuole fraction (Figure 4d-g) allow us to conclude that organelles in the purified fraction are representative of the organelles present in whole cells. Enrichment of α-mannosidase activity combined with undetectable succinate dehydrogenase and lactate dehydrogenase activity in the pure vesicle-vacuole fraction coupled with the CLSM and TEM images of the same fraction allow us to conclude that the organelles purified using the high density sucrose cushion represent a pure or nearly pure organelle fraction.
Supplementary Material
A. parasiticus was grown for 36 h under aflatoxin inducing conditions and protoplasts prepared and purified as described in Methods. Protoplast samples were fixed with 2.5% glutaraldehyde overnight at 4°C on a rotating platform shaker. Samples were post-fixed in 1% buffered osmium tetroxide overnight at 4°C, dehydrated in an acetone series (30–100%), and then infiltrated and polymerized in Poly/Bed 812 resin for 24h at 60°C. Resin blocks were sectioned using a Power Tome-XL ultramicrotome (Boeckeler Instruments, Tucson, AZ). Protoplast sections (70 nm thick) were mounted on formvar coated copper grids and stained with 1% uranyl acetate and lead citrate. Images were generated with a JEOL 100CX transmission electron microscope. a: TEM image of a protoplast. b: Magnified image of the larger inset in c. TEM image of the smaller inset in panel a. Grey arrowheads indicate lipid bilayer boundaries and black arrowheads indicate irregular boundaries. Bar in panel a = 1μm, bar in panel b = 100nm and bar in panel c = 50nm.
Release of vesicles and vacuoles from a protoplast after addition of triton X 100 (lysis) solution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akao T, Yamaguchi M, Yahara A. Cloning and expression of 1,2-alpha-mannosidase gene (fmanIB) from filamentous fungus Aspergillus oryzae: in vivo visualization of the FmanIBp-GFP fusion protein. Biosci Biotechnol Biochem. 2006;70:471–479. doi: 10.1271/bbb.70.471. other authors. [DOI] [PubMed] [Google Scholar]
- Berg V. Plant Physiology Manual. 1995 [Google Scholar]
- Bergman A. Laboratory Investigations in Cell & Molecular Biology. 3 edn Wiley, NY: 1990. [Google Scholar]
- Bethke PC, Hillmer S, Jones RL. Isolation of Intact Protein Storage Vacuoles from Barley Aleurone (Identification of Aspartic and Cysteine Proteases) Plant Physiol. 1996;110:521–529. doi: 10.1104/pp.110.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch M, Prebble E, Mosquera J, Anderson M. Protoplast preparation. 2001 [Google Scholar]
- Boller T, Kende H. Hydrolytic Enzymes in the Central Vacuole of Plant Cells. Plant Physiol. 1979;63:1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudet AM, Canut H, Alibert G. Isolation and Characterization of Vacuoles from Melilotus alba Mesophyll. Plant Physiol. 1981;68:1354–1358. doi: 10.1104/pp.68.6.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw RE. From protoplasts to gene clusters. Mycologist. 2006;20:133. [Google Scholar]
- Campbell CH, Rome LH. Coated vesicles from rat liver and calf brain contain lysosomal enzymes bound to mannose 6-phosphate receptors. J Biol Chem. 1983;258:13347–13352. [PubMed] [Google Scholar]
- Forster C, Marienfeld S, Wilhelm R, Kramer R. Organelle purification and selective permeabilisation of the plasma membrane: two different approaches to study vacuoles of the filamentous fungus Ashbya gossypii. FEMS Microbiol Lett. 1998;167:209–214. doi: 10.1111/j.1574-6968.1998.tb13230.x. [DOI] [PubMed] [Google Scholar]
