Abstract
Aims
Oxidative stress accompanies inflammatory and vascular diseases. The objective of this study was to explore whether reactive oxygen species can activate shedding of platelet receptors and thus suppress platelet function.
Methods and results
Hydrogen peroxide and glucose oxidase were chosen to model oxidative stress in vitro. We demonstrate that oxidative damage activated tumour necrosis factor-α-converting enzyme (TACE) and induced shedding of its targets, glycoprotein (GP) Ibα and GPV, in murine and human platelets. Also, 12-HpETE, a peroxide synthesized in the platelet lipoxygenase pathway, induced TACE-mediated receptor cleavage. The TACE activation was independent of platelet activation, as α-granule secretion, activation of αIIbβ3, or phosphatidylserine expression was not observed. TACE activation induced by hydrogen peroxide was dependent on p38 mitogen-activated protein kinase signalling, whereas protein kinase C, phosphoinositide 3-kinase, and caspases were not involved. Inhibition of p38 cytoplasmic targets, phospholipase A2 and heat shock protein 27, did not prevent shedding, whereas blocking 12-lipoxygenase or Src kinase slightly inhibited TACE activation. The loss of the GPIbα receptor induced by oxidative stress rendered platelets unable to incorporate into a growing thrombus in vivo.
Conclusion
Oxidative stress can render platelets functionally less active by shedding key adhesion receptors via the activation of p38. This suggests that oxidative injury of platelets may attenuate their function.
Keywords: TACE, ADAM17, Oxidative stress, Platelets, GPIbα
1. Introduction
Platelets are recruited to the site of vessel injury where they form a preliminary plug and activate other haemostatic mechanisms. Rapid platelet attachment to the vascular wall is required for haemostasis, and glycoprotein (GP) Ibα is a pivotal receptor for this process. Expressed in a complex with GPIX and GPV, GPIbα binds different substrates, predominantly von Willebrand factor. There are virtually no platelet–vessel wall interactions in mutant mice lacking the extracellular domain of GPIbα.1,2 Platelets from patients with genetic deficiency of GPIbα (Bernard–Soulier syndrome) demonstrate functional abnormalities.3 Therefore, normal expression and function of the GPIb-V-IX complex is a prerequisite for physiological haemostasis.
GPIbα can be eliminated from the platelet surface by different mechanisms. It is either internalized during platelet activation4 or shed by cleavage by tumour necrosis factor-α (TNF-α)-converting enzyme (TACE/ADAM17),5 and a potential role of Ca2+-dependent protease calpain has also been reported.6 TACE can be activated by direct stimulation of protein kinase C by phorbol 12-myristate 13-acetate (PMA).7 Mitochondrial damage induced by carbonyl cyanide m-chlorophenylhydrazone (CCCP), a process mimicking platelet ageing, results in the activation of ADAM17 and shedding of its membrane targets.5 In addition, the inhibition of calmodulin also results in the upregulation of TACE.8 ADAM17-dependent cleavage of GPIbα results in the shedding of the extracellular domain of the receptor, glycocalicin. Glycocalicin can normally be found in blood plasma.9 Although the physiological role of glycocalicin is not fully understood, shedding of GPIbα leads to dramatic attenuation of platelet function.5
Oxidative stress accompanies various vascular diseases.10 As neutrophils constitute a major source of reactive oxygen species (ROS) in the blood, oxidative stress participates in the pathogenesis of inflammatory diseases. Platelets and endothelial cells are also able to synthesize ROS upon stimulation,11,12 and it has been shown in vitro that a growing thrombus can generate oxygen radicals.13 Platelets are able to produce superoxide anion14 and more stable hydrogen peroxide (H2O2).15 Platelets also synthesize endogenous peroxides derived from long-chain fatty acids, such as 12-HpETE.16,17 Consequently, platelets could be influenced by ROS in multiple types of pathology based on inflammation, endothelial damage, or thrombosis.
Vascular oxidative stress is thought to be associated with elevated thrombosis18 due to its direct influence on platelets and suppression of bioavailability of anti-thrombotic nitric oxide (NO).19 For example, oxidative stress was suggested to be involved in increased arterial thrombosis in mice with hyperhomocysteinaemia.20 On the other hand, oxidative stress was shown to precede spontaneous intracranial haemorrhage in mice with hypertension,21 suggesting a potential contribution of ROS in bleeding. In the current study, we hypothesized that oxidative damage could directly suppress platelet functions and examined the effect of ROS on platelet receptor expression. We demonstrate that H2O2, glucose oxidase (GO), and 12-HpETE activate TACE, resulting in the shedding of GPIbα and GPV, components of a principal receptor complex involved in thrombosis. We also report that TACE activation in platelets by ROS is entirely mediated by p38 kinase.
