Abstract
Therapies targeting transforming growth factor β (TGFβ) signaling using neutralizing antibodies and small molecular inhibitors are in multiple clinical trails. However, TGFβ is known to work as both a tumor suppressor and a tumor promoter, and current knowledge does not provide sufficient information on what factors mediate this switch in function and when this switch occurs. Recent advances in multiple disciplines suggest that immune cells from the tumor host mayprovide the answer.
Transforming Growth Factor β: A Tumor Suppressor or a Tumor Promoter?
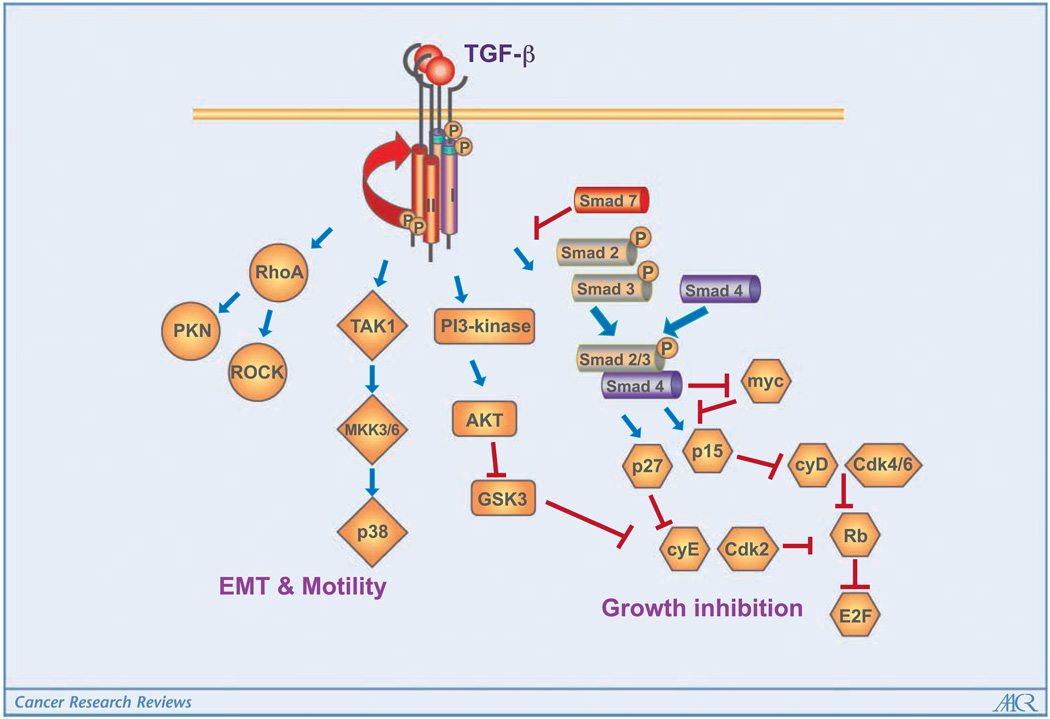
The transforming growth factor (TGF)-β ligands (TGFβ1, TGFβ2, and TGFβ3) signal through the type I and type II TGFβ receptors (TβRI and TβRII, respectively). Canonical signaling proceeds with phosphorylation of Smad2 and Smad3, which then combine with Smad4 to enter the nucleus to modulate transcription in cooperation with other transcription factors, coactivators, and corepressors. In addition, TGFβ binding to its receptors activates many noncanonical signaling pathways (Fig. 1).
Figure 1.
The TGFβ ligands signal through the type I and type II TGFβ receptors. Canonical signaling proceeds with phosphorylation of Smad2 and Smad3, which then combine with Smad4 to enter the nucleus to mediate growth inhibition. Smad7 is a negative mediator in this process. In addition, TGFβ binding to its receptors activates many noncanonical signaling pathways and regulates tumor cell migration and metastasis.
Alterations in TGFβ signaling have significant effects on tumor initiation and progression. However, TGFβ can function both as a tumor suppressor and as a tumor promoter, which is extensively reviewed by Bierie and Moses (1). The mechanisms determining when and how TGFβ switches from a tumor suppressor to a tumor promoter are a great challenge in the field. A variety of drugs including neutralizing antibodies and small molecular inhibitors have been developed to inhibit TGFβ signaling. However, the therapeutic effects of systemic inhibition of TGFβ signaling are likely dependent on the context and stage of tumor progression.
