Abstract
Control of acute murine cytomegalovirus (MCMV) infection is dependent upon both innate and adaptive immune responses, relying primarily upon natural killer (NK) and T-cell responses for control. Although CD28/B7 plays a clear role in T-cell responses in many antigen systems including some viral infections, the importance of co-stimulation during MCMV infection is unconfirmed. In addition, recent data suggest that CD28/B7 co-stimulation might also be important to Ly49H+ NK-cell expansion. We therefore hypothesized that CD28/B7 co-stimulation is critical to viral control after MCMV infection, and further that CD28/B7 co-stimulation plays a role in MCMV-specific T- and NK-cell responses. To test these hypotheses, we utilized C57BL/6 mice lacking the co-stimulatory molecules B7-1 and B7-2 or CD28. After primary infection with MCMV, viral titers are significantly elevated in mice lacking CD28 or B7 compared with wild-type mice. Impaired viral control is associated with significant defects in peripheral T-cell responses to MCMV, which appear to be dependent upon CD28/B7 co-stimulation. Abnormal hepatic T-cell responses in CD28−/− mice are preceded by impaired MCMV-specific Ly49H+ NK-cell responses. Cytokine evaluations confirm that CD28/B7 co-stimulation is not required for non-specific antiviral responses. We conclude that CD28-mediated co-stimulation is critical for early viral control during acute MCMV infection.
Introduction
Control of acute cytomegalovirus (CMV) infection is dependent upon both innate and adaptive immune responses. It has been demonstrated that natural killer (NK) cell responses are critical to early viral control following acute CMV infection in some mouse strains (7,13,14,51,62). Viral clearance is further dependent upon intact T-cell responses, with CMV inducing specific cytotoxic T-cell (CTL) responses in infected hosts (16,48,52,54–56,66,67). Optimal CTL responses require professional antigen-presenting cells (APCs), and APC/T-cell interactions are thus critical to CTL differentiation in infected hosts (1,19,41). Although adoptive transfer of anti-CMV antibody is protective during acute infection (5,22,30,32,61), humoral responses to murine CMV (MCMV) are slower to develop than cellular responses (9), and both B cells and antibody appear dispensable during acute infection (29,74). Thus defects in either NK- or T-cell responses have significant implications for viral control, which is clinically most obvious in patients with impaired NK- or T-cell immunity (10,63,65).
T-cell responses to CMV occur through clonally restricted antigen receptors, resulting in proliferation and clonal expansion of CMV-specific cells (55). Generally, optimal activation of T cells requires co-stimulation in addition to antigen-specific signals (59). One such co-stimulatory mechanism is functionalized by activating receptor CD28 expressed on T-cell surfaces. Ligands for CD28, namely B7-1 and B7-2 (hereafter referred to as B7 molecules), are prototypic co-stimulatory molecules expressed primarily on antigen-presenting cells (6,23,24,34–36,59,76). Thus optimal activation of T cells relies upon antigen presentation to the T-cell receptor (TCR), and is enhanced by co-stimulation via CD28/B7 ligation.
Although numerous studies demonstrate the importance of co-stimulation in varying in-vitro antigen systems, there are few data published on co-stimulation during anti-viral responses, and even fewer utilizing in-vivo models. Of the few studies done to date, CD28/B7 co-stimulation has been shown to have varying importance for T-cell regulation of other viruses (18,44,49,70,71, and reviewed in [8]), and there are no studies evaluating the importance of co-stimulation in control of CMV infection. Despite this, there are circumstantial data suggesting that co-stimulation is important to the control of MCMV. Included in MCMV's immune evasion repertoire are genes that interfere with expression of B7 molecules on APCs. MCMV has been shown to downregulate surface expression of both B7-1 (CD80) and B7-2 (CD86) co-stimulatory molecules in monocyte/macrophage and dendritic cells during infection (2,37,42). Given the known importance of these co-stimulatory proteins in development of adaptive T-cell responses in other systems, we hypothesized that if MCMV has evolved specific immune evasion mechanisms that downmodulate B7 molecule expression, then CD28/B7 co-stimulation must play a critical role in anti-viral defense to infection with MCMV.
In addition to antigen-specific T-cell responses, NK-cell subsets have recently been shown to expand in response to specific antigenic stimuli (21,26,58). One example is Ly49H+ NK-cell subset expansion in response to MCMV, which is triggered by activating receptor Ly49H binding to its recently described ligand MCMV protein m157 (4,12,21,25,51,64,73). Because this NK subset expansion is similar to adaptive T-cell responses, it has been postulated that co-stimulation might also be important to NK-cell expansion and effector function (26,45). Murine NK cells have been shown to express CD28, and current data suggest that CD28 activation is important for optimal NK proliferation by enhancing cytokine production in these cells (26,45). Additionally, NK-cell cytotoxicity is enhanced by either type of B7 molecule (26,39,75), although B7-stimulated NK cytolysis does not absolutely require CD28 (17,40). Taken together, these data suggest that co-stimulation could indeed play an important role in NK-cell subset expansion in response to MCMV infection.
To test these hypotheses, we utilized C57/BL6 mice lacking the co-stimulatory molecules B7-1 and B7-2 or CD28. After infection with MCMV, these mice demonstrate significant defects in viral control associated with impaired T- and NK-cell responses. Impaired cellular responses to infection do not appear to be consequent to defective cytokine responses after infection in knockout mice. Thus our study for the first time confirms that CD28/B7-mediated co-stimulation is critical for early viral control during acute MCMV infection, and provides evidence that co-stimulation plays a role in adaptive cellular responses to MCMV.
Materials and Methods
Mice
Male C57BL/6 mice deficient of both B7-1 (CD80) and B7-2 (CD86) (referred to as B7−/−) (11) or deficient of CD28 (referred to as CD28−/−) (26,60) were obtained from our colony. Age-matched male C57BL/6 mice 8–10 wk of age (Charles River Laboratories, Boston, MA) were used as controls. All animals were housed in a pathogen-free environment, adhering to the “Guide for the Care and Use of Laboratory Animals,” prepared by the National Research Council (National Institutes of Health Publication No. 86-23, revised 1985). After humane euthanasia, mice liver and spleen tissues were dissected aseptically, and underwent cellular isolation for flow cytometry analysis, viral titer analysis, or were frozen immediately in liquid nitrogen, then stored at −80°C for later analysis.
Viral infection
Primary CMV infection was achieved by IP injection of tissue culture passaged (once) through salivary gland–derived 5 × 104 PFU Smith strain MCMV (VR-1399/1981) obtained from the ATCC (Rockville, MD). Prior to infection (day 0) cohorts of 3 animals underwent analysis as controls. For infection experiments, 6 each of wild type, B7−/−, and CD28−/− mice were infected, with cohorts of 3 animals each euthanized at 2 and 6 days post-infection (dpi).
Antibodies and flow cytometry
Fluorescent dye–conjugated antibodies specific for CD3 (PerCP-Cy5.5), NK1.1 (PE), interferon-γ, and bromodeoxyuridine (BrdU, PRB-1) were all purchased from BD PharMingen (San Diego, CA). Cells isolated from livers or spleens of mice were stained with fluorescent dye–conjugated mAbs and were analyzed by flow cytometry (FACScalibur, Becton Dickinson, Mountain View, CA) (26). Intracellular IFN-γ was analyzed in splenic NK cells (not further stimulated ex vivo) using FITC-conjugated mAb to IFN-γ from BD PharMingen as previously described (27). Ly49H (APC) antibody was a kind gift from Dr. Wayne Yokoyama.
