Abstract
Aims
Cardiac myocytes depend on a delicate balance of glucose and free fatty acids as energy sources, a balance that is disrupted in pathological states such as diabetic cardiomyopathy and myocardial ischaemia. There are two families of cellular glucose transporters: the facilitated-diffusion glucose transporters (GLUT); and the sodium-dependent glucose transporters (SGLT). It has long been thought that only the GLUT isoforms, GLUT1 and GLUT4, are responsible for cardiac myocyte glucose uptake. However, we discovered that one SGLT isoform, SGLT1, is also an important glucose transporter in heart. In this study, we aimed to determine the human and murine cardiac expression pattern of SGLT1 in health and disease and to determine its regulation.
Methods and results
SGLT1 was largely localized to the cardiac myocyte sarcolemma. Changes in SGLT1 expression were observed in disease states in both humans and mouse models. SGLT1 expression was upregulated two- to three-fold in type 2 diabetes mellitus and myocardial ischaemia (P < 0.05). In humans with severe heart failure, functional improvement following implantation of left ventricular assist devices led to a two-fold increase in SGLT1 mRNA (P < 0.05). Acute administration of leptin to wildtype mice increased cardiac SGLT1 expression approximately seven-fold (P < 0.05). Insulin- and leptin-stimulated cardiac glucose uptake was significantly (P < 0.05) inhibited by phlorizin, a specific SGLT1 inhibitor.
Conclusion
Our data suggest that cardiac SGLT1 expression and/or function are regulated by insulin and leptin, and are perturbed in disease. This is the first study to examine the regulation of cardiac SGLT1 expression by insulin and leptin and to determine changes in SGLT1 expression in cardiac disease.
Keywords: Diabetes, Glucose, Insulin, Ischaemia, Leptin
1. Introduction
Cardiac function depends on the continuous supply of energy. Second only to free fatty acids, circulating glucose is one of the most important metabolic fuels for the heart. Under conditions of cardiac stress such as ischaemia, glucose utilization is activated relative to free fatty acids because of a decrease in cellular ATP content and release of ATP-mediated allosteric inhibition of the enzyme phosphofructokinase. This switch results in a greater amount of ATP production per mole of oxygen consumed. Similarly, end-stage heart failure is associated with a shift towards glucose utilization.1
Because of its hydrophilic nature, glucose is unable to pass the lipid bilayer of the cardiac myocyte sarcolemma by simple diffusion. At present, five facilitated-diffusion glucose transporters (GLUT1 through GLUT4 and GLUT7) are believed to exist in mammalian cells. In the mammalian heart, glucose transport is believed to be mediated mainly by two members of the GLUT family, GLUT1 and GLUT4.2 Whereas GLUT1 is regarded as a basal glucose transporter, GLUT4 is upregulated in response to insulin and mechanical work.3
In addition to the GLUT family, members of the sodium/glucose cotransporter (SGLT) family are widely present as they transport a variety of substrates (sugars, inositol, iodide, urea, and proline) into the cell using the electrochemical gradient of cations such as Na+.4 SGLT1 is found in small intestinal enterocytes and renal proximal tubule S3 cells, where it mediates glucose uptake.5 Although SGLT1 is highly expressed in the heart,6 its cardiac function has never been investigated. Moreover, whereas the expression of GLUT1 and GLUT4 in diseased hearts and their regulation by insulin and leptin have been well documented, little is known about SGLT1.
Therefore, the purpose of the present study was to investigate (i) the expression of SGLT1 in the normal human and murine heart; (ii) the change in expression of SGLT1 in heart disease; and (iii) whether insulin- and leptin-mediated regulation of cardiac glucose uptake is effected through SGLT1.
2. Methods
2.1. Mouse models
Male wildtype (WT) FVB, WT C57BL/6J, and leptin-deficient ob/ob mice at age 6–8 weeks were used as specified below for different experiments. C57BL/6J WT mice were subjected to coronary artery ligation (CAL) or sham surgeries as described previously7 and detailed below. Type 1 diabetes was induced by administration of streptozotocin (STZ) (Sigma, S0130) (75 mg/kg intraperitoneally) to 4 h fasted FVB WT mice for three consecutive days.8 After 4 weeks, these mice were sacrificed, blood was collected to confirm hyperglycaemia, and hearts excised. C57BL/6J WT mice were administered leptin (Pepro Tech) (10 mg/kg intraperitoneally),9 and sacrificed 30 min later for excision of cardiac tissue. FVB WT mice were administered insulin (Sigma) (10 U/kg subcutaneously) with glucose 2 g/kg intraperitoneally to avoid hypoglycaemia, and sacrificed 30 min later for excision of cardiac tissue. This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
2.2. Coronary artery ligation (CAL)
WT C57BL/6J mice were anesthetized with tribromoethanol (125 mg/kg intraperitoneally), intubated, ventilated, and a 0.7 cm incision made on the left chest through the fourth intercostal space as previously described.10 The pericardium was opened and the left anterior descending coronary artery was either completely ligated 1 mm below the left auricular margin with 7/0 prolene to induce myocardial infarction (MI), or loosely tied to generate sham-operated control mice. Mice were sacrificed and cardiac tissue harvested 30 days following surgery.
