Abstract
Background
Adults with Obesity (O) or Type 2 Diabetes (T2DM) are at higher risk for stroke and myocardial infarction. Increased carotid intima-media thickness (cIMT) and stiffness are associated with these adverse outcomes. We compared carotid arteries in youth who were lean (L), O or T2DM.
Methods and Results
Carotid ultrasound for cIMT, Young’s Elastic Modulus (YEM) and Beta Stiffness Index (β), anthropometric, laboratory, and BP were measured in 182 L, 136 O, and 128 T2DM youth; 10-24 years. Mean differences were evaluated by ANOVA. Independent determinates of cIMT, YEM and β were determined with General Linear Models. CV risk factors worsened from L to O to T2DM. T2DM had greater cIMT than lean and O for the common carotid and bulb. For the internal, both O and T2DM were thicker than L. The carotid arteries were stiffer O & T2DM as compared to L. Determinates of cIMT were Group, Group*age interaction, gender, SBP for common (r2 =0.17); age, race, and SBP for bulb (r2 =0.16); age, race, gender, SBP and total cholesterol for the internal (r2 =0.21). Age, SBP and DBP were determinates of all measures of carotid stiffness with gender adding to YEM (r2=0.23); BMI z score, Group and Group*age interaction contributing to β (r2 =0.31, all p<0.0001).
Conclusion
Youth with Obesity and T2DM diabetes have abnormalities in carotid thickness and stiffness only partially explained by traditional CV risk factors. These vascular changes should alert health care practitioners to address CV risk factors early to prevent an increase in the incidence of stroke and myocardial infarction.
Keywords: Carotid arteries, Elasticity, Obesity, Pediatrics, Risk factors
Introduction
Adults with Obesity or Type 2 Diabetes (T2DM) are at higher risk for stroke and myocardial infarction.1 Evidence of target organ damage in the carotid arteries (increased thickness and stiffness) is also associated with adverse CV outcomes even after adjusting for age.2 Since adults with obesity and T2DM are at risk for developing increased carotid intima-media thickness (cIMT)3 and carotid stiffness (cSTIFF),4, 5 we sought to determine if similar carotid structural and functional abnormalities exist in youth with obesity or T2DM as compared to lean controls.
Methods
Study Population
The study population consisted of 446 youth who were examined as part of an ongoing study of the cardiac and vascular effects of obesity and T2DM in adolescents and young adults (age 10-24 years, 65% non-Caucasian, 39% male). Diagnosis of T2DM (N for T2DM group = 128) was made by the primary provider. Chart review was performed to include only diabetic subjects who were islet cell antibody negative (glutamic acid decarboxylase, ICA 512, Insulin autoantibodies), had no evidence of other specific type of diabetes, and who were non-insulin requiring in the basal state to prevent diabetic ketoacidosis. All obese subjects underwent a 2-hour oral glucose tolerance test to rule out sub-clinical T2DM according to ADA guidelines.6 Pregnant females were excluded from the study.
Prior to enrollment in the study, written informed consent was obtained from subjects ≥18 years old or the parent or guardian for subjects < 18 years old. Written assent was also obtained for subjects < 18 years old according to the guidelines established by the Institutional Review Board at Cincinnati Children’s Hospital.
Data Collection
After a minimum 10 hour overnight fast, participants had questionnaire, anthropometric, blood pressure (BP), laboratory and carotid artery data collected. An average of 2 measures of height were obtained with a calibrated stadiometer (Veeder-Rood, Elizabethtown, NC) by trained personnel. Weight was also measured twice and averaged using a Health-O-Meter electronic scale. Body mass index was calculated as kilograms per meter squared. Lean (N for lean group = 182) was defined as BMI <85th% while obesity (N for obese group = 136) was defined as BMI > 95th% according to CDC growth charts.7 Blood pressure was measured according to the standards of the Fourth Report on BP in Children.8
Fasting plasma glucose was measured using a Hitachi model 704 glucose analyzer with intra-assay and inter-assay coefficients of variation (CV) of 1.2% and 1.6% respectively.9 Plasma insulin was measured by radioimmunoassay using an anti-insulin serum raised in guinea pegs, 125I labeled insulin (Linco, St. Louis, MO) and a double antibody method to separate bound from free tracer. This assay has a sensitivity of 2 pmol and has intra- and interassay CVs of 5% and 8%.10 Assays of fasting plasma lipid profiles were carried out in a laboratory which is NHLBI-CDC standardized with the LDL cholesterol concentration calculated using the Friedewald equation. C-reactive protein (CRP) was measured using a high sensitivity enzyme-linked immunoabsorbent assay. HbA1c was measured in red blood cells using HPLC methods. Duration of disease was measured from the date of diagnosis to the date of study.