Isolation of hepatic mononuclear leukocytes
Hepatic mononuclear cells (MNCs) were isolated as previously described (26). Briefly, livers were perfused through the portal vein with 10 mL phosphate-buffered saline (PBS) to remove blood cells. Individual livers were ground with a 3-mL syringe insert and suspended in 10 mL of medium. Single-cell suspensions were collected and centrifuged, and tissue debris was discarded. Cells were re-suspended in 2 mL 40% Percoll (Pharmacia, Uppsala, Sweden), and layered on 2.5 mL 70% Percoll in 15-mL conical tubes. MNCs were recovered at the interface between 40% and 70% Percoll after centrifugation at 1600 rpm at room temperature for 25 min. Remaining red blood cells in MNCs were lysed by ammonium chloride solution. Hepatic MNCs were then subjected to flow cytometry analysis.
BrdU incorporation assay in vivo
To determine cell proliferation in vivo, BrdU (1 mg/0.1 mL/mouse; Sigma, St Louis, MO) was injected IP into mice 3 h prior to acquiring liver and spleen tissues. Splenocytes and hepatic MNCs were isolated, stained with fluorescent-conjugated mAbs to CD3, NK1.1, and Ly49H, followed by intracellular staining with FITC-labeled mAb to BrdU, and were analyzed by four-color flow cytometry as previously described (26). Subsets of CD3+NK1.1−, CD3-NK1.1+Ly49H+, and CD3−NK1.1+Ly49H− cells were respectively gated to analyze BrdU incorporation. Ly49H monoclonal antibody was a gift from Dr. Wayne Yokoyama and biotinylated in our laboratory.
In vitro plaque assay
Spleen and liver tissues from 0, 2, and 6 dpi underwent in-vitro plaque assay (IVPA) analysis to determine viral titer, as well as real-time PCR to determine viral load. For IVPA, mouse embryo fibroblasts were grown to confluence in 6-well plates in DMEM (Gibco BRL, Carlsbad, CA). Following centrifugation (1000 × g for 10 min) of 5 mL of homogenized tissue, 1 mL of supernatant was placed in each well. These plates were centrifuged at 1000 g, and incubated at 37°C in 5% CO2 for 3–4 h. The plates were washed three times with PBS, then covered with 3 mL of 1% agar in DMEM. Following 6–7 days of incubation (37°C in 5% CO2), the plates were fixed in 10% formalin, stained with 1% crystal violet, and analyzed by low-power phase contrast microscopy for plaque formation. All IVPA experiments were performed in duplicate, and viral titers are reported as mean ± standard error of mean of both experiments.
IVPA techniques are time consuming and laborious, so we performed side-by-side comparisons of IVPA to real-time PCR to quantitate viral load in liver and spleen tissues. Linear regression analysis showed good correlation between IVPA and real-time PCR results (R2 0.89; p < 0.001, data not shown). A drawback to real-time PCR is that occasionally it was not sensitive enough to detect low viral copies, requiring the use of IVPA. Therefore when copy numbers are adequate enough to allow detection, real-time quantitative PCR is an accurate surrogate for IVPA to quantify viral load in tissues.
DNA isolation
DNA was extracted from tissues using QIAamp Tissue Kit (Qiagen GmbH, Hilden, Germany). DNA extracted from tissue homogenates was eluted in 100 μL of distilled water and stored at −20°C until analysis. DNA were amplified in a total volume of 25 μL with 200 nm of each primer and 1.0 U of Taq DNA polymerase (Gibco BRL) added in 2.5 μL of a PCR buffer (50 mM KCl, 20 mM Tris-HCl [pH 8.4], and 1.5 mM MgCl2).
Quantitative real-time PCR
Primer sets and fluorescence resonance energy transfer type probes were designed and optimized for MCMV GB and β-actin genes. Plasmids homologous to the sequences amplified by our PCR primers were cloned for these genes, and using serial dilutions of known plasmid concentrations, standard curves were constructed for quantitation. Regression equations had very high R2 values (.95 β-actin and .99 gB), and were used for subsequent copy number calculations. Real-time PCR results are expressed as copies of DNA/mRNA per copies of β-actin. All samples were analyzed in duplicate, and results are expressed as mean copies± standard error of mean. Sequences for the real-time primers/probes designed were as follows: β-actin forward: attgtgatggactccggtga, reverse: agctcatagctcttctccag, probe: cacccacactgtgcccatctac, CMV GB; forward: tgtactcgaagggagagct, reverse: cgttcaccaccgaagacac, and probe: cgcctcgaacgtgttcagcctg. For cytokine analyses, primers for IL-1, TNF-α and IFN-γ were obtained from Super Array (Biosciences). Sequences of other primers are as follows from 5′ to 3′: IL-2, forward: tcactcctcacagtgacctcaagt; reverse, agcgcttactttgtgctgtcct; IL-12α, forward: tgtcttagccagtcccgaaacct, reverse: gtgaagcaggatgcagagcttcatt; IL-15, forward: ccatctcgtgctacttgtgtttcct, reverse: caggacgtgttgatgaacatttggac; IL-7α, forward: atccacctcacacgaggcacaagt, reverse: caaacacgaagcagtttgggacc; IFN-α, forward: tgtctgatgcagcaggtgg, reverse: aagacagggctctccagac; GAPDH, forward: aactttggcattgtggaagg, and reverse: acacattgggggtaggaaca.
PCR reactions were carried out using a Smart-Cycler (Cepheid, Sunnyvale, CA), using the following program: initial denaturation 4 min at 94°C, 35 cycles of denaturation for 30 sec at 94°C, annealing for 30 sec at 53°C, elongation for 30 sec at 72°C, followed by final elongation for 7 min at 72°C, then hold at 4°C.
Statistics
Statistical analyses utilized one-way ANOVA or one- or two-tailed Student's t-test. p Values ≤0.05 were considered significant for all testing. Means are expressed as mean ± standard error. Statistical software used was GraphPad Prism (Version 4.03; GraphPad Software, San Diego, CA).
Results
CD28/B7 co-stimulation is critical for early viral control
To evaluate influences of the co-stimulatory molecules B7 and CD28 on early viral control, wild-type, B7−/−, and CD28−/−C57BL/6 mice (H-2b) were infected with MCMV. Viral titers were measured 2 and 6 dpi using both IVPA and quantitative real-time PCR for CMV DNA of tissue homogenates from spleen and liver.
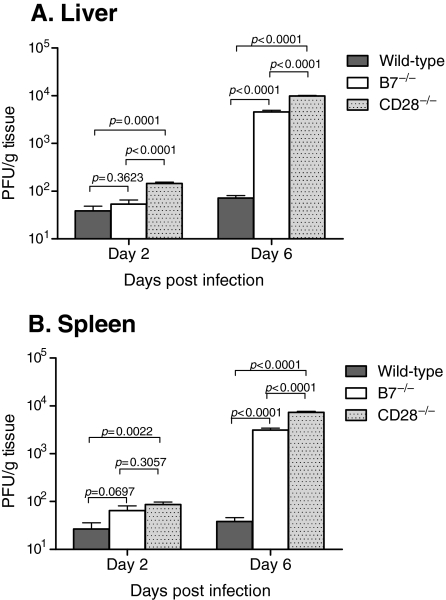
In both liver and spleen, there were significantly elevated MCMV titers (threefold) by day 2 in CD28−/− mice, while viral titers in B7−/− mice were not significantly different than wild-type mice (Fig. 1A and B). Previous studies have shown that 6 d after MCMV infection, MCMV-specific NK subset expansion peaks, and anti-viral T-cell expansion is beginning (20,21,58). In the current study viral titers in both B7−/− and CD28−/− mice were significantly elevated (∼2 logs) compared to wild-type mice in both liver and spleen 6 dpi (Fig. 1). Interestingly, CD28−/− mice had significantly higher viral titers (double) than B7−/− mice in both tissues by 6 dpi (Fig. 1A and B). In addition, hepatic titers were slightly but significantly higher than splenic titers at both time points (comparison not shown). Viral DNA loads from all tissues correlated very well with IVPA titers, further confirming IVPA results (R2 = 0.87; data not shown). We therefore conclude that both B7 and CD28 molecules play essential roles in early control of primary MCMV infection.