2.3. Human tissue
Failing human heart tissue from the University of Pittsburgh was obtained after Institutional Review Board approval and with the subjects’ informed consent. Non-failing control human heart samples (n = 5) were generously provided by Dr C. S. Moravec (Cleveland Clinic Foundation) and obtained under a protocol approved by the Institutional Review Board of the Cleveland Clinic Foundation. This study conforms with the principles outlined in the Declaration of Helsinki. Transmural samples of the lateral wall of the left ventricle (LV) were obtained from failing human hearts at the time of transplant (n = 26), or from the LV apex at the time of left ventricular assist device (LVAD) implantation and removal (paired samples). Subjects with LVADs had a history of either idiopathic dilated cardiomyopathy (n = 3) or ischaemic cardiomyopathy (n = 2). Human diabetic cardiomyopathy tissue was obtained from subjects with long-standing type 2 diabetes and end-stage heart failure in the absence of coronary artery disease and hypertension. Non-failing control human heart samples (n = 5) were recovered from the lateral wall of unmatched organ donors. All cardiac tissue was placed into cold cardioplegic solution (4°C) and rapidly transported to the laboratory, frozen in liquid nitrogen, and stored at −80°C. Table 1 lists all human cardiac tissue used in this study.
Table 1.
Sources of human cardiac tissue used
| Medications |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease or group | Sex | Age (years) | ACE-I | ARB | Beta-blocker | Aldosterone antagonist | Digoxin | Diuretic | Hydralazine | Nitrate | Anti-arrhythmic |
| Non-failing control heart | M | 39 | |||||||||
| Non-failing control heart | M | 50 | |||||||||
| Non-failing control heart | M | 59 | |||||||||
| Non-failing control heart | F | 43 | |||||||||
| Non-failing control heart | F | 60 | |||||||||
| Diabetic cardiomyopathy | M | 57 | Yes | Yes | Yes | Yes | |||||
| Diabetic cardiomyopathy | M | 58 | Yes | Yes | Yes | Yes | Yes | Yes | |||
| Diabetic cardiomyopathy | M | 62 | Yes | Yes | Yes | ||||||
| Diabetic cardiomyopathy | M | 64 | Yes | Yes | Yes | Yes | Yes | ||||
| Diabetic cardiomyopathy | M | 67 | Yes | Yes | Yes | Yes | Yes | Yes | |||
| Diabetic cardiomyopathy | F | 51 | Yes | Yes | Yes | Yes | Yes | ||||
| Ischaemic cardiomyopathy | M | 49 | Yes | Yes | Yes | Yes | Yes | Yes | |||
| Ischaemic cardiomyopathy | M | 56 | Yes | Yes | Yes | ||||||
| Ischaemic cardiomyopathy | M | 60 | Yes | Yes | Yes | ||||||
| Ischaemic cardiomyopathy | M | 68 | Yes | Yes | Yes | Yes | Yes | ||||
| Ischaemic cardiomyopathy | M | 69 | Yes | Yes | Yes | ||||||
| Ischaemic cardiomyopathy | M | 70 | Yes | Yes | Yes | Yes | |||||
| Ischaemic cardiomyopathy | M | 72 | Yes | Yes | Yes | ||||||
| Ischaemic cardiomyopathy | F | 44 | Yes | Yes | |||||||
| Ischaemic cardiomyopathy | F | 48 | Yes | Yes | Yes | ||||||
| Ischaemic cardiomyopathy | F | 69 | Yes | Yes | Yes | Yes | Yes | ||||
| Idiopathic dilated cardiomyopathy | M | 24 | |||||||||
| Idiopathic dilated cardiomyopathy | M | 57 | Yes | Yes | Yes | Yes | |||||
| Idiopathic dilated cardiomyopathy | M | 59 | Yes | Yes | Yes | Yes | |||||
| Idiopathic dilated cardiomyopathy | M | 62 | Yes | Yes | Yes | Yes | |||||
| Idiopathic dilated cardiomyopathy | M | 66 | Yes | Yes | Yes | ||||||
| Idiopathic dilated cardiomyopathy | F | 39 | Yes | Yes | Yes | ||||||
| Idiopathic dilated cardiomyopathy | F | 57 | Yes | Yes | |||||||
| Idiopathic dilated cardiomyopathy | F | 58 | Yes | Yes | Yes | ||||||
| Idiopathic dilated cardiomyopathy | F | 59 | Yes | Yes | |||||||
| Idiopathic dilated cardiomyopathy | F | 60 | Yes | Yes | Yes | Yes | |||||
| Left ventricular assist device | M | 36 | Yes | ||||||||
| Left ventricular assist device | M | 48 | Yes | Yes | Yes | ||||||
| Left ventricular assist device | M | 52 | Yes | Yes | |||||||
| Left ventricular assist device | M | 59 | |||||||||
| Left ventricular assist device | F | 37 | Yes | Yes | |||||||
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; F, female; M, male.
2.4. In vivo cardiac glucose uptake
Basal cardiac glucose uptake was measured in WT male FVB mice at age 6–8 weeks as described.11 In brief, mice were administered 2-deoxy-D-[1-14C]-glucose (2-[14C]DG) (10 µCi) intraperitoneally. After 30 min, mice were sacrificed and their hearts rapidly excised. Hearts were homogenized in 10 volumes of phosphate-buffered saline (PBS), and radioactivity in 20 µL of homogenate was measured in a liquid scintillation counter. Because 2-deoxy-D-glucose is phosphorylated but not further metabolized, it remains trapped inside cells. Thus, glucose uptake was estimated by determining cardiac radioactivity.