The evidence supporting TGFβ as a tumor suppressor comes from both human studies and mouse models. In a number of human cancers, mutations of the genes encoding TβRI and TβRII (Tgfbr1 and Tgfbr2, respectively) or decreased expression and phosphorylation of other components of this pathway have been reported. In mouse models, conditional knockout of Tgfbr2 in combination with expression of KrasG12D in pancreatic cancer (2) or with APC mutation in intestinal carcinomas (3) resulted in the development of much more aggressive tumors. Conditional deletion of Tgfbr2 in mammary epithelial cells that also express the polyoma middle T antigen (PyVmT) under the mouse mammary tumor virus promoter resulted in a shortened tumor latency and increased metastases (4). This observation is also true in several other mouse models including colon cancer and head and neck squamous cell carcinoma. Abrogation of TGFβ signaling specifically in stratified epithelia leads to destabilization of epithelial homeostasis and results in the development of spontaneous squamous cell carcinomas in the anogenital region (5). TGFβ suppresses tumor progression through inhibition of cell cycle progression; increased apoptosis; and suppression of expression of growth factors, cytokines, and chemokines.
TGFβ is often produced in large quantities by many tumor types and is known to be pro-oncogenic. A recent clinical study showed that high TGFβ activity is present in aggressive, highly proliferative gliomas and is associated with a poor prognosis in glioma patients (6). In colon cancer, mutation of the gene for the type II receptor in cancers with high levels of microsatellite instability points to a favorable outcome after adjuvant chemotherapy with fluorouracil-based regimens for stage III colon cancer (7). In mouse models as mentioned above, abrogation of TGFβ signaling in PyVmT mammary carcinomas enhances metastasis (4), and paradoxically, enhancement of TGFβ signaling by expression of a constitutively active TGFβ1 or TβRI in mammary epithelial cells in conjunction with c-Neu or PyVmT expression increases pulmonary metastases (8). Systemic inhibition of TGFβ signaling suppresses pulmonary metastases, indicating that TGFβ is a tumor promoter (8). Studies using genetically modified cancer cells and mouse tumor models also support TGFβ signaling in breast cancer bone metastases. The mechanisms of TGFβ as tumor promoter include dysregulation of cyclin-dependent kinase inhibitors; alteration in cytoskeletal architecture, which is often implicated in epithelial to mesenchymal transition; increases in proteases and extracellular matrix formation; decreased immunosurveillance; and increased angiogenesis.
What factors mediate this switch of TGFβ signaling in function from tumor suppressor to tumor promoter? When exactly does this switch occur? It has been postulated that changes in signal intensity and connectivity of Smad-dependent pathways and Smad-independent pathways may underlie the complex transition. In this hypothesis, whereas Smad-dependent signaling mediates the growth inhibition of TGFβ signaling, the Smad-independent pathways likely contribute to the tumor-promoting effect of the TGFβ signaling cascade. This includes oncogenic events such as amplification of MYC, activating mutations of RAS, and inactivating mutations of downstream effectors (retinoblastoma and cyclin-dependent kinase inhibitors). However, there are also data showing that Smad-dependent pathways are involved in tumor progression. For example, Smad signaling is responsible for lung metastasis through induction of angiopoietin-like 4 (9). High TGFβ-Smad activity is present in aggressive, highly proliferative gliomas and confers poor prognosis in glioma patients (6).
Effect of TGFβ on the Immune System of Tumor Host
One of the significant effects of TGFβ is to inhibit immunosurveillance mechanisms in the tumor host (10). TGFβ markedly and directly suppresses the transcription of genes encoding multiple key proteins of the “cytotoxic program” of CD8+ CTL, such as perforin and granzymes, cytotoxins that act through the granule exocytosis pathway (11). This inhibition of CD8 CTL is mediated by Gr-1CD11b myeloid cells through the production of TGFβ, which is regulated by natural killer T cells that secrete interleukin (IL)-3. Very interestingly, this induction of TGFβ production by myeloid cells plays a greater role in suppressing the immune response than production of TGFβ by the tumor itself because blocking production by these myeloid cells (by eliminating these cells or by blocking the upstream signal from natural killer T cells or IL-13) abrogated the suppression although the TGFβ production by the tumor itself was not affected (12). TGFβ also alters the polarization of the CD8+ cells in tumor-bearing mice, resulting in elevated IL-17, which suppressed apoptosis of tumor cells (13). TGFβ, coordinated with IL-21, induces CD4+CD25+ regulatory T cells, which counterbalance the effect of IL-6 that promotes proinflammatory IL-17–producing T cells (14). In addition, TGFβ is responsible for CD4+CD25+ regulatory T-cell inhibition of natural killer cell functions (15).