Carotid Ultrasonography
Carotid Ultrasound studies were performed by a single Registered Vascular Technologist who was blinded to subject group assignment. The carotid arteries were evaluated with high-resolution B-mode ultrasonography using a GE Vivid 7 ultrasound imaging system with a high resolution linear array vascular ultrasound variable frequency transducer centered at 7.5 MHz. For each subject, each carotid wall and segment was examined independently from continuous angles to identify the thickest cIMT. Multiple digital image loops were digitally transmitted using the Camtronic Medical System for off-line reading and analyses. A trace technique was employed to measure the maximum carotid thickness from leading edge (lumen-intima) to leading edge (media-adventitia). This technique was found to be more reproducible than point-point measurements (coefficient of variation for repeat readings 5.3-8.0% for trace versus 8.4-11.6% for point-to-point for the 3 carotid segments, unpublished data). Three segments were imaged with right and left sides averaged for the common carotid artery, the bifurcation (carotid bulb), and the internal carotid artery.
M-Mode measurements of the common carotid were also performed.11 An optimal 2-D image of the common carotid artery was obtained and the M-mode curser placed approximately 1 cm proximal to beginning of the carotid bulb. The maximal and minimal lumen diameters were read from the M-mode tracing for calculations of carotid stiffness.11 Calculations included Young’s Elastic Modulus (YEM) and Beta Stiffness Index (β).12
Statistical Analysis
All analyses were performed with Statistical Analyses Software (SAS, version 9.1.3)13 Average values for demographic, anthropometric, and laboratory data were obtained by BMI group. Variance stabilizing measures to transform non-normal values were performed as needed. Analysis of variance was performed to look for differences by BMI group, with Bonferroni correction for multiple comparisons as appropriate. Bivariate correlations were calculated between carotid outcome variables and all covariates overall and by BMI group. General Linear Models were constructed using important covariates from correlation analyses to elucidate independent determinates of cIMT and cSTIFF.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
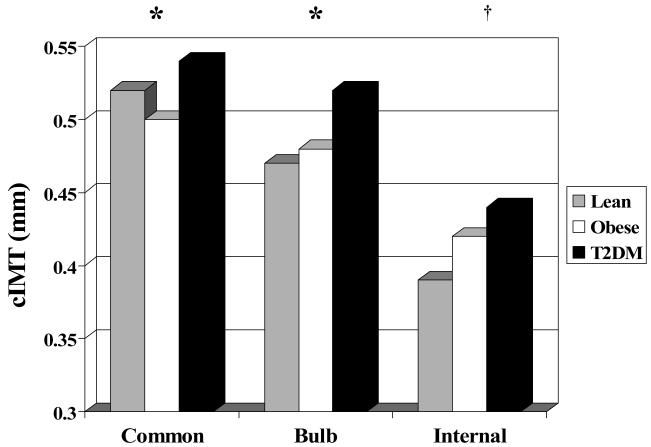
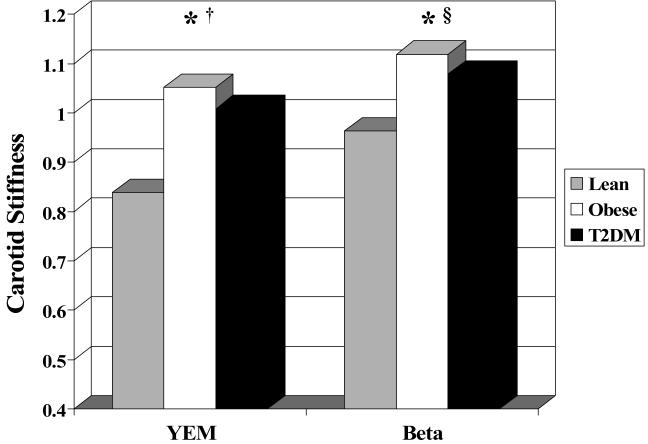
Groups did not differ by age, race or sex distribution. Traditional CV risk profile (WT, BP, HR, lipids, glucose, insulin, inflammation) worsened from lean to obese to T2DM (p values in Table 1). The proportion of subjects with BP in the pre- or true-hypertensive range differed significantly among groups when evaluated by analysis of variance. However, the absolute number of subjects with abnormal BP levels was low in the L (3.9% pre-, 2.8% true-) and O groups (8.1% pre-, 13.2% true-) with T having higher prevalence (10.5% pre-htn, 26.6% htn). Prevalence of abnormal DBP was lower (L: 2.8% pre-, 2.2% true-; O: 2.9% pre-, 7.4% true-; T2DM: 5.7% pre-, 14.5% true-). For all carotid segments, T2DM had significantly greater cIMT than lean subjects. T2DM had thicker cIMT than obese participants for the common and bulb but did not differ for the internal carotid where both obese and T2DM were thicker than lean subjects (Table 2 and Figure 1, all p≤0.05). Obese and T2DM groups had stiffer carotid arteries with higher YEM and β than the lean group (Table 2 and Figure 2).
Table 1.
