Abstract
Aims
Nuclear factor-κB (NF-κB) plays a critical role in cell growth and inflammation during the progression of cardiac hypertrophy and heart failure. Several members of nuclear receptor superfamily, including liver X receptors (LXRα and LXRβ), have been shown to suppress inflammatory responses, but little is known about their effects in cardiomyocytes.
Methods and results
We investigated LXR expression patterns in pressure overload-induced hypertrophic hearts and the hypertrophic growth of the LXRα-deficient hearts from mice (C57/B6) in response to pressure overload. The underlying mechanisms were also explored using cultured myocytes. We found that cardiac expression of LXRα was upregulated in pressure overload-induced left ventricular hypertrophy in mice. Transverse aorta coarctation-induced left ventricular hypertrophy was exacerbated in LXRα-null mice relative to control mice. A synthetic LXR ligand, T1317, suppressed cardiomyocyte hypertrophy in response to angiotensin II and lipopolysaccharide treatments. In addition, LXR activation suppressed NF-κB signalling and the expression of associated inflammatory factors. Overexpression of constitutively active LXRα and β in cultured myocytes suppressed NF-κB activity.
Conclusion
LXRs are negative regulators of cardiac growth and inflammation via suppressing NF-κB signalling in cardiomyocytes. This should provide new insights into novel therapeutic targets for treating cardiac hypertrophy and heart failure.
Keywords: LXRα, LXRβ, Cardiac hypertrophy, Inflammation, Angiotensin II, LPS, NF-κB, Cardiomyocytes
1. Introduction
Cardiac hypertrophy is a major risk factor in the progressive development of heart failure, a leading cause of death. The heart copes with a wide range of pathological stimuli, such as arterial hypertension, valvular heart disease, myocardial infarction, mitochondria DNA mutations, and sarcomeric dysfunction by compensatory growth.1,2 Hypertrophic growth of the heart may be an adaptive response and when it is exhausted, the heart fails. Inflammation plays a prominent role in the progression of cardiac hypertrophy and heart failure. Activation of nuclear factor-κB (NF-κB) is intricately linked to cardiomyocyte hypertrophy,3 largely by dramatic induction of inflammatory factors such as tumour necrosis factor-α (TNF-α), interleukin 6 (IL-6), monocyte chemotactic protein-1 (MCP-1), and others. Furthermore, mice with cardiac overexpression of TNF-α develop hypertrophic cardiomyopathy.4 Although it has been well documented that angiotensin II (AngII) is a growth factor for many cells including cardiomyocytes, emerging evidence has also suggested that AngII induces inflammation via activating NF-κB in cardiomyocytes.5 Intervention of AngII-induced cell growth and inflammatory responses is a potential therapeutic target for cardiac hypertrophy and heart failure. However, it has been difficult to identify tractable compounds for interfering myocardial inflammation and progression of cardiac hypertrophy to heart failure.
A handful of small molecules targeting to a variety of nuclear receptors have recently emerged as anti-inflammatory compounds.6 Liver X receptors (LXRs) are among these potential targets that may help to control inflammatory responses. LXRs were initially identified as members of the Class 2 superfamily of nuclear receptors by cDNA library screening for nuclear receptor homologs.7–9 Two LXR isoforms, LXRα (NR1H3) and LXRβ (NR1H2), have been identified in mammals and they share a high degree of amino acid similarity.10 Both LXRα and LXRβ function as heterodimers with the retinoid X receptor, and subsequently bind to specific DNA response elements within the regulatory regions of their target genes.9 LXRs are sterol-responsive transcription factors that regulate expression of genes involved in cholesterol metabolism and homeostasis in various tissues and cells.9,11,12 However, it remains unclear if LXRs play any roles in myocardial lipid metabolism. On the other hand, LXRs are also involved in inflammation and immune response. LXRα knockout (KO) and LXRα/LXRβ double KO, but not LXRβ KO mice, are more susceptible to intracellular pathogen Listeria monocytogenes, suggesting that LXRα plays an important protective role against this infection.13 T1317, a synthetic ligand that targets both LXRα and LXRβ, suppresses lipopolysaccharide (LPS)-induced expression of several proinflammatory proteins, such as IL-6 in mouse peritoneal macrophages.14 LPS injection induces a greater increase in hepatic synthesis of TNF-α and a higher circulating IL-6 level in LXRα/LXRβ double KO than in wild-type mice. Furthermore, T1317 reduces the secretion of TNF-α by Th1 lymphocytes.15 The mechanism through which LXR agonists suppress the transcription of proinflammatory genes may include inhibition of NF-κB.16,17 However, it remains unclear if LXRs in the heart play any role in the development of cardiac hypertrophy.
In this study, we provide evidence to support that cardiac LXRs are negative regulators of cardiac hypertrophy via suppressing NF-κB signalling.