2. Methods
2.1. Animals
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and all experimental procedures were approved by the Animal Care and Use Committee of the Immune Disease Institute. C57BL/6J wild-type mice were from The Jackson Laboratory (Bar Harbor, ME, USA). TACE+/ΔZn mutant mice (C57BL/6J/129Sv background) were kindly provided by Amgen (Seattle, WA, USA). TACEΔZn/ΔZn chimeras were generated as described.5
2.2. Reagents and antibodies
The reagents and antibodies were purchased as follows: antibodies against mouse P-selectin, αIIb, human GPIbα, annexin V, and z-VAD-FMK from BD Pharmingen, (San Jose, CA, USA), antibodies against mouse GPIbα, GPV, GPVI, GPIX, α5β1, and the activated form of αIIbβ3 from Emfret Analytics (Wuerzburg, Germany), aristolochic acid and 12-HpETE from Cayman Chemical (Ann Arbor, MI, USA), cinnamyl-3,4-dihydroxy-a-cyanocinnamate (CDC), esculetin, and cytochalasin D from Biomol (Plymouth Meeting, PA, USA), KRIBB3 (an inhibitor of HSP27), prostacyclin (PGI2), PGE1, thrombin, apyrase, wortmannin, and GO from Sigma (St Louis, MO, USA), TAPI-1, SB203580, PD98059, PP2, BAPTA-AM, and protein kinase C inhibitor Ro31-8220 from EMD Chemicals, Inc., Gibbstown, NJ, USA, and calcein acetoxymethyl ester (calcein AM) and Calcein Red Orange AM from Molecular Probes (Eugene, OR, USA).
2.3. Blood collection and preparation of washed platelets
2.3.1. Murine
Whole blood from the retro-orbital venous plexus was collected into Eppendorf tubes with heparin (7.5 U/mL final concentration). Blood was centrifuged at 200g for 5 min at room temperature (RT) to obtain platelet-rich plasma (PRP). PRP was incubated for 5 min with PGI2 (0.1 µg/mL) and centrifuged at 850g for 3.5 min. Platelet pellet was then resuspended in Tyrode's–HEPES buffer (137 mM NaCl, 2 mM KCl, 12 mM NaHCO3, 0.3 mM NaH2PO4, 5.5 mM glucose, 5 mM HEPES, 0.35% bovine serum albumin), and washed once prior to use in experiments.
2.3.2. Human
The investigation conforms with the principles outlined in the Declaration of Helsinki. We obtained informed consent from all donors and approval from the Immune Disease Institute Institutional Review Board. Whole ACD-stabilized blood was obtained from healthy donors who had not taken any medication for at least 10 days. PRP was prepared by centrifugation (120g, 10 min, RT). Platelets were washed at 750g in the presence of PGE1 (0.5 µg/mL) and apyrase (1 U/mL) and resuspended in Tyrode's–HEPES buffer (free of apyrase and PGE1).
2.4. Platelet exposure to oxidative stress and FACS analysis
Murine or human platelets were adjusted to the concentration of 1 × 105/μL and 1.5 × 105/μL, respectively. GO, H2O2, or 12-HpETE was added in the indicated concentrations, and platelets were incubated for up to 1 h (for murine platelets) and up to 6 h (for human platelets) at 37°C. Inhibitors of signalling pathways were added to the appropriate samples 15 min before the application of oxidative stress. Samples of platelets (5 µL) were mixed with fluorescently labelled mAbs (2 µg/mL final concentration for BD Pharmingen mAbs, clone HIP1, and 1:5 dilution for Emfret Analytics, clone Xia.G5, mAbs) and incubated 5 min at RT in the dark. The reaction was terminated by dilution in 500 µL PBS and the samples were analysed by FACS (Becton Dickinson) within 1 h.
2.5. Western blot for glycocalicin
After incubation with H2O2, samples of platelet supernatant or platelets (lysed in 1% Triton in the presence of protease inhibitor cocktail) were mixed with 2× sodium dodecyl sulphate sample buffer, boiled, and run on SDS–PAGE (7.5%) under reducing conditions. Proteins were then transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% milk (30 min) and incubated with 2 µg/mL anti-GPIb mAb (18 h, 4°C). After three washes with TBST, the membrane was incubated with secondary Ab conjugated with horseradish peroxidase (1 µg/mL). Proteins were visualized by enhanced chemiluminescence.
The mAb used for western blotting (clone Xia.G7) recognizes the extracellular domain of GPIbα. The molecular weight of intact GPIbα recognized by the mAb is 150 kDa, and the extracellular domain is 130 kDa. Therefore comparison of bands in platelet lysates and platelet supernatant makes it possible to distinguish whether the intact receptor or just the extracellular domain is shed.
2.6. Aggregometry
To assess platelet aggregation, light transmission was measured by a Chrono-Log four-channel optical aggregation system (Chrono-Log, Havertown, PA, USA). Washed platelets were resuspended in modified Tyrode's–HEPES buffer containing 1 mM CaCl2 at a concentration of 1.5 × 105 platelets/μL (human platelets) and 1 × 105/μL (murine platelets). Thrombin was added at the final concentration of 1 U/mL, and light transmission was recorded for 12 min.
2.7. In vivo thrombosis model
Intravital microscopy was performed as described.22 Briefly, intact or H2O2-treated platelets were differentially labelled with calcein-green or calcein-orange (1 µg/mL, 10 min, RT) and adjusted to the concentration of 2.5 × 107 and 7 × 107, respectively, in 100 µL of Tyrode's–HEPES buffer. Both types of labelled platelets were infused into 4-week-old male mice anaesthetized with avertin. A drop of blood (50 µL) was drawn, and an equal number of circulating platelets of both types were verified by FACS before starting the procedure. The mesentery was exposed through a midline abdominal incision and injury was induced by the application of 10% FeCl3 on a filter paper for 5 min. Arterioles were monitored until blood flow was arrested for >30 s. The whole process was recorded and the number of platelets interacting with the vessel wall 6 min after FeCl3 application was counted for 1 min.