- Hong S-Y. Analysis of transport and sub-cellular localization of aflatoxin biosynthetic enzymes, Ver-1 and Nor-1, using EGFP fusions in Aspergillus parasiticus. Michigan State University. Dept. of Food Science and Human Nutrition; 2008. p. 311. 2008. [Google Scholar]
- Hoppert M, Gentzsch C, Schorgendorfer K. Structure and localization of cyclosporin synthetase, the key enzyme of cyclosporin biosynthesis in Tolypocladium inflatum. Arch Microbiol. 2001;176:285–293. doi: 10.1007/s002030100324. [DOI] [PubMed] [Google Scholar]
- Hutchins MU, Klionsky DJ. Vacuolar localization of oligomeric alpha-mannosidase requires the cytoplasm to vacuole targeting and autophagy pathway components in Saccharomyces cerevisiae. J Biol Chem. 2001;276:20491–20498. doi: 10.1074/jbc.M101150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, Igarashi Y, Yamaguchi A. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- Kuznetsov A. V. a. G., E. Laboratory Protocol- Lactate Dehydrogenase. Mitochondrial Physiology Network: OROBOROS INSTRUMENTS. 2006 [Google Scholar]
- Lee LW, Chiou CH, Klomparens KL, Cary JW, Linz JE. Subcellular localization of aflatoxin biosynthetic enzymes Nor-1, Ver-1, and OmtA in time-dependent fractionated colonies of Aspergillus parasiticus. Arch Microbiol. 2004;181:204–214. doi: 10.1007/s00203-003-0643-3. [DOI] [PubMed] [Google Scholar]
- Lendenfeld T, Ghali D, Wolschek M, Kubicek-Pranz EM, Kubicek CP. Subcellular compartmentation of penicillin biosynthesis in Penicillium chrysogenum. The amino acid precursors are derived from the vacuole. J Biol Chem. 1993;268:665–671. [PubMed] [Google Scholar]
- Liang S-H. The function and expression of the ver-1 gene and localization of the ver-1 protein involved in aflatoxin B1 biosynthesis in Aspergillus parasiticus. leaves: Michigan State University. Dept. of Food Science and Human Nutrition; 1996. p. xi, 135. 1996. [Google Scholar]
- Martinoia E, Heck U, Boller T, Wiemken A, Matile P. Some properties of vacuoles isolated from Neurospora crassa slime variant. Arch Microbiol. 1979;120:31–34. doi: 10.1007/BF00413268. [DOI] [PubMed] [Google Scholar]
- Mathieu Y, Guern J, Kurkdjian A, Manigault P, Manigault J, Zielinska T, Gillet B, Beloeil JC, Lallemand JY. Regulation of Vacuolar pH of Plant Cells: I. Isolation and Properties of Vacuoles Suitable for P NMR Studies. Plant Physiol. 1989;89:19–26. doi: 10.1104/pp.89.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycock CJ, Nahorski SR. The validity of mitochondrial marker enzymes in rat heart. J Mol Cell Cardiol. 1971;3:229–241. doi: 10.1016/0022-2828(71)90043-5. [DOI] [PubMed] [Google Scholar]
- Reuter L. WSU Cell Biology Lab Manual. 1995 [Google Scholar]
- Rieder SE, Emr SD. Isolation of subcellular fractions from the yeast Saccharomyces cerevisiae. Curr Protoc Cell Biol. 2001 doi: 10.1002/0471143030.cb0308s08. Chapter 3, Unit 3 8. [DOI] [PubMed] [Google Scholar]
- Robert S, Zouhar J, Carter C, Raikhel N. Isolation of intact vacuoles from Arabidopsis rosette leaf-derived protoplasts. Nat Protoc. 2007;2:259–262. doi: 10.1038/nprot.2007.26. [DOI] [PubMed] [Google Scholar]
- Roze LV, Arthur AE, Hong SY, Chanda A, Linz JE. The initiation and pattern of spread of histone H4 acetylation parallel the order of transcriptional activation of genes in the aflatoxin cluster. Mol Microbiol. 2007;66:713–726. doi: 10.1111/j.1365-2958.2007.05952.x. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Ohnishi M, Sazuka T. Isolation of intact vacuoles and proteomic analysis of tonoplast from suspension-cultured cells of Arabidopsis thaliana. Plant Cell Physiol. 2004;45:672–683. doi: 10.1093/pcp/pch099. other authors. [DOI] [PubMed] [Google Scholar]
