Abstract
Aims
Vascular endothelial growth factor (VEGF) has been well documented to stimulate cell proliferation and differentiation; however, we have observed that copper (Cu)-induced regression of heart hypertrophy was VEGF-dependent. The present study was undertaken to test the hypothesis that Cu causes alterations in the distribution of VEGF receptors (VEGFRs) in hypertrophic cardiomyocytes so that it switches the signalling pathway from stimulation of cell growth to reversal of cell hypertrophy.
Methods and results
Primary cultures of neonatal rat cardiomyocytes were exposed to phenylephrine (PE) at a final concentration of 100 µM in cultures for 48 h to induce cell hypertrophy. The hypertrophic cardiomyocytes were exposed to copper sulfate at a final concentration of 5 µM in cultures for 24 h with a concomitant presence of PE. Flow cytometry, gene silencing, and ELISA procedures were used to analyse the changes in VEGFRs and their relationship with regression of cardiomyocyte hypertrophy. Cu did not change the concentration of VEGF in culture media, but increased the ratio of VEGFR-1 to VEGFR-2 two-fold. Gene silencing of VEGFR-2, in the absence of Cu addition, reversed PE-induced cardiomyocyte hypertrophy, which was suppressed by an anti-VEGF antibody. Gene silencing of VEGFR-1 blocked Cu-induced regression of cell hypertrophy and decreased the activity of cGMP-dependent protein kinase-1 (PKG-1). A PKG-1 antagonist, Rp-8-pCPT-cGMPS, blocked both Cu- and VEGFR-2 gene silencing-induced regression of cardiomyocyte hypertrophy.
Conclusion
Enhanced VEGFR-1 signalling is involved in Cu regression of cardiomyocyte hypertrophy, and the PKG-1 pathway is likely associated with VEGFR-1.
Keywords: VEGF, VEGFR, PKG-1, Copper, Cardiomyocyte, Hypertrophy
1. Introduction
Previous studies have shown that copper (Cu) addition to primary cultures of neonatal rat cardiomyocytes at a physiologically relevant concentration reverses cell hypertrophy induced by phenylephrine (PE) and this reversal is vascular endothelial growth factor (VEGF)-dependent.1 This observation recaptures the in vivo observation that dietary supplementation of physiologically relevant levels of Cu reverses cardiac hypertrophy induced by pressure overload in a mouse model, which is also VEGF-dependent.2 However, there is a fundamental distinction between the in vivo observation and the result obtained from cardiomyocytes in cultures. In the in vivo studies, VEGF stimulation of coronary angiogenesis is a major factor for the regression of cardiac hypertrophy,2–4 but the lack of blood vessels in cell cultures indicates a direct effect of VEGF on cardiomyocytes in the regression of cell hypertrophy.
VEGF triggers cellular responses through its receptors on the cell membrane. Binding of VEGF promotes the receptors to dimerize and become activated through autophosphorylation, leading to signalling transduction cascades.5 There are three VEGF receptors (VEGFRs) and each receptor functions differently. Activation of VEGFR-2 by VEGF in cells devoid of VEGFR-1 results in a mitogenic response, whereas the activation of VEGFR-1 in cells lacking of VEGFR-2 does not induce cell proliferation.6,7 Extensive studies performed in endothelial cells suggest that VEGFR-2 mediates most of the known cellular responses to VEGF such as embryonic vasculogenesis and tumor angiogenesis.8 The function of VEGFR-1 has not been fully understood, although it is suggested to regulate VEGFR-2 signalling negatively or positively.9–12 It has been shown that VEGFR-2 activates mitogen-activated protein kinase (MAPK) signalling pathway, whereas VEGFR-1 cannot activate this pathway,13 suggesting that the signalling transduction cascades induced by these two receptors are different. It is important to note that most of the studies of VEGF and its receptors focus on endothelial cells, although VEGFRs were found in neonatal rat cardiomyocytes.14
In cardiomyocytes, VEGF stimulates cell growth.15–17 A decoy VEGFR-2 blocks cardiac growth induced by Akt1 activation,3,18 indicating the link between the VEGFR-2 and the Akt1 signalling pathway. However, in the hypertrophic myocardium or cardiomyocytes in cultures, VEGF causes regression of hypertrophy.1–3 This suggests that VEGF has a dual function in cardiomyocytes, stimulating cell growth under physiological or stress conditions and reducing the size of cardiomyocytes under hypertrophic conditions.
The expression of the dual function of VEGF would be mediated by VEGFRs. The link of VEGFR-2 to the growth stimulation pathway suggests that other receptors would link to the regression pathway. In cardiomyocytes, a cGMP-dependent protein kinase-1 (PKG-1) pathway has been defined to be involved in the inhibition of myocardial growth19,20 or regression of cardiac hypertrophy.21 We hypothesize that in Cu-treated hypertrophic cardiomyocytes, the distribution of VEGFRs would be altered, leading to a switch from cell growth stimulation to regression of hypertrophy or the activation of the PKG-1 pathway. In this study, we specifically addressed changes in the ratio of VEGFR-1 to VEGFR-2 in Cu-induced regression of hypertrophy in cultured cardiomyocytes. We also defined the link between VEGFR-1 and PKG-1 pathways and demonstrated that enhanced VEGFR-1 signalling pathway is an important mechanism by which Cu causes regression of cardiomyocyte hypertrophy, a pathway involving PKG-1 signalling transduction.
