Abstract
Heart failure (HF) is a complex clinical syndrome with multiple aetiologies. Current treatment options can slow the progression to HF, but overall the prognosis remains poor. Clinical studies suggest that high dietary intake of the ω-3 polyunsaturated fatty acids (ω-3PUFA) found in fish oils (eicosapentaenoic and docosahexaenoic acids) may lower the incidence of HF, and that supplementation with pharmacological doses prolongs event-free survival in patients with established HF. The mechanisms for these potential benefits are complex and not well defined. It is well established that fish oil supplementation lowers plasma triglyceride levels, and more recent work demonstrates anti-inflammatory effects, including reduced circulating levels of inflammatory cytokines and arachidonic acid-derived eicosanoids, and elevated plasma adiponectin. In animal studies, fish oil favourably alters cardiac mitochondrial function. All of these effects may work to prevent the development and progression of HF. The ω-3PUFA found in plant sources, α-linolenic acid, may also be protective in HF; however, the evidence is not as compelling as for fish oil. This review summarizes the evidence related to use of ω-3PUFA supplementation as a potential treatment for HF and discusses possible mechanisms of action. In general, there is growing evidence that supplementation with ω-3PUFA positively impacts established pathophysiological targets in HF and has potential therapeutic utility for HF patients.
Keywords: α-Linolenic acid, Cardiac, Docosahexaenoic acid, Eicosapentaenoic acid, Inflammation, Metabolism
1. Introduction
Despite aggressive treatment, heart failure (HF) continues to have substantial morbidity and mortality, and is on the rise in most regions of the world.1 Current medical therapies for HF are mainly aimed at suppressing neurohormonal over-activation and treating haemodynamic symptoms. These pharmacotherapies improve clinical symptoms and slow progression of contractile dysfunction and expansion of left ventricular (LV) chamber volume, but nevertheless HF progression continues and prognosis for even optimally treated patients remains poor.2–6 Thus, there is a need for novel adjunctive therapies that act independently of current neurohormonally and haemodynamically oriented drugs. Nutritional approaches, such as dietary supplementation with ω-3 polyunsaturated fatty acids (ω-3PUFA), are particularly attractive because they could work additively with established therapies while not exerting negative haemodynamic effects.7,8
There is extensive evidence in support of the concept that a high intake of ω-3PUFA from fish oil—eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—exerts cardioprotective effects in terms of coronary artery disease and sudden cardiac death.9–12 On the basis of this evidence, current dietary guidelines recommend a daily intake of 1 g of EPA + DHA for primary and secondary prevention of coronary heart disease,11,13,14 whereas higher pharmacological doses of 3–4 g/day are recommended for the treatment of hypertriglyceridaemia.15,16 Recent epidemiological and experimental studies suggest that ω-3PUFA of marine origin may prevent the development and progression of HF17–19 at relatively low intakes that can be achieved solely through high consumption of oily fish (∼0.5 g/day of EPA + DHA). The GISSI-HF trial found that a low dose of EPA + DHA (∼0.85 g/day) significantly reduced mortality compared with placebo in HF patients.20 ω-3PUFA supplementation can favourably affect inflammation, plasma lipid profile,21,22 blood pressure,23 and cardiac mitochondrial function,24,25 all of which could prevent the development and progression of HF. These effects operate via multiple signalling pathways, beginning with the enrichment of cell membrane phospholipids with EPA and DHA. This can influence membrane function, modulating ion channels and receptors and the eicosanoid pathways. ω-3PUFA are ligands for a variety of nuclear transcription factors and thus they can impact the inflammatory response and lipid metabolism.
This review presents an overview of the current knowledge of ω-3PUFA in HF. The potential effects of ω-3PUFA supplementation on the prevention and treatment of HF are reviewed, and established and potential mechanisms of action of ω-3PUFA are discussed.
2. Metabolism of ω-3PUFA
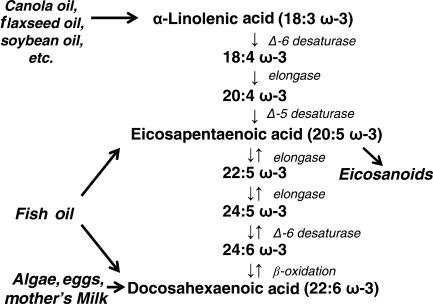
ω-3PUFA are one of the two classes of essential fatty acids, which cannot be synthesized de novo by mammals because the necessary enzymes to place a double bond at the ω-3 position are absent.26 Thus, ω-3PUFA must be obtained in adequate amounts from the diet. The most widely available ω-3PUFA for humans is α-linolenic acid (ALA, 18:3ω-3) which is abundant in some vegetable oils such as flaxseed oil, canola oil, and soybean oil. ALA can be converted by chain elongation and desaturation into long-chain ω-3PUFA containing 20 or more carbon atoms, EPA 20:5ω-3 and DHA 22:6ω-3 (Figure 1).27 Approximately 1–5% of dietary ALA is converted to EPA, and an additional <0.1% is converted to DHA, in young men.28 In women, this conversion is somewhat greater29 and is reduced by ∼50% with a Western diet rich in ω-6PUFA.30 Both EPA and DHA are found in fish oil, whereas DHA is high in eggs, mother's milk, and some strains of algae.
Figure 1.
The pathway for metabolism of ALA, EPA, and DHA.
This review will focus mainly on the effects of EPA and DHA, as they have generally been shown to be more potent and effective than ALA in reducing cardiovascular disease risk factors and clinical events, and in impacting the underlying biochemical mechanisms.31,32 Clinical fish oil supplements are a mixture of EPA and DHA, with a range of formulations varying from a ratio of ∼30:70 EPA to DHA, to a ratio of 70:30. Unfortunately, there are few data that assess the effects of EPA and DHA given separately, or comparing various EPA:DHA ratios. Thus, in the subsequent discussion, we will refer to EPA + DHA in general.