FIG. 1.
Effect of co-stimulation upon viral titers during acute MCMV infection. Wild-type C57BL/6, B7−/−, or CD28−/− knockout mice were infected with Smith MCMV (5 × 104 PFU) or receiving vehicle (PBS), and liver (A) and spleen (B) tissues were acquired 2 or 6 dpi. Tissues were analyzed by plaque assay to determine viral titers, and results are reported in plaque-forming units (PFU) per gram of tissue. Each data bar represents 3 mice. The data shown are representative of three comparable experiments.
CD28 and B7 are required for T-cell expansion in response to MCMV
CD8 T-cells are known to be vitally important to viral control following MCMV infection (16,53,55,56). Because CD28/B7 co-stimulation is known to be variably important to T-cell responses in other models of viral infection (8,18,44,49), one possible explanation for the observed differences in viral titers was that T cells in these knockout mice do not respond normally to MCMV without co-stimulation. To test this hypothesis, we evaluated CD3+ NK1.1− T-cell expansion in liver and splenic tissue following acute MCMV infection using flow cytometry to measure total numbers and cell division.
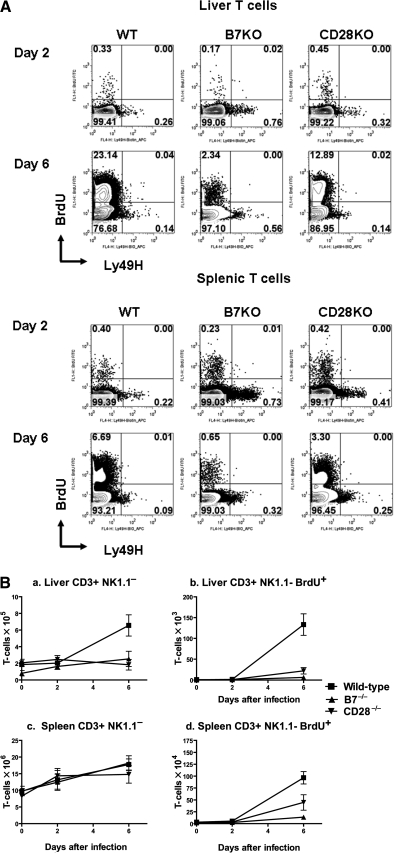
As previously published, wild-type mice showed significant expansion of hepatic T-cell counts by 6 dpi, which corresponded with increased BrdU incorporation (Fig. 2A and B). In contrast, B7−/− and CD28−/− mice had significantly lower hepatic T-cell counts at 6 dpi (p = 0.04, p = 0.03; Fig. 2A and B). Although this corresponded to impaired hepatic T-cell division, as suggested by impaired BrdU incorporation, which was significantly lower in B7−/− and CD28−/− mice than in wild-type mice (p = 0.01, p = 0.002; Fig. 2B), it is also possible that low hepatic T-cell counts could be consequent to impaired hepatic T-cell migration or T-cell death.
FIG. 2.
Co-stimulation in T-cell responses during acute MCMV infection. Wild-type C57BL/6 (WT), B7−/− (B7KO), or CD28−/− knockout (CD28KO) mice infected with Smith MCMV (5 × 104 PFU) or vehicle had liver and spleen tissues removed 2 or 6 dpi. Three hours prior to organ removal, each mouse received IP 1.0 mg of BrdU. Single splenocyte preparations or hepatic MNCs were prepared, stained with mAb to CD3 (PerCP-Cy5.5), NK1.1 (APC), and Ly49H (PE), followed by intracellular staining with mAb to BrdU (FITC) as described in materials and methods, and analyzed by flow cytometry. CD3+NK1.1− T cells were gated and analyzed for BrdU and Ly49H expression. Note that a small portion of CD3+NK1.1-BrdU− T cells expressed Ly49H, consistent with our previous observation (unpublished data). (A) Representative flow cytometric plots of liver and spleen from mice at 2 or 6 dpi. (B) T-cell numbers for each group at various time points post infection from a representative experiment. Each data point represents mean ± standard error for 3 mice. The experiment was repeated twice with similar results.
In contrast to liver, overall splenic T-cell counts in B7−/− and CD28−/− mice were comparable to those of wild-type mice (p = 0.88, p = 0.36; Fig. 2A and B). Curiously, this was observed despite significant impairment of T-cell division in B7−/− and CD28−/− mice as demonstrated by decreased BrdU incorporation (p = 0.0007, p = 0.05; Fig. 2A and B). Despite maintenance of normal numeric T-cell responses, knockout mice have dramatically elevated splenic viral titers compared to wild-type mice 6 dpi (Fig. 1B). One possible explanation for these observations is that small changes in T-cell numbers driven by antigen-specific T-cell proliferation could be obscured in the spleen, as this is a primary lymphoid organ. Similar analyses of CD3+NK1.1+ cells (NKT) showed no significant expansion, and no significant differences between wild-type and knockout mice (data not shown). From these studies we conclude that CD28/B7 co-stimulation is critical to T-cell division and subsequent peripheral expansion after infection with MCMV, and additionally that both B7 and CD28 influence accumulation of T cells in the liver.
CD28, but not B7, is required for expansion of Ly49H+ NK cells in liver
Robbins et al. have recently shown that early T-cell responses and viral control of MCMV are dependent upon preceding NK-cell responses (57). Therefore we considered the possibility that diminished T-cell proliferative responses, and elevated viral titers in spleen and liver, could also be additionally consequent to abnormal NK-cell responses in mutant mice. NK-cell responses to MCMV infection in normal mice begin at 2 dpi as non-specific responses, and peak at 6 dpi, with subset expansion of Ly49H+ cells (20,21,58). We therefore hypothesized that the impairment of early viral control observed in CD28−/− and B7−/− mice could be consequent to inadequate NK-cell expansion, and compared expansion between Ly49H+ and Ly49H− NK cells in livers after MCMV infection.
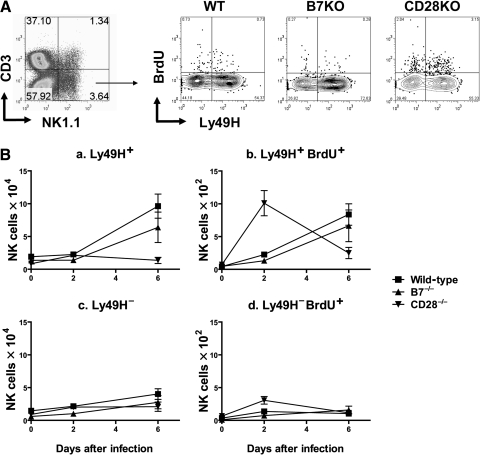
MNCs isolated from livers after MCMV infection were evaluated for Ly49H+ and Ly49H− NK-cell expansion by flow cytometry as shown in Fig. 3A, and summarized in Fig. 3B. Confirming previously published findings, Fig. 3B-a shows significant expansion from baseline of Ly49H+ NK cells in wild-type mice at day 2 of MCMV infection (p = 0.01) that rise more than fivefold by day 6 of infection (p < 0.01) (20,21,58). This expansion is due to Ly49H+ NK-cell division, as confirmed by significant increases of BrdU incorporation in this subset at 2 and 6 dpi in wild-type mice (Fig. 3B-b; p < 0.01 for both time points, compared to baseline). In contrast, non-specific Ly49H− NK-cell expansion and division were modest (Fig. 3B-c and d), which has also been consistently described by others (20,21,58).
FIG. 3.
