Abstract
Aims
Mitochondrial fusion and fission are essential processes for preservation of normal mitochondrial function. We hypothesized that fusion proteins would be decreased in heart failure (HF), as the mitochondria in HF have been reported to be small and dysfunctional.
Methods and results
Expression of optic atrophy 1 (OPA1), a mitochondrial fusion protein, was decreased in both human and rat HF, as observed by western blotting. OPA1 is important for maintaining normal cristae structure and function, for preserving the inner membrane structure and for protecting cells from apoptosis. Confocal and electron microscopy studies demonstrated that the mitochondria in the failing hearts were small and fragmented, consistent with decreased fusion. OPA1 mRNA levels did not differ between failing and normal hearts, suggesting post-transcriptional control. Simulated ischaemia in the cardiac myogenic cell line H9c2 cells reduced OPA protein levels. Reduction of OPA1 expression with shRNA resulted in increased apoptosis and fragmentation of the mitochondria. Overexpression of OPA1 increased mitochondrial tubularity, but did not protect against simulated ischaemia-induced apoptosis. Cytochrome c release from the mitochondria was increased both with reduction in OPA1 and with overexpression of OPA1.
Conclusion
This is the first report, to our knowledge, of changes in mitochondrial fusion/fission proteins in cardiovascular disease. These changes have implications for mitochondrial function and apoptosis, contributing to the cell loss which is part of the downward progression of the failing heart.
Keywords: Apoptosis, Heart failure, Ischaemia, Mitochondria, Fission, Fusion
1. Introduction
Apoptosis is an important mechanism of cell death in both heart failure (HF) and cardiac ischaemia; however, the underlying mechanisms by which the heart loses myocytes in HF are not completely understood. Mitochondria have a critical role in regulating apoptosis. Cardiac myocytes function at a high metabolic state, requiring large amounts of high-energy phosphates, and as a consequence, mitochondria are abundant in these cells. Mitochondrial fission and fusion, described relatively recently and most extensively in yeast, occur constantly and are thought to be critical for normal mitochondrial function.1–3 If fission is interrupted, large networks of fused mitochondria occur. If fusion fails, the mitochondria become small and fragmented. Abnormalities in fission and fusion can lead to apoptosis,4,5 which is an important mechanism of cardiac myocyte loss in HF.6–8
Optic atrophy 1 (OPA1) is a protein that is essential for normal mitochondrial fusion. In the nervous system, which has a high metabolic rate similar to the heart, mutations in OPA1 are associated with inherited neuropathies, but studies on OPA1 and cell function outside the nervous system are limited.9,10 Fission and fusion proteins have not been systematically studied in the heart. We hypothesized that both HF and ischaemia would be associated with abnormalities of fission and fusion that would contribute to cardiac cell death.
Three GTPases are involved in fusion, OPA1, MFN1, and MFN2, and two proteins in fission, DRP1 and Fis1. Mutations in these proteins are associated with inherited neuropathies, such as Charcot–Marie–Tooth disease.11 Reduction of OPA1, MFN1, or MFN2 results in impaired mitochondrial function. MFN1 and MFN2 are able, at least in part, to accommodate for a deficiency in one or the other; in contrast, deficiency of OPA1 is associated with mitochondrial respiratory dysfunction.12 The dynamin-related protein OPA1, located on the inner mitochondrial membrane, protects against apoptosis by preventing release of cytochrome c from the mitochondria.13 Because of the important functions of fission/fusion proteins and their mitochondrial location, we investigated the effect of HF on OPA1 and related proteins using explanted human hearts and a rat model of HF. As ischaemic cardiomyopathy (ICM) was associated with a decrease in OPA1, simulated ischaemia in cultured cells was used to investigate the underlying mechanisms.
2. Methods
See Supplementary material online for full Methods.
2.1. HF models
Two models of the failing heart were used: the explanted failing human heart removed at transplant and a high coronary ligation rat model of HF, as previously reported.14 HF human heart samples were the gift of Dr Guillermo Torre-Amione. This tissue bank has IRB approval at Methodist Hospital, Houston, TX, USA. The protocols involving human heart samples were approved by the University of California, Davis IRB as exempt, category 4. Failing heart samples came from adults identified as having dilated cardiomyopathy (DCM) or ICM. Significant coronary disease was excluded in those hearts identified as DCM. Normal, adult human heart samples were collected from brain dead donors by a private company (T-Cubed). The rat HF model produces mild to moderate HF at 9 weeks. Left ventricular size and fractional shortening were measured by echo as previously described.14 The animal protocol was approved by the University of California, Davis Animal Research Committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The left ventricle was used for all studies.
2.2. Electron microscopy
Standard transmission electron microscopy and immuno-electron microscopy were performed as previously described.15 Digital images of sequential fields were collected for analysis.
2.3. Mitochondrial measurements
Digital images of samples were collected from sequential fields during electron microscope (EM) analysis. To determine the population and size of the mitochondria, the EM images were analysed with Photoshop CS3, using the counting and area analysis function, in an approach similar to that reported by other investigators.16,17 For area measurement, the mitochondria were circled by the lasso tool and then the area of the circles were calculated and converted to their actual values using the scale bar.