The ω-6PUFA are the other class of essential fatty acids, comprised mainly of linoleic acid (18:2ω-6) and its metabolite, arachidonic acid (AA, 20:4ω-6).33 AA and EPA are substrates for cyclooxygenase (COX) and lipoxygenase involved in the production of eicosanoids (Figure 1).34 Both ω-3 and ω-6PUFA and their metabolites influence cellular homeostasis.35 It is the structural differences between these classes of PUFA that determine the specific properties associated with each, and a balanced intake of both ω-6 and ω-3PUFA is necessary to support optimal cardiovascular function,36 with optimal ratio of ω-6 to ω-3PUFA considered to be 1:1 to 4:1.34,37 In the diet consumed in developed countries (i.e. the ‘Western diet’), the ratio of ω-6 to ω-3PUFA can increase to 20:1–30:1,38 which is well above what is considered to be the healthy range, and can be an independent risk factor for developing HF and coronary artery disease.36,39
3. Metabolic effects of ω-3PUFA
3.1. ω-3PUFA lower triglyceride synthesis
It is well established that daily doses of ∼1 g EPA + DHA or greater lower serum triglycerides in humans,22,40 with similar effects in experimental animals at equivalent relative doses.21 In a meta-analysis of 72 placebo-controlled trials, Harris22 reported reductions of 25–35% in serum triglycerides with doses of 3–4 g/day of EPA + DHA, with greater reduction seen in those with higher triglyceride concentrations (>500 mg/dL). This effect is associated with modest increases of 5–10% in LDL cholesterol, and increases of 1–3% in HDL. Across studies, EPA + DHA lowered triglyceride levels in a dose-dependent fashion, with each 1 g/day increase in EPA + DHA associated with a ∼8 mg/dL decrease in serum triglyceride concentration.16
Several mechanisms have been proposed for the reduction in serum triglyceride levels seen with EPA + DHA. First, EPA + DHA reduce hepatic triglyceride synthesis due to reduction of substrate (fatty acids) availability. De novo fatty acid synthesis is regulated by the transcription factor sterol regulatory element-binding protein (SREBP)-1c, which stimulates synthesis of acetyl-CoA carboxylase-1 and fatty acid synthase, key lipogenic enzymes.41 In fish oil-fed mice, a decrease in triglyceride levels was associated with less mRNA expression for SREBP-1c in the liver.42 A similar effect was observed in EPA-treated HepG2 human hepatoma cells.43 SREBP-1c expression is under the regulation of other transcription factors,44 such as liver X receptor (LXR) and fernesoid X receptor (FXR), and both may be a target of EPA and DHA.45,46 Inhibition of LXR and/or stimulation of FXR by EPA + DHA may lead to suppression of SREBP-1c and SREBP-1c-regulated lipogenic genes. On the other hand, rat studies have shown that EPA and/or DHA increase peroxisomal and mitochondrial fatty acid β-oxidation,47 leaving less substrate for triglyceride synthesis. The expression of key genes involved in β-oxidation is controlled by the peroxisome proliferator-activated receptor (PPAR) α41 which is activated by ω-3PUFA.48 In addition, EPA and DHA have been shown to lower serum levels of non-esterified free fatty acids (NEFA),18,19 leading to decreased delivery of fatty acids to the liver. EPA and DHA also lower triglyceride levels via inhibition of the activity of diacylglycerol acyltransferase and phosphatidic acid phosphohydrolase,47 critical SREBP-1c-regulated enzymes involved in triglyceride synthesis.44 Finally, EPA and DHA can enhance VLDL triglyceride clearance via increased activity of lipoprotein lipase, a PPAR-regulated triglyceride hydrolase present in the capillary endothelium of various tissues. ω-3PUFA supplementation in humans resulted in an increase in gene expression49 of lipoprotein lipase in adipose tissue and increase in plasma lipoprotein lipase activity.50,51 Taken together, favourable effects of EPA and DHA on serum triglycerides and NEFA levels are attributable to modulation of nuclear transcription factors that control the expression of enzymes involved in triglyceride metabolism.
3.2. PPARs and mitochondrial function
Activation of PPARα, γ, and β/δ increase transcription of genes encoding proteins involved in the transport and metabolism of fatty acids. ω-3PUFA are potent activators of PPARs;48,52,53 thus, dietary supplementation with either ALA or EPA + DHA should induce expression of key proteins involved in cardiac lipid metabolism and may prevent the deterioration in mitochondrial function and depression in fatty acid oxidation that are classically observed in advanced HF.54–58 Our recent studies in rats subjected to pressure overload showed that dietary EPA + DHA did not significantly increase mRNA for PPARα target genes in the heart, including uncoupling protein 3, carnitine palmitoyltransferase-1, pyruvate dehydrogenase kinase 4, and medium chain acyl-CoA dehydrogenase.18 This finding indicates that cardiac activation of PPARs may not be an important mechanism for the cardioprotective effects of EPA + DHA.
Supplementation with EPA + DHA could exert a protective effect on the heart through improvement in mitochondrial function and the efficiency of ATP generation.59,60 Rats fed fish oil high in EPA + DHA showed a decrease in myocardial oxygen consumption without a decrease in LV power generation, resulting in greater LV mechanical efficiency in isolated perfused hearts studied under aerobic conditions and during post-ischaemic reperfusion.59,60 This phenomenon was observed over a wide range of LV filling pressures and workloads.59 It is proposed that this effect is due to changes in mitochondrial membrane phospholipid composition and improved efficiency of ATP generation;24,59,60 however, the precise mechanisms are not known.
4. Anti-inflammatory effect of ω-3PUFA
HF is associated with increased levels of inflammatory mediators such as eicosanoids61–63 and cytokines.64–68 Evidence from knockout mice suggests that both eicosanoids and cytokines are involved in myocyte hypertrophy, apoptosis, and ventricular remodelling leading to HF.69,70
4.1. Eicosanoid production
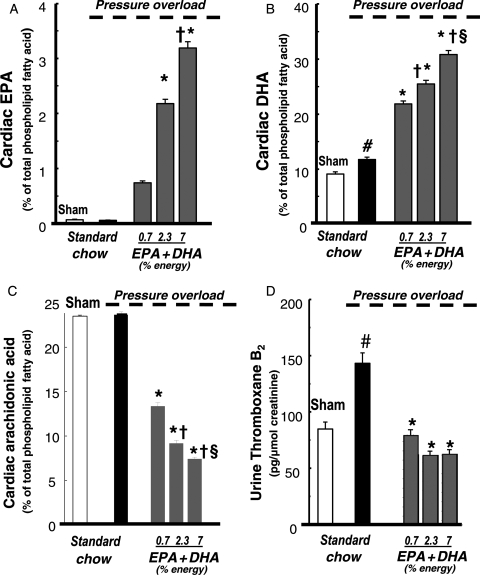
In humans consuming a typical Western diet, AA is the predominant PUFA in cell membrane phospholipids (∼20–25% of total membrane fatty acids).71,72 AA is a substrate for COX and lipoxygenase and thus is the precursor to pro-inflammatory prostaglandins, thromboxanes, and leukotrienes.73 Increased consumption of EPA + DHA results in a dose-dependent increase in their incorporation into membrane phospholipids in healthy hearts and in hearts subjected to chronic arterial pressure overload to induce cardiac hypertrophy and dysfunction19,74 (Figure 2). In addition, there is proportional decrease in AA in phospholipid in myocardium (Figure 2), erythrocytes,74 and inflammatory cells.75,76 Since less substrate is available for AA-derived eicosanoids, EPA + DHA supplementation decreases in vivo production of prostaglandin PGE2, 77,78 thromboxane A2, 19,79 and leukotriene B4.80 In addition, EPA becomes an alternative substrate for COX and lipoxygenase leading to production of anti-inflammatory eicosanoids, such as prostaglandin PGE3, thromboxane A3, and leukotriene B5.81 Recent studies found that EPA and DHA also generate resolvins, an anti-inflammatory eicosanoids in the COX-2 pathway.82,83 In addition, when rats subjected to pressure overload were supplemented with EPA + DHA, the pressure overload-induced increase in thromboxane B2 and 6-keto prostaglandin F1 in the urine was prevented.19 The fall in thromboxane B2, a breakdown product of prothrombotic, vasoconstrictive thromboxane A2, was likely due to the decreased incorporation of AA into cardiac phospholipids (Figure 2).19 Thus, EPA and DHA decrease production of pro-inflammatory AA-derived eicosanoids in the heart, which likely exerts a beneficial effect in HF.