Characteristics of the study population
| Variable | Lean | Obese | T2DM | |||
|---|---|---|---|---|---|---|
| N = 182 | N = 136 | N = 128 | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| Age (yrs) | 17.8 | 3.5 | 17.9 | 3.3 | 18.8 | 3.2 |
| Gender (% Female) | 59% | 67% | 60% | |||
| Race (% Non-Caucasian) | 62% | 65% | 63% | |||
| Height (m) *,† | 165.7 | 10.8 | 167.1 | 10.3 | 169.7 | 10.2 |
| Weight (kg)*,‡ | 59.5 | 11.9 | 102.6 | 21.2 | 105.8 | 29.9 |
| BMI (kg/m2)*,‡ | 21.4 | 2.6 | 36.5 | 6.5 | 36.6 | 9.4 |
| SystolicBP (mmHg)*,†,‡ | 110.2 | 9.5 | 119.1 | 10.4 | 124.6 | 12.5 |
| DiastolicBP (mmHg)*,†,‡ | 66.2 | 6.9 | 68.7 | 7.3 | 73.3 | 8.8 |
| Mean Pressure (mmHg)*,†,‡ | 77.6 | 7.8 | 83.6 | 7.6 | 88.3 | 9.9 |
| Pulse Pressure (mmHg)*,‡ | 44.0 | 7.0 | 50.4 | 7.3 | 51.3 | 9.1 |
| Heart Rate (beats/min)*,†,‡ | 63.7 | 11.3 | 67.4 | 10.2 | 72.3 | 11.7 |
| Total Cholesterol (mg/dl)*,†,‡ | 159.6 | 27.7 | 174.3 | 35.3 | 186.2 | 39.7 |
| LDL-Cholesterol (mg/dl)*,‡ | 88.5 | 23.3 | 108.6 | 32.1 | 113.8 | 37.3 |
| HDL-Cholesterol (mg/dl)*,‡ | 57.0 | 13.1 | 46.3 | 9.6 | 45.1 | 11.7 |
| Triglycerides (mg/dl)*,†,‡ | 69.8 | 32.2 | 100.9 | 62.8 | 145.5 | 100.9 |
| Glucose (mg/dl)*,† | 89.7 | 6.4 | 93.8 | 8.9 | 169.0 | 89.9 |
| Insulin (micromol/L)*,†,‡ | 11.5 | 4.7 | 23.9 | 18.3 | 29.9 | 23.5 |
| HbA1c (%)*,† | 5.4 | 0.5 | 5.5 | 0.7 | 8.5 | 3.3 |
| C-Reactive Protien(mg/L)*,‡ | 1.10 | 3.49 | 5.41 | 6.39 | 6.27 | 7.74 |
P≤0.05 for: Leans < T2DM
P≤0.05 for: Obese < T2DM
P≤0.05 for: Lean < Obese
Variance stabilizing transformations applied as needed prior to conducting ANOVA.
Table 2.
Carotid ultrasound parameters
| Variable | Lean | Obese | T2DM | |||
|---|---|---|---|---|---|---|
| N = 182 | N = 136 | N = 128 | ||||
| Mean | SD | SD | SD | |||
| Common carotid intima media thickness (mm)*,† | 0.52 | 0.08 | 0.50 | 0.09 | 0.54 | 0.10 |
| Bulb intima media thickness (mm)*,† | 0.47 | 0.08 | 0.48 | 0.11 | 0.52 | 0.13 |
| Internal carotid intima media thickness (mm)*,‡,§ | 0.39 | 0.08 | 0.42 | 0.09 | 0.44 | 0.10 |
| Beta stiffness coefficient(unitless)‡,| | 0.96 | 0.27 | 1.12 | 0.44 | 1.08 | 0.48 |
| Young’s elastic modulus (mmHg/mm),‡ | 83.8 | 24.6 | 105.2 | 40.5 | 100.8 | 47.1 |
P≤0.05 for Lean < T2DM
P≤0.05 for Obese < T2DM
P≤0.05 for Lean < Obese
Trends p≤0.1 Obese < T2DM
Lean < T2DM
Variance stabilizing transformations applied prior to conducting ANOVA.
Figure 1.
Carotid intima-medial thickness (cIMT) by BMI group. *P<0.05 Lean & Obese < T2DM; † P<0.05; Lean < Obese & T2DM.
Figure 2.
Carotid stiffness by BMI group. * P< 0.05 Lean < T2DM; † P<0.05 Lean < Obese; § p<0.1 Lean < T2DM. Young’s elastic pressure modulus (mmHg/mm) divided by 100 for graphing purposes. Beta stiffness index (Beta) is unitless.
Correlation analyses revealed that age, BP, LDL-C correlated with all outcome measures. BMI was significant for all but YEM. Glucose was significant for all cIMT segments. CRP correlated with stiffness measures and all cIMT but common carotid. HDL-C only correlated with internal cIMT. TG correlated with internal cIMT and YEM (all p <0.05). Correlations did not differ substantially by BMI group.