2. Methods
2.1. Cell culture
Primary cultures of neonatal rat cardiomyocytes were established according to a procedure published previously.1 The cultures were obtained from 1- to 3-day-old Sprague–Dawley rats and the purity of cardiomyocytes was determined by quantitative analysis by flow cytometry of the cell population containing α-sarcomeric actin labelled with fluorescent antibody, which was >95%. This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996). The animal procedure was approved by the Institutional Animal Care and Use Committee at the University of Louisville, which is certified by the American Association of Accreditation for Laboratory Animal Care.
2.2. Experimental procedure
Cell hypertrophy was induced by PE (Sigma-Aldrich) at a final concentration of 100 µM for 48 h in serum-free media, then Cu in the form of copper sulfate (CuSO4) was added directly to the cultures at a final concentration of 5 µM for an additional 24 h in the presence of PE in cultures. At the end of Cu treatment or 72 h culturing, the media were collected for VEGF secretion analysis. Cells were collected by trypsinization, suspended in PBS buffer, and counted using a haemocytometer. Protein content was measured using a Bradford method (Bio-Rad, Hercules, CA, USA) and normalized by the cell number. For the flow cytometry analysis of VEGFRs, the harvested cells were subjected to fixation and incubation with different markers before analysis as described below. To determine the role of different VEGFR in the regression of cardiomyocyte hypertrophy, siRNA targeting VEGFR-1 or VEGFR-2 was applied as described below. To assess the role of VEGF and PKG-1, we used a monoclonal anti-VEGF antibody (R&D Systems, Inc., Minneapolis, MN, USA) at a final concentration of 2 ng/mL, and a PKG-1α antagonist, Rp-8-pCPT-cGMPS (Sigma-Aldrich), at a final concentration of 0.5 µM in cultures. VEGF antibody or PKG antagonist was added 1 h prior to the addition of Cu or VEGFR-2 siRNA.
2.3. Cell size determination
Cell size was determined by a flow cytometer as described previously.1 Briefly, the cells were trypsinized, suspended in PBS, and directly passed through the flow cytometer. For each sample, apoptotic cells were excluded as reported previously,1 and viable 30 000 cells were recorded and analysed through forward scatter light, which is proportional to the cell size. The data were quantitatively analysed using the software FlowJo. In addition, cell volume was further determined using a microslide field finder (Fisher Scientific, Pittsburgh, PA, USA) following the procedure described previously.1 Briefly, cardiomyocytes in cultures were trypsinized, suspended in PBS, and loaded onto the microslide field finder. The diameters of ∼150 viable cells from each microslide were assessed and recorded. The cell volume was calculated using the equation for the volume of a sphere: V= (4/3)πr3, where V is the cell volume, π = 3.14, and r the radius.
2.4. Quantitative analysis of VEGF secretion
VEGF levels in collected media were determined by a Quantikine VEGF Immunoassay kit (R&D Systems) following the manufacturer's instruction. The absorbance of standards and samples was measured spectrophotometrically at 450 nm with a wavelength of 540 nm using a microplate reader. VEGF concentrations were calculated (in pg/mL) on the basis of the standard curve. The lower limit of the ELISA kit for VEGF measurement was 10 pg/mL. Each sample was measured in duplicate, and VEGF concentrations were normalized by the number of cells in each sample. For each cell culture supernatant, the inter- and intra-assay coefficients of variation range from 5.1 to 10.4% and 1.8 to 7.2%, respectively.
2.5. Immunochemical staining of VEGFRs
To examine alterations in the distribution of VEGFRs in cardiomyocytes after the treatment with PE and/or Cu, cells were cultured in an 8-well chamber slide and treated as described in Section 2.2. After Cu treatment for 24 h, cells were fixed in 100% methanol, followed by incubation with polyclonal anti-VEGFR-1 antibody (Santa Cruz), and subsequently with Alexa Fluor 488 conjugated goat anti-rabbit IgG. After washing, the culture chamber was incubated with monoclonal anti-VEGFR-2 antibody (Santa Cruz) followed by incubation with Alexa Fluor 594 conjugated goat anti-mouse IgG. Fluorescence was visualized by a fluorescent microscope (Nikon Instruments, Melville, NY, USA). Images were acquired by a Nikon digital camera DXM 1200 (Nikon Instruments) using a Nikon ACT-1 software.
2.6. Flow cytometric analysis of VEGFRs
A quantitative analysis of VEGFRs in cardiomyocytes under different treatments was done by a flow cytometric assay. Briefly, the cells were trypsinized, washed with PBS, and fixed in 75% ethanol at 4°C for at least 18 h. Immunofluorescence staining with an Alexa Fluor 488 conjugated antibody to VEGFR-1 (1:50, Santa Cruz), and Alexa Fluor 594 conjugated antibody to VEGFR-2 (1:50, Santa Cruz), was performed on the fixed cardiomyocytes. Receptor density was determined by the intensity of fluorescence using the software FlowJo based on the data collection from 30 000 cells per sample. The data were plotted in two-dimensional fluorescence intensity vs. cell number.