Very interestingly, publications from different laboratories, including our own, show that Gr-1+CD11b+ immature myeloid cells are the major source for high level of TGFβ in the tumor host (12, 16, 17). These Gr-1+CD11b+ cells have been known as myeloid immune suppressor cells or myeloid-derived suppressor cells since 1979. They inhibit natural killer cell, B-cell, and T-cell functions through the production of arginase and reactive oxygen species; they also inhibit functional maturation of dendritic cells and promote type II macrophage development, and thus represent one mechanism of tumor escape from immune system control and compromise the efficacy of cancer immunotherapy (18). There are two major subpopulations of these cells: mononuclear cells (precursor for macrophages) and low-density polymorphonuclear cells (immature neutrophils), and both populations suppressed antigen-specific T-cell responses, but through distinct effector molecules and signaling pathways (19). Recently, Gr-1+CD11b+ cells were found to directly disrupt the binding of specific peptide-MHC dimers to CD8-expressing T cells through nitration of tyrosines in a T-cell receptor-CD8 complex. This process makes CD8-expressing T cells unable to bind peptide-MHC and to respond to the specific peptide, although they retain their ability to respond to nonspecific stimulation (20).
TGFβ Orchestrates the Inflammatory Reaction in the Tumor Microenvironment
Host-derived inflammatory cells infiltrate into tumor tissues and provide growth factors, proangiogenic factors, proteases, as well as adhesion molecules that facilitate tumor cell proliferation, angiogenesis, invasion, and metastasis. One of the known mechanisms through which TGFβ mediates inflammatory reaction is the chemokines and chemokine receptors [e.g., CXC chemokine (CXCR)-4 and stromal cell–derived factor 1 (SDF-1)] and the recently reported CXC chemokine ligand 5 (CXCL5; ref. 17). These molecules play vital roles in the recruitment of host inflammatory cells into the tumor microenvironment. In addition, TGFβ also mediates nuclear factor-κB signaling, a master regulator of inflammation reaction. Tumor-infiltrating receptor activator of nuclear factor-κB ligand–expressing cells activate nuclear IκB kinase α and inhibit the transcription of tumor metastasis suppressor Maspin, thereby promoting tumor metastasis (21). TGFβ1 negatively regulates nuclear factor-κB activation in the gut through Smad7 (22). Inflammation by Helicobacter infection in Smad3-deficient mice caused the development of colon cancer (23). Furthermore, TGFβ cross-talks with inflammatory pathways through the modulation of IL-1 (24). In addition to epithelial cells, TGFβ signaling in stromal cells has significant effect on tumor development and growth. Loss of the TGFβ type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through up-regulation of TGFα-, macrophage-stimulating protein–,and hepatocyte growth factor–mediated signaling networks (25). TGFβ signaling also regulates endothelial cells, resulting in a significant effect on tumor angiogenesis.
In the distant premetastatic lung, TGFβ is one of the factors produced by tumor cells responsible for the production of the chemoattractants S100A8 and S100A9, which attract Mac1+ myeloid cells (26). Through this mechanism, tumor cells also activate mitogen-activated protein kinase p38 to acquire migratory activity with pseudopodia for invasion (invadopodia; ref. 26).
Gr-1+CD11b+ Myeloid Cells in the Switch of TGFβ Signaling from Tumor Suppressor to Tumor Promoter
Gr-1+CD11b+ myeloid cells are significantly overproduced in the bone marrow and spleens of tumor-bearing mice as well as in the peripheral blood of cancer patients. In addition to the immunosuppressive effect on tumor host, Gr-1+CD11b+ cells also infiltrate into tumors and promote tumor angiogenesis and metastasis by producing high levels of matrix metalloproteinases (MMP) and TGFβ (17, 27). Recently, these cells have been found to mediate tumor refractoriness to anti–vascular endothelial growth factor treatment (28).
Recent work from our laboratory showed that deletion of Tgfbr2 in mammary carcinoma cells results in increased CXCL5/CXCR2 and SDF-1/CXCR4 chemokine signals that enhance Gr-1+CD11b+ myeloid cell infiltration into the invasive front of the tumors (17). Once there, these Gr-1+CD11b+ cells directly promote tumor invasion and metastasis through increased production and function of MMPs. In addition, Gr-1+CD11b+ cell infiltration also results in increased TGFβ production in the tumor microenvironment. Our data show that autologous TGFβ signaling in mammary epithelial cells acts as a tumor suppressor, and when it is deleted or altered, it results in Gr-1+CD11b+ cell recruitment. This leads to increased MMP and TGFβ production, which enhances tumor invasion and metastasis. Thus, the switch of TGFβ signaling from tumor suppressor to tumor promoter involves an additional component, which is the recruitment of Gr-1+CD11b+ cells in the tumor microenvironment. This is supported by a recent publication in which CCR1+ myeloid cells (CD34+) are shown to be recruited to colon cancers with deletion of Smad4 and promote tumor invasion (29). Indeed, inflammatory cells (CD45 and BM8-positive cells) have been observed in head and neck tumors lacking TGFβ signaling (30). In TGFβ1-deficient mice, inflammation causes precancerous lesions to progress to colon cancer (31).