Effect of co-stimulation on LY49H+ NK-cell expansion in liver during acute MCMV infection. Wild-type C57BL/6, B7−/−, or CD28−/− knockout mice infected with Smith MCMV (5 × 104 PFU) had liver tissues removed 2 or 6 dpi. Three hours prior to organ removal, each mouse received BrdU. Hepatic MNCs were prepared and NK cells were enumerated and had division estimated by four-color flow cytometry. The MNCs were stained with fluorescent dye-conjugated mAbs to CD3 (PerCP-Cy5.5), NK1.1 (PE), and Ly49H (APC) for cell surface markers, and intracellular staining with mAb to BrdU (FITC) as described in materials and methods. Percentages of each subset (CD3−NK1.1+Ly49H+, CD3−NK1.1+Ly49H−, CD3−NK1.1+Ly49H+BrdU+, or CD3−NK1.1+Ly49H−BrdU+) were determined and then multiplied by numbers of bulk MNCs. (A) Representative flow cytometric plots at 2 dpi from CD3−NK1.1+ gated cells. (B) Mean cell numbers of CD3−NK1.1+ gated Ly49H+ and Ly49H− NK cells and BrdU incorporation in hepatic MNC over time. Each data point represents mean ± standard error of mean for 3 mice. This experiment was performed twice with comparable results.
As shown in Fig. 3B, CD28 appears to be important for MCMV-specific NK subset expansion/accumulation in liver tissue during MCMV infection. Unlike wild-type mice, CD28−/− mice do not develop Ly49H+ NK-cell expansion/accumulation, showing no significant increase in Ly49H+ NK counts above baseline (p > 0.05 for either time point; Fig. 3B-a). In fact, CD28−/− mice showed no significant difference between Ly49H+ (specific) and Ly49H− (non-specific) NK-cell counts at 6 dpi (p > 0.05; compare Fig. 3B-a and c). Curiously, this deficit in Ly49H+ NK cells occurred despite significantly higher division rates in Ly49H+ NK cells at 2 dpi in CD28−/− mice (p < 0.01; Fig. 3B-b). It is possible that this enhanced division was in part non-specific, as there was a concomitant similar, albeit smaller, elevation in Ly49H− NK-cell division at this same time (p < 0.05; Fig. 3B-d). Despite this, by 6 dpi, Ly49H+ NK counts were significantly lower than wild-type controls, and Ly49H− NK counts were no different than wild-type controls in liver tissues. Thus CD28 appears to be required for specific Ly49H+ NK-cell accumulation in the liver of infected mice, but does not appear to influence non-specific NK-cell responses to MCMV infection. It is unclear whether failed NK-cell expansion in CD28−/− mice livers reflects increased apoptosis in this population, and/or simply failure of normal recruitment of antigen-specific NK cells to the liver.
In contrast to CD28, B7 expression plays little or no role in expansion/accumulation of hepatic NK cells after infection. Fig. 3B-a shows that Ly49H+ NK cells in B7−/− mice significantly expand by 6 dpi compared to baseline. Thus wild-type and B7−/− mice showed no significant difference in Ly49H+ NK-cell counts at 6 dpi (p = 0.32), and BrdU incorporation in Ly49H+ NK cells was also similar between wild-type and B7−/− mice (Fig. 3B-b).
Neither CD28 nor B7 are required for expansion of splenic Ly49H+ NK cells
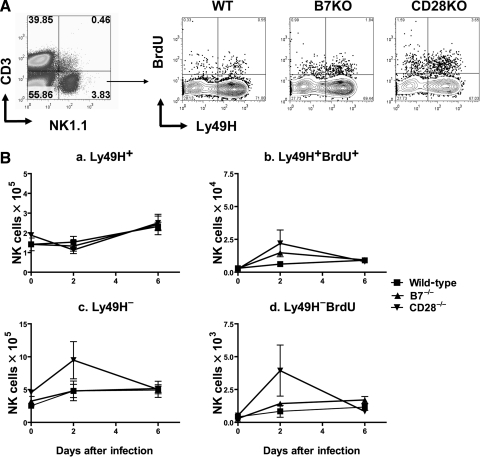
To explore whether co-stimulation is required for splenic NK-cell expansion, we evaluated Ly49H+ and Ly49H− NK cells after infection by flow cytometry. In contrast to liver, and similar to our T-cell findings, there was no significant difference in splenic Ly49H+ NK-cell numbers between wild-type, B7−/−, or CD28−/− mice at either 2 or 6 dpi (p = 0.56 and p =0.95, respectively; Fig. 4A and B), although splenic expansion of Ly49H+ NK cells was much more modest (∼twofold) than that seen in livers (>fivefold). In wild-type mice, MCMV-specific Ly49H+ NK cells showed a non-linear increase by day 6 (Fig. 4B), a result that has been previously published (20,21). The initial decline in splenic NK counts at 2 dpi has been previously postulated to be secondary to either redistribution of splenic NK cells to more peripheral sites of infection, or to apoptosis or consumption of these cells in the spleen (20).
FIG. 4.
Effect of co-stimulation on splenic NK-cell expansion during acute MCMV infection. Wild-type C57BL/6 (WT), B7−/− (B7KO), or CD28−/− knockout (CD28KO) mice infected with Smith MCMV (5 × 104 PFU) or vehicle had splenic tissues removed 2 or 6 dpi. Three hours prior to organ removal, each mouse received IP 1.0 mg BrdU. CD3−NK1.1+ gated splenocytes were enumerated and had division estimated by flow cytometry, as described in Fig. 3. (A) Representative flow cytometric plots 2 dpi. (B) Ly49H+ and Ly49H− NK-cell numbers and BrdU incorporation in spleens over time. Each data point represents mean ± standard error for 3 mice. This experiment was performed twice with comparable results.
Fig. 4B-b and c show the effects of B7 and CD28 on splenic NK-cell division following MCMV infection. Both B7−/− and CD28−/− mice show small but statistically significant increases in BrdU incorporation in Ly49H+ NK cells 2 dpi when compared to wild-type mice (p < 0.05). Nonetheless, this increased division corresponds to either no change or slight decreases in splenic Ly49H+ NK-cell numbers on day 2 (Fig. 4B-a). Although the hypothesis that these cells might be redistributing to other sites of infection is sensible, there is no evidence that this occurs on day 2 post infection in the liver, when liver counts of Ly49H+ NK cells are not significantly increased in either B7−/− or CD28−/− mice (Fig. 3B). Six days after infection, wild-type, B7−/−, and CD28−/− mice show comparable BrdU incorporation that is significantly elevated from baseline. This cell division correlates with modest increases in Ly49H+ NK-cell numbers in splenic tissues by 6 dpi (Fig. 4B-a and c). Importantly, when cell division rates for splenic Ly49H+ and Ly49H− NK cells are compared, there is identical BrdU incorporation in Ly49H− NK cells for wild-type and knockout mice (compare Fig. 4B-b and d). Thus it seems that non-specific NK-cell expansion is intact in knockout mice, and that splenic NK expansion in response to MCMV occurs by a non-specific mechanism that does not require CD28 or B7 co-stimulation.
Effects of co-stimulatory molecules on early cytokine production
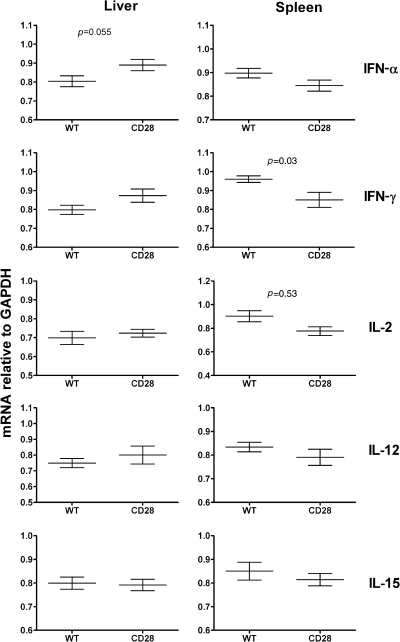
As an alternative hypothesis, it is possible that B7−/− or CD28−/− mice have some fundamental defect in early cytokine responses that could explain the observed defects in T- and NK-cell expansion independent of co-stimulation. To test this hypothesis, we examined cytokine mRNA in liver and splenic tissues from wild-type and CD28−/− mice, including IFN-α, IFN-γ, IL-1, IL-2, IL-12, IL-15, IL-17, and TNF-α, at both 2 and 6 dpi. There were no significant differences between CD28−/− and wild-type mice for any cytokine transcripts at 6 dpi in either tissue (data not shown). There were several differences noted at 2 dpi, which are illustrated in Fig. 5 and discussed below.