2. Methods
2.1. Experimental animals
Three-month-old male LXRα KO mice (C57/B6) (a generous gift from Dr David Mangelsdorf).12 Age- and gender-matched wild-type mice (C57/B6) (Charles River) were used as control. Mice were kept on a 12 h/12 h light/dark cycle in temperature-controlled rooms and had ad libitum access to water and standard laboratory chow. All experimental procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
2.2. Pressure overload-induced hypertrophy
The surgical procedure of transverse aortic coarctation (TAC) has been previously described.19 Briefly, mice were anaesthetized with xylazine (5 mg/kg s.c.) and ketamine (100 mg/kg i.m.). Anaesthesia was maintained by isoflurane inhalation (1.5–2.5%). After opening the chest, the transverse aorta was ligated between the truncus brachiocephalicus and the left common carotid artery by tying a 6-0 silk suture against a 25 gauge needle. Sham mice underwent the same procedure without ligation of the aorta. Mice were sacrificed 14 days after TAC or sham operation. Cardiac hypertrophy was assessed by heart weight to tibia length ratio.
2.3. Culture of cardiomyocytes
Rat neonatal cardiomyocytes (RNCM) were isolated by using a cardiomyocyte isolation system (Worthington) with minor modifications as described previously.13 Cardiomyocytes were then incubated for 24 h at 37°C with serum-free medium. Cardiomyocytes were fixed and stained with Haematoxylin and Eosin; cell surface area was calculated by measuring 50 random cells with Image Pro MediaCybernetics.
2.4. Transcript analyses
Real-time PCR was performed to quantify relative transcript levels in the various treatment groups. Applied Biosystems reagents were used for reverse transcription (MultiScribe MuLV reverse transcriptase) of RNA. A Roche LightCycler PCR system real-time was used to run PCR reactions with SYBP green PCR master mixes (Roche) containing 0.4 µM gene specific primers. Real-time PCR results from each gene/primer pair were normalized to results of β-actin and compared across conditions. The following primers were used: LXRα: up-GTC GTA GCA AAC CAC CAA G, low-GGT ATG AAG TGG CAA ATC G; LXRβ: up-TGA AGC GGC AAG AAG AGG AAC AGG, low-GGA CAC GAT GGC CAG CTC AGT AAA; atrial natriuretic factor (ANF): up-CAT CAC CCT GGG CTT CTT CCT, low-TGG GCT CCA ATC CTG TCA ATC; TNF-α: up-TGAACTTCGGGGTGATCGGTC, low-AGCCTTGTCCCTTGAAGAGGAAC; IL-6: up-CAC GAA GGC TGT GCT GTT T, low-ACT TGC TTC CCA CAC TGT TTG; MCP-1: up-TTAACGCCCCACTCACCTGCTG, low- CAATGTAGGCCGAGAGTGCTG; and β-actin: up-AGATTACTGCTCTGGCTCCTA, low-CAAAGAAAGGGTGTAAAACG.
2.5. Incorporation of [3H] leucine
To examine the effect of AngII on protein synthesis, the incorporation of [3H] leucine was measured essentially by the method of Thaik et al.18 Cultured RNCM were treated with AngII (1 µM) in the presence or in the absence of T1317 and co-incubated with [3H] leucine (1 µCi/mL) for 24 h. The cells were washed with PBS and then treated with 10% trichloroacetic acid at 4°C for 30 min to precipitate the proteins. The precipitates were then dissolved in NaOH (0.25 N). Aliquots were counted with scintillation counter.
2.6. Protein analyses
Total protein samples were extracted from left ventricles and subjected to SDS–PAGE and eletrotransferred to nitrocellulose membranes (Amersham). Western blot was performed using the Enhanced Chemiluminescence Detection System (Santa Cruz Biotechnology). A polyclonal antibody of anti-LXRα/β (Abcam) and a monoclonal antibody for pan-actin (SIGMA) were used. Protein content of TNF-α, MCP-1 in culture media of RNCM was measured by commercial cytokine assay service (AssayGate, Inc.) with Bead-Based Suspension Protein Arrays.
2.7. Transfection and luciferase assay
The H9C2 cells were purchased from the American Tissue Type Collection (CRL-1446). Cells were maintained in DMEM with 4 mmol/L l-glutamine adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 1.0 mmol/L sodium pyruvate, with 10% foetal bovine serum. Cells were grown on 24-well plates and allowed to become 60% confluent in complete medium. Transfection of p NF-κB -Luc, pANFα-Luc (Stratagene) was carried out using a transfection kit (Effectene® Transfection Reagent, Qiagen). After 24 h, cultured media were changed into serum-free media for 12 h. Then, cells were treated with T1317 (1, 5, and 10 µmol/L) or infected with adenoviral vectors for 23 h. Adenoviral vectors containing constitutively active forms of LXRα (ad-VP16-LXRα) and LXRβ (ad-VP16-LXRβ) were used.19,20 Ad-LacZ was used as control. Before harvesting, the cells were treated with AngII (1 µmol/L) for 15 min or LPS (10 ng/mL) for 5.5 h.
2.8. Statistics analyses
All quantitative results were presented as mean ± SEM. Data were analysed by one-factor or two-factor analysis of variance followed by post hoc analyses with Bonferroni test to detect differences between experimental groups using GraphPad Prism and QuickCalcs software (GraphPad Software Inc.).