2.7. Gene silencing of VEGFRs
Validated siRNA targeting rat VEGFR-1 and VEGFR-2 and negative mismatched control siRNA were purchased from Ambion, Inc. (Applied Biosystems, Cambridge, MA, USA). The siRNA sequences for VEGFR-2 were (from 5′ to 3′): GGGUAUCACUCAGACGACAtt (sense); UGUCGUCUGAGUGAUACCCag (antisense). The siRNA sequences for VEGFR-1 were (from 5′ to 3′): GAAGCGGUCUUCUUCCGAAtt (sense); UUCGGAAGAAGACCGCUUCag (antisense). The optimal transfection efficiency was determined from our preliminary study testing the range from 5 to 20 nM, and we selected the condition of siRNA causing an optimal silencing effect with minimal cytotoxicity. After PE treatment, cardiomyocytes were transfected with 10 nM annealed siRNA targeting rat VEGFR-1 or VEGFR-2 or negative mismatched siRNA in antibiotics-free media. Lipofectamine 2000 (Invitrogen) was used as the transfection reagent according to the manufacturer's instruction. After 48 h transfection, cells were trypsinized and collected for further analysis as described in Section 2.2.
2.8. cGMP-dependent protein kinase-1 activity
PKG-1 activity was assayed by a colorimetric analysis procedure (MBL, Woburn, MA, USA). The assay was performed 30 min after treatment with Cu in the experiment of VEGFR-1 siRNA-blocked regression of hypertrophic cardiomyocytes. Each sample was measured in duplicate and the instruction of the kit manufacture was closely followed.
2.9. Statistical analysis
Data were obtained from three separate experiments and are expressed as means ± SEM. A 2 × 2 factorial design was applied to the experiments with the results presented in Figures 1–4, and a 2 × 2 × 2 factorial design was applied to the experiments presented in Figures 4–7. The data were analysed according to the experimental design. After a significant interaction was detected by the analysis of variance based on the factorial design, the significance of the main effects was further determined by F-test. The level of significance was considered when P < 0.05.
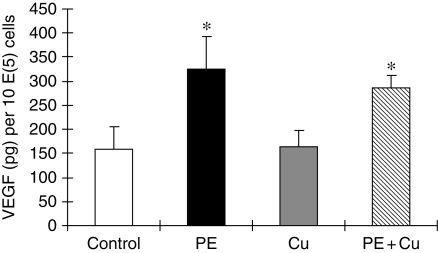
Figure 1.
Quantitative analysis of VEGF in culture media of primary cultures of neonatal rat cardiomyocytes. The cells were treated with 100 μM PE for 48 h in serum-free media, and then exposed to 5 µM CuSO4 for additional 24 h in the presence of PE. The assay procedure was described in Section 2. Each group of data were obtained from three independent experiments and each experiment contains triplicate samples for each treatment. Values are means ± SEM. *Significantly different from control group (P < 0.05).
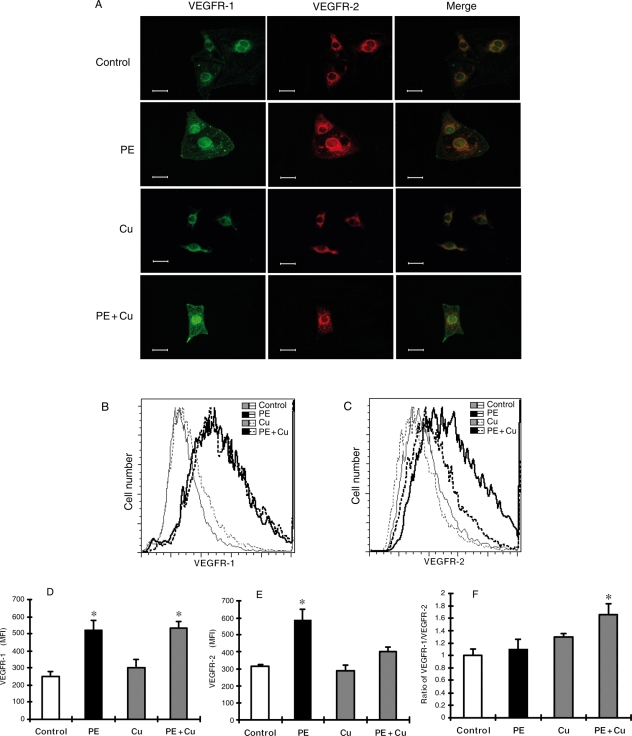
Figure 2.
(A) Representative fluorescence microscopic images of cells labelled with anti-VEGFR-1 or anti-VEGFR-2 antibodies, or the combination. After fixation, cells were incubated with polyclonal anti-VEGFR-1 antibody, and subsequently with Alexa Fluor 488 (green) conjugated goat anti-rabbit IgG. After washing, the cells were incubated with monoclonal anti-VEGFR-2 antibody followed by incubation with Alexa Fluor 594 (red) conjugated goat anti-mouse IgG. Images were acquired by a Nikon digital camera DXM 1200 using a Nikon ACT-1 software. ×400. (B–F) Flow cytometry analysis of VEGFR-1 and VEGFR-2 in cultured neonatal rat cardiomyocytes. At the end of Cu treatment as described in Figure 1, the cells were collected, fixed, and incubated with an Alexa Fluor 488 conjugated antibody to VEGFR-1 and Alexa Fluor 594 conjugated antibody to VEGFR-2. The cells were then subjected to flow cytometry analysis and 30 000 cells in each sample were counted. Receptor density was determined by the intensity of fluorescence using the software FlowJo based on the data collection from 30 000 cells per sample. The data were plotted in two-dimensional fluorescence intensity vs. cell number. (B) Representative histograms showing the change in the density of VEGFR-1 under different treatment conditions. (C) Representative histograms showing the change in the density of VEGFR-2 under different treatment conditions. (D) Quantitative analysis of the density of VEGFR-1 based upon the flow cytometric analysis. (E) Quantitative analysis of the density of VEGFR-2 based upon the flow cytometric analysis. Each group of data presented in (D) and (E) were obtained from three independent experiments and each experiment contains a triplicate samples for each treatment. (F) Ratio of VEGFR-1 to VEGFR-2 calculated from the data presented in (D) and (E). The values presented in the figures are means ± SEM. *Significantly different from control group (P < 0.05).