2.4. Apoptosis
Apoptosis was assessed using the Cell Death Detection assay (CDD, Roche), which measures DNA fragmentation, and the TUNEL assay (Roche). Cytochrome c release was investigated using cell fractionation.14
2.5. Short hairpin RNA and constructs
A short hairpin RNA (shRNA) against rat OPA1 was ligated into pENTR/U6. LacZ shRNA served as a control. The rat OPA1 full-length cDNA was introduced into pIRES-EGFP (Clontech) for OPA1 overexpression, whereas the empty vector (EGFP alone) was used as control. The pIRES-EGFP vector expresses both the subcloned genes, OPA1, and EGFP.
2.6. RNA and real-time PCR
RNA was isolated from heart samples and real-time PCR was performed as previously described.18
2.7. H9c2 cells (embryonic cardiac cell line)
Simulated ischaemia was carried out as previously described.19
2.8. Confocal microscopy
Mitochondria were labelled with organelle Lights Mito-Orange fluorescent protein (OFP) reagent (Invitrogen). To determine mitochondrial fusion activity, cells were visually scored into three morphological classifications: highly tubular—cells with more than 95% of the mitochondrial mass as tubules, tubular—cells with more than 40%, but less than 95% of the mitochondrial mass as tubules, fragmented—cells with spherical mitochondrial fragments and no more than 40% short tubules.
2.9. Statistics
Results were compared by ANOVA followed by a Student–Neuman–Keuls test for multiple comparisons. For normalized data an ANOVA on Ranks was used. The Student's t-test was used for comparisons involving two groups. Results are expressed as mean ± SEM. A value for P < 0.05 was considered significant.
3. Results
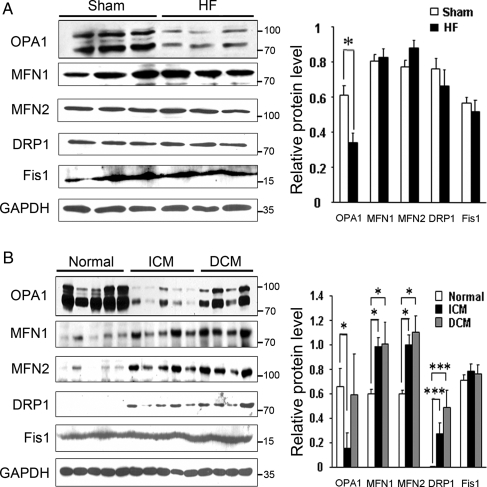
Functional studies in the HF rats showed a reduced fractional shortening of 14.2 ± 1.6% compared with 45.4 ± 2.6% in the controls (n = 5–6, P < 0.001). Left ventricular diastolic dimension was 1.2 ± 0.5 cm in HF vs. 0.80 ± 3.2 (n = 5–6, P < 0.005), consistent with HF. Expression of fission and fusion proteins was analysed by western blotting (Figure 1A). The fusion protein OPA1 was significantly decreased in HF rats (P < 0.05). There are five splice variants of OPA1. These fall between 80 and 100 kDa and are seen on the western (Figure 1A) as two doublets and a single band between the doublets. The bands had similar decreases in the HF hearts. There was no difference in the fusion proteins MFN1 and MFN2. Similarly, the fission proteins DRP1 and Fis1 were unchanged. For comparison, fission and fusion proteins were analysed in failing human hearts and five normal human hearts. OPA1 was markedly decreased in the hearts with ICM (Figure 1B, P < 0.001). MFN1 and MFN2 were both increased (P < 0.05). Five bands were present for OPA1 as a combination of doublets and a single band. The fission protein DRP1 was increased, whereas Fis1 was unchanged. In contrast, in DCM hearts OPA1 was not changed, but both MFN1 and MFN2 were increased (P < 0.05). DRP1 was increased (P < 0.001) and Fis1 unchanged. As OPA1 was decreased in both failing human and rat hearts, this suggests an important role for OPA1 in the progressive deterioration of the failing heart, particularly in ischaemia-induced HF.
Figure 1.
Expression of fission and fusion proteins in heart failure (HF). (A) Westerns from same blot showing relative expression of OPA1, MFN1, MFN2, DRP1, Fis1 in HF rat heart. GAPDH shown as control. (B) Expression of fission and fusion proteins in explanted human hearts compared with normal hearts. Same western shown for all five proteins and GAPDH (loading control). Graphs summarize the relative protein levels normalized to GAPDH. ICM, ischaemic cardiomyopathy; DCM, non-ischaemic cardiomyopathy. n = 3–5/group. *P < 0.05; ***P < 0.001.