- Squire RA. Ranking animal carcinogens: a proposed regulatory approach. Science. 1981;214:877–880. doi: 10.1126/science.7302565. [DOI] [PubMed] [Google Scholar]
- van den Broek WJ, Stunnenberg HG, Wennekes LM. Protoplasts from Aspergillus nidulans. Microbios. 1979;26:115–128. [PubMed] [Google Scholar]
- Vaughn LE, Davis RH. Purification of vacuoles from Neurospora crassa. Mol Cell Biol. 1981;1:797–806. doi: 10.1128/mcb.1.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, Goud B, Ruohola H, Novick PJ. Fractionation of yeast organelles. Methods Cell Biol. 1989;31:335–356. doi: 10.1016/s0091-679x(08)61618-0. [DOI] [PubMed] [Google Scholar]
- Wiemken A. Isolation of vacuoles from yeasts. Methods Cell Biol. 1975;12:99–109. doi: 10.1016/s0091-679x(08)60954-1. [DOI] [PubMed] [Google Scholar]
- Yabe K, Nakajima H. Enzyme reactions and genes in aflatoxin biosynthesis. Appl Microbiol Biotechnol. 2004;64:745–755. doi: 10.1007/s00253-004-1566-x. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T, Ohsumi Y, Anraku Y. Solubilization and purification of alpha-mannosidase, a marker enzyme of vacuolar membranes in Saccharomyces cerevisiae. J Biol Chem. 1988;263:5158–5163. [PubMed] [Google Scholar]
- Yu J, Chang PK, Ehrlich KC. Clustered pathway genes in aflatoxin biosynthesis. Appl Environ Microbiol. 2004;70:1253–1262. doi: 10.1128/AEM.70.3.1253-1262.2004. other authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R. The function, accumulation and localization of the Nor-1 protein involved in aflatoxin biosynthesis and the function of the fluPgene associated with sporulation in Aspergillus parasiticus. leaves: Michigan State University. Dept. of Food Science and Human Nutrition and Multidisciplinary Graduate Program in Environmental Toxicology; 1997. p. xiii, 224. 1997. [Google Scholar]
- Zigel M, Kobzeva N. Preparation of protoplasts and localization of ribonuclease, beta-1,3-glucanase and glucosoisomerase in the fungal mycelium of Penicillium brevi-compactum. Prikl Biokhim Mikrobiol. 1980;16:245–248. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. parasiticus was grown for 36 h under aflatoxin inducing conditions and protoplasts prepared and purified as described in Methods. Protoplast samples were fixed with 2.5% glutaraldehyde overnight at 4°C on a rotating platform shaker. Samples were post-fixed in 1% buffered osmium tetroxide overnight at 4°C, dehydrated in an acetone series (30–100%), and then infiltrated and polymerized in Poly/Bed 812 resin for 24h at 60°C. Resin blocks were sectioned using a Power Tome-XL ultramicrotome (Boeckeler Instruments, Tucson, AZ). Protoplast sections (70 nm thick) were mounted on formvar coated copper grids and stained with 1% uranyl acetate and lead citrate. Images were generated with a JEOL 100CX transmission electron microscope. a: TEM image of a protoplast. b: Magnified image of the larger inset in c. TEM image of the smaller inset in panel a. Grey arrowheads indicate lipid bilayer boundaries and black arrowheads indicate irregular boundaries. Bar in panel a = 1μm, bar in panel b = 100nm and bar in panel c = 50nm.
Release of vesicles and vacuoles from a protoplast after addition of triton X 100 (lysis) solution.