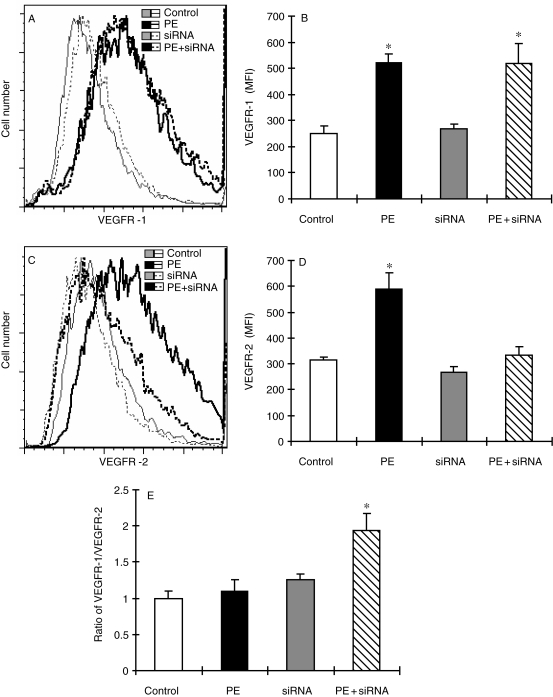
Figure 3.
Flow cytometry analysis of VEGFR-1 and VEGFR-2 in cultured neonatal rat cardiomyocytes treated with siRNA targeting VEGFR-2. The experimental procedure and treatment protocol were described in Section 2. At the end of siRNA treatment, the cells were collected, fixed, and incubated with an Alexa Fluor 488 conjugated antibody to VEGFR-1 and Alexa Fluor 594 conjugated antibody to VEGFR-2. The cells were then subjected to flow cytometry analysis and 30 000 cells in each sample were counted. Receptor density was determined by the intensity of fluorescence using the software FlowJo based on the data collection from 30 000 cells per sample. The data were plotted in two-dimensional fluorescence intensity vs. cell number. (A) Representative histograms showing the change in the density of VEGFR-1 under different treatment conditions. (B) Quantitative analysis of the density of VEGFR-1 based upon the flow cytometric analysis. (C) Representative histograms showing the change in the density of VEGFR-2 under different treatment conditions. (D) Quantitative analysis of the density of VEGFR-2 based upon the flow cytometric analysis. Each group of data presented in (B) and (D) were obtained from three independent experiments and each experiment contains a triplicate samples for each treatment. (E) Ratio of VEGFR-1 to VEGFR-2 calculated from the data presented in (B) and (D). The values presented in the figures are means ± SEM. *Significantly different from control group (P < 0.05).
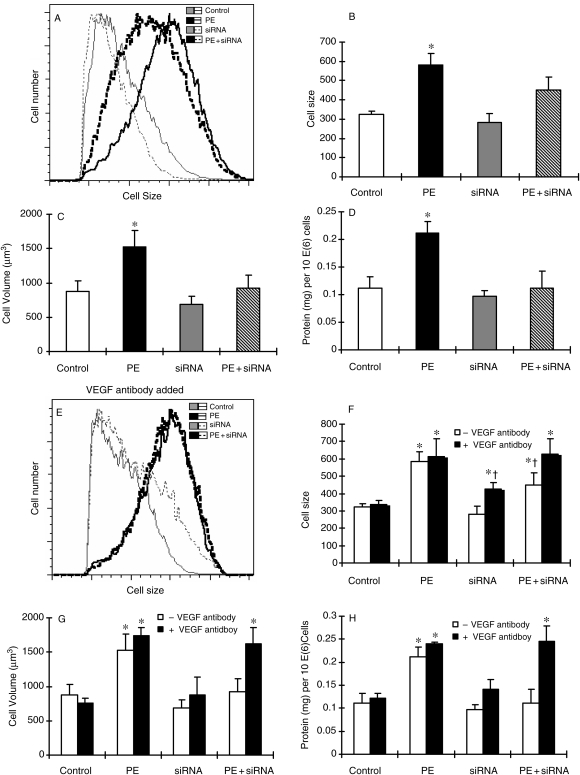
Figure 4.
Regression of PE-induced cell hypertrophy by siRNA targeting VEGFR-2 in primary cultures of neonatal rat cardiomyocytes, and the requirement of VEGF in the regression of cardiomyocyte hypertrophy induced by siRNA targeting VEGFR-2. The induction of cellular hypertrophy and the treatment with siRNA targeting VEGFR-2 were described in Section 2. (A) Representative flow cytometric histograms showing the change in cell size under different experimental conditions. (B) Quantitative analysis of cell size by flow cytometer. (C) Analysis of cell volume after siRNA treatment. Cell volume was calculated from cell diameter, which was determined by a microslide field finder. Approximately 150 cells from each group were randomly measured by the microslide field finder. (D) The protein content in the cells measured by a Bradford method and normalized by cell number. (E) Representative flow cytometric histograms showing the change in cell size under different experimental conditions; however, only the cells that were treated with anti-VEGF antibody were shown here (VEGF antibody added) and the cells were treated with anti-VEGF antibody 1 h prior to siRNA treatment. (F) Quantitative analysis of cell size by flow cytometer. (G) Analysis of cell volume, calculated from cell diameter, which was determined by a microslide field finder. Approximately 150 cells from each group were randomly measured by the microslide field finder. (H) The protein content in the cells measured by a Bradford method and normalized by cell number. Each group of data were obtained from three independent experiments and each experiment contains triplicate samples for each treatment. Values are means ± SEM. *Significantly different from control group and †significantly different from PE-treated group (P < 0.05).