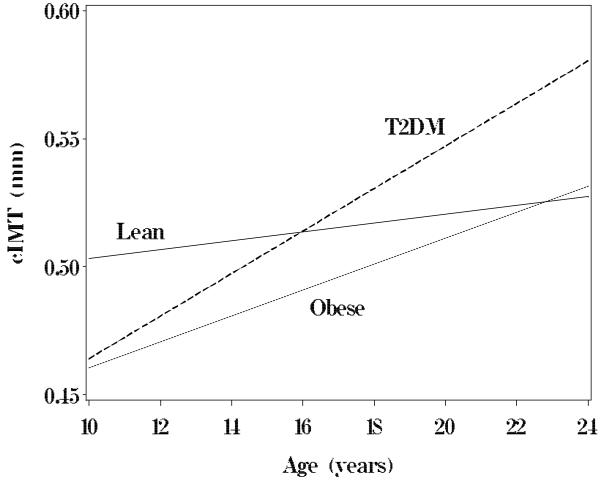
Multivariate linear regression was performed for all carotid outcome variables against the covariates of group, race, sex, age, height, BMI z score, HR, SBP, DBP, TChol, LDL-C, HDL-C, TG, Glucose, Insulin and hs-CRP (Table 3). Independent determinates of cIMT were Group, Group*age interaction, gender, SBP for common (r2 =0.17); age, race, and SBP for bulb (r2 =0.15); age, race, sex, SBP and total cholesterol for the internal (r2 =0.21) carotid arteries respectively. When stratified by group, general linear models and simple plots (Figure 3) revealed that the slope for the regression of age on common carotid IMT was flat for lean subjects indicating no significant increase over the age range in our study (lean cIMT = 0.49 + 0.0017*age, p=NS). However, there was a significant increase in cIMT with age for obese and T2DM with the slope of the regression steepest for T2DM (obese cIMT = 0.41 + 0.0051*age; T2DM cIMT =0.38 + 0.0083*age, both p < 0.002) suggesting an increased effect of age on cIMT progression in the presence of T2DM. Age and BP were determinates of all measures of carotid stiffness with BMI, Group and Group*age interaction contributing to β (r2 for YEM=0.23, β=0.31, all p<0.0001).
Table 3.
Determinants of Increased cIMT and Reduced Arterial Stiffness. Multiple Linear Regression with parameter estimates
| Variable | Common cIMT | Bulb cIMT | Internal cIMT | Beta Stiffness Coefficient | Young’s Elastic Modulus | |
|---|---|---|---|---|---|---|
| Intercept | -2.20 | -3.54 | 8.75 | 0.12 (NS) | 0.50 (NS) | |
| Group | Lean | 0.16 | 0.18 (NS) | |||
| Obese | 0.016 (NS) | 0.34 | ||||
| Age | 0.0051 | 0.00040 | -0.0008 | 0.0013 | 0.00052 | |
| Group*Age | Lean | -0.00045 | -0.00015 (NS) | |||
| Obese | -0.00016 (NS) | -0.00095 | ||||
| Sex | -0.069 | 0.25 | 0.17 | |||
| Race | 0.025 | -0.086 | ||||
| Body Mass Index z-score | 0.092 | 2.09 | ||||
| Systolic BP | 0.31 | 0.55 | -1.04 | 1.31 | -1.35 | |
| Diastolic BP | -1.65 | |||||
| Total Cholesterol | -0.24 | |||||
| R2 | 0.17 | 0.15 | 0.21 | 0.32 | 0.23 | |
All Models have p≤0.0001; Full model contained group, race, sex, age, height, BMI, HR, SBP, DBP, TChol, LDL-C, HDL-C, TG, Glucose, Insulin and hs-CRP. All parameter estimates listed have P≤0.05 unless specified as not significant (NS).
Figure 3.
Common carotid intima-medial thickness (cIMT) by age and group. P for slope not equal to 0 was <0.002 for Obese and T2DM, NS for Lean group.
Modeling was repeated using BP category (normal, pre- or true-hypertensive) instead of continuous BP variables. The results did not change substantially although the models tended to explain a smaller proportion of the variability in cIMT and cSTIFF. The importance of group as a covariate did not change except that group entered the model for internal carotid. Due to the small number of subjects in some of the BP category by group cells, it was felt that modeling with BP as a continuous variable was more robust.
Discussion
Our study showed that adolescents and young adults with T2DM have significantly thicker cIMT than lean controls for the all carotid artery segments. This is not surprising since our subjects with T2DM had higher CV risk factor levels. More important is the confirmation that increased thickness of the common carotid and bulb can also be found in uncomplicated obesity. Furthermore, beta stiffness coefficient and YEM were higher in both obese and T2DM subjects as compared to lean, when examined in an analysis of variance. These data demonstrate that early changes in vascular structural and function can be demonstrated in youth with obesity prior to the development of carbohydrate intolerance. Additional compromise is demonstrated in patients with established T2DM.
As expected, levels of CV risk factors increased from the lean to obese to T2DM groups and traditional CV risk factors correlated with cIMT and stiffness. In multivariate analyses, group was an independent determinate of cIMT for the common carotid and beta stiffness coefficient. Classification as obese or T2DM was not an independent determinant of cIMT in the bulb or internal carotid or YEM. This suggests that the effect of obesity and T2DM may impart additional influences on common carotid structure and some measures of carotid function that are not entirely explained by traditional CV risk factors. In addition, obesity and T2DM may be modifying the effect of risk factors as seen by the greater effect of age on common carotid cIMT in the obese and T2DM as compared to the lean group.