3. Results
3.1. LXRα is upregulated in hypertrophic heart induced by left ventricular pressure overload
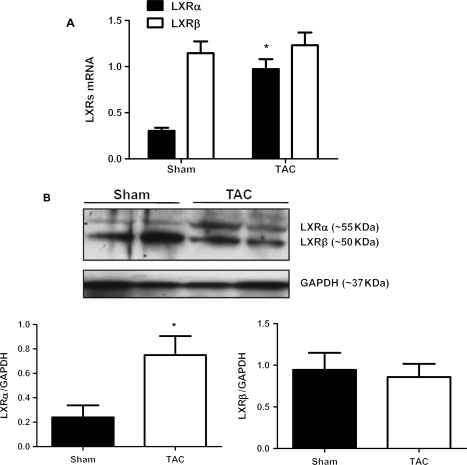
To examine whether cardiac expression of LXRs alters in response to hypertrophy, the relative expression levels of both LXR subtypes in the heart were examined by real-time PCR measurement on samples from mice with TAC and sham operation. As expected, LXRα and LXRβ were both expressed in the heart (Figure 1A). However, the expression of LXRβ was substantially more abundant (Figure 1A). Real-time PCR analyses revealed that transcript levels of LXRα, but not LXRβ, were markedly increased in RNA samples extracted from the left ventricles of TAC mice compared with those of sham mice (Figure 1A). Correspondingly, western blot revealed that LXRα protein was substantially more abundant in samples from TAC than those from sham mice (Figure 1B). These results suggest that LXRα may be involved in the adaptive responses in the development of cardiac hypertrophy.
Figure 1.
LXRs expression in pressure overload-induced cardiac hypertrophy. LXRα is upregulated in mouse hearts in response to transverse aortic coarctation (TAC). Wild-type mice (C57/B6) at their ages of 3-month-old were used for 2 weeks of TAC procedures. Age- and sex-matched sham-operated mice were served as control. (A) Real-time PCR analyses on transcript levels of LXRα and LXRβ in RNA samples extracted from mouse hearts after 2 weeks of TAC and sham operations. Data are expressed as mean ± SEM (n = 6, *P < 0.05 vs. sham). (B) Western blotting analyses on protein expression of LXRα and LXRβ in left ventricles of mice after 2 weeks of TAC and sham operation. Representative images and quantitative results are shown. Data are expressed as mean ± SEM (n = 4, *P < 0.05 vs. sham).
3.2. Pressure overload induces exacerbated cardiac hypertrophy in LXRα null mice
We further assessed the in vivo relevance of LXR signalling in regulating the pathogenesis of cardiac hypertrophy. The 2 weeks TAC procedure in mice led to cardiac hypertrophy with increased heart mass and elevated ANF mRNA expression in the left ventricle of both LXRα KO and wild-type controlled mice when compared with sham controls (Figure 2). However, heart mass and ANF mRNA were increased more markedly in LXRα KO than in WT mice (Figure 2A and B). These results indicate that LXRα deficiency in the heart could lead to exacerbated cardiac hypertrophy in response to pressure overload.
Figure 2.
Effect of LXRα deficiency on pressure overload-induce cardiac hypertrophy. Pressure overload-induced cardiac hypertrophy was exacerbated in LXRα KO mice compared with that of WT mice. Wild-type mice (C57/B6) and LXRα KO mice at their ages of 4-month-old were used for 2 weeks of TAC procedures. Age- and sex-matched sham-operated mice were served as control. (A) Ratio of heart weight to tibial length in LXRα KO and wild-type mice after 2 weeks of TAC or sham procedures. Data are expressed as mean ± SEM (n = 12, *P < 0.05 vs. sham, #P < 0.05 vs. WT). (B) Real-time PCR analyses on transcript levels of ANF in RNA samples extracted from mouse hearts after 2 weeks of TAC and sham operations. Data are expressed as mean ± SEM (n = 6, *P < 0.05 vs. sham; #P < 0.05 vs. WT).