2.8. Statistical analysis
Data are expressed as mean ± SD. The results were calculated using Student's t-test or ANOVA followed by Tukey's post hoc test, and the difference between groups was considered significant at P < 0.05.
3. Results
3.1. Oxidative stress induces TACE-dependent shedding of GPIbα and GPV on platelets
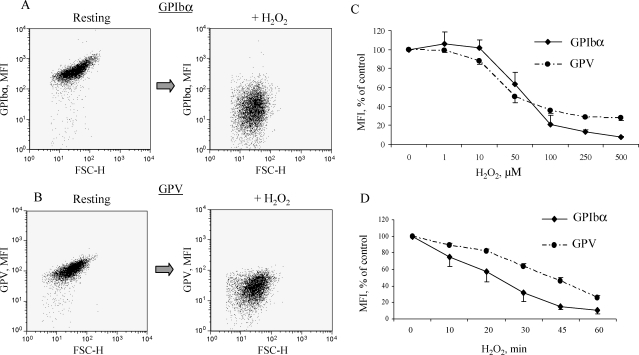
To address the effect of ROS on platelet receptor expression, we incubated washed murine platelets with H2O2, a relatively stable reactive oxygen compound used to mimic oxidative stress in vitro.23 The presence of H2O2 resulted in a marked decrease in GPIbα and GPV expression levels on the platelet membrane (Figure 1A and B) as determined by mean fluorescence intensity (MFI). Similar to H2O2, GO (10 mU/mL, 1 h, 37°C), which enzymatically generates H2O2,24 when added to washed platelets, induced complete disappearance of GPIbα from the platelet membrane (not shown). Next, we evaluated the dose-dependency of the H2O2 effect. The decrease in MFI became statistically significant at 50 µM H2O2 for GPIbα and 10 µM for GPV, and the lowest dose inducing the effect close to maximal was 100 µM (Figure 1C). This H2O2 concentration has also been used to explore H2O2 effects on vascular cells,25 and therefore we utilized it in our further experiments.
Figure 1.
H2O2 induces loss of GPIbα and GPV from the platelet membrane. Washed murine platelets were treated with H2O2 at increasing concentrations for the indicated periods of time at 37°C. GPIbα and GPV expression was evaluated by FACS. Representative dot plots for GPIbα (A) and for GPV (B) expression on control and H2O2-treated platelets (100 µM, 1 h). Dose dependency (C) and time course (D) of receptor loss determined as percentage mean fluorescence intensity (MFI) of control platelets. Data represent mean ± SD of six to eight independent experiments.
Because GPIbα shedding induced by, for example, PMA (a PKC agonist) occurs rapidly (10 min),5 we assessed the temporal kinetics of the H2O2 effect. In contrast to PMA, H2O2-induced receptor downregulation occurred over a longer time period (1 h, Figure 1D), although a small but statistically significant MFI decrease was observed already at 20 min for GPIbα and 10 min for GPV staining.
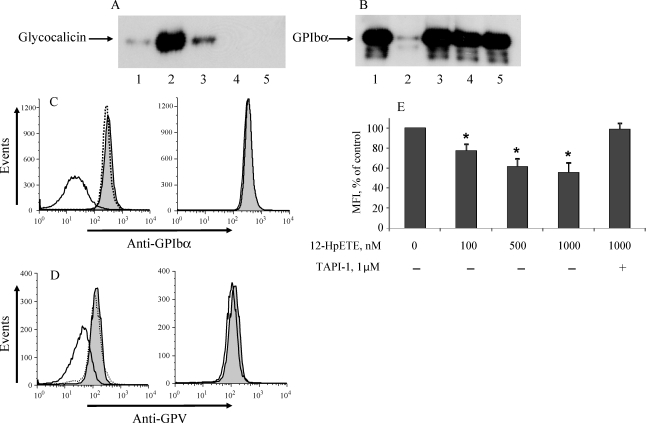
To examine whether H2O2 induces receptor shedding or internalization, we performed western blot to analyse both platelets and the supernatant after treatment with H2O2. Increased levels of glycocalicin appeared in the supernatant, and the extracellular domain of GPIbα disappeared from platelets following ROS treatment; this effect was completely blocked by an inhibitor of TACE, TAPI-1 (Figure 2A and B, lanes 1–3). H2O2 induced no shedding in TACEΔZn/ΔZn platelets (Figure 2A and B, lanes 4 and 5). Similar results were obtained by FACS for GPIbα and GPV (Figure 2C and D). Therefore, oxidative stress induces the shedding of the receptors from the platelet membrane in a TACE-dependent fashion.
Figure 2.
H2O2- and 12-HpETE-induced shedding of GPIbα and GPV is TACE-dependent. Murine platelets from wild-type (WT) or TACEΔZn/ΔZn chimeras were treated with H2O2 (100 µM, 1 h, 37°C) in the presence or absence of the TACE inhibitor, TAPI-1. Platelet supernatants (A) and lysates (B) were analysed by SDS–PAGE, transferred to a membrane, and blotted with specific anti-GPIbα mAbs. (1) WT, untreated; (2) WT, H2O2; (3) WT/TAPI-1, H2O2; (4) TACEΔZn/ΔZn, untreated; (5) TACEΔZn/ΔZn, H2O2. Platelets from the same samples were analysed by FACS for GPIbα expression. (C) GPIbα and (D) GPV, left panel represents WT platelets and right panel TACEΔZn/ΔZn platelets. The grey-shaded area represents resting platelets; the solid line, H2O2-treated platelets; and the dotted line, H2O2-treated WT platelets in the presence of TAPI-1. (E) Platelets were treated with 12-HpETE, a peroxide naturally produced by platelets, for 1 h in indicated concentrations in the absence or presence of TAPI-1 (1 µM). Surface expression of GPIbα was analysed by FACS, n = 5; Asterisk indicates statistically significant difference compared with untreated cells.