In additional experiments, the effect of insulin and leptin on cardiac glucose uptake was measured in the presence or absence of phlorizin, a specific SGLT1 inhibitor. Phlorizin (Sigma) was dissolved in a solution containing 10% ethanol, 15% DMSO, and 75% saline and was administered intraperitoneally at a dose of 0.4 g/kg. Control mice were administered vehicle without phlorizin. Thirty minutes following phlorizin, insulin (10 U/kg subcutaneously) with glucose (2 g/kg intraperitoneally), or leptin (10 mg/kg intraperitoneally) was administered. Ten minutes later, 2-[14C]DG was administered and glucose uptake measured as described above.
2.5. RNA isolation and real-time quantitative PCR (QPCR)
Total RNA was isolated from whole hearts with TRIzol (Invitrogen). Reverse transcriptase reactions were performed as described12 using the Superscript III First-Strand Kit (Invitrogen) for first-strand cDNA synthesis. Primers for real-time quantitative PCR (QPCR) analysis (Table 2) were designed using published sequence information, avoiding regions of homology with other genes. Ten nanogram of cDNA were analysed on an ABI PRISM 7700 using Absolute SYBR Green ROX PCR Master Mix (Thermo Scientific). Fold-change analysis was based on normalizing to cyclophilin transcript levels for murine cDNA and GAPDH transcript levels for human cDNA.
Table 2.
Real-time quantitative PCR primers used to quantify mRNA expression
| Murine SGLT1 | Sense | 5′-TCTGTAGTGGCAAGGGGAAG-3′ |
| Antisense | 5′-ACAGGGCTTCTGTGTCTTGG-3′ | |
| Murine cyclophilin | Sense | 5′-TGTGCCAGGGTGGTGACTT-3′ |
| Antisense | 5′-TCAAATTTCTCTCCGTAGATGGACTT-3′ | |
| Human SGLT1 | Sense | 5′-TCCTGCTTGCTATTTTCTGGA-3′ |
| Antisense | 5′-ATAATCGTGGGACAGTTGCTG-3′ | |
| Human GAPDH | Sense | 5′-TCAACGACCACTTTGTCAAGCTCA-3′ |
| Antisense | 5′-GCTGGTGGTCCAGGGGTCTTACT-3′ |
2.6. Immunoblot analysis
Extraction of total protein, and cytosolic protein and membrane protein fractions, and immunoblotting were performed as described previously.10,12,13 Protein concentrations were measured by the Bradford method (Bio-Rad). An equal amount (50 µg) of protein was separated by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS–PAGE). After electrophoresis, protein was transferred to PVDF membranes (Amersham). The membranes were then blocked in Tris-buffered saline Tween-20 (TBS-T; 10 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween-20) and 5% non-fat dry milk for 1 h, and subsequently washed and incubated with primary antibodies in TBS-T and 2% bovine serum albumin (BSA) at 4°C overnight. The following polyclonal antibodies and titres were used: murine SGLT1 (1:200, Santa Cruz #sc20582), human SGLT1 (1:200, Santa Cruz #sc47397), GLUT1 (1:200, Santa Cruz #sc1605), GLUT4 (1:1000, Cell Signaling #2299), and Na, K-ATPase α1 (1:1000, Cell Signaling #3010).
After washing with TBS-T, membranes were incubated with anti-rabbit (1:10000 dilution, Amersham, #NA934V) or anti-goat (1:2000 dilution, Santa Cruz, sc-2020) horseradish peroxidase conjugated secondary antibody for 1 h. Signal was detected by chemiluminescence using the ECL detection system (Amersham). Gel staining with Coomassie blue or immunoblotting for GAPDH was used as internal controls for loading of protein.
To demonstrate anti-murine SGLT1 antibody specificity in cardiac tissue, we performed peptide inhibition. The membrane was stripped in an acidic glycine buffer after exposure to film and re-probed with anti-SGLT1 antibody that had been pre-incubated with the immunizing peptide, followed by secondary antibody as above. This membrane was exposed to film for an identical time period as the membrane incubated with the antibody alone.14
2.7. Protein extraction by sucrose gradient
The preparation and fractionation of membranes from cardiac muscles was performed as previously described.15 Briefly, hearts were removed, minced, and homogenized on ice using a Polytron homogenizer in a buffer containing 20 mM HEPES, 250 mM sucrose, 1 mM EDTA, 5 mM benzamidine, 1 µM aprotinin A, 1 µM pepstatin, 1 µM leupeptin, and 1 mM phenylmethylsulfonyl fluoride (pH 7.4). The homogenate was centrifuged at 2000 g for 10 min and then at 9000 g for 20 min. The supernatant was then centrifuged at 180 000 g for 90 min. The 180 000 g pellet was re-suspended in PBS with protease inhibitors, loaded on a 10–30% (wt/wt) continuous sucrose gradient (3–4 mg of protein per 5 mL gradient), and centrifuged at 48 000 rpm for 55 min in a SW-50.1 rotor. Gradients were fractionated starting from the bottom of the tube. The gradients were divided into nine fractions and the protein content in each fraction was measured by Bradford reagent. A total of 100 µg of protein from each fraction was analysed by immunoblotting. All centrifugation was performed at 4°C. The β1 subunit of the Na+/K+ ATPase, a cell membrane marker, was measured by immunoblot to document adequate enrichment of membrane fractions.