Figure 5.
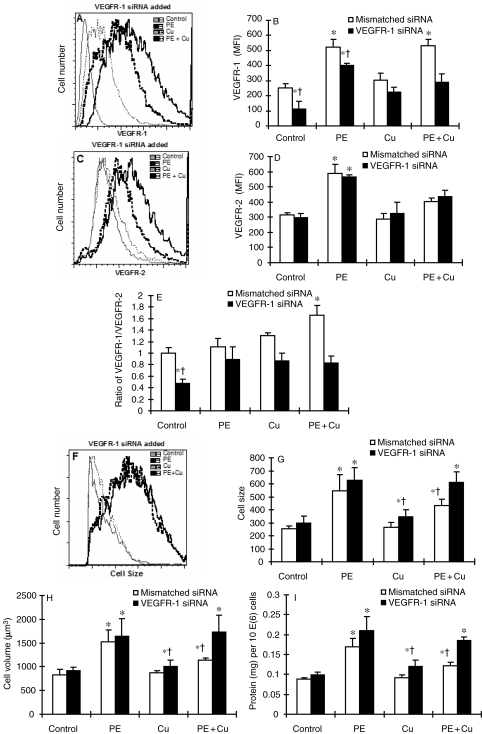
Effect of siRNA targeting VEGFR-1 on the distribution of VEGFR-1 and VEGFR-2 and the antagonistic effect of siRNA targeting VEGFR-1 on Cu-induced regression of cell hypertrophy in cultured neonatal rat cardiomyocytes. The experimental procedure and treatment protocol were described in Section 2. At the end of siRNA treatment, the cells were collected, fixed, and incubated with an Alexa Fluor 488 conjugated antibody to VEGFR-1 and Alexa Fluor 594 conjugated antibody to VEGFR-2. The cells were then subjected to flow cytometry analysis and 30 000 cells in each sample were counted. Receptor density was determined by the intensity of fluorescence using the software FlowJo based on the data collection from 30 000 cells per sample. The data were plotted in two-dimensional fluorescence intensity vs. cell number. (A) Representative histograms showing the change in the density of VEGFR-1 under different treatment conditions, but only shown the VEGFR-1 siRNA treated, not the mismatch siRNA treated. (B) Quantitative analysis of the density of VEGFR-1 based on the flow cytometric analysis. (C) Representative histograms showing the change in the density of VEGFR-2 under different treatment conditions in the presence of VEGFR-1 siRNA. (D) Quantitative analysis of the density of VEGFR-2 based upon the flow cytometric analysis. (E) Ratio of VEGFR-1 to VEGFR-2 calculated from the data presented in (C) and (D). (F) Representative flow cytometric histograms showing the change in cell size under different experimental conditions in the presence of VEGFR-1 siRNA (the results from mismatched siRNA were not included). (G) Quantitative analysis of cell size by flow cytometer. (H) Analysis of cell volume calculated from cell diameter, which was determined by a microslide field finder. Approximately 150 cells from each group were randomly measured by the microslide field finder. (I) The protein content in the cells measured by a Bradford method and normalized by cell number. Each group of data were obtained from three independent experiments and each experiment contains a triplicate samples for each treatment. Values are means ± SEM. *Significantly different from control group and †significantly different from PE-treated group (P < 0.05).
Figure 6.
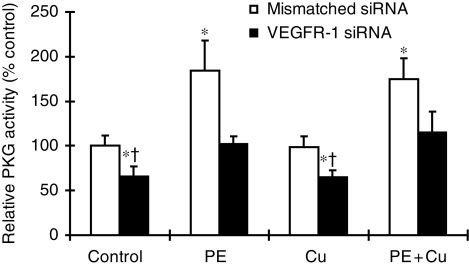
Inhibitory effect of siRNA targeting VEGFR-1 on PE-elevated PKG-1 activity. The experimental procedure was described in Section 2. Each group of data were obtained from three independent experiments and each experiment contains a triplicate samples for each treatment. Values are means ± SEM. *Significantly different from control group and †significantly different from PE-treated group (P < 0.05).
Figure 7.