Along with external ROS, platelets are also able to produce peroxides; a primary one is 12-HpETE, formed in the 12-lipoxygenase pathway.16 When co-incubated with platelets in nanomolar-to-micromolar concentrations, this peroxide also stimulated the shedding of GPIbα (Figure 2E). This effect was reversed by TACE inhibition, indicating that 12-HpETE, similar to H2O2, activates TACE. The shedding of GPV was also observed, although to a lesser extent than the shedding of GPIbα (to 84.5 ± 4.7% of control MFI for GPV compared with 61.1 ± 7.1% for GPIbα, at 1000 nM 12-HpETE).
After exposure to H2O2 (100 µM), GO (10 mU/mL), or 12-HpETE (1 µM), murine platelets retained an ability to aggregate normally in response to 1 U/mL thrombin (not shown), which suggests that the oxidative stress did not significantly impair their in vitro function.
3.2. Oxidative stress and the expression of other platelet receptors
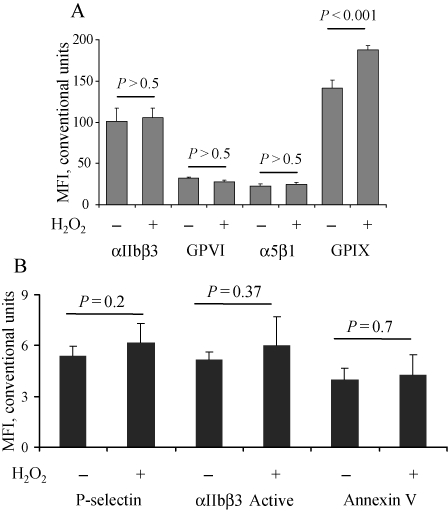
To determine whether oxidative stress promotes the shedding of other platelet receptors, we assessed the expression of other surface antigens. Incubation with H2O2 induced no significant change in the expression of αIIb and α5β1 integrins and GPVI (Figure 3A). The expression of GPIX was elevated. The reason for this effect remains unclear, although a possible explanation could be higher accessibility of binding sites on this receptor in the absence of the large glycosylated GPIbα extracellular domain.
Figure 3.
H2O2 does not induce platelet activation and shedding of other major platelet receptors. Murine platelets were treated with H2O2 (100 µM, 1 h), and the expression of GPVI, GPIX, αIIbβ3, and α5β1 integrin was determined by flow cytometry (A). To evaluate platelet activation, platelets were stained for surface expression of P-selectin, activated αIIbβ3, or phosphatidylserine (by annexin-V binding) (B). Data represent mean ± SD of four to six independent experiments.
Since platelet activation can change the level of receptor expression, we next evaluated the effect of oxidative stress on platelet activation markers. No increase in the surface expression of P-selectin, stored in α-granules, or phosphatidylserine, located on the inner platelet membrane layer, was observed after treatment with H2O2 (Figure 3B), indicating the lack of translocation of these molecules. ROS at the concentrations tested also did not activate αIIbβ3, as shown by the lack of binding of an antibody recognizing only the active form of the complex. Thus, the observed receptor shedding occurred without full platelet activation.
3.3. Oxidative damage limits platelet adhesion and ability to incorporate into a growing thrombus
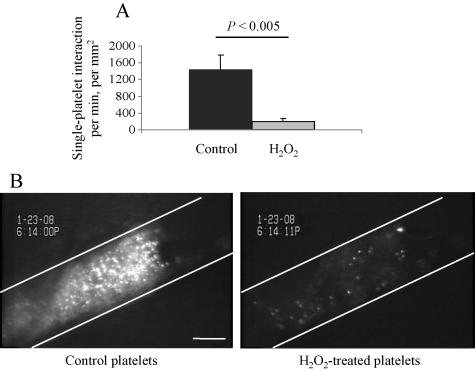
To evaluate how ROS-induced TACE activation affects platelet function in vivo, we injected recipient mice simultaneously with control and H2O2-pretreated murine platelets labelled with calcein AM and calcein Orange AM, respectively. In the FeCl3-induced arterial thrombosis model, the initial interaction of H2O2-treated platelets with vascular wall was substantially decreased (Figure 4A). The number of either transiently or firmly arrested platelets was decreased greater than seven-fold after H2O2 treatment compared with controls. When an occluding thrombus was formed, it contained a large number of control platelets, whereas only a few ROS-treated platelets were detected (Figure 4B).
Figure 4.
ROS-treated platelets do not adhere and incorporate into a thrombus in vivo. Washed murine platelets were labelled with calcein-green (control) or calcein-red (H2O2-treated, 100 µM, 1 h) and injected into a wild-type mouse. The amount of injected control and H2O2-treated cells was 2.5 × 107 and 7 × 107, respectively. Thrombosis was induced by the application of FeCl3 on mesenteric arterioles of 90–100 µm in diameter. (A) Number of adherent (transiently and firmly) platelets 6 min after FeCl3 application, counted for 1 min. (B) Representative photograph of an occlusive thrombus, rich with control but not with H2O2-treated platelets. White lines delineate the vessel. Bar 50 µm.