Figure 2.
Effect of EPA + DHA supplementation in rats with pressure overload induced by constriction of the abdominal aorta on the cardiac content of EPA (A), DHA (B), and AA (C). Urinary levels of thromboxane B2 (D), the primary breakdown product of AA-derived thromboxane A2. Data are the mean ± SEM; n = 8. #P < 0.05 vs. sham; *P < 0.05 vs. standard chow; †P < 0.05 vs. 0.7 EPA + DHA; §P < 0.05 vs. 2.3 EPA + DHA. From Duda et al.19
4.2. Cytokines
Supplementation with EPA and DHA reduces inflammation, as reflected in lower circulating levels of the pro-inflammatory cytokines TNFα, IL-1, and IL-6.77,84–87 The mechanisms for this effect again may be linked to the ability of ω-3PUFA to modulate transcription factors. Cytokine synthesis is regulated by nuclear transcription factor kappa B (NF-κB),88 which is activated in HF.89,90 In vitro, EPA prevented LPS-induced NF-κB activation and expression of TNFα mRNA.91 NF-κB activity is negatively regulated by PPARs in neonatal rat cardiac myocytes, where PPARα and γ agonists inhibited NF-κB activation and expression of TNFα mRNA in response to LPS.92 Thus, ω-3PUFA activation of PPARs may lead to suppression of NF-κB and NF-κB-regulated pro-inflammatory cytokines. In addition, it has been suggested that NF-κB activity is also modulated by adiponectin, a cardioprotective adipocyte-derived hormone.93
4.3. Adiponectin
Adiponectin exerts protective effects on the heart and vasculature.94–97 Its expression and secretion in adipose tissue are up-regulated by PPARγ agonists and EPA + DHA via a PPARγ-dependent mechanism.18,98 Circulating adiponectin levels are elevated in a dose-dependent fashion with supplementation with EPA and DHA in rats,18,19 mice,98,99 and humans.100 Adiponectin knockout mice have enhanced LV pathology in response to pressure overload, which can be rescued by adenovirus-mediated delivery of adiponectin.94 These protective effects of adiponectin have been attributed to the activation of AMPK94 and inhibition of Akt.101 However, changes in Akt and AMPK activation were not observed in our recent studies in rats subjected to pressure overload, where there was a large dose-dependent increase in adiponectin that was strongly associated with improved LV function and remodelling.18,19 As suggested above, the beneficial effect of adiponectin could be linked to its anti-inflammatory action, as elevated adiponectin levels are associated with reduced inflammation in humans.102 In addition, pharmacological inhibitors of the COX-2 pathway were found to reverse the inhibitory effects of adiponectin on LPS-induced TNFα production in cardiomyocytes, indicating that adiponectin may exert its effects in a COX-2-dependent manner.101
5. Other beneficial effects of ω-3PUFA
In patients, supplementation with EPA and DHA (dose ∼3.4 g/day) improved endothelial function, possibly through altered NO production.40,103 This effect could be responsible for the modest blood pressure-lowering effect of ω-3PUFA. A meta-analysis of 36 randomized trials showed a moderate reduction in systolic blood pressure by 2.1 mmHg (P < 0.001) and in diastolic blood pressure by 1.6 mmHg (P < 0.001) with a dose of ∼3.7 g/daily of EPA + DHA.23 These effects were larger in older and hypertensive subjects. In addition, a meta-analysis of 30 randomized trials found that fish oil (∼3.5 g/day of EPA + DHA) reduced heart rate by 2.5 bpm (P < 0.001) in subjects with baseline heart rate ≥69 bpm and treatment duration ≥12 weeks.104
6. ω-3PUFA and HF in humans: epidemiological and intervention studies
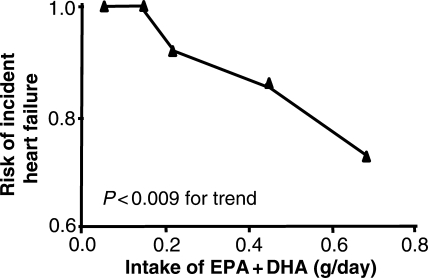
HF is not a simple disease but rather a complex clinical syndrome with multiple aetiologies. As discussed above, EPA and DHA favourably modulates various factors linked to the pathophysiology of HF, including triglyceride and fatty acid levels, cardiac metabolism, mitochondrial function, inflammatory response, and endothelial function. All of these effects should combine to attenuate the development and progression of HF. While high intake of EPA + DHA exerts cardioprotective effects in both primary and secondary coronary heart disease prevention,12,14,105 little is known regarding the effect of EPA + DHA intake of the incidence of HF. A large-scale epidemiological study conducted in the USA showed that dietary intake of EPA + DHA was negatively associated with the development of HF17 in older adults. Over a 12-year period, fish oil consumption was associated with a lower incident of HF, with 20% lower risk with intake 1–2 times/week, 31% lower risk with intake ≥3 times/week, compared with intake <1 time/month or less. Estimated dietary intake of marine ω-3PUFA was also inversely associated with HF, with 37% lower risk in the highest quintile of intake compared with the lowest (Figure 3). A similar association between consumption of EPA + DHA and reduction in the incidence of HF was recently observed in a Japanese population.106
Figure 3.
Effect for developing HF over a 12 years period among 4738 adults (>65 years) plotted as a function of estimated intake of EPA + DHA. Reprinted from Mozaffarian et al.,17 Copyright 2005, with permission from Elsevier.