3.3. The LXR agonist T1317 inhibits AngII- and LPS-induced cardiomyocyte hypertrophy
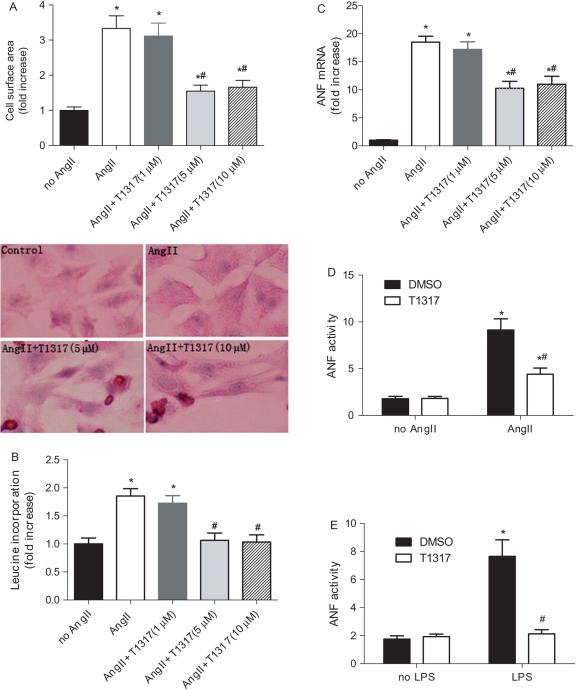
To investigate a potential role of LXRs as negative regulators of cardiomyocyte hypertrophy, we assessed the effect of LXR activation in AngII-induced cardiomyocyte hypertrophy. RNCM were cultured and treated with AngII (1 µmol/L) in the presence or absence of various doses of T1317 (1, 5, and 10 µmol/L). AngII induced cardiomyocyte growth with increased cell surface area, which was blunted by T1317 at the doses of 5 and10 µmol/L (Figure 3A and B). Whereas T1317 at various doses did not change the rate of leucine incorporation without AngII (data not shown), T1317 at the dose of 5 and 10 µmol/L inhibited leucine incorporation induced by AngII in cultured RNCM (Figure 3B). ANF is a well-defined hypertrophic marker in cardiac myocytes.21 AngII but not T1317 induced a robust upregulation of ANF mRNA expression (Figure 3C). T1317 (5 and 10 µmol/L) at least partially abolished AngII-induced upregulation of ANF transcript (Figure 3C). Further, T1317 treatment blunted more than 50% of the robust rise of ANF promoter activity in cultured H9C2 cells induced by AngII (Figure 3D). LPS, an endotoxin that is frequently used as inflammation inductor, can also induce pathological hypertrophic responses with upregulation of ANF in H9C2 cell.22 ANF promoter activity assay confirmed T1317 (10 µmol/L) substantially suppressed LPS-induced ANF activity compared with vehicle treatment (Figure 3E). These results suggest that T1317 may be an effective compound in inhibiting cardiac hypertrophy.
Figure 3.
Effect of LXR agonist T1317 on AngII-induced cardiac hypertrophy in RNCM. RNCM were stimulated with 1 µM AngII in the presence or absence of T1317 (1, 5, and 10 µmol/L) that was added 2 h before experiments. (A) Effects of AngII with and without T1317 on cell surface area of cardiomyocytes. Cell surface area was calculated by measuring 50 random cardiomyocytes with Image Pro. (n = 6, *P < 0.05 vs. no AngII, #P < 0.05 vs. AngII alone). Shown are representative images of cardiomyocytes with different treatments. (B) [3H] leucine incorporation was determined by co-incubating cardiomyocytes with 1.0 µCi/mL [3H] leucine for 12 h. Data are expressed as mean ± SEM (n = 6, *P < 0.05 vs. no AngII, #P < 0.05 vs. AngII alone). (C) Real-time PCR analysis of the mRNA levels of ANF in AngII-stimulated cardiomyocytes in the presence or absence of T1317. Data are expressed as mean ± SEM (n = 6, *P < 0.05 vs. no AngII, #P < 0.05 vs. AngII alone). (D) LXR-selective ligand T1317 (10 µmol/L) inhibited AngII-induced luciferase activities driving by ANF promoter in cultured H9C2 myocytes. Data are expressed as mean ± SEM (n = 6, *P < 0.05 vs. no AngII; #P < 0.05 vs. DMSO). (E) LXR-selective ligand T1317 (10 µmol/L) inhibited LPS-induced luciferase activities driving by ANF promoter in H9C2 myocytes. Data are expressed as mean ± SEM (n = 6, *P < 0.05 vs. no LPS; #P < 0.05 vs. DMSO).