2.8. Tissue fixation and immunofluorescence staining
FVB WT mouse cardiac tissue was processed for immunofluorescence of SGLT1 as described previously.16 Hearts were fixed for 4 h at room temperature in PBS containing 4% paraformaldehyde, 10 mM sodium periodate, 70 mM lysine, and 5% sucrose (PLP), washed in PBS, and quenched in NH4Cl.17 Tissues were cryoprotected in a solution of 30% sucrose in PBS overnight at 4°C. These tissues were embedded in OT compound (Tissue TEK, Sakura Finetek) and mounted on a cutting block. After being frozen in a Reichert Frigocut microtome, sections were picked up on Superfrost Plus slides (Fisher). Immunofluorescence staining was performed on 4 µm cryostat sections after SDS antigen retrieval.18 Slides were washed in PBS followed by incubation with a blocking solution containing 1% BSA in PBS-0.02% sodium azide for 15 min. Slides were then incubated with anti-murine SGLT1 antibody (1:600 dilution, Santa Cruz #sc20582) for 75 min at room temperature. Sections were then washed twice for 5 min in high-salt PBS (2.7% NaCl) and once in PBS, and then incubated for 1 h with a secondary antibody coupled to FITC (donkey anti-X IgG, Jackson Immunologicals). After repeating the same series of washes as above, the slides were mounted with Vectashield (Vector Labs). An incubation omitting the primary antibody (using DAKO reagent alone) was performed in parallel. The SGLT1 immunizing peptide used to produce this antibody was employed for peptide inhibition controls using methods previously described.14
2.9. Statistical analysis
Results are expressed as mean ± SE. Differences between two groups were compared by Student's t-test and among multiple groups by one-way ANOVA with post hoc Bonferroni test. A P-value <0.05 was considered significant.
3. Results
3.1. SGLT1 is expressed in murine and human cardiac myocytes
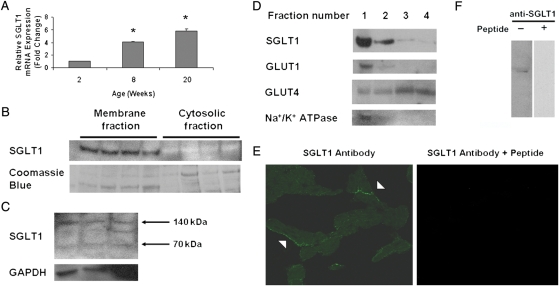
Although the presence of SGLT1 mRNA in the heart has been reported,6 protein expression in cardiac tissue has not been characterized. We first measured by QPCR relative SGLT1 mRNA expression in the WT FVB mouse heart at three different ages. Cardiac SGLT1 mRNA expression progressively increased from age 2 through 20 weeks (Figure 1A). By immunoblot, we determined that SGLT1 protein was present in murine (Figure 1B) and human cardiac tissue (Figure 1C). As in other tissues, SGLT1 appeared to be partially dimerized and/or subjected to post-translational modifications in the human heart.
Figure 1.
SGLT1 is expressed in murine and human cardiac myocytes. (A) Relative SGLT1 mRNA expression was assessed by QPCR in hearts harvested from male WT FVB mice at ages 2, 8, and 20 weeks (n = 5 per group). Data are expressed as mean ± SE. *P < 0.01 relative to 2-week-old hearts. (B) A representative immunoblot of membrane and cytosolic fractions of murine cardiac protein showed that SGLT1 was present only in the membrane fraction. Coomassie blue staining of the protein gel was used to document the relative quantity of protein loaded for the immunoblot. (C) A representative immunoblot of total human cardiac protein showed the presence of two (70 and 140 kDa) SGLT1 bands in all lanes, and an intermediate band in the rightmost lane. An immunoblot of GAPDH was used to document the relative quantity of protein loaded. (D) A representative immunoblot of murine cardiac protein fractions derived on a sucrose gradient showed colocalization of SGLT1, GLUT1, and Na+/K+ ATPase (a marker for the sarcolemma). (E) Immunofluorescence microscopy showed that SGLT1 was predominantly localized to the sarcolemma of cardiac myocytes from 8-week-old male WT FVB mice (left, arrowheads), a staining pattern that was significantly decreased by pre-incubation of the antibody with the immunizing peptide. (F) Further to demonstrate anti-murine SGLT1 antibody specificity, the SGLT1 band visualized by immunoblot was completely competed off by pre-incubation of the antibody with the peptide.
Because SGLT1 is expressed in the plasma membranes of renal and intestinal cells,19,20 we hypothesized that SGLT1 was localized to the sarcolemma in cardiac myocytes. To test this hypothesis, we first determined whether SGLT1 was membrane bound in cardiac myocytes. Total membrane (comprising both sarcolemmal and intracellular membranes) and cytosolic protein fractions from WT murine hearts were extracted and subjected to immunoblotting. SGLT1 protein was detected only in membrane fraction (Figure 1B). To determine whether SGLT1 was preferentially localized to the sarcolemma, cardiac protein was fractionated on a sucrose flotation gradient, and fractions analysed by immunoblot. SGLT1 was present in fractions rich in Na+/K+ ATPase, a marker for the sarcolemma (Figure 1D). Similarly, GLUT1, but not GLUT4, was predominantly localized to the sarcolemma. By immunofluorescence, we confirmed that SGLT1 was expressed in cardiac myocytes and was predominantly localized to the sarcolemma (Figure 1E). The specificity of the antibody used was confirmed by peptide inhibition in immunofluorescence labelling and immunoblotting (Figure 1E and F).