However, contradictory to the above observations, overexpression of TGFβ1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation (32). It is unclear what underlies these different observations, and whether there are different mechanisms of chemokine/chemokine receptor mechanisms involved for deletion of TGFβ signaling versus increased TGFβ signaling.
Are Immune Cells from Tumor Host the Answer for TGFβ Antagonism Therapy?
Although changes in signal intensity and connectivity of Smad-dependent and Smad-independent pathways have been postulated as a mechanism for the TGFβ switch from suppressor to promoter, the factors that initiate such changes in signaling are not known. The above studies and results suggest that tumor-infiltrating bone marrow–derived Gr-1+CD11b+ cells change the dynamics in the primary tumor microenvironment, which results in changes in the signaling cascade in tumor cells. These studies also point out that TGFβ produced by Gr-1+CD11b+ cells is a significant component of the tumor-promoting effect of TGFβ signaling, affecting the tumor microenvironment and host immune system (Fig. 2). In fact, in preclinical mouse models, the efficacy of the anti-TGFβ antibody 1D11 in suppressing metastasis was dependent on a synergistic combination of effects on both the tumor parenchyma and microenvironment. This includes enhancement of the CD8+ T-cell–mediated antitumor immune response, increased infiltration of natural killer cells and T cells at the metastatic site, and increased expression of an NKG2D ligand (Rae1γ) and of a death receptor (TNFRSF1A) on tumor cells (33). This line of understanding of TGFβ is particularly important because the therapeutic effect of TGFβ antagonism is largely dependent on when TGFβ switches from tumor suppressor to tumor promoter. Our studies suggest that Gr-1+CD11b+ cells may be used as a biomarker for patient selection in ongoing phase I/II clinical trials of TGFβ therapy. This is supported by recent findings that the immune/inflammatory responses are reliable markers in human hepatocellular carcinoma (34) and colorectal tumors predicting clinical outcome (35). These studies also point out a novel therapeutic strategy for advanced cancer, which is the prevention of the recruitment of MMP-expressing cells by chemokine receptor antagonists.
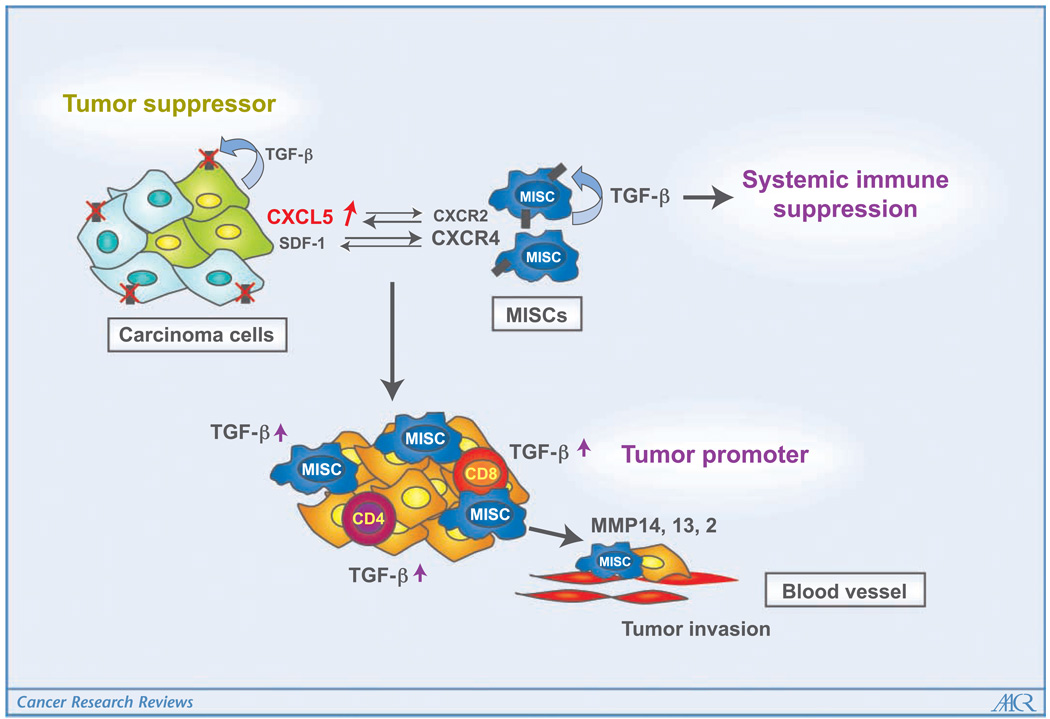
Figure 2.