FIG. 5.
Effect of co-stimulation of early cytokine production upon MCMV infection. Wild-type C57BL/6 or CD28−/− knockout mice infected with Smith MCMV (5 × 104 PFU) had liver and splenic tissues evaluated at 2 dpi. Total RNA was prepared and subjected to real-time PCR analysis for IL-1, IL-2, IL-12, IL-15, IL-17, IFN-γ, TNF-α, and IFN-α. There were no significant differences in IL-1, IL-17, or TNF-α transcripts between wild-type and CD28−/− mice in the liver or the spleen (not shown). Data shown are arbitrary transcript units (relative to GAPDH) for IFN-α, IFN-γ, IL-2, IL-12, and IL-15, in liver or spleen at 2 dpi. Significant differences are noted by p values in this figure. Each data point represents mean ± standard error of mean for 3–4 mice.
Several cytokines are known to be critical to T-cell responses to MCMV, including IFN-α and γ, IL-2, and IL-12 (43,57). There were no significant differences in IL-2 transcription in hepatic tissues at 2 or 6 dpi that might explain abnormal T-cell numbers and division in CD28−/− mice (Fig. 5). There were, however, elevated hepatic IFN-α transcripts observed in CD28−/− mice at 2 dpi that became comparable to wild-type by 6 dpi (Fig. 5). It has been previously shown that a lack of early viral control by NK cells leads to overexpression of IFN-α after MCMV infection, and that elevated IFN-α impairs T-cell responses to MCMV (57). Although there were decreased levels of IL-2 and IFN-γ transcripts observed in spleens of CD28−/− mice at 2 dpi compared to wild-type mice, these returned to normal by 6 dpi (not shown), and these deficits did not appear to influence overall splenic T-cell numbers after infection (Fig. 2).
Furthermore, there are several cytokines known to be critical in early NK responses to MCMV, including IFN-α, IL-2, IL-12, and IL-15 (45,46). Importantly, there were no significant differences in these cytokine transcripts in liver between wild-type and CD28−/− mice to explain defective hepatic Ly49H+ NK-cell expansion observed in CD28−/− mice (Fig. 5). IL-2, IL-12, and IL-15 levels were comparable in wild-type and CD28−/− mice, and as previously mentioned, day 2 liver IFN-α mRNA levels were actually higher in CD28−/− mice. Although IL-2 levels were lower in spleens at 2 dpi, this did not appear to influence splenic NK responses (Fig. 4). Finally, there were no defects in non-specific Ly49H− NK-cell expansion after infection in knockout mice when compared to wild-type mice in either liver (Fig. 3) or spleen (Fig. 4), which functionally confirms that non-specific responses to MCMV are intact in these knockout mice.
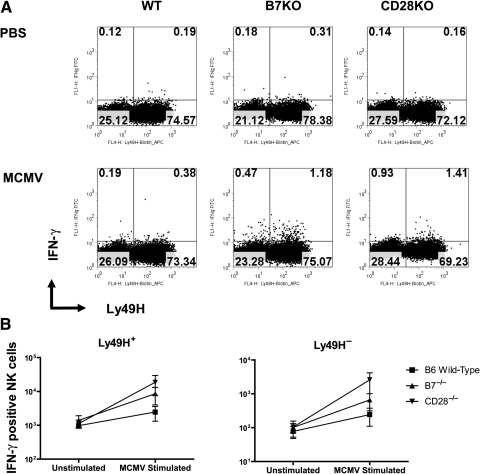
Because IFN-γ is a dominant cytokine produced by NK cells during MCMV infection (21), we examined whether co-stimulation influences early IFN-γ production by NK cells in response to MCMV infection. As shown in Fig. 6, numbers of Ly49H+ IFN-γ-producing NK-cells in spleens 2 days after infection are not impaired in knockout mice. One might anticipate early NK IFN-γ responses to be consequent to m157-Ly49H interaction, but this does not appear to be the case at 2 dpi, because Ly49H− NK cells showed the same fold increase in IFN-γ expression at 2 dpi (Fig. 6B). These findings also suggest that the decreased transcription of IFN-γ observed in whole spleen lysates (Fig. 5) is occurring outside the NK-cell compartment. Although wild-type splenic Ly49H+ NK cells expressing IFN-γ are numerically somewhat lower than those previously published at this time point, we did not stimulate our cells ex vivo to elicit this response. Unfortunately, hepatic NK cells were too few to allow similar analysis of IFN-γ-producing cells by flow cytometry (all were used in Ly49H enumeration and BrdU experiments), but transcripts of IFN-γ at 2 and 6 dpi were comparable in liver between wild-type and CD28−/− mice (Fig. 5).
FIG. 6.
Co-stimulation and Ly49H+ NK-cell IFN-γ responses during acute MCMV infection. Wild-type C57BL/6 (WT), B7−/− (B7KO), or CD28−/− (CD28KO) knockout mice infected with Smith MCMV (5 × 104 PFU) or receiving vehicle (PBS) had splenic tissues removed at 2 dpi. Single splenocytes were prepared, stained with mAb to CD3 (PerCP-Cy5.5), NK1.1 (PE), and Ly49H (APC), followed by intracellular staining with FITC-conjugated mAb to IFN-γ and analysis by four-color flow cytometry. CD3−NK1.1+ cells were gated as described in Fig. 3, and further analyzed for Ly49H+ and IFN-γ expression. Ly49H+ NK cells expressing IFN-γ were enumerated. (A) Representative of flow cytometric plots for Ly49H+IFN-γ+ NK cells from each group. (B) Numbers of IFN-γ-producing Ly49H+ and Ly49H− NK cells in each group. Each data point represents mean ± standard error of mean for 3 mice.
Interpreting these cytokine results is somewhat complicated by differences in viral titers in knockout mice, as well as a lack of cellular fraction–specific analyses. Differences in IFN-γ expression in splenocyte lysates that were not observed in NK cells highlight the possibility that splenocyte and hepatic lysates could mask subtle differences in cytokine production in individual cell subsets. Nonetheless, there appear to be no glaring defects in cytokine responses to infection in CD28 knockout mice, and taken together, these observations suggest that non-specific cytokine responses to MCMV are intact in CD28−/− mice.
Discussion
This study confirms that the co-stimulatory molecules B7 and CD28 are critical to host viral control during acute MCMV infection. Our viral titer data clearly demonstrate that both molecules are required for host immune control of virus replication following acute MCMV infection in both hepatic and splenic tissues. This influence in viral titers began as early as 2 dpi, becoming more dramatic at 6 dpi in mice lacking B7−/− or CD28−/−. Early responses to MCMV (days 1–2) are known to be largely a consequence of APC release of cytokines, resulting in non-specific NK-cell expansion (3,19,47), while later viral control (day 6) is dependent upon antecedent non-specific and MCMV specific NK-cell responses, as well as subsequently developing T-cell responses (14,16,33,48,50,55,57). Because of the known importance of co-stimulation in adaptive immune responses, we further explored influences of co-stimulation on T- and NK-cell responses in these knockout mice.