3.4. The LXR agonist T1317 inhibits AngII- and LPS-induced NF-κB activity
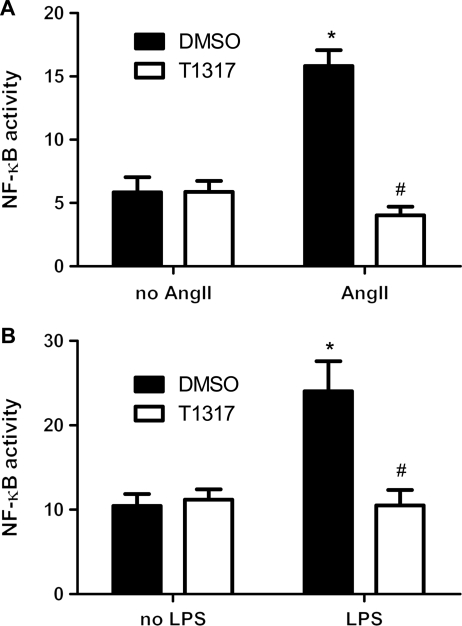
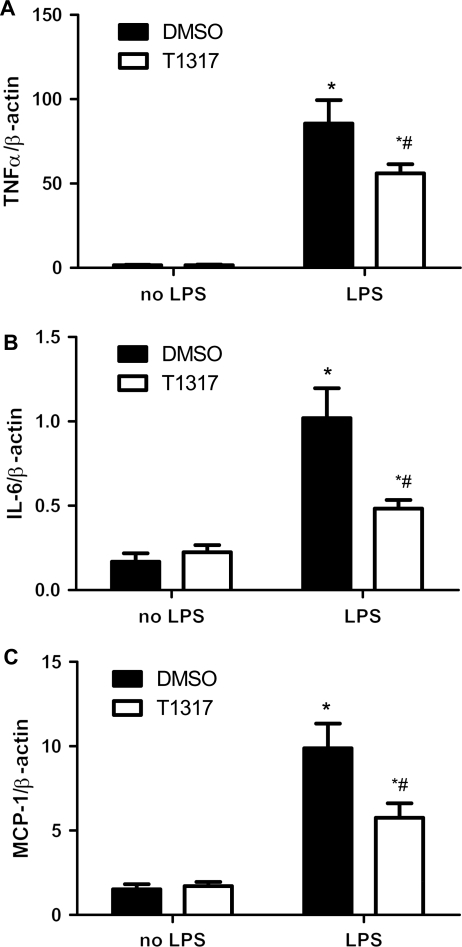
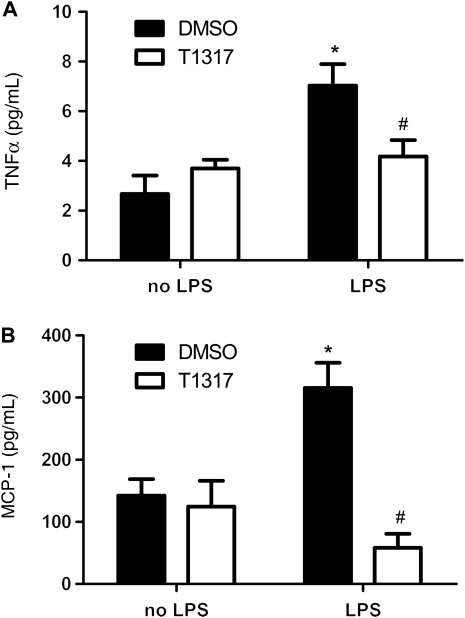
Since NF-κB signal pathway is involved in both inflammation and cardiac hypertrophy, we investigated the effect of T1317 on AngII- and LPS-induced NF-κB activity in cultured H9C2 myocytes. A luciferase reporter construct containing five copies of the NF-κB binding site (pNF-κB-TK-Luc) was transiently transfected in cultured H9C2 myocytes with the existence of T1317. Although T1317 did not induce basal change, luciferase assay revealed that T1317 suppressed both AngII- and LPS-induced upregulation of NF-κB activity (Figure 4A and B). Similarly, T1317 pretreatment substantially abolished LPS-induced TNF-α, IL-6, and MCP-1 transcript overexpression in cultured cardiomyocytes (Figure 5A–C). Furthermore, protein measurement revealed that the LPS-induced TNF-α and MCP-1 production in the cultured media of the above cardiomyocytes were similarly blunted by the treatment of T1317 (Figure 6A and B). Therefore, these results support that T1317 suppresses NF-κB activity to alleviate hypertrophic growth of cardiomyocytes under hypertrophic stimuli.
Figure 4.
Effect of LXR-selective ligand T1317 on NF-κB activity. (A) LXR-selective ligand T1317 (10 µmol/L) inhibited AngII-induced luciferase activities driving by NF-κB promoter in cultured H9C2 myocytes. Data are expressed as mean ± SEM (n = 4, *P < 0.05 vs. no AngII, #P < 0.05 vs. DMSO). (B) LXR-selective ligand T1317 (10 µmol/L) inhibited LPS-induced luciferase activities driving by NF-κB promoter in cultured H9C2 myocytes. Data are expressed as mean ± SEM (n = 4, *P < 0.05 vs. no LPS, #P < 0.05 vs. DMSO).
Figure 5.
Effect of T1317 on LPS-induced NF-κB target gene expression in cultured RNCM. Real-time PCR analyses on transcript level of (A) TNF-α, (B) IL-6, and (C) MCP-1 in RNA samples extracted from RNCM treated with LPS (10 ng/mL) in the presence and absence of T1317 (10 µmol/L). Data are expressed as mean ± SEM (n = 4, *P < 0.05 vs. no AngII, #P < 0.05 vs. DMSO).
Figure 6.
Effect of T1317 on LPS-induced MCP-1 and TNF-α production in media of RNCM. (A) MCP-1 and (B) TNF-α levels in media of RNCM treated with LPS (10 ng/mL) in the presence and absence of T1317 (10 µmol/L) were measured by commercial cytokine assay service (AssayGate, Inc.) with bead-based suspension protein arrays. Data are expressed as mean ± SEM (n = 4, *P < 0.05 vs. no LPS, #P < 0.05 vs. DMSO).