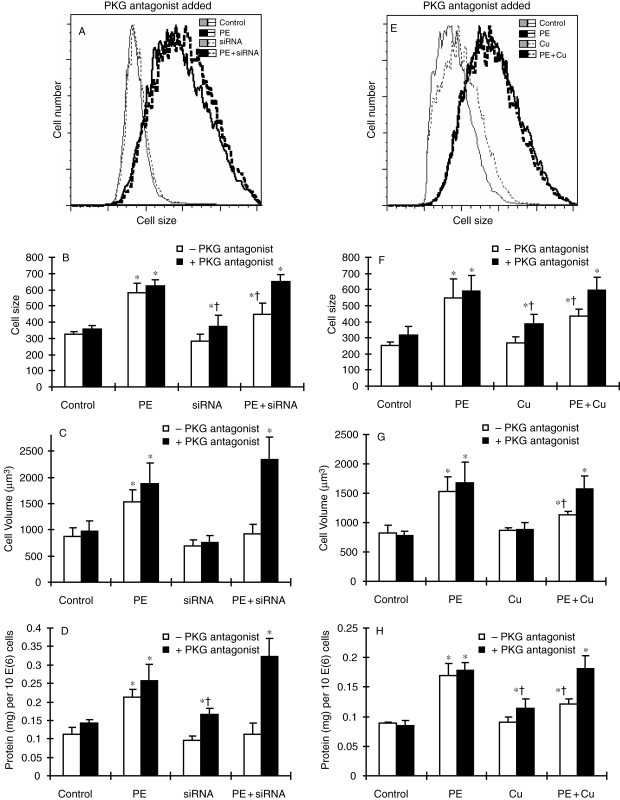
Antagonistic effect of PKG-1 inhibitor, Rp-8-pCPT-cGMPS, on regression of cell hypertrophy induced by siRNA targeting VEGFR-2 (A–D) or Cu supplementation (E–H). The induction of cell hypertrophy and the treatment with siRNA targeting VEGFR-2 or Cu supplementation were described in Section 2. (A and E) Representative flow cytometric histograms showing the change in cell size under different experimental conditions in the presence of PKG-1 inhibitor only (PKG antagonist added). (B and F) Quantitative analysis of cell size by flow cytometer. (C and G) Analysis of cell volume calculated from cell diameter, which was determined by a microslide field finder. Approximately 150 cells from each group were randomly measured by the microslide field finder. (D and H) The protein content in the cells measured by a Bradford method and normalized by cell number. Each group of data were obtained from three independent experiments and each experiment contains a triplicate samples for each treatment. Values are means ± SEM. *Significantly different from control group and †significantly different from PE-treated group (P < 0.05).
3. Results
3.1. The increase in the ratio of VEGFR-1 to VEGFR-2 by Cu treatment in hypertrophic cardiomyocytes
Cardiomyocyte hypertrophy was induced by PE at a final concentration of 100 µM in cultures for 48 h in serum-free media. These cells were then treated with Cu by a direct addition of CuSO4 into cultures at a final concentration of 5 µM. PE treatment increased VEGF concentrations in culture media, but Cu treatment affected neither basic nor PE-elevated VEGF concentrations in culture media (Figure 1). Then, we investigated the effect of Cu on the density of VEGFRs. PE increased the density of both VEGFR-1 (green) and VEGFR-2 (red) (Figure 2). Cu addition did not change the density of VEGFR-1, but markedly reduced that of VEGFR-2 in hypertrophic cardiomyocytes, so that the merge of the two fluorescent labelling showed more green colour (VEGFR-1) than the orange observed under PE only treatment condition (Figure 2). Quantitative analysis by flow cytometer confirmed the effect of Cu on the density of VEGFRs, and the ratio of VEGFR-1 to VEGFR-2 was significantly increased as shown in Figure 2.
3.2. Reversal of PE-induced cardiomyocyte hypertrophy by gene silencing of VEGFR-2
To determine whether the increase in the ratio of VEGFR-1/VEGFR-2 was related to Cu-induced reversal of cardiomyocyte hypertrophy, we used siRNA targeting VEGFR-2 to increase the ratio of VEGFR-1/VEGFR2, as shown in Figure 3. The VEGFR-2 gene silencing-induced increase in the ratio of VEGFR-1 to VEGFR-2 was as the same as that induced by Cu treatment, which was associated with reversal of cardiomyocyte hypertrophy (Figure 4), an effect that was comparable to that induced by Cu treatment.1 In addition, the VEGFR-2 gene silencing-induced regression of cardiomyocyte hypertrophy was also VEGF-dependent, as shown that anti-VEGF antibody blocked the reversal by VEGFR-2 gene silencing of cardiomyocyte hypertrophy (Figure 4).
3.3. Suppression of Cu-induced reversal of cardiomyocyte hypertrophy by gene silencing of VEGFR-1
The increase in the ratio of VEGFR-1/VEGFR-2 accompanied with the reversal of PE-induced cardiomyocyte hypertrophy indicates the determinant role of VEGFR-1 in Cu regression of cardiomyocyte hypertrophy. To confirm this, we used an siRNA targeting VEGFR-1 to examine the effect of VEGFR-1 suppression on Cu reversal of cardiomyocyte hypertrophy. Gene silencing of VEGFR-1 inhibited the response of the cells to PE-induced VEGFR-1 elevation, thereby suppressed the increase in the ratio of VEGFR1/VEGFR2 (Figure 5). Importantly, this VEGFR-1 gene silencing blocked Cu reversal of PE-induced cardiomyocyte hypertrophy, as shown in Figure 5.
3.4. Association of PKG-1 with VEGFR-1-mediated reversal of cardiomyocyte hypertrophy by Cu treatment
Activation of PKG-1 signalling pathway has been shown to be involved in the regression of cardiac hypertrophy.21 The link between PKG-1 and VEGFR-1 was examined by (i) the effect of VEGFR-1 gene silencing on PKG-1 activity and (ii) the effect of a PKG-1 antagonist on Cu- or VEGFR-2 gene silencing-induced regression of cardiomyocyte hypertrophy. The result presented in Figure 6 shows that PE treatment significantly elevated PKG-1 activity and Cu addition did not change PKG-1 activity, but VEGFR-1 gene silencing significantly attenuated PE-induced PKG-1 activation. Importantly, a PKG-1 antagonist, Rp-8-pCPT-cGMPS, blocked both Cu- and VEGFR-2 gene silencing-induced reversal of cardiomyocyte hypertrophy, as shown in Figure 7.