Adult studies have demonstrated a strong association between obesity and cIMT. The Multi-Ethnic Study of Atherosclerosis examined a large cohort (N = 6814, 45 - 84 years) of CV disease free subjects and found obese subjects of both genders were more likely to have common and internal carotid cIMT greater than the 80th percentile.3 Although the obese subjects were more likely to report hypertension or diabetes, the association between adiposity and cIMT persisted after adjustment for traditional CV risk factors.3 Developing adiposity in childhood has also been linked to carotid thickness as an adult.14 In the Muscatine Study, childhood BMI was a significant determinate of cIMT measured as an adult (33-42 years) for women.15 The Bogalusa Heart Study found that cumulative levels of BMI in childhood were associated with adult cIMT in both genders even after controlling for adult BMI.16
Cross-sectional studies have demonstrated a relationship between obesity and common carotid IMT during childhood.17-22 Iannuzi studied 100 children with BMI > 95th percentile (CDC) who were healthy without any obesity-related comorbidities (hypertension, dyslipidemia, glucose intolerance or diabetes).23 Common carotid IMT was thicker in obese youth compared to age-matched controls even after controlling for traditional CV risk factors. However, when glucose was entered into the model instead of HOMA, the difference in cIMT by obesity group reached only marginal significance.23 In a similar study, Reinehr found glucose to be an independent determinate of cIMT in obese and lean children along with BMI, SBP and high sensitivity CRP.24 These data suggest a similar relationship between worsening carbohydrate intolerance and thicker cIMT as seen in adults. Mangee studied a larger cohort (N = 228) and also found increased cIMT in obese as compared to lean children. However, only the common carotid was evaluated and no multivariate modeling was performed.25 In a study of Chinese youth, both the internal and common carotid arteries were thicker in obese subjects. However, the bulb was not examined in the study nor was multivariate regression performed. Furthermore, the average BMI in this small study (N = 61, BMI 27.7 kg/m2) was lower than in our cohort (BMI 36.5 kg/m2) suggest that their findings may not be generalizable to more obese American youth.26 One recent study did measure all three carotid segments in lean and obese youth, but only on the left side. Furthermore, their multivariate models contained incomplete lipid data (only 12 of 30 controls) and did not include BP as a covariate.27 Our data extend the observations on the effect of obesity on all segments of the carotid artery and provide insight on independent determinants of cIMT in youth using multivariate models containing all traditional CV risk factors.
Similar to data on cIMT, truncal subcutaneous fat accumulation measured as an adolescent was associated with increased cSTIFF in adulthood (average age 36 years).28 In a larger study of healthy adults (N = 2255, age 24-39 years), the relationship remained even after adjusting for number of CV risk factors.29 Cross-sectional studies of children have also demonstrated increased cSTIFF in obese subjects with the metabolic syndrome as compared to obese children without this CV risk factor clustering even after adjusting for age, gender and CRP.30 Tounian, et al found obese youth to have stiffer carotid arteries when compared to lean controls but they did not find significant relationship between cSTIFF and CV risk factors.31 Our data includes a larger cohort than the study by Tounian which may explain our ability to demonstrate relationships between CV risk factors and carotid stiffness in both univariate and multivariate models. These data suggest that a spectrum of vascular abnormalities may develop as an individual develops obesity prior to progressing to metabolic derangement and final development of type 2 diabetes.
Substantial data are available describing vascular changes in adults with T2DM. Diabetic subjects have both thicker32, 33 and stiffer34 carotid arteries than healthy controls. Thickness is affected by BP, age, duration of diabetes, glycemic control and degree of adiposity.32, 35 Insulin resistance modifies carotid stiffness in adults with T2DM.34 Arterial stiffness is also affected by carbohydrate intolerance. Data from the Atherosclerosis Risk in Communities Study show higher carotid stiffness with increasing concentrations of fasting glucose in non-diabetic middle aged subjects.36 This was also seen in the Rotterdam Study of older adults.37 Our observation of increasing severity of carotid abnormalities across the obese to the diabetic subjects also suggests a graded effect of diminishing metabolic control on vascular damage.
In youth, substantial evidence is available demonstrating abnormalities in carotid ultrasound in subjects with type 1 diabetes. Most are small studies with fewer than 100 subjects and the majority image only the common carotid artery.38-40 Although the majority demonstrate significantly thicker cIMT in youth with T1DM as compared to controls, many are unable to demonstrate relationships between CV risk factors and cIMT. This may be due to absence of risk factor data 41, 42 or study designs where subjects were intentionally or coincidentally matched by risk factor levels.43, 44 Similar to our data, a few studies did find univariate correlations between cIMT and BMI, BP and lipids but did not perform multivariate analyses to evaluate independent determinates.25, 45, 46 The narrow range of differences in BP and cholesterol levels between controls and children with T1DM is not surprising given the normal, low BMI levels in cases and controls in these papers. Our data is the first to examine the effect of T2DM on cIMT. Furthermore, it includes 2 controls (1 lean and 1 obese) for each subject with T2DM providing a wider range of CV risk factor levels increasing our power to demonstrate independent associations between T2DM, CV risk factors and cIMT.
Stiffer vessels are also found in youth with type 1 diabetes. Poor glycemic control as manifest by higher HbA1c levels, correlated with carotid distensibility in one study.47 Another found environmental agents such as exposure to tobacco or frequent respiratory infections to be related to carotid compliance.48 Our data are unique in showing the effect of T2DM on vascular compromise in a larger group of adolescents and young adults.