The first trial with a clinical outcome with EPA + DHA supplementation in HF patients was reported last year.20 The GISSI-HF study was a randomized, double-blind, placebo-controlled trial to investigate whether low-dose supplementation with EPA + DHA would reduce hospitalization and morbidity and mortality in a large population of symptomatic HF patients. Patients with New York Heart Association class II–IV HF (ejection fraction 33 ± 8%, and 63% of patients NYHA Class II) were randomly assigned to fish oil (0.85 g/day of EPA + DHA) (n = 3494) or placebo (n = 3481) and followed for a median of 3.9 years. The fish oil supplement used in this study is approved in Europe and the USA for the treatment of hypertriglyceridaemia and contains EPA and DHA at a ratio of 45:55. The primary endpoints of the trial were all-cause mortality and time to death or admission to hospital for cardiovascular reasons. The treatment was well tolerated and showed a significant reduction in mortality [adjusted hazard ratio (HR) 0.91, P = 0.041] and admission to hospital for cardiovascular reasons (adjusted HR 0.92, P = 0.009). While the benefit was modest, it is important to keep in mind that the dose was low, and the study population was aggressively treated with β-blockers (65%) and ACE inhibitors or ARBs (93%). The dose of EPA + DHA used in the GISSI-HF trial was only 25% of the recommended dose for the treatment of hypertriglyceridaemia,107 which suggests that future trials should evaluate much higher doses.
HF is characterized by abnormalities of cardiac repolarization which increase the risk of sudden death. In HF patients who die, almost 50% of the deaths are sudden.108 Numerous experimental and clinical studies have demonstrated anti-arrhythmic effect of EPA + DHA109–111 and a decreased incidence of sudden death with ω-3PUFA supplementation.12,105 The effect of ω-3PUFA on sudden cardiac death has not been investigated in an HF population. The influence of ω-3PUFA on cardiac electrophysiology is outside the focus of this review, and the reader is referred to recent review papers for more details.12,112–114
7. ω-3PUFA in animal models of HF
Experimental studies also support and help in the understanding of the favourable effect of EPA + DHA on HF. Takahashi et al.115 found that dietary supplementation with fish oil attenuated cardiac hypertrophy with improved cardiac function and prolonged life in mice with genetic systemic carnitine deficiency. These mice develop HF secondary to the inability to oxidize fatty acids. They also noted that supplementation with fish oil altered the myocardial concentration of various diacylglycerols and prevented diacylglycerol-induced activation of protein kinase C, which has been linked to cardiac hypertrophy and development of HF.116
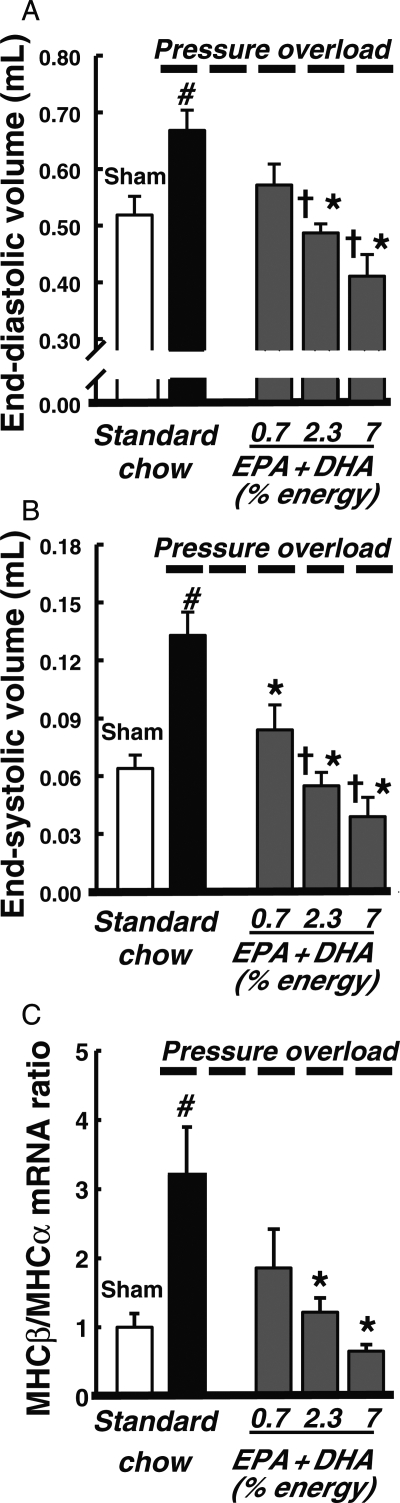
More recently, the cardioprotective effects of fish oil supplementation have been reported in a rat model of pressure overload-induced HF.18,19 Fish oil supplementation prevented LV remodelling and contractile dysfunction in a dose-dependent fashion (Figure 4).19 These doses corresponded to a human intake of ∼1.6, 5.1, and 15.5 g/day of EPA + DHA (calculated assuming an energy intake of 2000 kcal/day). These beneficial effects correlated with the increase in plasma concentration of adiponectin and suppressed inflammation as was seen by a decrease in urinary thromboxane B2 and plasma TNFα. In addition, this study reported cardioprotective effects only with supplementation with ω-3PUFA derived from fish, but not from a vegetable source (ALA).19 These effects are probably a result of the changes in phospholipids from cardiac membranes that were observed with dietary treatment. The fish oil diets increased EPA and DHA incorporation into cardiac phospholipids and decreased AA content to a greater extent than the ALA diets.19 As noted above, the composition of cardiac membranes can alter downstream eicosanoid signalling.
Figure 4.