3.4. ROS-induced platelet receptor cleavage is mediated by p38 MAP kinase
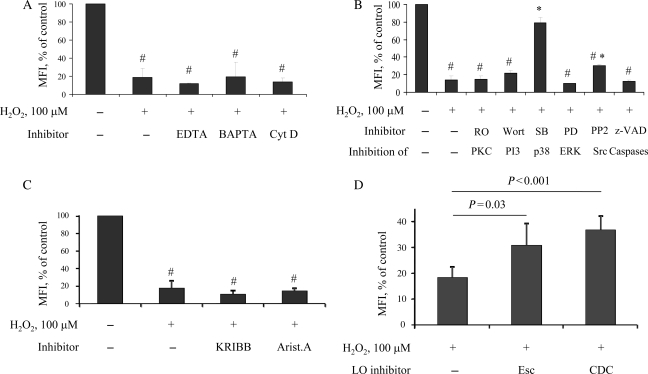
At present, only limited information exists regarding the mechanisms of TACE activation in platelets. To address this question, we utilized a pharmacological approach. Chelating extracellular Ca2+ with EDTA or intracellular Ca2+ with BAPTA-AM did not prevent the shedding of GPIbα (Figure 5A). Similarly, cytochalasin D, an inhibitor of actin polymerization, failed to block the effect of H2O2. These results suggest that neither Ca2+-dependent mechanisms nor platelet actin cytoskeleton was involved. TACE activation was also independent of PKC, PI3-kinase, and caspases, pathways that could be activated by ROS26,27 (Figure 5B). The inhibition of Src kinase only slightly reduced the receptor cleavage, whereas blocking p38 MAP kinase completely abrogated shedding. Neutralizing another MAP kinase, ERK, did not prevent H2O2-induced receptor shedding (Figure 5B).
Figure 5.
Signal transduction pathways involved in ROS-induced TACE activation. Murine platelets were pre-treated with the indicated inhibitors for 15 min, and then H2O2 (100 µM) was added for 1 h. GPIbα expression was detected by FACS. The Y axis represents percentage of mean fluorescence intensity (MFI) compared with untreated cells, whose MFI was taken as 100%. #P < 0.05 vs. untreated platelets; *P < 0.05 vs. H2O2 only. (A) EDTA, 2 mM; BAPTA, 50 µM; Cytochalasin D, 5 µg/mL. (B) RO31-8220 (a PKC inhibitor), 2 µg/mL; wortmannin (a PI3 kinase inhibitor), 1 µM; SB203580 (an inhibitor of p38 MAPK), 20 µM; PD98059 (an inhibitor of ERK), 50 µM; PP2 (an Src inhibitor), 20 µM; z-VAD (general caspase inhibitor), 20 µM. (C) KRIBB3 (an HSP27 phosphorylation inhibitor), 10 µg/mL; aristolochic acid (PLA2 inhibitor), 20 µM. (D) Murine platelets were pre-treated with lipoxygenase inhibitors esculetin (40 µM) or CDC (30 µM) for 15 min, followed by incubation with H2O2 (100 µM) for 1 h; n = 6.
Two cytoplasmic targets for p38 have been described: PLA2 and HSP27 (via MAPK-activated protein kinase-2).28,29 Both molecules are part of the platelet physiology; therefore, they are good candidates for downstream molecules involved in TACE activation. However, blocking PLA2 or inhibiting HSP27 phosphorylation did not prevent receptor cleavage (Figure 5C). Thus, it appears that oxidative stress activates p38 in platelets, which in turn upregulates TACE activity through a yet unidentified pathway.
Interestingly, neutralization of 12-lipoxygenase by two independent inhibitors resulted in moderate but statistically significant attenuation of the ROS effect (Figure 5D), which suggests the involvement of this pathway in TACE activation in platelets.
3.5. ROS induce receptor shedding from human platelets
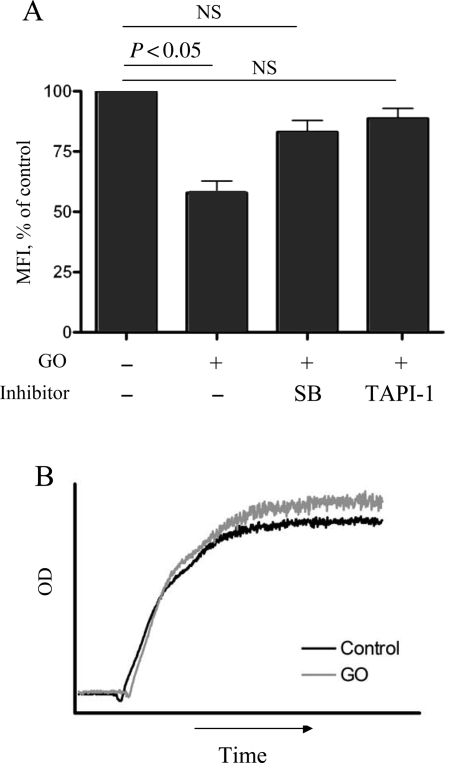
We next addressed the ability of ROS to reduce receptor expression on human platelets. In human platelets, bolus of H2O2 activated TACE only at non-physiological doses of 1 mM and above (not shown), which was substantially higher than the concentration effective with murine cells. Therefore, we used GO (25 mU/mL), an enzyme that mimics oxidative stress by producing H2O2.24 After 6 h of incubation, GPIbα expression decreased to almost 50% of the initial level (Figure 6A). Platelet viability after incubation with GO was confirmed by normal aggregation in response to thrombin (Figure 6B). Shedding was reversed by TAPI-1, suggesting the involvement of TACE. The effect of GO was inhibited by SB203580, indicating again that p38 kinase is implicated in TACE activation induced by the oxidative damage.