Limitations
Our cross-sectional design does not allow us to determine the time sequence for development of vascular changes as an individual progresses from uncomplicated obesity to metabolic syndrome and finally type 2 diabetes. This cohort also has a somewhat narrow age range which may have limited our ability to detect increase in common carotid cIMT in the lean subjects. The T2DM group was also slightly older with higher BP and cholesterol levels than the lean and obese groups. However, multivariate analyses controlling for these covariates were performed to statistically control for these disparities. Although we found a significant difference in cIMT between groups, the absolute magnitude of the differences were small. Therefore the utility of cIMT measurement for risk stratification for pediatric patients may be diminished by imprecision of the methodology and biologic variability. Furthermore, usage of ultrasound screening is limited by lack of normative data across ages, genders and race/ethnicity. Further refinement of ultrasound techniques and collection of additional data in normal children is needed to advance the field.
Conclusions
We conclude that adolescents and young adults with obesity and T2DM diabetes are at risk for early atherosclerotic changes in the carotid arteries. The abnormalities are only partially explained by traditional CV risk factors such as age and BP, as presence of obesity or diabetes contributed independently to carotid structure and function. These findings are particularly disturbing as the prevalence of obesity-related metabolic syndrome and T2DM in youth is increasing across the globe49 and may lead to a parallel increase in adverse CV outcomes. Therefore, pediatric health care practitioners should continue to screen for abnormalities in CV risk factors especially in children with elevated BMI or T2DM. Comprehensive life-style interventions to reduce obesity must be applied now if we are to prevent a projected decline in life-expectancy for our youth.50
Acknowledgements
We would like to acknowledge the work of the entire T2CVD team. We would also like to thank the participants of the T2DVD study and their families without whose support this study would not be possible.
Funding sources:
This study was supported by NIH (NHLBI) R01 HL076269 (CV Disease in Adolescents with Type 2 Diabetes).
Footnotes
Disclosures:
The authors have no other conflicts of interests to report.
Journal Subject Codes:
[59] Doppler Ultrasound Imaging of Brain and Arteries
[113] Obesity
[135] Risk factors
[190] Type 2 Diabetes Mellitus
References
- 1.Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the third national health and nutrition examination survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- 2.Ali YS, Rembold KE, Weaver B, Wills MB, Tatar S, Ayers CR, Rembold CM. Prediction of major adverse cardiovascular events by age-normalized carotid intimal medial thickness. Atherosclerosis. 2006;187:186–190. doi: 10.1016/j.atherosclerosis.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Burke GL, Bertoni AG, Shea S, Tracy R, Watson KE, Blumenthal RS, Chung H, Carnethon MR. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:928–935. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA. Obesity is associated with increased arterial stiffness from adolescence until old age. Journal of Hypertension. 2005;23:1839–1846. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- 5.van Dijk RA, Bakker SJ, Scheffer PG, Heine RJ, Stehouwer CD. Associations of metabolic variables with arterial stiffness in type 2 diabetes mellitus: focus on insulin sensitivity and postprandial triglyceridaemia. European Journal of Clinical Investigation. 2003;33:307–315. doi: 10.1046/j.1365-2362.2003.01137.x. [DOI] [PubMed] [Google Scholar]
- 6.ADA Type 2 diabetes in children and adolescents. American Diabetes Association. Diabetes Care. 2000;23:381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention http://www.cdc.gov/
- 8.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 9.Goodman E, Daniels SR, Morrison JA, Huang B, Dolan LM. Contrasting prevalence of and demographic disparities in the World Health Organization and National Cholesterol Education Program Adult Treatment Panel III definitions of metabolic syndrome among adolescents. J Pediatr. 2004;145:445–451. doi: 10.1016/j.jpeds.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 10.Martin LJ, Woo JG, Daniels SR, Goodman E, Dolan LM. The relationships of adiponectin with insulin and lipids are strengthened with increasing adiposity. J Clin Endocrinol Metab. 2005;90:4255–4259. doi: 10.1210/jc.2005-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Association of carotid atherosclerosis and left ventricular hypertrophy. JACC. 1995;25:83–90. doi: 10.1016/0735-1097(94)00316-i. [DOI] [PubMed] [Google Scholar]
- 12.Cavallini MC, Roman MJ, Blank SG, Pini R, Pickering TG, Devereux RB. Association of the auscultatory gap with vascular disease in hypertensive patients. Ann Intern Med. 1996;124:877–883. doi: 10.7326/0003-4819-124-10-199605150-00003. [DOI] [PubMed] [Google Scholar]