Effect of EPA + DHA supplementation on LV end-diastolic (A) and end-systolic (B) volumes, and the ratio of myosin heavy chain (MHC)-β to MHC-α, as established molecular marker of HF (C) at 11 weeks in rats with pressure overload-induced HF. Data are the mean ± SEM; n = 9–11. #P < 0.05 vs. sham; *P < 0.05 vs. standard chow; †P < 0.05 vs. 0.7 EPA + DHA. From Duda et al.19
8. Effects of ALA in HF
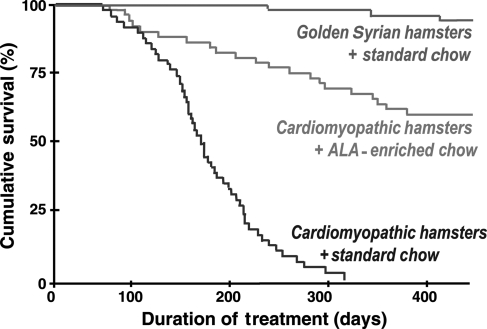
High intake of ALA prevents coronary heart disease37,117–119 and a recent animal study suggests that it may have favourable effects in HF.120 Dietary ALA is associated with fewer coronary events,121–123 lower triglycerides,124 decreased thromboxane B2,125 and inhibition of cytokine production,126,127 all of which should positively influence outcomes in HF. Fiaccavento et al.120 recently showed that high intake of ALA from flaxseed oil (∼11% of energy intake as ALA) significantly increased survival time compared with the standard diet in cardiomyopathic hamsters (Figure 5). Histological examination at 150 days of treatment showed that the cardiomyopathic hamsters fed the ALA diet had preserved sarcolemmal and mitochondrial membrane integrity, less fibrosis, and normal mRNA expression for myosin heavy chain isoforms, atrial natriuretic peptide, and transforming growth factor-β1. In contrast, animals fed the standard diet exhibited the typical HF pathology compared with normal hamsters. When cardiomyopathic hamsters were fed the high ALA diet, LV peak systolic and end-diastolic pressures and peak positive and negative dP/dt were not different from normal hamsters and were significantly improved compared with the cardiomyopathic hamsters fed the standard diet. It is important to note that the cardiomyopathic hamster is an atypical genetic model that has limited relevance to most human HF; nevertheless, there is clearly a dramatic positive effect with the ALA-enriched diet in this model.
Figure 5.
Survival rates of cardiomyopathic Syrian hamsters and golden Syrian hamsters. Statistically significant differences are observed between HF animals (cardiomyopathic Syrian hamsters) fed the standard chow and both healthy controls (golden Syrian hamsters) (P < 0.01) and HF animals fed the ALA-enriched diet. Reprinted from Fiaccavento et al.,120 with permission from the American Society for Investigative Pathology.
We recently evaluated the effect of ALA in the early stages of pressure overload-induced HF in rats using ALA intakes of 0.7, 2.3, and 7% of energy intake, and observed prevention of the increase in cardiomyocyte apoptosis at the highest dose of ALA.19 On the other hand, we did not see a consistent positive benefit on LV function, unlike the strong favourable effect observed with equivalent doses of EPA + DHA. It is important to keep in mind that our study only addressed the early stages of the progression of HF (initial 12 weeks of pressure overload) and thus our neutral results should be interpreted with caution. The effects of high intake of ALA on apoptosis may not translate into improved LV function and survival until much later (∼18–24 weeks). In addition, the study by Fiaccavento et al. showed a strong positive effect on survival at with ∼11% of energy intake as ALA, suggesting that perhaps a higher dose is needed.
9. Conclusion and future directions
At present, there is growing evidence that ω-3PUFA supplementation positively impacts established pathophysiological mechanisms in HF and thus has potential for preventing and treating HF. The effects of EPA + DHA on inflammation, membrane phospholipid composition, and mitochondrial function in the failing heart are not well characterized and require extensive study. The use of alternative models of HF, such as myocardial infarction-induced HF, could provide further insight into understanding how EPA + DHA affects membrane structure/function, inflammatory pathways, mitochondrial function, and clinically relevant endpoints. In addition, while protective effects have been observed with ALA supplementation, they are poorly understood and deserve thorough attention. ALA has advantages over EPA and DHA, in that high intake of ALA is possible at low cost through diet alone, thus from a public health standpoint ALA has major advantages over EPA or DHA.
The evaluation of ω-3PUFA in HF patients should be a primary component of future research in this field. At present, while there is encouraging evidence for the use of of ω-3PUFA in the treatment of HF, clear results from pivotal trials are lacking. The findings of the recent GISSI trial, which showed modest reduction in mortality and cardiovascular-related hospital admissions in HF patients with a low dose of EPA + DHA (0.85 g/day),20 indicate that additional trials with a higher dose (>3 g/day) are warranted. There is woefully little data from patients regarding the mechanism of action of ω-3PUFA in HF, thus future trials should include assessment of inflammatory markers, membrane phospholipids, and cardiac energetics.
Conflict of interest: none declared.
Funding
W.C.S. was supported by NIH grants HL074237 and HL091307. M.K.D. was supported by Foundation for Polish Science grant HOM/2008/2B.
References
- 1.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2005;46:e1–e81. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Coats AJ. Angiotensin type-1 receptor blockers in heart failure. Prog Cardiovasc Dis. 2002;44:231–242. doi: 10.1053/pcad.2002.31585. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation. 1996;94:2807–2816. doi: 10.1161/01.cir.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 4.Colucci WS, Packer M, Bristow MR, Gilbert EM, Cohn JN, Fowler MB, et al. Carvedilol inhibits clinical progression in patients with mild symptoms of heart failure. US Carvedilol Heart Failure Study Group. Circulation. 1996;94:2800–2806. doi: 10.1161/01.cir.94.11.2800. [DOI] [PubMed] [Google Scholar]
- 5.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–448. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- 7.Stanley WC, Hoppel CL. Mitochondrial dysfunction in heart failure: potential for therapeutic interventions? Cardiovasc Res. 2000;45:805–806. doi: 10.1016/s0008-6363(99)00419-8. [DOI] [PubMed] [Google Scholar]
- 8.Sharma N, Okere IC, Duda MK, Chess DJ, O'shea KM, Stanley WC. Potential impact of carbohydrate and fat intake on pathological left ventricular hypertrophy. Cardiovasc Res. 2007;73:257–268. doi: 10.1016/j.cardiores.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 2006;98:3i–18i. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Das UN. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukot Essent Fatty Acids. 2000;63:351–362. doi: 10.1054/plef.2000.0226. [DOI] [PubMed] [Google Scholar]