Figure 6.
ROS activate TACE in human platelets. (A) GO (25 mU/mL) was added to washed human platelets, and platelets were incubated for 6 h at 37°C in the presence or absence of TAPI-1 (1 µM) or SB203580 (20 µM). The expression of GPIbα was determined by FACS; n = 5. (B) Human platelets were incubated with GO (25 mU/mL) for 6 h, then Ca2+ (1 mM) was added, and aggregation in the presence of 1 U/mL thrombin was recorded. Representative graph of five experiments is shown.
Thus, similar to murine platelets, human platelets respond to oxidative stress by activating TACE and shedding GPIbα.
4. Discussion
Oxidative stress has been considered pro-thrombotic due to the ability of some ROS (for example, superoxide anion) to stimulate platelet aggregation and release30 and to reduce the bioavailability of NO.17 In contrast, we show here that the direct effect of ROS on platelets results in the activation of TACE and cleavage of its target receptors, such as GPIbα and GPV, from the plasma membrane. As GPIbα is critically important for thrombosis1 and H2O2-treated platelets are unable to incorporate into a thrombus in vivo (Figure 4), our results suggest that oxidative stress may potentially limit platelet function.
In contrast to rapid shedding induced by the artificial agent PMA, ROS-promoted cleavage required 1 h to proceed. This finding suggests that oxidative stress is not likely to limit rapidly growing thrombi at sites of strong injury but may do so when thrombi develop slowly and persist for a long time. Platelet, neutrophil, or endothelium-derived ROS might trigger platelet membrane receptor downregulation, thus attenuating further platelet recruitment. This mechanism may be relevant in certain thrombosis-related diseases where thrombi may be pacified, such as angina pectoris, which are more prone to chronic thrombus development rather than rapid, acute thrombus formation resulting, for example, from atherosclerotic plaque rupture.
Although thrombosis is elevated in some severe inflammatory diseases, some pro-inflammatory cytokines can be anti-thrombotic. For example, it has been shown that systemic administration of TNF-α exerts an anti-thrombotic activity in an NO-dependent fashion.31 TNF-α activates neutrophils32 and was shown to induce the production of superoxide in platelets.33 Consequently, inflammation could trigger thrombosis-limiting mechanisms by the cooperative action of two factors: NO that comes from the vessel wall, and ROS from endothelium or blood cells, thus diminishing the expression of platelet adhesion receptors. Importantly, 12-HpETE, intracellular peroxide produced in the 12-lipoxygenase pathway, was also able to activate TACE and shed the receptors. This finding suggests that even in the absence of external sources of ROS, the oxidative stress-triggered anti-thrombotic mechanism may slowly develop over the life of a thrombus.
The metalloproteinase TACE is known to mediate the shedding of GPIbα and GPV in platelets.5 The activation of TACE by ROS has recently been reported in monocytic cell line and neurons.34,35 In the present work, we show that oxidative damage can activate TACE in platelets. Although TACE activation was entirely dependent on p38 kinase, known cytoplasmic targets of p38, PLA2 and HSP27 were not involved. It is known that the p38/HSP27 axis mediates actin reorganization induced by oxidative damage.36 The fact that HSP27 was not involved, in conjunction with the lack of an effect by cytochalasin D, suggests that TACE activation is independent of the actin cytoskeleton. Our results point to the possibility that p38 directly phosphorylates TACE in platelets, as was demonstrated for ERK using an in vitro kinase assay.37 Alternatively, it is also possible that p38 activates TACE via some other intermediate molecule.
We demonstrate that oxidative stress promotes receptor shedding not only in murine but also in human platelets. Shedding from human platelets was slow, similar to that observed in previous studies in which TACE activation and receptor shedding was stimulated by CCCP, an agent inducing mitochondrial injury.5
A potentially important aspect of oxidative damage is the defect in platelet function that progressively develops during platelet storage. Stored platelets shed GPIbα as a part of the storage-induced damage called platelet storage lesion. The shed protein accumulates in platelet concentrates as glycocalicin.38,39 In parallel, stored platelets progressively lose their adhesive capacity.40 This process could be mediated at least in part by accumulating ROS, and the cleavage of GPIbα and GPV can underlie deteriorated platelet function after storage. These speculations are further supported by recent findings that in vitro aged platelets (in a CCCP-induced mitochondrial injury model) rapidly shed GPIbα in a TACE-dependent fashion.5 Such injured platelets have an impaired post-transfusion recovery. The inhibition of metalloproteinases prevented receptor shedding and prolonged platelet lifespan in the circulation. The disruption of mitochondrial potential also occurs in platelets during in vivo ageing.41 Therefore, oxidative stress could be implicated in platelet storage lesion, and anti-oxidants could be beneficial for maintaining platelet function during storage.