3.5. Adenoviral-mediated overexpression of constitutively active LXRα and LXRβ suppressed NF-κB activity
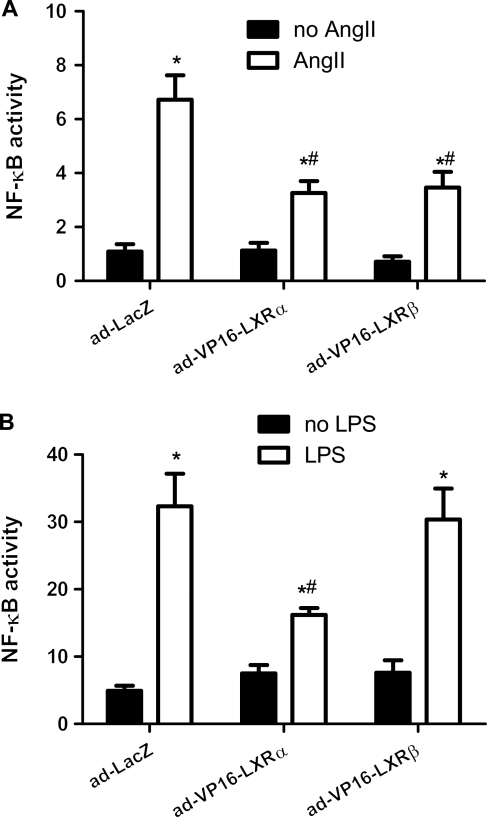
We further examined whether there was any differentiated effect of each LXR subtype on their capacity to suppress NF-κB activity. Adenoviral vectors ad-VP16-LXRα and ad-VP16-LXRβ were used to mediate overexpression of the constitutively active forms of LXRα and LXRβ in cultured myocytes. Ad-LacZ was used as control. After treated with adenoviral (10 PFU) for 24 h, VP16-LXRα, VP16-LXRβ, and LacZ overexpression induced similar basal activity of NF-κB reporter. VP16-LXRα overexpression substantially mitigated AngII-induced upregulation of NF-κB activity (Figure 7A). Similar suppression of VP16-LXRα overexpression on LPS-induced NF-κB activity was also revealed (Figure 7B). On the other hand, VP16-LXRβ overexpression only suppressed AngII- but not LPS-induced NF-κB activity (Figure 7A and B). Therefore, it implicates that both LXRs suppress NF-κB activity and hence may serve as negative regulators of hypertrophic growth.
Figure 7.
Effect of overexpression of constitutively active LXR on NF-κB activity. Cultured H9C2 myocytes were transfected with a pNF-κB reporter/luciferase vector and co-transfected with a control vector. Cells were transfected with ad-VP16-LXRα, ad-VP16-LXRβ, or ad-LacZ. NF-κB activities induced by (A) AngII (1 µmol/L) and (B) LPS (10 ng/mL) in the above treated cells were measured. Relative quantitation of luciferase activities represents activities of NF-κB. Data are expressed as mean ± SEM (n = 6, *P < 0.05 vs. no AngII or no LPS, #P < 0.05 vs. Ad-LacZ + AngII or Ad-LacZ + LPS).
4. Discussion
In the present study, we investigated the effect of LXR activation on cardiac hypertrophy. Our findings indicate that LXRα mRNA level is significantly increased by TAC-induced left ventricular hypertrophy. TAC induces exacerbated cardiac hypertrophy in LXRα KO mice compared with wild-type control. LXR ligand, T1317, suppresses AngII- and LPS-induced hypertrophied responses by blocking NF-κB signalling. Both LXRα and LXRβ appear to mediate the inhibitory effect of T1317 on NF-κB signalling, whereas LXRα may be more responsive to more varieties of hypertrophic stimuli.
Emerging evidence indicates that LXRs play important roles in lipid and glucose metabolisms, regulate cell proliferation and differentiation, and inflammation (see review23). Recent evidence shows that LXRs are negative regulators of inflammatory responses in various systems of the body.14,23 However, it is unknown whether LXRs play a similar anti-inflammation role in the heart. Our current findings suggest that LXR activation may represent a negative regulation mechanism in cardiomyocytes under hypertrophic stimuli. Together with the finding that LXRα expression rose in response to pressure overload-induced cardiac hypertrophy in the in vivo heart, it becomes plausible that LXR signalling may be essential for the heart to cope with hypertrophic growth stimuli. As a matter of fact, TAC induced greater left ventricular hypertrophy in LXRα KO than in wild-type mice. This result proves that LXRα signalling may be involved in negatively regulating hypertrophic growth in response to pathological stimuli. Nevertheless, it remains unclear whether LXRβ is also required for the heart to resist to pathological growth. With the exacerbated hypertrophic response in the LXRα KO heart, endogenous LXRβ alone appears not sufficient to prevent pressure overload-induced hypertrophic growth of an in vivo heart. The role of LXRs in lipogenic gene expression has been well documented. Since the LXRα KO mice had no detectable cardiac phenotypic changes in their life time (data not shown), it is not likely that a pre-existed condition of the LXRα KO heart exacerbated hypertrophic response. On the other hand, the current study does not exclude the possibility that combined changes in inflammatory and lipid metabolism mediating the anti-hypertrophic role of LXRs. Further studies on the role of LXRs in fatty acid and glucose metabolism of the heart should be insightful.