- 11.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:e20–e30. doi: 10.1161/01.atv.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 12.Mozaffarian D. Fish and n-3 fatty acids for the prevention of fatal coronary heart disease and sudden cardiac death. Am J Clin Nutr. 2008;87:1991S–1996S. doi: 10.1093/ajcn/87.6.1991S. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 14.GISSI-Prevezione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 15.Weber P, Raederstorff D. Triglyceride-lowering effect of omega-3 LC-polyunsaturated fatty acids–a review. Nutr Metab Cardiovasc Dis. 2000;10:28–37. [PubMed] [Google Scholar]
- 16.Jacobson TA. Role of n-3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. Am J Clin Nutr. 2008;87:1981S–1990S. doi: 10.1093/ajcn/87.6.1981S. [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Duda MK, O'shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, et al. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007;76:303–310. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duda MK, O'shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, et al. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovascular Res. 2009;81:319–327. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, et al. Gissi-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 21.Harris WS. n-3 fatty acids and serum lipoproteins: animal studies. Am J Clin Nutr. 1997;65:1611S–1616S. doi: 10.1093/ajcn/65.5.1611S. [DOI] [PubMed] [Google Scholar]
- 22.Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65:1645S–1654S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 23.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–1499. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Pepe S, Tsuchiya N, Lakatta EG, Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–H158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- 25.McMillin JB, Bick RJ, Benedict CR. Influence of dietary fish oil on mitochondrial function and response to ischemia. Am J Physiol. 1992;263:H1479–H1485. doi: 10.1152/ajpheart.1992.263.5.H1479. [DOI] [PubMed] [Google Scholar]
- 26.Roche HM. Unsaturated fatty acids. Proc Nutr Soc. 1999;58:397–401. doi: 10.1017/s002966519900052x. [DOI] [PubMed] [Google Scholar]
- 27.Jump DB. The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem. 2002;277:8755–8758. doi: 10.1074/jbc.R100062200. [DOI] [PubMed] [Google Scholar]
- 28.Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br J Nutr. 2002;88:355–363. doi: 10.1079/BJN2002662. [DOI] [PubMed] [Google Scholar]
- 29.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–420. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- 30.Gerster H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int J Vitam Nutr Res. 1998;68:159–173. [PubMed] [Google Scholar]
- 31.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 33.Salem NR, Pawlosky R, Wegher B, Hibbeln J. In vivo conversion of linoleic acid to arachidonic acid in human adults. Prostaglandins Leukot Essent Fatty Acids. 1999;60:407–410. doi: 10.1016/s0952-3278(99)80021-0. [DOI] [PubMed] [Google Scholar]
- 34.Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70:560S–569S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- 35.Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24:597–615. doi: 10.1146/annurev.nutr.24.012003.132106. [DOI] [PubMed] [Google Scholar]
- 36.Harris WS. The omega-6/omega-3 ratio and cardiovascular disease risk: uses and abuses. Curr Atheroscler Rep. 2006;8:453–459. doi: 10.1007/s11883-006-0019-7. [DOI] [PubMed] [Google Scholar]
- 37.Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr. 2006;83:1526S–1535S. doi: 10.1093/ajcn/83.6.1526S. [DOI] [PubMed] [Google Scholar]
- 38.Robinson JG. Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. Am J Cardiol. 2006;98:39i–49i. doi: 10.1016/j.amjcard.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 39.Harris WS, Assaad B, Poston WC. Tissue omega-6/omega-3 fatty acid ratio and risk for coronary artery disease. Am J Cardiol. 2006;98:19i–26i. doi: 10.1016/j.amjcard.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Harris WS, Rambjor GS, Windsor SL, Diederich D. n-3 fatty acids and urinary excretion of nitric oxide metabolites in humans. Am J Clin Nutr. 1997;65:459–464. doi: 10.1093/ajcn/65.2.459. [DOI] [PubMed] [Google Scholar]
- 41.Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 42.Le Jossic-Corcos C, Gonthier C, Zaghini I, Logette E, Shechter I, Bournot P. Hepatic farnesyl diphosphate synthase expression is suppressed by polyunsaturated fatty acids. Biochem J. 2005;385:787–794. doi: 10.1042/BJ20040933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaima N, Sugawara T, Goto D, Hirata T. Trans geometric isomers of EPA decrease LXRalpha-induced cellular triacylglycerol via suppression of SREBP-1c and PGC-1beta. J Lipid Res. 2006;47:2712–2717. doi: 10.1194/jlr.M600273-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura MT, Cheon Y, Li Y, Nara TY. Mechanisms of regulation of gene expression by fatty acids. Lipids. 2004;39:1077–1083. doi: 10.1007/s11745-004-1333-0. [DOI] [PubMed] [Google Scholar]
- 45.Pawar A, Botolin D, Mangelsdorf DJ, Jump DB. The role of liver X receptor-alpha in the fatty acid regulation of hepatic gene expression. J Biol Chem. 2003;278:40736–40743. doi: 10.1074/jbc.M307973200. [DOI] [PubMed] [Google Scholar]
- 46.Claudel T, Inoue Y, Barbier O, Duran-Sandoval D, Kosykh V, Fruchart J, et al. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125:544–555. doi: 10.1016/s0016-5085(03)00896-5. [DOI] [PubMed] [Google Scholar]
- 47.Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17:387–393. doi: 10.1097/01.mol.0000236363.63840.16. [DOI] [PubMed] [Google Scholar]
- 48.Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 49.Khan S, Minihane AM, Talmud PJ, Wright JW, Murphy MC, Williams CM, et al. Dietary long-chain n-3 PUFAs increase LPL gene expression in adipose tissue of subjects with an atherogenic lipoprotein phenotype. J Lipid Res. 2002;43:979–985. [PubMed] [Google Scholar]
- 50.Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J Lipid Res. 2003;44:455–463. doi: 10.1194/jlr.M200282-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Harris WS, Lu G, Rambjor GS, Walen AT, Ontko JA, Cheng Q, et al. Influence of n-3 fatty acid supplementation on the endogenous activities of plasma lipases. Am J Clin Nutr. 1997;66:254–260. doi: 10.1093/ajcn/66.2.254. [DOI] [PubMed] [Google Scholar]
- 52.Sethi S, Ziouzenkova O, Ni H, Wagner DD, Plutzky J, Mayadas TN. Oxidized omega-3 fatty acids in fish oil inhibit leukocyte-endothelial interactions through activation of PPAR alpha. Blood. 2002;100:1340–1346. doi: 10.1182/blood-2002-01-0316. [DOI] [PubMed] [Google Scholar]