In conclusion, we have demonstrated that ROS activate TACE in platelets in a p38-dependent fashion. This leads to the shedding of GPIbα and GPV and dramatic impairment of platelet adhesion to the vessel wall and incorporation in a thrombus. Thus, the effect of oxidative stress on platelets could represent a mechanism for limiting platelet function.
Funding
This work was supported by National Heart, Lung and Blood Institute of the National Institutes of Health (grant PO1 HL056949 to D.D.W.). M.C. was supported by a fellowship from the Fondation pour la Recherche Médicale.
Acknowledgements
We thank Lesley Cowan and Emily Waters for help in preparing the manuscript.
Conflict of interest: none declared.
References
- 1.Bergmeier W, Piffath CL, Goerge T, Cifuni SM, Ruggeri ZM, Ware J, et al. The role of platelet adhesion receptor GPIbalpha far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc Natl Acad Sci USA. 2006;103:16900–16905. doi: 10.1073/pnas.0608207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard–Soulier syndrome. Blood. 2002;100:2102–2107. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- 3.Lopez JA, Andrews RK, Afshar-Kharghan V, Berndt MC. Bernard–Soulier syndrome. Blood. 1998;91:4397–4418. [PubMed] [Google Scholar]
- 4.Bergmeier W, Rackebrandt K, Schroder W, Zirngibl H, Nieswandt B. Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood. 2000;95:886–893. [PubMed] [Google Scholar]
- 5.Bergmeier W, Piffath CL, Cheng G, Dole VS, Zhang Y, von Andrian UH, et al. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates GPIbalpha shedding from platelets in vitro and in vivo. Circ Res. 2004;95:677–683. doi: 10.1161/01.RES.0000143899.73453.11. [DOI] [PubMed] [Google Scholar]
- 6.McGowan EB, Yeo KT, Detwiler TC. The action of calcium-dependent protease on platelet surface glycoproteins. Arch Biochem Biophys. 1983;227:287–301. doi: 10.1016/0003-9861(83)90373-9. [DOI] [PubMed] [Google Scholar]
- 7.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 8.Rabie T, Strehl A, Ludwig A, Nieswandt B. Evidence for a role of ADAM17 (TACE) in the regulation of platelet glycoprotein V. J Biol Chem. 2005;280:14462–14468. doi: 10.1074/jbc.M500041200. [DOI] [PubMed] [Google Scholar]
- 9.Coller BS, Kalomiris E, Steinberg M, Scudder LE. Evidence that glycocalicin circulates in normal plasma. J Clin Invest. 1984;73:794–799. doi: 10.1172/JCI111273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heistad DD. Oxidative stress and vascular disease: 2005 Duff lecture. Arterioscler Thromb Vasc Biol. 2006;26:689–695. doi: 10.1161/01.ATV.0000203525.62147.28. [DOI] [PubMed] [Google Scholar]
- 11.Maresca M, Colao C, Leoncini G. Generation of hydrogen peroxide in resting and activated platelets. Cell Biochem Funct. 1992;10:79–85. doi: 10.1002/cbf.290100203. [DOI] [PubMed] [Google Scholar]
- 12.Chen K, Keaney J. Reactive oxygen species-mediated signal transduction in the endothelium. Endothelium. 2004;11:109–121. doi: 10.1080/10623320490482655. [DOI] [PubMed] [Google Scholar]
- 13.Gorog P, Kovacs IB. Lipid peroxidation by activated platelets: a possible link between thrombosis and atherogenesis. Atherosclerosis. 1995;115:121–128. doi: 10.1016/0021-9150(94)05506-e. [DOI] [PubMed] [Google Scholar]
- 14.Marcus AJ, Silk ST, Safier LB, Ullman HL. Superoxide production and reducing activity in human platelets. J Clin Invest. 1977;59:149–158. doi: 10.1172/JCI108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finazzi-Agro A, Menichelli A, Persiani M, Biancini G, Del Principe D. Hydrogen peroxide release from human blood platelets. Biochim Biophys Acta. 1982;718:21–25. doi: 10.1016/0304-4165(82)90004-6. [DOI] [PubMed] [Google Scholar]
- 16.Hamberg M, Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci USA. 1974;71:3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman JE. Oxidative stress and platelets. Arterioscler Thromb Vasc Biol. 2008;28:s11–s16. doi: 10.1161/ATVBAHA.107.159178. [DOI] [PubMed] [Google Scholar]
- 18.Madamanchi NR, Hakim ZS, Runge MS. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost. 2005;3:254–267. doi: 10.1111/j.1538-7836.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- 19.Loscalzo J. Oxidative stress in endothelial cell dysfunction and thrombosis. Pathophysiol Haemost Thromb. 2002;32:359–360. doi: 10.1159/000073600. [DOI] [PubMed] [Google Scholar]