4. Discussion
The present study followed our previous observation that Cu reversal of PE-induced hypertrophy in primary cultures of neonatal rat cardiomyocytes is VEGF-dependent1 to provide mechanistic insights into the Cu action. The results presented here show that Cu at the physiologically relevant level used in the present study did not increase VEGF production in the primary cultures of neonatal rat cardiomyocytes. However, our previous study has shown that anti-VEGF antibody blocked the reversal by Cu of cardiomyocyte hypertrophy.1 This VEGF-dependent action of Cu suggested that although Cu did not cause increases in VEGF production, it would change the downstream events that are VEGF-dependent. Such downstream events include VEGFRs. The results obtained indeed demonstrated that Cu increased the ratio of VEGFR-1 to VEGFR-2, and this increase was highly responsible for the reversal of cardiomyocyte hypertrophy.
The first evidence that showed the cause–effect relationship between the increase in the ratio of VEGFR-1 to VEGFR-2 and the reversal of cardiomyocyte hypertrophy was obtained from the experiment using siRNA targeting VEGFR-2 to elevate the ratio of VEGFR-1 to VEGFR-2. This VEGFR-2 gene silencing-induced increase in the ratio of VEGFR-1 to VEGFR-2 was thus differentiated from the action of Cu, but reproduced the effect of Cu-induced reversal of cardiomyocyte hypertrophy. The second evidence was obtained from the study showed that anti-VEGF antibody also effectively blocked the reversal of cardiomyocyte hypertrophy induced by VEGFR-2 gene silencing, indicating the importance of interaction between VEGF and the VEGFR-1. The third evidence was that gene silencing of VEGFR-1 blocked Cu-induced reversal of cardiomyocyte hypertrophy, further indicating the role of VEGFR-1 in mediating Cu-induced reversal of cardiomyocyte hypertrophy.
There are several studies that have demonstrated different functions between VEGFR-2 and VEGFR-1. In particular, VEGFR-2 is more responsible for cell growth stimulation triggered by VEGF in endothelial cells. Activation of VEGFR-2 by VEGF in cells devoid of VEGFR-1 results in a mitogenic response.6,7 In addition, VEGFR-2 is more predominant in most of the known cellular responses to VEGF such as embryonic vasculogenesis and tumour angiogenesis.8 The link between VEGFR-2 and its downstream pathways is more related to growth stimulation such as MAPK signalling pathway. The Akt1 pathway is a critical component in the process of myocardial hypertrophy and it has been shown that VEGFR-2 is linked to Akt1, as demonstrated by the fact that decoy VEGFR-2 blocks cardiac growth induced by Akt1 activation.3,18
Although previous studies have not provided a comprehensive understanding of the role of VEGFR-1 in cellular function, many studies have pointed out a counter action of VEGFR-1 relative to VEGFR-2, so that it was suggested that the function of VEGFR-1 is more related to a negative regulation of the action of VEGFR-2.9–12 The present study produced evidence that shows VEGFR-1 is associated with PKG-1 activation. The link of VEGFR-1 to this important pathway in regression of heart hypertrophy provides an explanation of the VEGFR-1-dependent reversal of cardiomyocyte hypertrophy. There would be multiple pathways that are triggered by VEGFR-1 activation. However, the PKG-1 signalling pathway is the most studied and important in relation to regression of heart hypertrophy. Our observation that suppression of VEGFR-1 reduced the activity of PKG-1 suggests that the activation of PKG-1 at least in part is VEGFR-1-dependent. Conversely, the PKG-1 antagonist Rp-8-pCPT-cGMPS effectively blocked both Cu- and VEGFR-2 gene silencing-induced reversal of cardiomyocyte hypertrophy. Taken together, these results demonstrate that VEGFR-1 is likely involved in Cu-induced reversal of cardiomyocyte hypertrophy, a pathway that is associated with PKG-1 activation.
Activation of PKG-1 in hypertrophic myocardium has been observed in pressure overload-induced heart hypertrophy.21 In a mouse model of cardiac pressure overload induced by transverse aorta constriction (TAC), heart hypertrophy was observed after TAC for 3 weeks. PKG-1 activity was significantly elevated in the hypertrophic myocardium.21 This activation would be considered as a counter action to heart hypertrophy induced by pressure overload. However, the PKG-1 activation was not sufficient to prevent the development of heart hypertrophy.21 But a treatment with an oral phosphodiesterase-5A inhibitor, sildenafil, blocked the intrinsic catabolism of cGMP leading to further activation of PKG-1 with an accompanied regression of heart hypertrophy. In the study presented here, we observed that PE treatment enhanced PKG-1 activity, but Cu did not affect the PE-induced activation of PKG-1. However, Cu inhibited VEGFR-2, which is linked to the Akt-1 signalling pathway. Along with this, inhibition was the increase in the ratio of VEGFR-1/VEGFR-2, thus making the VEGFR-1 downstream signalling pathway overshadows that of VEGFR-2. Therefore, although Cu did not increase the level of PKG-1 activity, the signalling pathway switching from VEGFR-2 to VEGFR-1 would make the activity of PKG-1 become dominant, leading to activation of the hypertrophic reversal signalling cascades.