- 53.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lei B, Lionetti V, Young ME, Chandler MP, D' Agostino C, Kang E, et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol. 2004;36:567–576. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 56.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 57.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 58.Davila-Roman VG, Vedala G, Herrero P, de las FL, Rogers JG, Kelly DP, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 59.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–2308. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- 60.Pepe S, McLennan PL. (n-3) Long chain PUFA dose-dependently increase oxygen utilization efficiency and inhibit arrhythmias after saturated fat feeding in rats. J Nutr. 2007;137:2377–2383. doi: 10.1093/jn/137.11.2377. [DOI] [PubMed] [Google Scholar]
- 61.Castellani S, Paladini B, Paniccia R, Di Serio C, Valloti B, Ungar A, et al. Increased renal formation of thromboxane A2 and prostaglandin F2 alpha in heart failure. Am Heart J. 1997;133:94–100. doi: 10.1016/s0002-8703(97)70253-9. [DOI] [PubMed] [Google Scholar]
- 62.Zamorano B, Carmona MT. Prostaglandin-E2 and cyclic adenosine 3′-5′ monophosphate levels in the hypertrophied rat heart. Biol Res. 1992;25:85–89. [PubMed] [Google Scholar]
- 63.Newman WH, Frankis MB, Halushka PV. Increased myocardial release of prostacyclin in dogs with heart failure. J Cardiovasc Pharmacol. 1983;5:194–201. doi: 10.1097/00005344-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Packer M. Is tumor necrosis factor an important neurohormonal mechanism in chronic heart failure? Circulation. 1995;92:1379–1382. doi: 10.1161/01.cir.92.6.1379. [DOI] [PubMed] [Google Scholar]
- 65.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 66.Aukrust P, Gullestad L, Ueland T, Damas JK, Yndestad A. Inflammatory and anti-inflammatory cytokines in chronic heart failure: potential therapeutic implications. Ann Med. 2005;37:74–85. doi: 10.1080/07853890510007232. [DOI] [PubMed] [Google Scholar]
- 67.Sharma R, Bolger AP, Li W, Davlouros PA, Volk HD, Poole-Wilson PA, et al. Elevated circulating levels of inflammatory cytokines and bacterial endotoxin in adults with congenital heart disease. Am J Cardiol. 2003;92:188–193. doi: 10.1016/s0002-9149(03)00536-8. [DOI] [PubMed] [Google Scholar]
- 68.Aukrust P, Yndestad A, Ueland T, Damas JK, Gullestad L. Anti-inflammatory trials in chronic heart failure. Heart Fail Monit. 2006;5:2–9. [PubMed] [Google Scholar]
- 69.Hara A, Yuhki K, Fujino T, Yamada T, Takayama K, Kuriyama S, et al. Augmented cardiac hypertrophy in response to pressure overload in mice lacking the prostaglandin I2 receptor. Circulation. 2005;112:84–92. doi: 10.1161/CIRCULATIONAHA.104.527077. [DOI] [PubMed] [Google Scholar]
- 70.Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, et al. Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 2007;115:1398–1407. doi: 10.1161/CIRCULATIONAHA.106.643585. [DOI] [PubMed] [Google Scholar]
- 71.Lemaitre RN, King IB, Raghunathan TE, Pearce RM, Weinmann S, Knopp RH, et al. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation. 2002;105:697–701. doi: 10.1161/hc0602.103583. [DOI] [PubMed] [Google Scholar]
- 72.Luostarinen R, Boberg M, Saldeen T. Fatty acid composition in total phospholipids of human coronary arteries in sudden cardiac death. Atherosclerosis. 1993;99:187–193. doi: 10.1016/0021-9150(93)90021-l. [DOI] [PubMed] [Google Scholar]
- 73.Jenkins CM, Cedars AM, Gross RW. Eicosanoid signalling pathways in the heart. Cardiovasc Res. 2009;82:240–249. doi: 10.1093/cvr/cvn346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, et al. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 75.Gibney MJ, Hunter B. The effects of short- and long-term supplementation with fish oil on the incorporation of n-3 polyunsaturated fatty acids into cells of the immune system in healthy volunteers. Eur J Clin Nutr. 1993;47:255–259. [PubMed] [Google Scholar]
- 76.Healy DA, Wallace FA, Miles EA, Calder PC, Newsholme P. Effect of low-to-moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids. 2000;35:763–768. doi: 10.1007/s11745-000-0583-1. [DOI] [PubMed] [Google Scholar]
- 77.Mantzioris E, Cleland LG, Gibson RA, Neumann MA, Demasi M, James MJ. Biochemical effects of a diet containing foods enriched with n-3 fatty acids. Am J Clin Nutr. 2000;72:42–48. doi: 10.1093/ajcn/72.1.42. [DOI] [PubMed] [Google Scholar]
- 78.LeBlanc CJ, Horohov DW, Bauer JE, Hosgood G, Mauldin GE. Effects of dietary supplementation with fish oil on in vivo production of inflammatory mediators in clinically normal dogs. Am J Vet Res. 2008;69:486–493. doi: 10.2460/ajvr.69.4.486. [DOI] [PubMed] [Google Scholar]
- 79.Nordøy A, Hatcher L, Goodnight S, Fitzgerald GA, Conner WE. Effects of dietary fat content, saturated fatty acids, and fish oil on eicosanoid production and hemostatic parameters in normal men. J Lab Clin Med. 1994;123:914–920. [PubMed] [Google Scholar]
- 80.Mehta JL, Lopez LM, Lawson D, Wargovich TJ, Williams LL. Dietary supplementation with omega-3 polyunsaturated fatty acids in patients with stable coronary heart disease. Effects on indices of platelet and neutrophil function and exercise performance. Am J Med. 1988;84:45–52. doi: 10.1016/0002-9343(88)90007-1. [DOI] [PubMed] [Google Scholar]
- 81.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 82.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Serhan CN. Systems approach with inflammatory exudates uncovers novel anti-inflammatory and pro-resolving mediators. Prostaglandins Leukot Essent Fatty Acids. 2008;79:157–163. doi: 10.1016/j.plefa.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am J Clin Nutr. 2001;73:539–548. doi: 10.1093/ajcn/73.3.539. [DOI] [PubMed] [Google Scholar]
- 85.Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004;134:1806–1811. doi: 10.1093/jn/134.7.1806. [DOI] [PubMed] [Google Scholar]
- 86.Lennie TA, Chung ML, Habash DL, Moser DK. Dietary fat intake and proinflammatory cytokine levels in patients with heart failure. J Card Fail. 2005;11:613–618. doi: 10.1016/j.cardfail.2005.06.434. [DOI] [PubMed] [Google Scholar]
- 87.Mehra MR, Lavie CJ, Ventura HO, Milani RV. Fish oils produce anti-inflammatory effects and improve body weight in severe heart failure. J Heart Lung Transplant. 2006;25:834–838. doi: 10.1016/j.healun.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 88.Valen G, Yan ZQ, Hansson GK. Nuclear factor kappa-B and the heart. J Am Coll Cardiol. 2001;38:307–314. doi: 10.1016/s0735-1097(01)01377-8. [DOI] [PubMed] [Google Scholar]
- 89.Wong SC, Fukuchi M, Melnyk P, Rodger I, Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-kappaB in myocardium of patients with congestive heart failure. Circulation. 1998;98:100–103. doi: 10.1161/01.cir.98.2.100. [DOI] [PubMed] [Google Scholar]