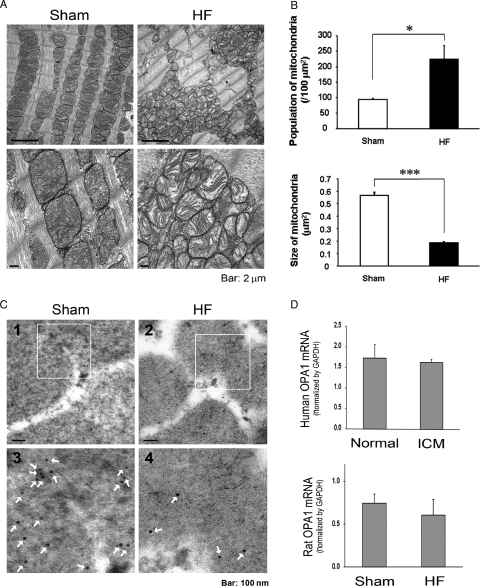
EM was used to analyse fission and fusion changes in rat HF. The mitochondria in the HF rat heart were disorganized and smaller (Figure 2A). The absolute number of mitochondria per area was significantly increased and the individual mitochondrial cross-sectional areas were significantly decreased in the rat HF hearts (P < 0.05 and P < 0.001, respectively, Figure 2B). Immuno-electron microscopy demonstrated that there was less OPA1 in rat HF mitochondria compared with sham controls (Figure 2C). These results are consistent with depressed fusion and implicate the reduction in OPA1 as a possible mechanism of apoptosis and cell loss.12,20
Figure 2.
(A) Representative EM of sham-operated control and HF rat hearts. (B) Graphs summarize the mitochondria per area (left panel) and average mitochondrial size (right panel). n = 3 hearts/group. (C) (1) Sham-operated control heart showing mitochondrial OPA1 by immuno-EM. (2) Representative image of HF rat heart showing reduced mitochondrial OPA1. (3 and 4) Show enlargement of area outlined in panels (1 and 2). Arrows—5 nM gold particle-antibody bound to OPA1. (D) Upper panel—real-time PCR was done to compare OPA1 mRNA in failing vs. normal human heart samples. Lower panel—real-time PCR for OPA1 mRNA in failing rat hearts vs. sham-operated controls. Results normalized to GAPDH. n = 3–5/group. Bar = 100 nM. *P < 0.05; ***P < 0.001.
To further investigate the underlying aetiology for decreased OPA1 in HF, OPA1 mRNA levels were determined by real-time PCR. OPA1 mRNA levels did not differ between human ICM hearts and normal controls (Figure 2D). Similarly, rat HF hearts had the same amount of OPA1 mRNA as controls.
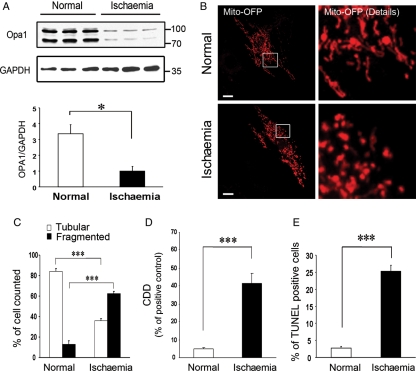
In vivo models of HF showed a decrease in OPA1 and an increase in small mitochondria, which would be seen with a loss of the fusion/fission balance. To further investigate this, H9c2 cells, a cardiac myogenic cell line, were used as a model of ischaemic injury. The cells were subjected to simulated ischaemia for 10 h, depriving the cells of oxygen and metabolic substrates. Ischaemia/reoxygenation was sufficient to depress expression of OPA1 to less than one-third of control (P < 0.05, Figure 3A). Studies of the mitochondria using OFP, a mitochondrial localizing fluorescent probe, showed loss of the normal mitochondrial network with simulated ischaemia (Figure 3B). Mitochondria were scored as tubular/connected vs. fragmented (Figure 3C) by a reviewer blinded to treatment group. In normal cells, less than 20% of the mitochondria were fragmented, increasing to over 60% following simulated ischaemia (P < 0.05). These mitochondrial changes correlated with an increase in DNA fragmentation by the CDD (Figure 3D, P < 0.001) and TUNEL assays (Figure 3E, P < 0.001).
Figure 3.
(A) Western showing expression of the two main OPA1 variants in normal and post-simulated ischaemia H9c2 cells. GAPDH shown in lower panel. Graph summarizes the relative OPA1 expression normalized to GAPDH. (B) Cells were transfected with an OFP construct. OFP is a fluorescent protein, which localizes to the mitochondria. Normal cells show tubular network, which is lost post-simulated ischaemia (lower panel). (C) Images of cells were collected and analysed for the amount of tubular vs. fragmented mitochondrial network (see Methods for details). (D) Apoptosis post-simulated ischaemia as determined by CDD assay. (E) Graph shows percent of cells that were TUNEL positive. n = 3–12/group. *P < 0.05; ***P < 0.001.