- 13.SAS Institute . SAS OnlineDoc, Version 9.1.3. SAS Institute; Cary, NC: 2002. [Google Scholar]
- 14.Li SX, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood - The Bogalusa Heart Study. JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 15.Davis P, Dawson J, Riley W, Laurer R. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: the Muscatine Study. Circulation. 2001;104:2815–2819. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 16.Freedman DS, Patel DA, Srinivasan SR, Chen W, Tang R, Bond MG, Berenson GS. The contribution of childhood obesity to adult carotid intima-media thickness: the Bogalusa Heart Study. Int J Obes (Lond) 2008;32:749–756. doi: 10.1038/sj.ijo.0803798. [DOI] [PubMed] [Google Scholar]
- 17.Stabouli S, Kotsis V, Papamichael C, Constantopoulos A, Zakopoulos N. Adolescent Obesity is Associated with High Ambulatory Blood Pressure and Increased Carotid Intimal-Medial Thickness. J Pediatr. 2005;147:651–656. doi: 10.1016/j.jpeds.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Schiel R, Beltschikow W, Radon S, Kramer G, Perenthaler T, Stein G, Schiel R, Beltschikow W, Radon S, Kramer G, Perenthaler T, Stein G. Increased carotid intima-media thickness and associations with cardiovascular risk factors in obese and overweight children and adolescents. European Journal of Medical Research. 2007;12:503–508. [PubMed] [Google Scholar]
- 19.Atabek ME, Pirgon O, Kivrak AS. Evidence for association between insulin resistance and premature carotid atherosclerosis in childhood obesity. Pediatric Research. 2007;61:345–349. doi: 10.1203/pdr.0b013e318030d206. [DOI] [PubMed] [Google Scholar]
- 20.Giannini C, de Giorgis T, Scarinci A, Ciampani M, Marcovecchio ML, Chiarelli F, Mohn A. Obese related effects of inflammatory markers and insulin resistance on increased carotid intima media thickness in pre-pubertal children. Atherosclerosis. 2008;197:448–456. doi: 10.1016/j.atherosclerosis.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, Lam CW, Metreweli C, Celermajer DS, Sung RYT, Leung SSF, Lam CWK. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2004;28:852–857. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 22.Wunsch R, de Sousa G, Toschke AM, Reinehr T. Intima-media thickness in obese children before and after weight loss. Pediatrics. 2006;118:2334–2340. doi: 10.1542/peds.2006-0302. [DOI] [PubMed] [Google Scholar]
- 23.Iannuzzi A, Licenziati MR, Acampora C, Salvatore V, Auriemma L, Romano ML, Panico S, Rubba P, Trevisan M. Increased carotid intima-media thickness and stiffness in obese children. Diabetes Care. 2004;27:2506–2508. doi: 10.2337/diacare.27.10.2506. [DOI] [PubMed] [Google Scholar]
- 24.Reinehr T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R. Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism. 2006;55:113–118. doi: 10.1016/j.metabol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Mangge H, Schauenstein K, Stroedter L, Griesl A, Maerz W, Borkenstein M. Low grade inflammation in juvenile obesity and type 1 diabetes associated with early signs of atherosclerosis. Experimental & Clinical Endocrinology & Diabetes. 2004;112:378–382. doi: 10.1055/s-2004-821023. [DOI] [PubMed] [Google Scholar]
- 26.Zhu W, Huang X, He J, Li M, Neubauer H. Arterial intima-media thickening and endothelial dysfunction in obese Chinese children. European Journal of Pediatrics. 2005;164:337–344. doi: 10.1007/s00431-005-1642-y. [DOI] [PubMed] [Google Scholar]
- 27.Demircioglu F, Kocyigit A, Arslan N, Cakmakci H, Hizli S, Sedat AT. Intima-media thickness of carotid artery and susceptibility to atherosclerosis in obese children with nonalcoholic fatty liver disease. Journal of Pediatric Gastroenterology & Nutrition. 2008;47:68–75. doi: 10.1097/MPG.0b013e31816232c9. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira I, Twisk JWR, van Mechelen W, Kemper HCG, Seidell JC, Stehouwer CDA. Current and adolescent body fatness and fat distribution: relationships with carotid intima-media thickness and large artery stiffness at the age of 36 years. Journal of Hypertension. 2004;22:145–155. doi: 10.1097/00004872-200401000-00024. [DOI] [PubMed] [Google Scholar]
- 29.Juonala M, Jarvisalo MJ, Maki-Torkko N, Kahonen M, Viikari JSA, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;112:1486–1493. doi: 10.1161/CIRCULATIONAHA.104.502161. [DOI] [PubMed] [Google Scholar]
- 30.Iannuzzi A, Licenziati MR, Acampora C, Renis M, Agrusta M, Romano L, Valerio G, Panico S, Trevisan M. Carotid artery stiffness in obese children with the metabolic syndrome. American Journal of Cardiology. 2006;97:528–531. doi: 10.1016/j.amjcard.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 31.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, Girardet JP, Bonnet D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 32.Taniwaki H, Kawagishi T, Emoto M, Shoji T, Kanda H, Maekawa K, Nishizawa Y, Morii H. Correlation between the intima-media thickness of the carotid artery and aortic pulse-wave velocity in patients with type 2 diabetes. Vessel wall properties in type 2 diabetes. Diabetes Care. 1999;22:1851–1857. doi: 10.2337/diacare.22.11.1851. [DOI] [PubMed] [Google Scholar]