3.2. Murine and human cardiac SGLT1 expression is perturbed in disease states
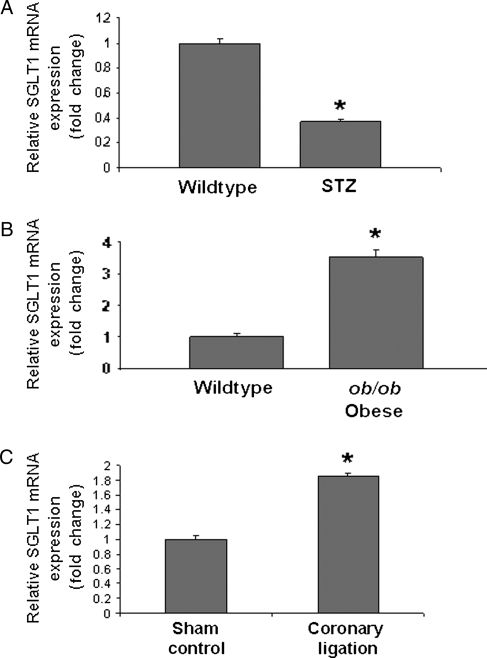
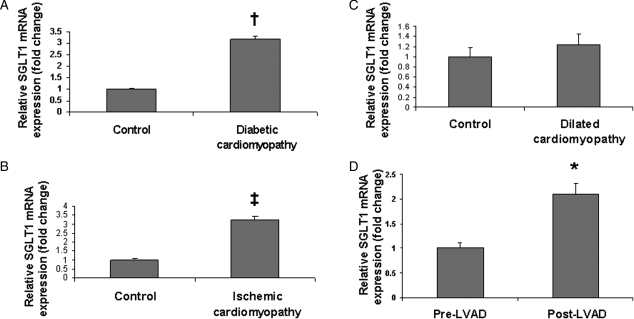
We hypothesized that changes in cardiac SGLT1 expression may be associated with cardiac diseases that are characterized by changes in energy substrate utilization. Therefore, we determined SGLT1 mRNA expression by QPCR in ischaemic and diabetic murine and human hearts.1 Cardiac SGLT1 expression was significantly decreased in STZ diabetic mice, a model of type 1 diabetes (Figure 2A), but increased in both obese ob/ob mice, a model of type 2 diabetes (Figure 2B), and WT mice after CAL (Figure 2C). These observations were replicated in human cardiac tissue. SGLT1 expression was increased in subjects with end-stage cardiomyopathy secondary to type 2 diabetes (Figure 3A) and ischaemia (Figure 3B) undergoing cardiac transplantation. However, no change in SGLT1 expression was observed in idiopathic dilated cardiomyopathy (Figure 3C). In failing human hearts, a significant increase in SGLT1 expression relative to baseline was observed following implantation of a LVAD and functional recovery (Figure 3D).
Figure 2.
Perturbations in levels of cardiac SGLT1 mRNA expression as measured by QPCR in diseased murine hearts relative to control. Cardiac SGLT1 expression was significantly (A) decreased in streptozotocin (STZ) treated (type 1 diabetic) FVB mice, (B) increased in ob/ob (type 2 diabetic) mice, and (C) increased in WT C57BL/6J mice after coronary artery ligation. n = 4–6 per group. Data are expressed as mean ± SE. *P < 0.05.
Figure 3.
Perturbations in levels of cardiac SGLT1 mRNA expression as measured by QPCR in diseased human hearts. Significant increases in SGLT1 expression were observed in subjects with end-stage cardiomyopathy secondary to (A) long-standing type 2 diabetes and (B) coronary artery disease, relative to age- and sex-matched control subjects. (C) No change in SGLT1 expression was observed in idiopathic dilated cardiomyopathy. (D) In failing hearts, implantation of left ventricular assist devices (LVAD) resulted in an increase in SGLT1 expression, which correlated with improved contractile function. Paired samples from the same subjects were used. n = 5–6 per group. Data are expressed as mean ± SE. *P < 0.05; †P < 0.01; ‡P < 0.001 relative to control or baseline.
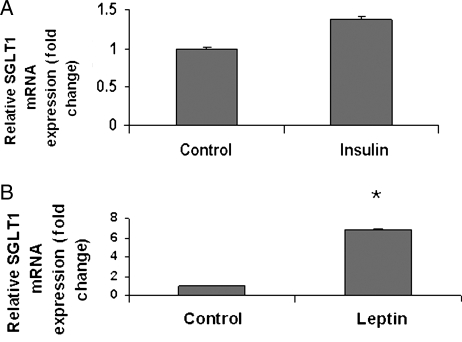
3.3. Leptin, but not insulin, increased cardiac SGLT1 expression
Because insulin and leptin, a neurohormone responsible for signalling of caloric excess, are important regulators of cardiac glucose uptake, we determined the effects of these hormones on cardiac SGLT1 mRNA expression in the WT murine heart. No change in SGLT1 expression was observed 30 min following insulin administration (Figure 4A). However, leptin increased SGLT1 expression approximately seven-fold relative to control (Figure 4B).
Figure 4.
Acute effects of (A) insulin in WT FVB mice and (B) leptin in WT C57BL/6J mice on cardiac SGLT1 mRNA expression as measured by QPCR 30 min following administration. Leptin, but not insulin, significantly increased SGLT1 expression. n = 3–5 per group. Data are expressed as mean ± SE. *P < 0.05; NS, not significant.