The finding here provides further insights into Cu regression of heart hypertrophy, as reported previously.2 Clinically, regression of heart hypertrophy by Cu supplementation would be a simple and less expensive therapy for heart disease. The Cu level used in the present study would be equivalent to the level of Cu found in conventional mineral supplements (2 mg Cu/day), which is much lower than the tolerable upper intake level of 10 mg/day for adults 19 years of age or older.22 In addition, the current US-Canadian Dietary Reference Intakes for Cu is 0.9 mg/day,22 a relatively big margin for dietary Cu supplementation.
There are several limitations in the present study. We did not examine how Cu regulates the expression or differential distribution of VEGFRs. This is an important question and the answer to this question will help further understand the role of Cu in regression of heart hypertrophy. From our observation that Cu suppressed PE-elevated VEGFR-2 levels, it would suggest that Cu would not cause overexpression of VEGFR-1. Then the question needs to be addressed is how Cu reduces VEGFR-2, suppressing its expression or enhancing its degradation? Our future studies will address this question. Another limitation of this study is that the mechanism by which VEGFR-1 regulates PKG-1 activation has not been defined. This mechanistic insight is important for further understanding the signalling pathway leading to regression of cardiomyocyte hypertrophy, which will be an important focus in our future studies.
In conclusion, the present study demonstrates that Cu-induced regression of cardiomyocyte hypertrophy is related to a switch of signalling pathways from VEGFR-2 to VEGFR-1. Although both pathways are VEGF-dependent, the data suggest that VEGFR-1 is associated with signalling pathways linked to PKG-1, which has been demonstrated both in vivo and in vitro to be associated with cardiomyocyte growth inhibition and regression of cardiomyocyte hypertrophy. It remains to be elucidative that how Cu regulates the differential distribution between VEGFR-1 and VEGFR-2 in hypertrophic cardiomyocytes and how VEGFR-1 interacts PKG-1 leading to the activation of hypertrophic reversal signalling pathways.
Funding
This work was supported in part by National Institutes of Health (HL63760 to Y.J.K.).
Acknowledgements
The authors are indebted to Zhanxiang Zhou and Wenke Feng for technical advices.
Conflict of interest: none declared.
References
- 1.Zhou Y, Jiang Y, Kang YJ. Copper reverses cardiomyocyte hypertrophy through vascular endothelial growth factor-mediated reduction in the cell size. J Mol Cell Cardiol. 2008;45:106–117. doi: 10.1016/j.yjmcc.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Y, Reynolds C, Xiao C, Feng W, Zhou Z, Rodriguez W, et al. Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J Exp Med. 2007;204:657–666. doi: 10.1084/jem.20061943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao RL, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh K, Shiojima I. Cardiac growth and angiogenesis coordinated by intertissue interactions. J Clin Invest. 2007;117:3176–3179. doi: 10.1172/JCI34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruch C, Skiniotis G, Steinmetz MO, Walz T, Ballmer-Hofer K. Structure of a VEGF-VEGF receptor complex determined by electron microscopy. Nat Struct Mol Biol. 2007;14:249–250. doi: 10.1038/nsmb1202. [DOI] [PubMed] [Google Scholar]
- 6.Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene. 1995;10:135–147. [PubMed] [Google Scholar]
- 7.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 8.Reynolds AR, Reynolds LE, Nagel TE, Lively JC, Robinson SD, Hicklin DJ, et al. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004;64:8643–8650. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- 9.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, et al. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- 10.Zeng H, Dvorak HF, Mukhopadhyay D. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) peceptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2001;276:26969–26979. doi: 10.1074/jbc.M103213200. [DOI] [PubMed] [Google Scholar]
- 11.Rahimi N, Dayanir V, Lashkari K. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J Biol Chem. 2000;275:16986–16992. doi: 10.1074/jbc.M000528200. [DOI] [PubMed] [Google Scholar]
- 12.Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 13.Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem. 1997;272:32521–32527. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi N, Seko Y, Noiri E, Tobe K, Kadowaki T, Sabe H, et al. Vascular endothelial growth factor induces activation and subcellular translocation of focal adhesion kinase (p125FAK) in cultured rat cardiac myocytes. Circ Res. 1999;84:1194–1202. doi: 10.1161/01.res.84.10.1194. [DOI] [PubMed] [Google Scholar]
- 15.Shimojo N, Jesmin S, Zaedi S, Otsuki T, Maeda S, Yamaguchi N, et al. Contributory role of VEGF overexpression in endothelin-1-induced cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H474–H481. doi: 10.1152/ajpheart.00922.2006. [DOI] [PubMed] [Google Scholar]
- 16.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, et al. P53-induced inhibition of HIF-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 17.Shyu KG, Liou JY, Wang BW, Fang WJ, Chang H. Carvedilol prevents cardiac hypertrophy and overexpression of hypoxia-inducible factor-1α and vascular endothelial growth factor in pressure-overloaded rat heart. J Biomed Sci. 2005;12:409–420. doi: 10.1007/s11373-005-3008-x. [DOI] [PubMed] [Google Scholar]
- 18.Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–893. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci USA. 2002;99:11363–11368. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wollert KC, Fiedler B, Gambaryan S, Smolenski A, Heineke J, Butt E, et al. Gene transfer of cGMP-dependent protein kinase I enhances the antihypertrophic effects of nitric oxide in cardiomyocytes. Hypertension. 2002;39:87–92. doi: 10.1161/hy1201.097292. [DOI] [PubMed] [Google Scholar]
- 21.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 22.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]