It is not entirely surprising that co-stimulation is critical to MCMV control, given recent data demonstrating the importance of B7 during herpes simplex virus and Epstein-Barr virus infection (44,70,71). Further data supporting this hypothesis include MCMV targeting of DC responsiveness and co-stimulation as part of its immune evasion armamentarium (2,37,41,42). B7 proteins enhance NK-mediated killing, and conversely, B7-1–deficient APCs display significant reductions in their ability to promote T-cell activation (23,39,75). Thus circumstantially, it seemed likely that co-stimulation via B7 and CD28 would be critical to MCMV control after acute infection. Despite these circumstantial data, there are other viruses, such as lymphocytic choriomeningitis virus, that actually develop vigorous CD8 responses without co-stimulation (49). Thus ours are the first data of which we are aware to definitively prove that co-stimulation is required for early control of CMV infection.
Antigen presentation and co-stimulation are known to be critical in development of normal T-cell responses in many antigen systems. Interestingly, MCMV has been shown to cause “immune paralysis” in DCs, which manifests as reduced release of IL-2 and IL-12, and impairment of T-cell activation (2,28). This impairment has recently been attributed to viral genes m138 and m147.5, which cause downregulation of the co-stimulatory molecules B7-1 (CD80) and B7-2 (CD86) (37,42) on DCs. Our studies confirm the importance of CD28/B7 co-stimulation in the development of T-cell responses to MCMV in vivo, as knockout mice showed impaired hepatic T-cell expansion and accumulation. This may be a direct consequence of diminished T-cell division, as there was significantly lower T-cell BrdU incorporation following MCMV infection in both B7−/− and CD28−/− mice compared to wild-type mice. It is possible that poor expansion of T cells in both B7−/− and CD28−/− mice is a consequence of early abnormalities in splenic IL-2 or IFN-γ transcription, but this will require further study. Overall, it is likely that this impaired T-cell response at least contributes to the elevated viral titers seen at 6 dpi.
Much like APC/T-cell interactions, APC/NK interactions have also been shown to be critical in development of NK-cell responses to MCMV. Early during MCMV infection, APCs produce IFN-α/β and IL-12, causing IFN-γ-dependent non-specific NK expansion in vivo (3,19,47). In addition to these non-specific NK responses, MCMV-specific Ly49H+ NK subset expansion occurs 4–8 days after infection, consequent to stimulatory receptor Ly49H+ activation (20), presumably activated by binding ligand m157 (12,64,73). This subset expansion has been reported to peak at 6 dpi in both liver and spleen tissue (3,20,21,58), and we reproduced these results in wild-type mice. Recent work has shown that interaction between CD8α+ DC and NK cells is important for expansion of this MCMV-specific Ly49H+ NK subset in response to acute infection (3). Altogether these observations by others are quite novel, demonstrating for the first time that NK cells, long considered to be central to “innate” immunity, actually have “adaptive” characteristics.
Because of these adaptive characteristics, and the obvious importance of APC/NK interactions, we postulated that similar to T cells, co-stimulation might be important to expansion of MCMV-specific NK-cell subsets. Indeed, CD28−/− mice had significantly reduced hepatic Ly49H+ NK subset expansion, which correlated with significant impairment in Ly49H+ NK-cell division at 6 dpi. In contrast, B7 appeared dispensable to MCMV-specific NK-subset expansion, as B7−/− mice developed normal hepatic Ly9H+ NK counts and BrdU incorporation. Nonetheless it was conceivable that deficits in Ly49H+ NK-subset expansion in CD28−/− mice could be explained by some fundamental defect in innate responses in these mice. Importantly, there were no significant differences in IFN-α, IFN-γ, IL-12, or IL-15 transcripts after infection that might explain impaired NK-subset expansion in CD28−/− mice. Thus, although not definitively proven, our data suggest that CD28 co-stimulation enhances subset expansion of peripheral NK cells. This provocative observation will require further study.
The mechanism by which CD28 might participate in hepatic NK-cell expansion is currently not known. MCMV-specific NK expansion is somewhat unique in that it does not depend upon MHC-I antigen presentation, but instead relies upon direct recognition of m157 (an MHC-I homologue) expressed on the surface of infected cells by activating receptor Ly49H+ (72). We speculate that just as MHC/TCR signaling and antigen-specific T-cell expansion is enhanced by CD28/B7 co-stimulation, m157/Ly49H signaling may be similarly enhanced, and therefore lack of CD28 stimulation leads to the depressed NK responses seen in CD28−/− mice. Unfortunately, this hypothesis doesn't fit neatly with our results, as B7−/− mice do not show the same effect. This is in contrast to the utter dependence of peripheral T-cell responses upon both co-stimulatory molecules. At best, we can consider that peripheral NK-cell subset expansion is influenced by co-stimulatory molecule CD28, but appears independent of co-stimulatory ligand B7. If true, this also suggests that CD28 might recognize a different B7 family ligand in B7−/− mice that facilitates MCMV-specific NK-subset expansion.
Although co-stimulation appears to be important to splenic T-cell division in response to MCMV infection, the decreased T-cell division observed in knockout mice did not negatively impact overall splenic T-cell counts. This could simply be because splenocytes responsive to MCMV comprise a small percentage of the splenocyte population, or that day 6 is too early to detect significant differences in T-cell counts. Alternatively, splenic expansion of T cells after MCMV infection might not be solely reliant on increased cell division co-stimulated by B7 or CD28 molecules, but may occur by some other mechanism, such as resistance to activation-induced T-cell death. In contrast, splenic NK-cell expansion appears to be altogether independent of co-stimulatory influence, occurring primarily by non-specific expansion, as shown by equal expansion and division of both Ly49H+ and Ly49H− NK cells in wild-type and knockout mice. This is consistent with previously published hypotheses that splenic NK-cell expansion occurs by a mechanism different than that seen in the liver (68,69). Although this issue of splenic NK expansion has become somewhat controversial (38,68,69), it would appear that whatever the mechanism, splenic NK- and T-cell expansion occurs independent of co-stimulation by B7 or CD28. It is interesting to note that development of relatively “normal” MCMV-specific NK-cell and T-cell numbers in splenic tissues was associated with better viral control in spleen tissues compared to liver (comparison not shown). Nonetheless, splenic viral titers were still much higher in both B7−/− and CD28−/− than wild-type mice, suggesting that there is some important immune interaction missing in these knockout mice.
Interestingly, at almost every time point, CD28−/− mice had significantly higher viral titers than B7−/− mice. While this could be explained simply by CD28 being relatively more important to viral control, it is also possible that a lack of CD28 molecules could have additional consequences. CD28−/− mice still express B7 molecules, which can also bind CTLA-4 and PD-L1, known inhibitory receptors (15,23,31,40). It is therefore possible that absence of CD28 could cause unopposed B7/CTLA-4 or PD-L1 binding and signaling, downregulating adaptive responses to MCMV. This could explain the differences in viral titers between CD28−/− and B7−/− mice observed in livers as early as 2 dpi, and in both liver and spleen by 6 dpi. Alternatively, because T-cell responses are in part dependent upon preceding NK-cell responses (57), it is also possible that B7−/− mice, which develop normal NK but abnormal T-cell responses, have slightly better viral control than CD28−/− mice, which have sequential impairment of both NK- and T-cell responses. Robbins et al. have shown that poor viral control by NK cells leads to higher IFN-α levels after infection (57), which we observed in CD28−/− mice at 2 dpi. These hypotheses are not mutually exclusive, are both consistent with previously published data, and likely explain the differences in viral titers observed in our knockout mice.