3.4. Increased cardiac glucose uptake in response to insulin and leptin is mediated at least in part by SGLT1
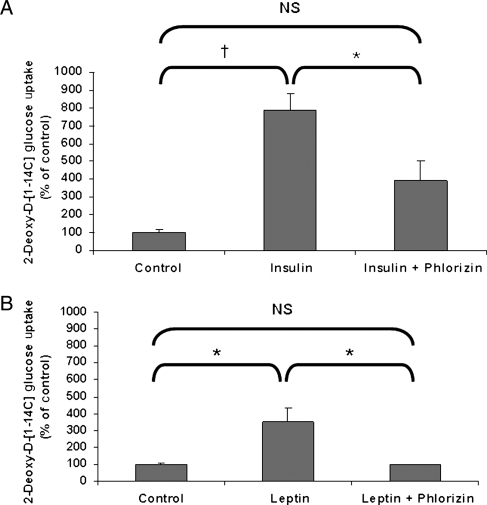
Stimulation of cardiac glucose uptake by insulin and leptin has long been thought to be mediated by GLUT1 and GLUT4. Our discovery of cardiac SGLT1 expression changes in response to leptin suggested that SGLT1 may play a functional role in modulating cardiac glucose uptake in response to hormonal stimuli. Significantly increased cardiac glucose uptake was observed 30 min following administration of insulin (10 U/kg subcutaneously), which was inhibited by phlorizin, a specific SGLT1 inhibitor (Figure 5A). Similarly, significantly increased cardiac glucose uptake was observed following administration of leptin (10 mg/kg intraperitoneally), which was completely inhibited by phlorizin (Figure 5B). These data suggest that SGLT1 mediates at least a proportion of, and possibly all of, the increase in cardiac glucose uptake in response to insulin and leptin.
Figure 5.
Increased cardiac glucose uptake in WT FVB mice in response to exogenous insulin and leptin administration is dependent on SGLT1. (A) Increased glucose uptake observed after insulin administration was inhibited by phlorizin, a SGLT1 inhibitor. (B) Increased glucose uptake observed after leptin administration was completely inhibited by phlorizin (n = 3 per group). Data are expressed as mean ± SE. *P < 0.05; †P < 0.01; NS, not significant.
4. Discussion
Cardiac myocytes depend on a delicate balance of glucose and free fatty acids as energy sources, a balance that is disrupted in pathological states such as diabetes and myocardial ischaemia.21 Under-stressed conditions such as ischaemia, glucose utilization is activated, resulting in an increase in the amount of ATP generated per mole of oxygen consumed. Therefore, elucidation of the role of SGLT1 in cardiac glucose uptake in health and in disease may inform the development of novel treatments. Classically, it has long been thought that only the GLUT isoforms, GLUT1 and GLUT4, are responsible for glucose uptake in cardiac myocytes.3 However, we have determined that SGLT1 is highly expressed in murine and human cardiac myocytes, with preferential localization in the sarcolemma; SGLT1 expression is altered in diabetic and ischaemic cardiomyopathy; SGLT1 expression may be regulated in part by leptin; and SGLT1 mediates at least part of the increased cardiac glucose uptake in response to insulin and leptin.
One previous study determined unexpectedly high expression of SGLT1 mRNA in the human heart, approximately 10-fold greater than in kidney tissue.6 However, expression of the SGLT1 protein and its cellular localization in heart were not examined. The current study is the first to report the presence of the sodium/glucose cotransporter system in the cardiac myocyte sarcolemma, by both membrane protein fractionation studies and immunofluorescence microscopy. In protein fractions, SGLT1 colocalizes with GLUT1, which is normally localized to the sarcolemma, and with Na+/K+ ATPase, a marker for the sarcolemma; and to a lesser extent with GLUT4, which can be translocated from intracellular stores to the sarcolemma when required. Interestingly, at least in mice, cardiac SGLT1 expression appears to increase with age. The basis for this age dependence is uncertain.
Previous reports22–24 suggest that functional SGLT1 is an oligomer, resulting from homodimerization, or from heterodimerization with RS1, a regulatory protein with a molecular weight very close to that of SGLT1. Our immunoblots of human cardiac protein exhibited two (70 and 140 kDa) SGLT1 bands in all hearts, which could reflect dimerization, but also an intermediate band in at least one heart. Although the identity of the intermediate band is unknown, we speculate that it may represent post-translational modifications in some hearts. For example, it is known that SGLT1 can be phosphorylated. Two SGLT1 bands were also observed previously in rat coronary endothelial cells.25 However, our immunoblots of murine cardiac protein exhibited only monomeric SGLT1. It is unclear at present whether there are species or tissue-specific differences in dimerization and/or post-translational modification.
We next considered whether functional changes in SGLT1 may contribute to the pathophysiology of cardiac diseases, particularly those characterized by increased consumption of glucose. Protein expression of GLUT1 and GLUT4 is decreased in diabetic hearts without a corresponding decrease in cardiac glucose uptake,26 suggesting the presence of other functional cardiac glucose transporters. We observed increased cardiac SGLT1 expression both in human subjects with end-stage cardiomyopathy secondary to type 2 diabetes and in ob/ob obese mice, a model of type 2 diabetes which exhibits altered myocardial glucose metabolism. Conversely, decreased cardiac expression of SGLT1 was observed in STZ-treated mice, a model of type 1 diabetes. Although the mechanism of divergent SGLT1 expression in type 1 and type 2 diabetes is uncertain, we speculate that increased SGLT1 may be related to chronic hyperinsulinaemia in type 2 diabetes. However, it should be noted that in our study, acute insulin exposure led only to a mild, statistically insignificant increase in SGLT1 expression in WT murine hearts. It is possible that increased SGLT1 in the diabetic heart is an adaptive change in response to a reduction in cardiac GLUT1 and GLUT4 expression.