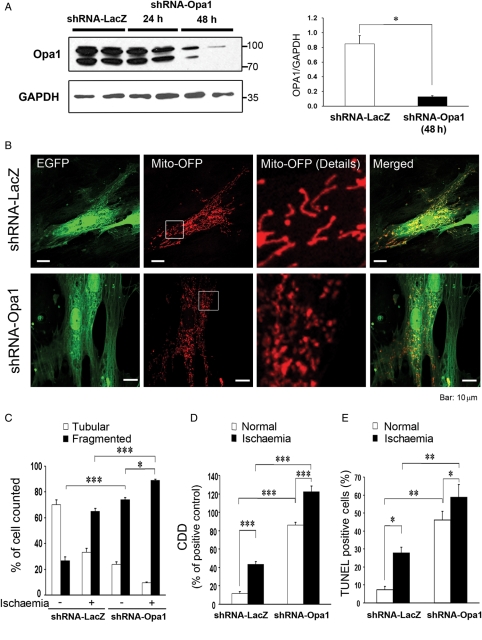
An OPA1 shRNA was designed to investigate the effect of isolated reduction of OPA1. LacZ shRNA was used as a control. The sequences were screened against GenBank for unexpected matches. The shRNA-OPA1 decreased OPA1 by more than 80% at 48 h in H9c2 cells (Figure 4A, P < 0.05). The constructs for shRNA also expressed EGFP, which was used to identify transfected cells. EGFP-positive cells treated with shRNA-OPA1 showed increased mitochondrial fragmentation compared with cells transfected with shRNA-LacZ (Figure 4B and C). Simulated ischaemia increased mitochondrial fragmentation. Control cells after simulated ischaemia showed levels of mitochondrial fragmentation similar shRNA-OPA1 treated cells. Simulated ischaemia plus shRNA-OPA1 treatment resulted in fragmentation of 90% of the mitochondria. OPA1 reduction resulted in apoptosis with increased DNA fragmentation and TUNEL-positive nuclei (Figure 4D and E, P < 0.001 and P < 0.01). As would be expected, simulated ischaemia increased apoptosis in control cells, but to an even greater extent in cells with reduced OPA1 (Figure 4D and E).
Figure 4.
Effect of OPA1 shRNA on the mitochondrial network and cell viability. (A) Western shows OPA1 expression after 48 h of treatment with OPA1 shRNA or to LacZ shRNA (control). Lower panel—GAPDH as control. Right panel shows relative OPA1 expression in control cells (expressing shRNA-LacZ) vs. shRNA-OPA1 cells. Values normalized to GAPDH. (B) Confocal microscopy showing mitochondrial network after OFP expression in cells transfection with shRNA-LacZ or shRNA-OPA1. shRNA constructs also expressed EGFP, so cells with shRNA were clearly identifiable. Left panel shows EGFP, second panel shows OFP expression in the mitochondria, right panel shows enlarged image of mitochondria and far right panel shows merged image of OFP and EGFP. (C) Images of cells were collected and analysed for the amount of tubular vs. fragmented mitochondrial network (see Methods for details). (D) Apoptosis post-simulated ischaemia by CDD assay. (E) Graph shows percent of cells that were TUNEL positive. n = 3–12/group. *P < 0.05; **P < 0.01; ***P < 0.001.
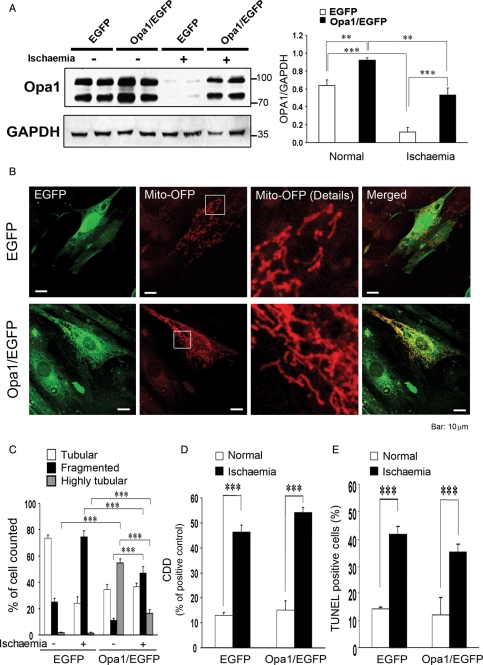
Reduction in OPA1 resulted in increased fragmentation of mitochondria, increased apoptosis, and increased sensitivity to simulated ischaemia. To determine whether increased OPA1 would be protective, OPA1 was co-expressed with EGFP in H9c2 cells using an OPA1-pIRES-EGFP vector with a ribosomal re-entry site. Control cells were transfected with the empty vector and thus expressed EGFP alone. The amount of OPA1 was increased by 50% (P < 0.01, Figure 5A). With ischaemia, OPA1 levels dropped in cells expressing EGFP alone and in those expressing OPA1 and EGFP; however, OPA1 levels were significantly higher after ischaemia in the cells overexpressing OPA1. At baseline, cells overexpressing OPA1 showed increased mitochondrial tubularity compared with cells transfected with EGFP alone (Figure 5C). With simulated ischaemia, less mitochondrial fragmentation occurred in the cells overexpressing OPA1 (Figure 5C). However, the increase in OPA1 did not prevent ischaemia-induced apoptosis (Figure 5D and E).
Figure 5.
(A) Western demonstrating the changes in OPA1 with overexpression and following ischaemia. Cells expressed either OPA1 and EGFP or EGFP alone. Graph summarizes multiple experiments. (B) Confocal immunocytochemistry of cells overexpressing OPA1 and EGFP or EGFP alone. Mito-OFP was used as a mitochondrial marker. Greater tubularity is visible with OPA1 overexpression (lower panel). (C) Mitochondrial morphology was quantified, as described in Methods. Effect of OPA1 plus EGFP overexpression vs. EGFP alone. (D) Effect of OPA1 overexpression on apoptosis (DNA fragmentation) after simulated ischaemia. (E) Percent of cells undergoing apoptosis (TUNEL) after simulated ischaemia. Comparison of OPA1 overexpressing cells vs. EGFP alone. n = 4–12/group. **P < 0.01; ***P < 0.001.