It has been well established that NF-κB activation is involved in the hypertrophic response of cardiomyocytes.23,24 The heart produces several inflammatory cytokines, including TNF-α, under various pathological stimuli that activate NF-κB signalling.25 Targeted therapies to reduce cardiac TNF-α production can improve cardiac performance in post-ischaemic and failing hearts.26 Elevated levels of circulating cytokines have been demonstrated in patients with heart failure.27 Our current results show for the first time that a synthetic LXR ligand, T1317, exerted anti-hypertrophic effect in cardiomyocytes. The anti-hypertrophic effects of T1317 were demonstrated by its capacity in preventing AngII-induced cardiomyocyte enlargement, protein synthesis, and ANF-transcriptional activity. Moreover, T1317 suppressed AngII- and LPS-induced hypertrophic and inflammatory responses. Therefore, the anti-hypertrophic effect of T1317 appears to be involved in signalling pathways associated with inflammatory responses. Indeed, we found that T1317 suppressed NF-κB activity in cultured embryonic heart cells, suggesting that LXRs inhibit cardiac hypertrophy and inflammation via the NF-κB signalling pathway. Furthermore, we provide evidence to support the inhibitory effect of T1317 treatment on LPS-induced expression of NF-κB target genes in cultured cardiomyocytes. This finding is consistent with previous studies on many other cell types. Since T1317 is a synthetic ligand that can activate both LXRα and LXRβ, we further explored the direct effects of each subtype of LXRs on AngII- and LPS-induced inflammation and hypertrophy in cultured cardiomyocytes by assessing the effects of adenoviral-mediated overexpression of constitutively active forms of LXRα and LXRβ. The VP16-LXRα and VP16-LXRβ fusion proteins encoded by these vectors show enhanced constitutive activity.19,20 It is not immediately clear why only the constitutively active LXRα, but not LXRβ, directly inhibits LPS-induced NF-κB signalling. Since overexpression of the VP16-LXRβ also blunted AngII-induced NF-κB activation, it is plausible that both subtypes of LXRs are potentially involved in negative regulation of inflammation and hypertrophic growth of the heart. However, LXRα may be more responsive and plays an even broader and more active role in regulating stresses exerted onto the heart.
While a majority of studies on various tissues and cells consistently confirm the anti-inflammation effect of LXR activation, a recent study unveiled that LXR activation potentiates LPS response in cultured human macrophages by promoting activation of the Toll-like receptor (TLF)-4 signalling pathway.28 However, we could not detect any change in TLF-4 expression in cultured cardiomyocytes treated with T1317 at various time points (data not shown). Therefore, it is likely that LXR may not regulate TLF-4 signalling in cardiomyocytes as it does in macrophages. A recent study demonstrated that LXR activation suppresses AngII receptor I (ATI) expression in smooth muscle cells.29 However, the acute effect of LXR on inhibiting NF-κB signalling excludes this possibility. Furthermore, there is no detectable cardiac hypertrophy in the LXRα null mice. It is not likely that the lack of LXRα in the heart triggers elevated ATI expression. Therefore, it appears that the activated LXR exerts anti-hypertrophic effect via direct suppression of NF-κB signalling.
Taken together, the present study has demonstrated that LXR activation exerts anti-hypertrophy effect in cardiac myocytes via inhibiting NF-κB signalling in response to pathological stimuli. This finding should provide new insights into the role of LXR signalling in the heart and how it is involved in negative regulation of pathological growth of the heart. With similar findings in regard to the LXRs' anti-inflammation roles on many other tissues and cell types, the current findings may support further preclinical trial on the use of synthetic ligands targeting to LXRs for the treatment of cardiac disorders.
Conflict of interest: none declared.
Funding
This work was supported by grants from NIH (1R01HL085499, 1R01HL084456, and R21 AT003734).