- 33.Wagenknecht LE, D’Agostino RB, Jr, Haffner SM, Savage PJ, M R. Impaired glucose tolerance, type 2 diabetes, and carotid wall thickness: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 1998;21:1812–1818. doi: 10.2337/diacare.21.11.1812. [DOI] [PubMed] [Google Scholar]
- 34.van Dijk RA, Bakker SJ, Scheffer PG, Heine RJ, Stehouwer CD. Associations of metabolic variables with arterial stiffness in type 2 diabetes mellitus: focus on insulin sensitivity and postprandial triglyceridaemia. Eur J Clin Invest. 2003;33:307–315. doi: 10.1046/j.1365-2362.2003.01137.x. [DOI] [PubMed] [Google Scholar]
- 35.Selvin E, Coresh J, Golden SH, Boland LL, Brancati FL, Steffes MW. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28:1965–1973. doi: 10.2337/diacare.28.8.1965. [DOI] [PubMed] [Google Scholar]
- 36.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995;91:1432–1443. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 37.van Popele NM, Elizabeth Hak A, Mattace-Raso FUS, Bots ML, van der Kuip DAM, Reneman RS, Hoeks APG, Hofman A, Grobbee DE, Witteman JCM. Impaired fasting glucose is associated with increased arterial stiffness in elderly people without diabetes mellitus: the Rotterdam Study. J Am Geriatr Soc. 2006;54:397–404. doi: 10.1111/j.1532-5415.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- 38.Jarvisalo MJ, Putto-Laurila A, Jartti L, Lehtimaki T, Solakivi T, Ronnemaa T, Raitakari OT. Carotid artery intima-media thickness in children with type 1 diabetes. Diabetes. 2002;51:493–498. doi: 10.2337/diabetes.51.2.493. [DOI] [PubMed] [Google Scholar]
- 39.Peppa-Patrikiou M, Scordili M, Antoniou A, Giannaki M, Dracopoulou M, Dacou-Voutetakis C. Carotid atherosclerosis in adolescents and young adults with IDDM. Relation to urinary endothelin, albumin, free cortisol, and other factors. Diabetes Care. 1998;21:1004–1007. doi: 10.2337/diacare.21.6.1004. [DOI] [PubMed] [Google Scholar]
- 40.Rabago Rodriguez R, Gomez-Diaz RA, Tanus Haj J, Avelar Garnica FJ, Ramirez Soriano E, Nishimura Meguro E, Aguilar-Salinas CA, Wacher NH, Rabago Rodriguez R, Gomez-Diaz RA, Tanus Haj J, Avelar Garnica FJ, Ramirez Soriano E, Nishimura Meguro E, Aguilar-Salinas CA, Wacher NH. Carotid intima-media thickness in pediatric type 1 diabetic patients. Diabetes Care. 2007;30:2599–2602. doi: 10.2337/dc07-0922. [DOI] [PubMed] [Google Scholar]
- 41.Krantz JS, Mack WJ, Hodis HN, Liu CR, Liu CH, Kaufman FR. Early onset of subclinical atherosclerosis in young persons with type 1 diabetes. J Pediatr. 2004;145:452–457. doi: 10.1016/j.jpeds.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 42.Stakos DA, Schuster DP, Sparks EA, Wooley CF, Osei K, Boudoulas H. Cardiovascular effects of type 1 diabetes mellitus in children. Angiology. 2005;56:311–317. doi: 10.1177/000331970505600311. [DOI] [PubMed] [Google Scholar]
- 43.Atabek ME, Kurtoglu S, Pirgon O, Baykara M. Arterial wall thickening and stiffening in children and adolescents with type 1 diabetes. Diabetes Research & Clinical Practice. 2006;74:33–40. doi: 10.1016/j.diabres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Atabek ME, Pirgon O, Kurtoglu S, Imamoglu H. Evidence for an association between type 1 diabetes and premature carotid atherosclerosis in childhood. Pediatr Cardiol. 2006;27:428–433. doi: 10.1007/s00246-006-1199-1. [DOI] [PubMed] [Google Scholar]
- 45.Abdelghaffar S, El Amir M, El Hadidi A, El Mougi F. Carotid intima-media thickness: an index for subclinical atherosclerosis in type 1 diabetes. Journal of Tropical Pediatrics. 2006;52:39–45. doi: 10.1093/tropej/fmi071. [DOI] [PubMed] [Google Scholar]
- 46.Dalla Pozza R, Bechtold S, Bonfig W, Putzker S, Kozlik-Feldmann R, Netz H, Schwarz HP. Age of onset of type 1 diabetes in children and carotid intima medial thickness. Journal of Clinical Endocrinology & Metabolism. 2007;92:2053–2057. doi: 10.1210/jc.2006-2868. [DOI] [PubMed] [Google Scholar]
- 47.Parikh A, Sochett EB, McCrindle BW, Dipchand A, Daneman A, Daneman D. Carotid artery distensibility and cardiac function in adolescents with type 1 diabetes. J Pediatr. 2000;137:465–469. doi: 10.1067/mpd.2000.109002. [DOI] [PubMed] [Google Scholar]
- 48.Odermarsky M, Andersson S, Pesonen E, Sjoblad S, Yla-Herttuala S, Liuba P. Respiratory infection recurrence and passive smoking in early atherosclerosis in children and adolescents with type 1 diabetes. European Journal of Clinical Investigation. 2008;38:381–388. doi: 10.1111/j.1365-2362.2008.01952.x. [DOI] [PubMed] [Google Scholar]
- 49.Molnar D. The prevalence of the metabolic syndrome and type 2 diabetes mellitus in children and adolescents. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S70–74. doi: 10.1038/sj.ijo.0802811. [DOI] [PubMed] [Google Scholar]
- 50.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]