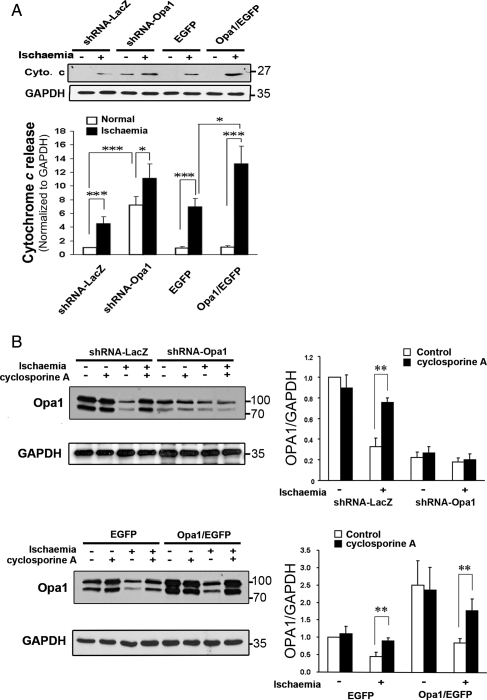
To further investigate the effect of OPA1 protein levels on the mitochondria and apoptosis, cytochrome c release was compared among cells treated with shRNA to OPA1 or LacZ, cells overexpressing OPA1 and EGFP or EGFP alone. Reduction in OPA1 with shRNA resulted in the release of cytochrome c from the mitochondria at baseline (Figure 6A). Simulated ischaemia resulted in greater release of cytochrome c in the OPA1 shRNA treated cells compared with cells treated with shRNA-LacZ. Unexpectedly, cells overexpressing OPA1 had release of cytochrome c at baseline, which was increased after simulated ischaemia, compared with cells overexpressing EGFP alone. Thus, overexpression of OPA1 was not protective against apoptosis, and in fact induced apoptosis.
Figure 6.
(A) Effect of changes in OPA1 expression on cytochrome c release with ischaemia—Cytochrome c release was compared with shRNA treatment vs. OPA1 overexpression. Representative western compares release from the treatment groups with and without simulated ischaemia. Graph summarizes multiple experiments. Densitometric values for all were normalized to control with shRNA-LacZ. Reduction in OPA1 with shRNA caused cytochrome c release and this increased with simulated ischaemia. OPA1 overexpression increased the release of cytochrome c. (B) Effect of CsA (500 nM) on OPA1 levels during simulated ischaemia. Upper panels demonstrate protective effect of CsA on OPA1 levels in simulated ischaemia, whereas it has no effect in ischaemia if OPA1 already reduced with shRNA. Lower panels show that even with OPA1 overexpression CsA protects OPA1 during simulated ischaemia. n = 4/group. *P < 0.05; **P < 0.01.
As shown above, simulated ischaemia reduced expression of OPA1 (Figure 5A). Cyclosporin A (CsA) reduces mitochondrial injury in ischaemia/reperfusion and blocks the mitochondrial permeability transition pore (PTP).21,22 To investigate the role of mitochondrial injury and the PTP in OPA1 reduction, cells were pretreated with CsA prior to simulated ischaemia. Pretreatment with CsA blocked the reduction of OPA1 that occurred with simulated ischaemia (Figure 6B). In the absence of ischaemia, CsA had no effect on OPA1 levels and also did not affect levels of OPA1 in cells treated with OPA1 shRNA. CsA protected the increased OPA1 (in cells overexpressing OPA1) from simulated ischaemia (Figure 6B).
4. Discussion
OPA1 protein levels were decreased in both rat and human ischaemic HF, but mRNA levels were unaltered suggesting post-transcriptional regulation of OPA1 expression. OPA1 levels were overall unchanged in human DCM hearts, although some hearts showed a marked reduction in OPA1 that reflects the heterogeneity of this disease classification.8,23–25 The mitochondria in the failing rat hearts were small and fragmented compared with normal hearts, consistent with decreased fusion.12,20 OPA1 levels decreased in response to simulated ischaemia, but overexpression of OPA1 during simulated ischaemia did not prevent apoptosis. Reduction of OPA1 with shRNA increased mitochondrial fragmentation and decreased tubularity of the mitochondria compared with the increased tubularity with overexpression of the protein. These changes were associated with increased apoptosis at baseline, and a further increase in apoptosis with simulated ischaemia. Most significantly, cytochrome c release was greater both with reduction in OPA1 and with overexpression of OPA1. CsA treatment inhibited the reduction in OPA1 in ischaemia.