Conclusion
In conclusion, the co-stimulatory molecules B7 and CD28 are critical to viral control in mice during acute CMV infection, as absence of either molecule leads to significant elevations in viral titers. We feel that the differences in viral titers observed in knockout mice are likely a direct consequence of impaired T-cell expansion, with more subtle contributions from impaired MCMV-specific NK-cell expansion in CD28−/− mice. Whether CD28/B7 co-stimulation is critical for NK-subset expansion remains unproven, but this provocative observation certainly deserves further study. These data thus support the hypothesis that downregulation of co-stimulatory molecules by MCMV is an important immune evasion strategy during acute infection.
Acknowledgments
This work was supported by National Institutes of Health grant RO1 gM066115 (C.H.C.), Strategic Initiative Grant, Ohio State University (J.X.G.), American Cancer Society IRG 112367 (J.X.G.), and the Immunology Program Award, Ohio State University (J.X.G.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Andoniou CE. van Dommelen SL. Voigt V, et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nature Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 2.Andrews DM. Andoniou CE. Granucci F. Ricciardi-Castagnoli P. Degli-Esposti MA. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nature Immunol. 2001;2:1077–1084. doi: 10.1038/ni724. [DOI] [PubMed] [Google Scholar]
- 3.Andrews DM. Scalzo AA. Yokoyama WM. Smyth MJ. Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nature Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 4.Arase H. Mocarski ES. Campbell AE. Hill AB. Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 5.Araullo-Cruz TP. Ho M. Armstrong JA. Protective effect of early serum from mice after cytomegalovirus infection. Infect Immun. 1978;21:840–842. doi: 10.1128/iai.21.3.840-842.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azuma M. Ito D. Yagita H. Okumura K. Phillips JH. Lanier LL. Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366:76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 7.Bancroft GJ. Shellam GR. Chalmer JE. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981;126:988–994. [PubMed] [Google Scholar]
- 8.Bertram EM. Dawicki W. Watts TH. Role of T cell co-stimulation in anti-viral immunity. Semin Immunol. 2004;16:185–196. doi: 10.1016/j.smim.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Bickerstaff AA. Zimmerman PD. Wing BA. Taylor F. Trgovcich J. Cook CH. A flow cytometry-based method for detecting antibody responses to murine cytomegalovirus infection. J Virol Methods. 2007;142:50–58. doi: 10.1016/j.jviromet.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biron CA. Byron KS. Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells [see comment] N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 11.Borriello F. Sethna MP. Boyd SD, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 12.Bubic I. Wagner M. Krmpoti A, et al. Gain of virulence caused by loss of a gene in murine cytomegalovirus. J Virol. 2004;78:7536–7544. doi: 10.1128/JVI.78.14.7536-7544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukowski JF. Woda BA. Habu S. Okumura K. Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131:1531–1538. [PubMed] [Google Scholar]
- 14.Bukowski JF. Woda BA. Welsh RM. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984;52:119–128. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butte MJ. Keir ME. Phamduy TB. Sharpe AH. Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavanaugh VJ. Deng Y. Birkenbach MP. Slater JS. Campbell AE. Vigorous innate and virus-specific cytotoxic T-lymphocyte responses to murine cytomegalovirus in the submaxillary salivary gland. J Virol. 2003;77:1703–1717. doi: 10.1128/JVI.77.3.1703-1717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers BJ. Salcedo M. Ljunggren H-G. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1) Immunity. 1996;5:311–317. doi: 10.1016/s1074-7613(00)80257-5. [DOI] [PubMed] [Google Scholar]
- 18.Christensen JE. Christensen JP. Kristensen NN. Hansen NJV. Stryhn A. Thomsen AR. Role of CD28 co-stimulation in generation and maintenance of virus-specific T cells. Int Immunol. 2002;14:701–711. doi: 10.1093/intimm/dxf037. [DOI] [PubMed] [Google Scholar]
- 19.Dalod M. Hamilton T. Salomon R. Salazar-Mather TP. Henry SC. Hamilton JD. Biron CA. Dendritic cell responses to early murine cytomegalovirus infection: Subset functional specialization and differential regulation by interferon α/β. J Exp Med. 2003;197:885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dokun AO. Chu DT. Yang L. Bendelac AS. Yokoyama WM. Analysis of in situ NK cell responses during viral infection. J Immunol. 2001;167:5286–5293. doi: 10.4049/jimmunol.167.9.5286. [DOI] [PubMed] [Google Scholar]
- 21.Dokun AO. Kim S. Smith HR. Kang HS. Chu DT. Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nature Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 22.Farrell HE. Shellam GR. Protection against murine cytomegalovirus infection by passive transfer of neutralizing and non-neutralizing monoclonal antibodies. J Gen Virol. 1991;72:149–156. doi: 10.1099/0022-1317-72-1-149. [DOI] [PubMed] [Google Scholar]
- 23.Freeman GJ. Borriello F. Hodes RJ, et al. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993;262:907–909. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- 24.Freeman GJ. Gribben JG. Boussiotis V, et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 25.French AR. Sjolin H. Kim S, et al. DAP12 signaling directly augments proproliferative cytokine stimulation of NK cells during viral infections. J Immunol. 2006;177:4981–4990. doi: 10.4049/jimmunol.177.8.4981. [DOI] [PubMed] [Google Scholar]
- 26.Gao J-X. Liu X. Wen J. Caligiuri MA. Stroynowski I. Zheng P. Liu Y. Two-signal requirement for activation and effector function of natural killer cell response to allogeneic tumor cells. Blood. 2003;102:4456–4463. doi: 10.1182/blood-2003-07-2480. [DOI] [PubMed] [Google Scholar]
- 27.Gao J-X. Zhang H. Bai X-F, et al. Perinatal blockade of B7-1 and B7-2 inhibits clonal deletion of highly pathogenic autoreactive T cells. J Exp Med. 2002;195:959–971. doi: 10.1084/jem.20011948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granucci F. Vizzardelli C. Pavelka N, et al. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol. 2001;2:882–888. doi: 10.1038/ni0901-882. [DOI] [PubMed] [Google Scholar]
- 29.Jonjic S. Pavic I. Polic B. Crnkovic I. Lucin P. Koszinowski UH. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med. 1994;179:1713–1717. doi: 10.1084/jem.179.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klenovsek K. Weisel F. Schneider A, et al. Protection from CMV infection in immunodeficient hosts by adoptive transfer of memory B cells. Blood. 2007;110:3472–3479. doi: 10.1182/blood-2007-06-095414. [DOI] [PubMed] [Google Scholar]
- 31.Krummel MF. Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawson CM. Grundy JE. Shellam GR. Antibody responses to murine cytomegalovirus in genetically resistant and susceptible strains of mice. J Gen Virol. 1988;69:1987–1998. doi: 10.1099/0022-1317-69-8-1987. [DOI] [PubMed] [Google Scholar]
- 33.Lawson CM. O'Donoghue H. Reed WD. The role of T cells in mouse cytomegalovirus myocarditis. Immunology. 1989;67:132–134. [PMC free article] [PubMed] [Google Scholar]