- 20.Dayal S, Wilson KM, Leo L, Arning E, Bottiglieri T, Lentz SR. Enhanced susceptibility to arterial thrombosis in a murine model of hyperhomocysteinemia. Blood. 2006;108:2237–2243. doi: 10.1182/blood-2006-02-005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakisaka Y, Miller JD, Chu Y, Baumbach GL, Wilson S, Faraci FM, et al. Oxidative stress through activation of NAD(P)H oxidase in hypertensive mice with spontaneous intracranial hemorrhage. J Cereb Blood Flow Metab. 2008;28:1175–1185. doi: 10.1038/jcbfm.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauhan AK, Motto DG, Lamb CB, Bergmeier W, Dockal M, Plaimauer B, et al. Systemic antithrombotic effects of ADAMTS13. J Exp Med. 2006;203:767–776. doi: 10.1084/jem.20051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishida M, Maruyama Y, Tanaka R, Kontani K, Nagao T, Kurose H. G. alpha(i) and G alpha(o) are target proteins of reactive oxygen species. Nature. 2000;408:492–495. doi: 10.1038/35044120. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Saengsirisuwan V, Sloniger JA, Teachey MK, Henriksen EJ. Oxidant stress and skeletal muscle glucose transport: roles of insulin signaling and p38 MAPK. Free Radic Biol Med. 2006;41:818–824. doi: 10.1016/j.freeradbiomed.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 25.Edwards DH, Li Y, Griffith TM. Hydrogen peroxide potentiates the EDHF phenomenon by promoting endothelial Ca2+ mobilization. Arterioscler Thromb Vasc Biol. 2008;28:1774–1781. doi: 10.1161/ATVBAHA.108.172692. [DOI] [PubMed] [Google Scholar]
- 26.Waldron RT, Rey O, Zhukova E, Rozengurt E. Oxidative stress induces protein kinase C-mediated activation loop phosphorylation and nuclear redistribution of protein kinase D. J Biol Chem. 2004;279:27482–27493. doi: 10.1074/jbc.M402875200. [DOI] [PubMed] [Google Scholar]
- 27.Lopez JJ, Salido GM, Gomez-Arteta E, Rosado JA, Pariente JA. Thrombin induces apoptotic events through the generation of reactive oxygen species in human platelets. J Thromb Haemost. 2007;5:1283–1291. doi: 10.1111/j.1538-7836.2007.02505.x. [DOI] [PubMed] [Google Scholar]
- 28.Kramer RM, Roberts EF, Um SL, Borsch-Haubold AG, Watson SP, Fisher MJ, et al. p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. Evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J Biol Chem. 1996;271:27723–27729. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- 29.Saklatvala J, Rawlinson L, Waller RJ, Sarsfield S, Lee JC, Morton LF, et al. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J Biol Chem. 1996;271:6586–6589. doi: 10.1074/jbc.271.12.6586. [DOI] [PubMed] [Google Scholar]
- 30.Handin RI, Karabin R, Boxer GJ. Enhancement of platelet function by superoxide anion. J Clin Invest. 1977;59:959–965. doi: 10.1172/JCI108718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cambien B, Bergmeier W, Saffaripour S, Mitchell HA, Wagner DD. Antithrombotic activity of TNF-alpha. J Clin Invest. 2003;112:1589–1596. doi: 10.1172/JCI19284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menegazzi R, Cramer R, Patriarca P, Scheurich P, Dri P. Evidence that tumor necrosis factor alpha (TNF)-induced activation of neutrophil respiratory burst on biologic surfaces is mediated by the p55 TNF receptor. Blood. 1994;84:287–293. [PubMed] [Google Scholar]
- 33.De Biase L, Pignatelli P, Lenti L, Tocci G, Piccioni F, Riondino S, et al. Enhanced TNF alpha and oxidative stress in patients with heart failure: effect of TNF alpha on platelet O2- production. Thromb Haemost. 2003;90:317–325. doi: 10.1160/TH03-02-0105. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Oliver P, Lancaster JJ, Schwarzenberger PO, Joshi MS, Cork J, et al. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 2001;15:303–305. doi: 10.1096/fj.00-0371fje. [DOI] [PubMed] [Google Scholar]
- 35.Pietri M, Schneider B, Mouillet-Richard S, Ermonval M, Mutel V, Launay JM, et al. Reactive oxygen species-dependent TNF-alpha converting enzyme activation through stimulation of 5-HT2B and alpha1D autoreceptors in neuronal cells. FASEB J. 2005;19:1078–1087. doi: 10.1096/fj.04-3631com. [DOI] [PubMed] [Google Scholar]
- 36.Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- 37.Diaz-Rodriguez E, Montero JC, Esparis-Ogando A, Yuste L, Pandiella A. Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735: a potential role in regulated shedding. Mol Biol Cell. 2002;13:2031–2044. doi: 10.1091/mbc.01-11-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George JN. Platelet membrane glycoproteins: alteration during storage of human platelet concentration. Thromb Res. 1976;8:719–724. doi: 10.1016/0049-3848(76)90253-x. [DOI] [PubMed] [Google Scholar]
- 39.Sano M, Williams S, Smith N, Horne M, Gralnick HR. Plasma glycocalicin in platelet concentrates: relationship to other parameters of the storage lesion. Thromb Res. 1998;92:195–198. doi: 10.1016/s0049-3848(98)00128-5. [DOI] [PubMed] [Google Scholar]
- 40.Boomgaard MN, Gouwerok CW, Homburg CH, de Groot G, IJsseldijk MJ, de Korte D. The platelet adhesion capacity to subendothelial matrix and collagen in a flow model during storage of platelet concentrates for 7 days. Thromb Haemost. 1994;72:611–616. [PubMed] [Google Scholar]
- 41.Pereira J, Soto M, Palomo I, Ocqueteau M, Coetzee LM, Astudillo S, et al. Platelet aging in vivo is associated with activation of apoptotic pathways: studies in a model of suppressed thrombopoiesis in dogs. Thromb Haemost. 2002;87:905–909. [PubMed] [Google Scholar]