Ischaemia increases cardiac glucose utilization, requiring a greater capacity for glucose transport across the sarcolemma. In canine hearts subjected to low-flow ischaemia, there is a significant translocation of both GLUT1 and GLUT4 from the intracellular pool to the sarcolemma.27 Several pharmacological agents with demonstrated anti-ischaemic effects have also recently been shown to act by stimulating glucose metabolism in heart.28 In our study, we observed a several-fold increase in SGLT1 expression in both human ischaemic cardiomyopathy and murine hearts subjected to CAL. We observed increased SGLT1 expression associated with the functional recovery in failing human hearts after LVAD insertion, suggesting that upregulation of SGLT1 may be an adaptive response to injury.
Although SGLT1 expression was upregulated by acute administration of leptin, no change was observed with insulin. The role of SGLT1 in the hormonal modulation of cardiac glucose uptake was further addressed by administration of insulin and leptin in WT mice in the presence and absence of phlorizin, a specific inhibitor of SGLT1. Phlorizin inhibited insulin- and leptin-induced increases in cardiac glucose uptake partially to completely. Therefore, SGLT1 appears to be responsible for at least part of, and possibly all of, insulin-stimulated and leptin-stimulated cardiac glucose uptake. Similar to our study, phlorizin was shown to inhibit insulin-stimulated glucose uptake in rat skeletal muscle.25 Although studies to identify the mechanism of increased SGLT1 activity after insulin administration have not yet been performed, insulin may promote trafficking of SGLT1 to the sarcolemma, or may directly stimulate SGLT1 activity. Of note, insulin activates protein kinase C (PKC), and phosphorylation of SGLT1 by PKC29 leads to recruitment of SGLT1 to the plasma membrane.4,30
The relative importance of SGLT1 relative to GLUT1 and GLUT4 in normal and diseased hearts remains uncertain. Mice lacking cardiac GLUT4 exhibited an increase in basal cardiac glucose transport, with a corresponding increase in GLUT1 expression.31 Similar upregulation of SGLT1 as a compensatory mechanism is plausible. Further studies examining the expression and function of SGLT1 in GLUT1 and GLUT4 knockout mice at baseline and in the presence of stressors are warranted.
In conclusion, our data show that SGLT1 is expressed in cardiac myocytes, with preferential localization in the sarcolemma. SGLT1 expression is increased in type 2 diabetes and ischaemia, but decreased in type 1 diabetes. SGLT1 appears to be at least in part responsible for increased cardiac glucose uptake following exposure to insulin and leptin, and leptin appears to act by directly increasing SGLT1 expression. To our knowledge, this is the first study to examine the regulation of SGLT1 by insulin and leptin in the normal heart, and demonstrate changes in SGLT1 expression in disease states. Further studies will be required to determine the therapeutic value of modulation of SGLT1 expression, cellular localization, and activity.
Funding
This work was supported by an American Heart Association Scientist Development Grant (F.A.), an American Heart Association Postdoctoral Fellowship (S.K.B.), and National Institutes of Health Grants P30 DK079307 ‘Pittsburgh Kidney Research Center’ and K08 HD045524 (N.M.P.-S.). F.A. is a Doris Duke Charitable Foundation Clinical Scientist.
Conflict of interest: none declared.
Acknowledgements
From the University of Pittsburgh, we thank Charles F. McTiernan, Ph.D., Fan Gong, Ph.D., and Christy Smolak for providing technical advice and assistance. From the University of Pittsburgh Medical Center, we thank the research nurse coordinators within the Cardiovascular Institute and Division of Cardiothoracic Surgery for assistance in patient enrolment and clinical data, and the cardiothoracic surgeons and their support staff for assistance in acquisition of surgical specimens. From the Cleveland Clinic Foundation, we thank Christine S. Moravec, Ph.D. (Department of Cardiovascular Medicine, Kaufman Center for Heart Failure), the cardiac transplant teams, Department of Thoracic and Cardiovascular Surgery, residents in the Department of Pathology, and Life Banc of Northeastern Ohio for helping to obtain control cardiac tissues for research.