4.1. Differences in HF models
Three models of HF were used. The rat coronary ligation model is similar to human ischaemic HF, which is secondary to coronary artery disease. Both models involve ischaemia, and ischaemia is likely a key cause of OPA1 loss, as discussed below. Human DCM, the third model, is a heterogenous disease classification where the distinguishing characteristic is the absence of significant coronary disease.24,25 The human DCM hearts did not show reduced OPA1 levels; this may be secondary to the absence of ischaemia in this model. Both human models had an increase in MFN1 and MFN2 as well as in DRP1. None of these were changed in the rat model. Increased DRP1 can cause apoptosis, but translocation to the mitochondria is necessary for this effect.26,27 Mitochondrial fusion in mammalian cells is controlled by MFN1, MFN2, and OPA1, and elimination of any of these proteins induces mitochondrial fragmentation.20 The increase in MFN1 and MFN2 may be in compensation for the reduction in OPA1. Regulation of the MFN1, MFN2, and DRP1 expression may differ between rat and human, or the much longer duration of the human disease (years vs. weeks), and concomitant use of multiple medications may alter gene expression. Virtually all humans with HF are treated with powerful medications including angiotensin-converting enzyme inhibitors and beta-blockers, which may influence gene expression.28
4.2. OPA1 localization and function
OPA1 is essential for normal fusion.9 In addition, OPA1 has a role in maintaining cristae structure within the mitochondria, in maintaining inner membrane (IM) integrity and IM potential, and in preventing release of cytochrome c from the cristae.13 The effect of changes in OPA1 on mitochondrial energetics remains unclear. There have been two different approaches: (i) reduction in OPA1 levels with RNAi or knockout (heterozygote, homozygote embryonic lethal) and (ii) the study of patient (with OPA1 mutations)—derived cells, primarily fibroblasts. A number of studies are summarized in Table 1. In mouse embryonic fibroblasts (MEF) cells, a 90% reduction in OPA1 resulted in loss of ΔΨm (mitochondrial membrane potential) and severe reduction in endogenous respiration.12 More recently, Drosophila that are heterozygous for knockout of OPA1 have been reported to have decreased complex II and III function, but normal complex I and IV function.29 These flies also have increased reactive oxygen species, decreased resistance to oxidative stress, and decreased life span. Antioxidants improved the reduction in life span in males, but not females.
Table 1.
Summary of studies on OPA1 and mitochondrial function
| Model/cell type | OPA1 | Endpoints | |
|---|---|---|---|
| Chen et al.12 | Primary MEF | RNAi reduction in OPA1 | RNAi-loss of ΔΨm, severe reduction in endogenous respiration/O2 consumption |
| Tang et al.29 | Drosophila | Heterozygote for OPA1 knockout | Decreased resistance to oxidative stress; decreased lifespan improved by antioxidants in males only, increased reactive oxygen species; reduced complex II and III function |
| Lodi et al.30 | 31p-MRS, skeletal mm function in vivo in patients | c.2708-2711delTTAG truncated protein lacking 56 C-terminal aa | Prolonged phosphocreatine post-exercise recovery time |
| Zanna et al.9 | Cultured skin fibroblasts | c.2708-2711delTTAG and four other mutations two c.2819-2A>C | Decreased complex I activity and mGPD activity |
| Spinazzi et al.31 | Fibroblasts and myotubes from biopsy | c1410_1443 + 4del38 truncated 481 aa protein, not detected by commercial abs | Abnormal morphology, normal OXPHOS activity, normal ΔΨm, normal complex I activity in fibroblasts |
| Myorov et al.32 | Lymphoblastic cell lines—transformed leucocytes from patients with OPA1 mutations | Six mutations, one c.2708delTTAG | No difference in energetics, wide range in function, particularly complex I |
| Chevrollier et al.33 | Cultured fibroblasts from skin biopsies | Six different OPA1 mutations | Complex IV decreased function, increased F1-ATPase activity, reduced ΔΨm, reduced OXPHOS efficiency |
MEF, mouse embryonic fibroblasts; p-MRS, phosphorus magnetic resonance spectroscopy; Aa, amino acids; C-terminal, carboxyl terminus; OXPHOS, oxidative phosphorylation; ΔΨm, mitochondrial membrane potential.
In a second approach, investigators have examined the effect of various OPA1 mutations on mitochondrial function (Table 1). One problem is that the mutations vary widely from point mutations involving a single amino acid substitution to truncated proteins. It is not known whether these truncated proteins retain any function. An in vivo study demonstrated that the c.2708-2711delTTAG mutation resulted in prolonged recovery of phosphocreatine after exercise.30 Other studies have looked at cells cultured from skin biopsies and lymphoblastic cell lines derived from patient leucocytes.9,31–33 As summarized (Table 1), the genetic mutations involved and results are both heterogeneous. Mitochondrial energetics ranged from reduced to normal.9,31–33 It is difficult to arrive at a unifying conclusion as to the role of OPA1 in mitochondrial energetics from these data. Furthermore, cultured fibroblasts are not high-energy cells, and any defects may be less apparent. In the current study, OPA1 was decreased in the mitochondria in ischaemic HF, and based on the studies in the Drosophila and MEF cells, this might be expected to impair function, consistent with the decrease in ATP levels seen in HF.12,29 To better understand the function of OPA1, further studies are needed on the effect of changing OPA1 levels in intact organs as well as in cells. Other cell types with high-energy requirements, such as heart or muscle cells, need to be studied. Only with more focused work will we have a clear picture of the role of OPA1 in mitochondrial function in the mammalian cell.