- 90.Matsumori A, Sasayama S. The role of inflammatory mediators in the failing heart: immunomodulation of cytokines in experimental models of heart failure. Heart Fail Rev. 2001;6:129–136. doi: 10.1023/a:1011457910659. [DOI] [PubMed] [Google Scholar]
- 91.Zhao Y, Joshi-Barve S, Barve S, Chen LH. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23:71–78. doi: 10.1080/07315724.2004.10719345. [DOI] [PubMed] [Google Scholar]
- 92.Takano H, Nagai T, Asakawa M, Toyozaki T, Oka T, Kumoro I, et al. Peroxisome proliferator-activated receptor activators inhibit lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal rat cardiac myocytes. Circ Res. 2000;87:596–602. doi: 10.1161/01.res.87.7.596. [DOI] [PubMed] [Google Scholar]
- 93.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 94.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 97.Ouchi N, Shibata R, Walsh K. Targeting adiponectin for cardioprotection. Expert Opin Ther Targets. 2006;10:573–581. doi: 10.1517/14728222.10.4.573. [DOI] [PubMed] [Google Scholar]
- 98.Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924–928. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 99.Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar MJ, Hensler M, et al. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49:394–397. doi: 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- 100.Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. 2007;27:1918–1925. doi: 10.1161/ATVBAHA.106.136853. [DOI] [PubMed] [Google Scholar]
- 101.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 102.Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, et al. Association between adiponectin and mediators of inflammation in obese women. Diabetes. 2003;52:942–947. doi: 10.2337/diabetes.52.4.942. [DOI] [PubMed] [Google Scholar]
- 103.Morgan DR, Dixon LJ, Hanratty CG, El Sherbeeny N, Hamilton PB, McGrath LT, et al. Effects of dietary omega-3 fatty acid supplementation on endothelium-dependent vasodilation in patients with chronic heart failure. Am J Cardiol. 2006;97:547–551. doi: 10.1016/j.amjcard.2005.08.075. [DOI] [PubMed] [Google Scholar]
- 104.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 105.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 106.Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, et al. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women: The JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 107.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 108.Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J. 1988;115:869–875. doi: 10.1016/0002-8703(88)90891-5. [DOI] [PubMed] [Google Scholar]
- 109.McLennan PL. Myocardial membrane fatty acids and the antiarrhythmic actions of dietary fish oil in animal models. Lipids. 2001;36:S111–S114. doi: 10.1007/s11745-001-0692-x. [DOI] [PubMed] [Google Scholar]
- 110.McLennan PL, Bridle TM, Abeywardena MY, Charnock JS. Comparative efficacy of n-3 and n-6 polyunsaturated fatty acids in modulating ventricular fibrillation threshold in marmoset monkeys. Am J Clin Nutr. 1993;58:666–669. doi: 10.1093/ajcn/58.5.666. [DOI] [PubMed] [Google Scholar]
- 111.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 112.Leaf A, Xiao YF, Kang JX, Billman GE. Membrane effects of the n-3 fish oil fatty acids, which prevent fatal ventricular arrhythmias. J Membr Biol. 2005;206:129–139. doi: 10.1007/s00232-005-0789-9. [DOI] [PubMed] [Google Scholar]
- 113.Matthan NR, Jordan H, Chung M, Lichtenstein AH, Lathrop DA, Lau J. A systematic review and meta-analysis of the impact of omega-3 fatty acids on selected arrhythmia outcomes in animal models. Metabolism. 2005;54:1557–1565. doi: 10.1016/j.metabol.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 114.Leaf A, Xiao YF, Kang JX, Billman GE. Prevention of sudden cardiac death by n-3 polyunsaturated fatty acids. Pharmacol Ther. 2003;98:355–377. doi: 10.1016/s0163-7258(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 115.Takahashi R, Okumura K, Asai T, Hirai T, Murakami H, Murakami R, et al. Dietary fish oil attenuates cardiac hypertrophy in lipotoxic cardiomyopathy due to systemic carnitine deficiency. Cardiovasc Res. 2005;68:213–223. doi: 10.1016/j.cardiores.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 116.Bayer AL, Heidkamp MC, Patel N, Porter M, Engman S, Samarel AM. Alterations in protein kinase C isoenzyme expression and autophosphorylation during the progression of pressure overload-induced left ventricular hypertrophy. Mol Cell Biochem. 2003;242:145–152. [PubMed] [Google Scholar]
- 117.Harris WS. Alpha-linolenic acid: a gift from the land? Circulation. 2005;111:2872–2874. doi: 10.1161/CIRCULATIONAHA.105.545640. [DOI] [PubMed] [Google Scholar]
- 118.Campos H, Baylin A, Willett WC. Alpha-linolenic acid and risk of nonfatal acute myocardial infarction. Circulation. 2008;118:339–345. doi: 10.1161/CIRCULATIONAHA.107.762419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Djousse L, Arnett DK, Carr JJ, Eckfeldt JH, Hopkins PN, Province MA, et al. Dietary linolenic acid is inversely associated with calcified atherosclerotic plaque in the coronary arteries: the National Heart, Lung, and Blood Institute Family Heart Study. Circulation. 2005;111:2921–2926. doi: 10.1161/CIRCULATIONAHA.104.489534. [DOI] [PubMed] [Google Scholar]
- 120.Fiaccavento R, Carotenuto F, Minieri M, Masuelli L, Vecchini A, Bei R, et al. Alpha-linolenic acid-enriched diet prevents myocardial damage and expands longevity in cardiomyopathic hamsters. Am J Pathol. 2006;169:1913–1924. doi: 10.2353/ajpath.2006.051320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De LM, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 122.Hu FB, Stampfer MJ, Manson JE, Rimm EB, Wolk A, Colditz GA, et al. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr. 1999;69:890–897. doi: 10.1093/ajcn/69.5.890. [DOI] [PubMed] [Google Scholar]
- 123.Baylin A, Kabagambe EK, Ascherio A, Spiegelman D, Campos H. Adipose tissue alpha-linolenic acid and nonfatal acute myocardial infarction in Costa Rica. Circulation. 2003;107:1586–1591. doi: 10.1161/01.CIR.0000058165.81208.C6. [DOI] [PubMed] [Google Scholar]
- 124.Djousse L, Hunt SC, Arnett DK, Province MA, Eckfeldt JH, Ellison RC. Dietary linolenic acid is inversely associated with plasma triacylglycerol: the National Heart, Lung, and Blood Institute Family Heart Study. Am J Clin Nutr. 2003;78:1098–1102. doi: 10.1093/ajcn/78.6.1098. [DOI] [PubMed] [Google Scholar]
- 125.Guivernau M, Meza N, Barja P, Roman O. Clinical and experimental study on the long-term effect of dietary gamma-linolenic acid on plasma lipids, platelet aggregation, thromboxane formation, and prostacyclin production. Prostaglandins Leukot Essent Fatty Acids. 1994;51:311–316. doi: 10.1016/0952-3278(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 126.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 127.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]