How TGFβ signaling switches from being tumor suppressive to tumor promoting is unknown. Host-derived immature myeloid Gr-1+CD11b+ cells are recruited into the tumor microenvironment with deletion of the type II TGFβ receptor gene in mammary carcinomas through CXCL5/CXCR2 and SDF-1/CXCR4. In addition, Gr-1+CD11b+ cells express high levels of MMPs and TGFβ1, which promote tumor invasion and immune suppression. The effects of Gr-1+CD11b+ cells on the tumor microenvironment and host immunosurveillance constitute a tumor-promoting mechanism of TGFβ signaling.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Bierie B, Moses HL. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 2.Ijichi H, Chytil A, Gorska AE, et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-β signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz NM, Upton M, Rojas A, et al. Transforming growth factor β receptor type II inactivation induces the malignant transformation of intestinal neoplasms initiated by Apc mutation. Cancer Res. 2006;66:9837–9844. doi: 10.1158/0008-5472.CAN-06-0890. [DOI] [PubMed] [Google Scholar]
- 4.Forrester E, Chytil A, Bierie B, et al. Effect of conditional knockout of the type II TGF-β receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005;65:2296–2302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 5.Guasch G, Schober M, Pasolli HA, et al. Loss of TGFβ signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–327. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruna A, Darken RS, Rojo F, et al. High TGFβ-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe T, Wu TT, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–1206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arteaga CL. Inhibition of TGFh signaling in cancer therapy. Curr Opin Genet Dev. 2006;16:30–37. doi: 10.1016/j.gde.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Padua D, Zhang XH, Wang Q, et al. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahl SM, Wen J, Moutsopoulos N. TGF-β: a mobile purveyor of immune privilege. Immunol Rev. 2006;213:213–227. doi: 10.1111/j.1600-065X.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 11.Trapani JA. The dual adverse effects of TGF-β secretion on tumor progression. Cancer Cell. 2005;8:349–350. doi: 10.1016/j.ccr.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nam JS, Terabe M, Kang MJ, et al. Transforming growth factor β subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–3923. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiringhelli F, Menard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-β-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-β-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Huang J, Ren X, et al. Abrogation of TGFβ signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marx J. Cancer immunology. Cancer’s bulwark against immune attack: MDS cells. Science. 2008;319:154–156. doi: 10.1126/science.319.5860.154. [DOI] [PubMed] [Google Scholar]
- 19.Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T-cell suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 20.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo JL, Tan W, Ricono JM, et al. Nuclear cytokine-activated IKKα controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 22.Monteleone G, Mann J, Monteleone I, et al. A failure of transforming growth factor-β1 negative regulation maintains sustained NF-κB activation in gut inflammation. J Biol Chem. 2004;279:3925–3932. doi: 10.1074/jbc.M303654200. [DOI] [PubMed] [Google Scholar]
- 23.Maggio-Price L, Treuting P, Zeng W, et al. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;66:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu T, Tian L, Han Y, et al. Dose-dependent cross-talk between the transforming growth factor-β and interleukin-1 signaling pathways. Proc Natl Acad Sci U S A. 2007;104:4365–4370. doi: 10.1073/pnas.0700118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiratsuka S, Watanabe A, Aburatani H, et al. Tumour-mediated up-regulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Debusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 28.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b(+)Gr1(+) myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura T, Kometani K, Hashida H, et al. SMAD4-deficient intestinal tumors recruit CCR1(+) myeloid cells that promote invasion. Nat Genet. 2007;39:467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 30.Lu SL, Herrington H, Reh D, et al. Loss of transforming growth factor-β type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006;20:1331–1342. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engle SJ, Ormsby I, Pawlowski S, et al. Elimination of colon cancer in germ-free transforming growth factor β1-deficient mice. Cancer Res. 2002;62:6362–6366. [PubMed] [Google Scholar]
- 32.Lu SL, Reh D, Li AG, et al. Overexpression of transforming growth factor β1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res. 2004;64:4405–4410. doi: 10.1158/0008-5472.CAN-04-1032. [DOI] [PubMed] [Google Scholar]
- 33.Nam JS, Terabe M, Mamura M, et al. An anti-transforming growth factor β antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008;68:3835–3843. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in heaptocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]