References
- 1.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 2.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Ha T, Gao X, Kelley J, Williams DL, Browder IW, et al. NF-kappaB activation is required for the development of cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H1712–H1720. doi: 10.1152/ajpheart.00124.2004. [DOI] [PubMed] [Google Scholar]
- 4.Higuchi Y, Chan TO, Brown MA, Zhang J, DeGeorge BR, Jr, Funakoshi H, et al. Cardioprotection afforded by NF-kappaB ablation is associated with activation of Akt in mice overexpressing TNF-alpha. Am J Physiol Heart Circ Physiol. 2006;290:H590–H598. doi: 10.1152/ajpheart.00379.2005. [DOI] [PubMed] [Google Scholar]
- 5.Wu L, Iwai M, Li Z, Li JM, Mogi M, Horiuchi M. Nifedipine inhibited angiotensin II-induced monocyte chemoattractant protein 1 expression: involvement of inhibitor of nuclear factor kappa B kinase and nuclear factor kappa B-inducing kinase. J Hypertens. 2006;24:123–130. doi: 10.1097/01.hjh.0000198031.30095.d1. [DOI] [PubMed] [Google Scholar]
- 6.Hong C, Tontonoz P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr Opin Genet Dev. 2008;18:461–467. doi: 10.1016/j.gde.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song C, Kokontis JM, Hiipakka RA, Liao S. Ubiquitous receptor: a receptor that modulates gene activation by retinoic acid and thyroid hormone receptors. Proc Natl Acad Sci USA. 1994;91:10809–10813. doi: 10.1073/pnas.91.23.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teboul M, Enmark E, Li Q, Wikstrom AC, Pelto-Huikko M, Gustafsson JA. OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc Natl Acad Sci USA. 1995;92:2096–2100. doi: 10.1073/pnas.92.6.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 10.Alberti S, Steffensen KR, Gustafsson JA. Structural characterisation of the mouse nuclear oxysterol receptor genes LXRalpha and LXRbeta. Gene. 2000;243:93–103. doi: 10.1016/s0378-1119(99)00555-7. [DOI] [PubMed] [Google Scholar]
- 11.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 12.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 13.Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 15.Walcher D, Kummel A, Kehrle B, Bach H, Grub M, Durst R, et al. LXR activation reduces proinflammatory cytokine expression in human CD4-positive lymphocytes. Arterioscler Thromb Vasc Biol. 2006;26:1022–1028. doi: 10.1161/01.ATV.0000210278.67076.8f. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda T, Kanno M, Kawamoto M, Yuge O, Ninomiya Y. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 gene expression by 22(R)-hydroxycholesterol requires de novo protein synthesis in activated macrophages. J Steroid Biochem Mol Biol. 2005;97:376–383. doi: 10.1016/j.jsbmb.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thaik CM, Calderone A, Takahashi N, Colucci WS. Interleukin-1 beta modulates the growth and phenotype of neonatal rat cardiac myocytes. J Clin Invest. 1995;96:1093–1099. doi: 10.1172/JCI118095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laffitte BA, Joseph SB, Chen M, Castrillo A, Repa J, Wilpitz D, et al. The phospholipid transfer protein gene is a liver X receptor target expressed by macrophages in atherosclerotic lesions. Mol Cell Biol. 2003;23:2182–2191. doi: 10.1128/MCB.23.6.2182-2191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, et al. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci USA. 2001;98:507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadoshima J, Jahn L, Takahashi T, Kulik TJ, Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem. 1992;267:10551–10560. [PubMed] [Google Scholar]
- 22.Liu CJ, Cheng YC, Lee KW, Hsu HH, Chu CH, Tsai FJ, et al. Lipopolysaccharide induces cellular hypertrophy through calcineurin/NFAT-3 signaling pathway in H9c2 myocardiac cells. Mol Cell Biochem. 2008;313:167–178. doi: 10.1007/s11010-008-9754-0. [DOI] [PubMed] [Google Scholar]
- 23.Wang YY, Dahle MK, Agren J, Myhre AE, Reinholt FP, Foster SJ, et al. Activation of the liver X receptor protects against hepatic injury in endotoxemia by suppressing Kupffer cell activation. Shock. 2006;25:141–146. doi: 10.1097/01.shk.0000191377.78144.d9. [DOI] [PubMed] [Google Scholar]
- 24.Cook SA, Novikov MS, Ahn Y, Matsui T, Rosenzweig A. A20 is dynamically regulated in the heart and inhibits the hypertrophic response. Circulation. 2003;108:664–667. doi: 10.1161/01.CIR.0000086978.95976.41. [DOI] [PubMed] [Google Scholar]
- 25.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 26.Gurevitch J, Frolkis I, Yuhas Y, Lifschitz-Mercer B, Berger E, Paz Y, et al. Anti-tumor necrosis factor-alpha improves myocardial recovery after ischemia and reperfusion. J Am Coll Cardiol. 1997;30:1554–1561. doi: 10.1016/s0735-1097(97)00328-8. [DOI] [PubMed] [Google Scholar]
- 27.Raymond RJ, Dehmer GJ, Theoharides TC, Deliargyris EN. Elevated interleukin-6 levels in patients with asymptomatic left ventricular systolic dysfunction. Am Heart J. 2001;141:435–438. doi: 10.1067/mhj.2001.113078. [DOI] [PubMed] [Google Scholar]
- 28.Fontaine C, Rigamonti E, Nohara A, Gervois P, Teissier E, Fruchart JC, et al. Liver X receptor activation potentiates the lipopolysaccharide response in human macrophages. Circ Res. 2007;101:40–49. doi: 10.1161/CIRCRESAHA.106.135814. [DOI] [PubMed] [Google Scholar]
- 29.Imayama I, Ichiki T, Patton D, Inanaga K, Miyazaki R, Ohtsubo H, et al. Liver X receptor activator downregulates angiotensin II type 1 receptor expression through dephosphorylation of Sp1. Hypertension. 2008;51:1631–1636. doi: 10.1161/HYPERTENSIONAHA.107.106963. [DOI] [PubMed] [Google Scholar]