- 34.Linsley PS. Brady W. Grosmaire L. Aruffo A. Damle NK. Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linsley PS. Brady W. Urnes M. Grosmaire LS. Damle NK. Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linsley PS. Clark EA. Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci USA. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loewendorf A. Kruger C. Borst EM. Wagner M. Just U. Messerle M. Identification of a mouse cytomegalovirus gene selectively targeting CD86 expression on antigen-presenting cells. J Virol. 2004;78:13062–13071. doi: 10.1128/JVI.78.23.13062-13071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loh J. Chu DT. O'Guin AK. Yokoyama WM. Virgin HW., IV Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol. 2005;79:661–667. doi: 10.1128/JVI.79.1.661-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luque I. Reyburn H. Strominger JL. Expression of the CD80 and CD86 molecules enhances cytotoxicity by human natural killer cells. Human Immunol. 2000;61:721–728. doi: 10.1016/s0198-8859(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Fontecha A. Assarsson E. Carbone E. Karre K. Ljunggren H-G. Triggering of murine NK cells by CD40 and CD86 (B7-2) J Immunol. 1999;162:5910–5916. [PubMed] [Google Scholar]
- 41.Mathys S. Schroeder T. Ellwart J. Koszinowski UH. Messerle M. Just U. Dendritic cells under influence of mouse cytomegalovirus have a physiologic dual role: to initiate and to restrict T cell activation. J Infect Dis. 2003;187:988–999. doi: 10.1086/368094. [DOI] [PubMed] [Google Scholar]
- 42.Mintern JD. Klemm EJ. Wagner M, et al. Viral interference with B7-1 costimulation: A new role for murine cytomegalovirus Fc receptor-1. J Immunol. 2006;177:8422–8431. doi: 10.4049/jimmunol.177.12.8422. [DOI] [PubMed] [Google Scholar]
- 43.Mocikat R. Braumuller H. Gumy A, et al. Natural killer cells activated by MHC class ILow targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19:561–569. doi: 10.1016/s1074-7613(03)00264-4. [DOI] [PubMed] [Google Scholar]
- 44.Müller A. Schmitt L. Raftery M. Schönrich G. Paralysis of B7 co-stimulation through the effect of viral IL-10 on T cells as a mechanism of local tolerance induction. Eur J Immunol. 1998;28:3488–3498. doi: 10.1002/(SICI)1521-4141(199811)28:11<3488::AID-IMMU3488>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 45.Nandi D. Gross JA. Allison JP. CD28-mediated costimulation is necessary for optimal proliferation of murine NK cells. J Immunol. 1994;152:3361–3369. [PubMed] [Google Scholar]
- 46.Nguyen KB. Salazar-Mather TP. Dalod MY, et al. Coordinated and distinct roles for IFN-α/β, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 47.Orange J. Biron C. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 48.Pahl-Seibert M-F. Juelch M. Podlech J. Thomas D. Deegen P. Reddehase MJ. Holtappels R. Highly protective in vivo function of cytomegalovirus IE1 epitope-specific memory CD8 T cells purified by T-cell receptor-based cell sorting. J Virol. 2005;79:5400–5413. doi: 10.1128/JVI.79.9.5400-5413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardigon N. Bercovici N. Calbo S. Santos-Lima EC. Liblau R. Kourilsky P. Abastado JP. Role of co-stimulation in CD8 + T cell activation. Int Immunol. 1998;10:619–630. doi: 10.1093/intimm/10.5.619. [DOI] [PubMed] [Google Scholar]
- 50.Podlech J. Holtappels R. Pahl-Seibert MF. Steffens HP. Reddehase MJ. Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J Virol. 2000;74:7496–7507. doi: 10.1128/jvi.74.16.7496-7507.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quinnan GV. Manischewitz JE. The role of natural killer cells and antibody-dependent cell-mediated cytotoxicity during murine cytomegalovirus infection. J Exp Med. 1979;150:1549–1554. doi: 10.1084/jem.150.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinnan GV. Manischewitz JE. Ennis FA. Cytotoxic T lymphocyte response to murine cytomegalovirus infection. Nature. 1978;273:541–543. doi: 10.1038/273541a0. [DOI] [PubMed] [Google Scholar]
- 53.Quinnan GV. Manischewitz JE. Ennis PA. Role of cytotoxic T lymphocytes in murine cytomegalovirus infection. J Gen Virol. 1980;47:503–508. doi: 10.1099/0022-1317-47-2-503. [DOI] [PubMed] [Google Scholar]
- 54.Reddehase MJ. Keil GM. Koszinowski UH. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur J Immunol. 1984;14:56–61. doi: 10.1002/eji.1830140111. [DOI] [PubMed] [Google Scholar]
- 55.Reddehase MJ. Mutter W. Munch K. Buhring HJ. Koszinowski UH. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61:3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reddehase MJ. Weiland F. Munch K. Jonjic S. Luske A. Koszinowski UH. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robbins SH. Bessou G. Cornillon A, et al. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathogens. 2007;3:e123. doi: 10.1371/journal.ppat.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robbins SH. Tessmer MS. Mikayama T. Brossay L. Expansion and contraction of the NK cell compartment in response to murine cytomegalovirus infection. J Immunol. 2004;173:259–266. doi: 10.4049/jimmunol.173.1.259. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 60.Shahinian A. Pfeffer K. Lee KP, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 61.Shanley JD. Jordan MC. Stevens JG. Modification by adoptive humoral immunity of murine cytomegalovirus infection. J Infect Dis. 1981;143:231–237. doi: 10.1093/infdis/143.2.231. [DOI] [PubMed] [Google Scholar]
- 62.Shellam GR. Allan JE. Papadimitriou JM. Bancroft GJ. Increased susceptibility to cytomegalovirus infection in beige mutant mice. Proc Natl Acad Sci USA. 1981;78:5104–5108. doi: 10.1073/pnas.78.8.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simmons RL. Matas AJ. Rattazzi LC. Balfour HH., Jr Howard JR. Najarian JS. Clinical characteristics of the lethal cytomegalovirus infection following renal transplantation. Surgery. 1977;82:537–546. [PubMed] [Google Scholar]
- 64.Smith HRC. Heusel JW. Mehta IK, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spector SA. Wong R. Hsia K. Pilcher M. Stempien MJ. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J Clin Invest. 1998;101:497–502. doi: 10.1172/JCI1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Starr SE. Allison AC. Role of T lymphocytes in recovery from murine cytomegalovirus infection. Infect Immun. 1977;17:458–462. doi: 10.1128/iai.17.2.458-462.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sylwester AW. Mitchell BL. Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8 + T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tay C. Welsh R. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tay CH. Yu LYY. Kumar V. Mason L. Ortaldo JR. Welsh RM. The role of LY49 NK cell subsets in the regulation of murine cytomegalovirus infections. J Immunol. 1999;162:718–726. [PubMed] [Google Scholar]
- 70.Thebeau LG. Morrison LA. B7 costimulation plays an important role in protection from herpes simplex virus type 2-mediated pathology. J Virol. 2002;76:2563–2566. doi: 10.1128/jvi.76.5.2563-2566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thebeau LG. Vagvala SP. Wong YM. Morrison LA. B7 costimulation molecules expressed from the herpes simplex virus 2 genome rescue immune induction in B7-deficient mice. J Virol. 2007;81:12200–12209. doi: 10.1128/JVI.01224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tripathy SK. Smith HRC. Holroyd EA. Pingel JT. Yokoyama WM. Expression of m157, a murine cytomegalovirus-encoded putative major histocompatibility class I (MHC-I)-like protein, is independent of viral regulation of host MHC-I. J Virol. 2006;80:545–550. doi: 10.1128/JVI.80.1.545-550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Voigt V. Forbes CA. Tonkin JN. Degli-Esposti MA. Smith HRC. Yokoyama WM. Scalzo AA. Murine cytomegalovirus m157 mutation and variation leads to immune evasion of natural killer cells. Proc Natl Acad Sci USA. 2003;100:13483–13488. doi: 10.1073/pnas.2233572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waisman A. Croxford AL. Demircik F. New tools to study the role of B cells in cytomegalovirus infections. Med Microbiol Immunol. 2008;197:145–149. doi: 10.1007/s00430-008-0088-z. [DOI] [PubMed] [Google Scholar]
- 75.Wilson JL. Charo J. Martin-Fontecha A, et al. NK cell triggering by the human costimulatory molecules CD80 and CD86. J Immunol. 1999;163:4207–4212. [PubMed] [Google Scholar]
- 76.Wu Y. Guo Y. Huang A. Zheng P. Liu Y. CTLA-4-B7 interaction is sufficient to costimulate T cell clonal expansion. J Exp Med. 1997;185:1327–1336. doi: 10.1084/jem.185.7.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]