References
- 1.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 2.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res. 1997;33:243–257. doi: 10.1016/s0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 3.Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res. 2008;79:249–258. doi: 10.1093/cvr/cvn116. [DOI] [PubMed] [Google Scholar]
- 4.Wright EM, Hirsch JR, Loo DD, Zampighi GA. Regulation of Na+/glucose cotransporters. J Exp Biol. 1997;200:287–293. doi: 10.1242/jeb.200.2.287. [DOI] [PubMed] [Google Scholar]
- 5.Turk E, Zabel B, Mundlos S, Dyer J, Wright EM. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature. 1991;350:354–356. doi: 10.1038/350354a0. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Cryan EV, D'Andrea MR, Belkowski S, Conway BR, Demarest KT. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1) J Cell Biochem. 2003;90:339–346. doi: 10.1002/jcb.10631. [DOI] [PubMed] [Google Scholar]
- 7.McGaffin KR, Sun CK, Rager JJ, Romano LC, Zou B, Mathier MA, et al. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc Res. 2008;77:54–63. doi: 10.1093/cvr/cvm023. [DOI] [PubMed] [Google Scholar]
- 8.Keck M, Romero-Aleshire MJ, Cai Q, Hoyer PB, Brooks HL. Hormonal status affects the progression of STZ-induced diabetes and diabetic renal damage in the VCD mouse model of menopause. Am J Physiol Renal Physiol. 2007;293:F193–F199. doi: 10.1152/ajprenal.00022.2007. [DOI] [PubMed] [Google Scholar]
- 9.Kaibara A, Moshyedi A, Auffenberg T, Abouhamze A, Copeland EM, 3rd, Kalra S, et al. Leptin produces anorexia and weight loss without inducing an acute phase response or protein wasting. Am J Physiol. 1998;274:R1518–R1525. doi: 10.1152/ajpregu.1998.274.6.R1518. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee SK, Ramani R, Saba S, Rager J, Tian R, Mathier MA, et al. A PRKAG2 mutation causes biphasic changes in myocardial AMPK activity and does not protect against ischemia. Biochem Biophys Res Commun. 2007;360:381–387. doi: 10.1016/j.bbrc.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 11.Zhao H, Yakar S, Gavrilova O, Sun H, Zhang Y, Kim H, et al. Phloridzin improves hyperglycemia but not hepatic insulin resistance in a transgenic mouse model of type 2 diabetes. Diabetes. 2004;53:2901–2909. doi: 10.2337/diabetes.53.11.2901. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad F, Banerjee SK, Lage ML, Huang XN, Smith SH, Saba S, et al. The role of cardiac troponin T quantity and function in cardiac development and dilated cardiomyopathy. PLoS ONE. 2008;3:e2642. doi: 10.1371/journal.pone.0002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, et al. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 14.Pastor-Soler N, Bagnis C, Sabolic I, Tyszkowski R, McKee M, Van Hoek A, et al. Aquaporin 9 expression along the male reproductive tract. Biol Reprod. 2001;65:384–393. doi: 10.1095/biolreprod65.2.384. [DOI] [PubMed] [Google Scholar]
- 15.Zhou M, Sevilla L, Vallega G, Chen P, Palacin M, Zorzano A, et al. Insulin-dependent protein trafficking in skeletal muscle cells. Am J Physiol. 1998;275:E187–E196. doi: 10.1152/ajpendo.1998.275.2.E187. [DOI] [PubMed] [Google Scholar]
- 16.Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol. 2008;294:C488–C494. doi: 10.1152/ajpcell.00537.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breton S, Tyszkowski R, Sabolic I, Brown D. Postnatal development of H+ ATPase (proton-pump)-rich cells in rat epididymis. Histochem Cell Biol. 1999;111:97–105. doi: 10.1007/s004180050339. [DOI] [PubMed] [Google Scholar]
- 18.Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS) Histochem Cell Biol. 1996;105:261–267. doi: 10.1007/BF01463929. [DOI] [PubMed] [Google Scholar]
- 19.Runembert I, Queffeulou G, Federici P, Vrtovsnik F, Colucci-Guyon E, Babinet C, et al. Vimentin affects localization and activity of sodium-glucose cotransporter SGLT1 in membrane rafts. J Cell Sci. 2002;115:713–724. doi: 10.1242/jcs.115.4.713. [DOI] [PubMed] [Google Scholar]
- 20.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- 21.Saunders J, Mathewkutty S, Drazner MH, McGuire DK. Cardiomyopathy in type 2 diabetes: update on pathophysiological mechanisms. Herz. 2008;33:184–190. doi: 10.1007/s00059-008-3115-3. [DOI] [PubMed] [Google Scholar]
- 22.Koepsell H, Spangenberg J. Function and presumed molecular structure of Na(+)-D-glucose cotransport systems. J Membr Biol. 1994;138:1–11. doi: 10.1007/BF00211064. [DOI] [PubMed] [Google Scholar]
- 23.Giudicelli J, Bertrand MF, Bilski S, Tran TT, Poiree JC. Effect of cross-linkers on the structure and function of pig-renal sodium-glucose cotransporters after papain treatment. Biochem J. 1998;330:733–736. doi: 10.1042/bj3300733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veyhl M, Spangenberg J, Puschel B, Poppe R, Dekel C, Fritzsch G, et al. Cloning of a membrane-associated protein which modifies activity and properties of the Na(+)-D-glucose cotransporter. J Biol Chem. 1993;268:25041–25053. [PubMed] [Google Scholar]
- 25.Elfeber K, Stumpel F, Gorboulev V, Mattig S, Deussen A, Kaissling B, et al. Na(+)-D-glucose cotransporter in muscle capillaries increases glucose permeability. Biochem Biophys Res Commun. 2004;314:301–305. doi: 10.1016/j.bbrc.2003.12.090. [DOI] [PubMed] [Google Scholar]
- 26.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 27.Young LH, Renfu Y, Russell R, Hu X, Caplan M, Ren J, et al. Low-flow ischemia leads to translocation of canine heart GLUT-4 and GLUT-1 glucose transporters to the sarcolemma in vivo. Circulation. 1997;95:415–422. doi: 10.1161/01.cir.95.2.415. [DOI] [PubMed] [Google Scholar]
- 28.Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation. 1997;95:313–315. doi: 10.1161/01.cir.95.2.313. [DOI] [PubMed] [Google Scholar]
- 29.Turk E, Kerner CJ, Lostao MP, Wright EM. Membrane topology of the human Na+/glucose cotransporter SGLT1. J Biol Chem. 1996;271:1925–1934. doi: 10.1074/jbc.271.4.1925. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch JR, Loo DD, Wright EM. Regulation of Na+/glucose cotransporter expression by protein kinases in Xenopus laevis oocytes. J Biol Chem. 1996;271:14740–14746. doi: 10.1074/jbc.271.25.14740. [DOI] [PubMed] [Google Scholar]
- 31.Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]