4.3. Splice variants and OPA1 loss
Both normal and failing hearts expressed five splice variants, although the two doublets are difficult to see clearly when the westerns are exposed long enough to visualize the fifth band. The presence of five splice variants is considered to be the standard, though many of these studies were in cultured cell lines.20 The number of published splice variants in mammalian cells varies, with a number of authors showing only two protein bands by western blotting.9,31 Our data showed differential changes in OPA1 splice variants in neither the failing heart samples nor during simulated ischaemia. Although OPA1 expression was decreased in ischaemic HF, the decrease was across all splice variants. Opening of the PTP caused OPA1 cleavage in isolated mitochondria, and the key mediating event was loss of ATP;34 in the current study, CsA, which blocks PTP opening, reduced the decrease in OPA1 seen with ischaemia.35 We found no difference in mRNA levels for OPA1 between normal and HF hearts, supporting the concept that ischaemia promotes the loss of OPA1 protein post-translationally, most likely through degradation by proteases activated by ATP loss during ischaemia.34
4.4. OPA1 and apoptosis
Reduction in OPA1 increased apoptosis both at baseline and after simulated ischaemia. Reduction of OPA1 in a cancer cell line increased mitochondrial fragmentation and the sensitivity to apoptotic stimuli.4 Unexpectedly, OPA1 overexpression did not protect against apoptosis: instead, it amplified cytochrome c release. Increased OPA1 did result in greater tubularity of the mitochondria. Others have reported that overexpression of OPA1 is anti-apoptotic.13,36 However, both of these studies used isolated mitochondria and one also used intact 293T cells. Yamaguchi et al.36 recently proposed that OPA1 forms a reverse purse string that prevents cytochrome c release from the cristae when loose. The current work used a physiological injury and assayed cytochrome c release from mitochondria in situ. Our results suggest that OPA1 is needed for function, but more OPA1 does not protect the mitochondria. Possibly, excess OPA1 interferes with the purse string at the base of the cristae, but others did not find this to be the case.36 The differences in results may be secondary to different cell types, 293T vs. H9c2 cells. CsA, which blocks PTP opening, mitochondrial calcium overload and the drop in ATP, prevented the decrease in OPA1 seen in cells with normal and high levels of OPA1 after ischaemia.21,35 Thus, CsA protects the mitochondria against ischaemic injury, but OPA1 does not, even though it is necessary for normal mitochondrial function.
4.5. Study limitations
Experimental sample sizes ranged from 3 to 12. Thus, there is the possibility of a type II statistical error.
5. Conclusions
HF in both human and rat ICM was characterized by a marked decrease in OPA1. Loss of OPA1 leads to apoptosis, and in the current study decreasing OPA1 resulted in apoptosis. Ischaemia markedly depressed OPA1 levels, and this can lead to further apoptosis. Apoptotic cell death via reduction of OPA1 and mitochondrial fusion may contribute to HF progression, which is characterized by progressive loss of cardiac myocytes. Much remains to be understood about OPA1's anti-apoptotic role and its role in mitochondrial energetics.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the National Institutes of Health [grant numbers HL077281, HL079071] and a Merit Award from the Department of Veterans Affairs (all to A.A.K.).
Conflict of interest: none declared.
References
- 1.Chan DC. Mitochondria: dynamic organelles in disease, aging and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Meeusen S, Nunnari J. How mitochondria fuse. Curr Opin Cell Biol. 2005;17:389–394. doi: 10.1016/j.ceb.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Chan DC. Dissecting mitochondrial fusion. Dev Cell. 2006;11:592–594. doi: 10.1016/j.devcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Jeong S, Karbowski M, Smith CL, Youle RJ. Roles of mammalian mitochondrial fission and fusion mediators Fis1, Drp1 and OPA1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, et al. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci, USA. 1999;96:8144–8149. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 8.Hilfiker-Kleiner D, Landmesser U, Drexler H. Molecular mechanisms in heart failure: focus on cardiac hypertrophy, inflammation, angiogenesis, and apoptosis. J Am Coll Cardiol. 2006;48:A56–A66. [Google Scholar]
- 9.Zanna C, Ghelli A, Porcelli AM, Karbowski M, Youle RJ, Schimpf S, et al. OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain. 2008;131:352–367. doi: 10.1093/brain/awm335. [DOI] [PubMed] [Google Scholar]
- 10.Amati-Bonneau P, Valentino ML, Reynier P, Gallardo ME, Bornstein B, Boissiere A, et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy ‘plus’ phenotypes. Brain. 2008;131:338–351. doi: 10.1093/brain/awm298. [DOI] [PubMed] [Google Scholar]
- 11.Baloh RH, Schmidt RE, Pestronk A, Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci. 2007;27:422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 13.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon S, et al. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol. 2007;293:H2238–H2247. doi: 10.1152/ajpheart.00740.2007. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Knowlton AA. HSP60, Bax, apoptosis and the heart. J Cell Mol Med. 2005;9:51–58. doi: 10.1111/j.1582-4934.2005.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM, Jr, Klein JB, et al. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E896–E905. doi: 10.1152/ajpendo.00047.2004. [DOI] [PubMed] [Google Scholar]
- 17.Totland GK, Madsen L, Klementsen B, Vaagenes H, Kryvi H, Froyland L, et al. Proliferation of mitochondria and gene expression of carnitine palmitoyltransferase and fatty acyl-CoA oxidase in rat skeletal muscle, heart and liver by hypolipidemic fatty acids. Biol Cell. 2000;92:317–329. doi: 10.1016/s0248-4900(00)01077-7. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton KL, Lin L, Wang Y, Knowlton AA. Effect of ovariectomy on cardiac gene expression: inflammation and changes in SOCS genes expression. Physiol Genomics. 2007;32:254–263. doi: 10.1152/physiolgenomics.00039.2007. [DOI] [PubMed] [Google Scholar]
- 19.Voss MR, Gupta S, Stice JP, Baumgarten G, Lu L, Tristan JM, et al. Effect of mutation of amino acids 246–251 (KRKHKK) in HSP72 on protein synthesis and recovery from hypoxic injury. Am J Physiol Heart Circ Physiol. 2005:H687–H692. doi: 10.1152/ajpheart.00872.2004. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Chan DC. New insights into mitochondrial fusion. FEBS Lett. 2007;581:2168–2173. doi: 10.1016/j.febslet.2007.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akao M, O'Rourke B, Kusuoka H, Teshima Y, Jones SP, Marban E. Differential actions of cardioprotective agents on the mitochondrial death pathway. Circ Res. 2003;92:195–202. doi: 10.1161/01.res.0000051862.16691.f9. [DOI] [PubMed] [Google Scholar]
- 22.Ow YL, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 23.Donahue MP, Marchuk DA, Rockman HA. Redefining heart failure: the utility of genomics. J Am Coll Cardiol. 2006;48:1289–1298. doi: 10.1016/j.jacc.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 24.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 25.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy: from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Bras M, Yuste VJ, Roúe G, Barbier S, Sancho P, Virely C, et al. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol Cell Biol. 2007;27:7073–7088. doi: 10.1128/MCB.02116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Men X, Wang H, Li M, Cai H, Xu S, Zhang W, et al. Dynamin-related protein 1 mediates high glucose induced pancreatic beta cell apoptosis. Int J Biochem Cell Biol. 2009;41:879–890. doi: 10.1016/j.biocel.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 28.Chen FC, Brozovich FV. Gene expression profiles of vascular smooth muscle show differential expression of mitogen-activated protein kinase pathways during captopril therapy of heart failure. J Vasc Res. 2008;45:445–454. doi: 10.1159/000126735. [DOI] [PubMed] [Google Scholar]
- 29.Tang S, Le PK, Tse S, Wallace DC, Huang T. Heterozygous mutation of OPA1 in Drosophila shortens lifespan mediated through increase reactive oxygen species production. PLOS One. 2009;4:e4492. doi: 10.1371/journal.pone.0004492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lodi R, Tonon C, Valentino M, Iotti S, Clementi V, Malucelli E, et al. Deficit of in vivo mitochondrial ATP production in OPA1-related dominant optic neuropathy. Ann Neurol. 2004;56:719–723. doi: 10.1002/ana.20278. [DOI] [PubMed] [Google Scholar]
- 31.Spinazzi M, Cazzola S, Bortolozzi M, Baracca A, Loro E, Casarin A, et al. A novel deletion in the GTPase domain of OPA1 causes defects in mitochondrial morphology and distribution, but not in function. Hum Mol Genet. 2008;17:3291–3302. doi: 10.1093/hmg/ddn225. [DOI] [PubMed] [Google Scholar]
- 32.Mayorov VI, Lowrey AJ, Biousse V, Newman NJ, Cline SD, Brown MD. Mitochondrial oxidative phosphorylation in autosomal dominant optic atrophy. BMC Biochem. 2008;9:22. doi: 10.1186/1471-2091-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chevrollier A, Guillet V, Loiseau D, Gueguen N, de Crescenzo MP, Verny C, et al. Hereditary optic neuropathies share a common mitochondrial coupling defect. Ann Neurol. 2008;63:794–798. doi: 10.1002/ana.21385. [DOI] [PubMed] [Google Scholar]
- 34.Baricault L, Segui B, Guegand L, Olichon A, Valette A, Larminat F, et al. OPA1 cleavage depends on decreased mitochondrial ATP level and bivalent metals. Exp Cell Res. 2007;313:3800–3808. doi: 10.1016/j.yexcr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Pagano A, Donati Y, Metrailler I, Barazzone Argiroffo C. Mitochondrial cytochrome c release is a key event in hyperoxia-induced lung injury: protection by cyclosporin A. Am J Physiol Lung Cell Mol Physiol. 2004;286:L275–L283. doi: 10.1152/ajplung.00181.2003. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi R, Lartigue L, Perkins G, Scott RT, Dixit A, Kushnareva Y, et al. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol Cell. 2008;31:557–569. doi: 10.1016/j.molcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.