Abstract
Studying the mechanisms of host survival resulting from viral encephalitis is critical to the development of vaccines. Here we have shown in several independent studies that high-dose treatment with neutralizing antibody prior to intranasal infection with Venezuelan equine encephalitis virus had an antiviral effect in the visceral organs and prolonged survival time of infected mice, even in the absence of alpha beta T cells. Nevertheless, the antibody treatment did not prevent the development of lethal encephalitis. In contrary, the adoptive transfer of primed CD4+ T cells is necessary to prevent lethal encephalitis in mice lacking alpha beta T cell receptor.
Keywords: Vaccine, Venezuelan equine encephalitis virus, animal models
1. Introduction
Studying the mechanisms of pathogen clearance and host survival resulting from viral encephalitis is critical to the development of vaccines and drug therapies against central nervous system (CNS)-invading zoonotic pathogens. Several alphaviruses (genus Alphavirus; family Togaviridae), namely eastern, western and Venezuelan equine encephalitis viruses (VEEV), cycle between mosquito vectors and vertebrate hosts and cause lethal encephalitis in horses and potentially humans [1]. Venezuelan equine encephalitis virus (VEEV) is a zoonotic alphavirus with multiple subtypes that has been responsible for significant morbidity and mortality during epizootic outbreaks in equines and humans [2]. During the most recent outbreak in South America in 1995, approximately 100,000 human cases of infection were reported with over 300 clinical cases diagnosed [3]. In addition to being a naturally emerging/re-emerging pathogen, VEEV can be transmitted via aerosol route, is therefore a potential biothreat agent, and work with this agent must be performed at Biosafety Level 3. Vaccines to limit infection and/or fatal encephalitis are currently under development using a variety of approaches [4–6]. The live attenuated vaccine strain, TC-83 was developed over four decades ago by serial passaging of the Trinidad Donkey (TrD) VEEV strain in guinea pig myocytes and remains the only available vaccine for humans (United States Food and Drug Administration Investigational New Drug status). However, residual pathogenicity has been a concern with this strain [4, 7, 8]. Recent new generation live-attenuated vaccines that incorporate the use of alphavirus vectors expressing VEEV proteins have been shown to be both safe and effective in protecting mice and hamsters from lethal disease [4, 9–11].
Various animal models have been used to examine the distinct phases of infection, including the development of VEEV-induced encephalitis. Both hamster and mouse models have been used to study the pathogenesis of the virus and the host immune response as well as to evaluate vaccine efficacy [12–14]. Infection in the mouse by peripheral (subcutaneous) route of exposure, which mimics natural infection, results in a biphasic disease pattern in which the virus initially replicates in lymphoid tissue and ultimately progresses into the CNS [4, 7, 9]. In contrast, intranasal (i.n.) and intracranial (i.c.) challenge result in earlier CNS infection. Once CNS infection is established, acute meningoencephalitis with neuronal cell death follows, which is uniformly fatal [12–14].
In previous studies, we utilized a variety of approaches, e.g., both loss and gain of function in the T cell compartment and loss of function in the B cell compartment, to investigate several specific immune effector mechanisms that may contribute to the host response to VEEV [10]. Specifically, we utilized inbred mice with selective immunodeficiencies in the T and B cell compartments (Suppl. Table 1). Our studies indicated that in contrast to γδTCR KO mice, αβ TCR KO mice are not protected from lethal encephalitis following i.n. VEEV challenge [10]. In addition, to assess the importance of virus-specific antibody response, we utilized mice deficient in mature B cells (μMT strain, mice with disruption in the IgM heavy chain) [15]. A minor proportion of μMT mice survive lethal challenge infection following vaccination suggesting that antibody is not absolutely required for protection from VEEV-mediated lethal encephalitis [10].
To test this hypothesis we have performed a variety of experiments utilizing passive antibody transfer in this model. Here we show in several independent studies that the systemic, high-dose treatment with neutralizing antibody prior to intranasal infection with VEEV had a profound antiviral effect in the visceral organs and prolonged survival time of infected mice even in the absence of αβ T cells. Nevertheless, the antibody treatment did not prevent the development of lethal encephalitis in this model.
Based on these findings, we further proposed that primed CD4+ or CD8+ T cells would enhance antiviral defense and promote survival if transferred into naïve mice. Accordingly, in several independent studies we performed adoptive transfer of CD4+ and CD8+ T cells into αβ T cell KO mice prior to challenge with VEEV. Transfer of CD4+ T cells, but not CD8+ T cells, resulted in protection from lethal encephalitis. The survival correlates with influx of CD3 positive cells into brains and reduction of infection to a level below the detection limit by day 28 after infection.
We believe that our data may have direct implications for vaccine design aimed at protecting against lethal VEEV upon intranasal delivery or, potentially, aerosol exposure. In addition, this is a useful model to study the acute response to VEEV infection of the brain as well as later repair process.
2. Methods
2.1. Mice
Animal studies were approved by the Institutional Animal Care and Use Committee at UTMB and were carried out according to National Institutes of Health (NIH) guidelines. The following B6 mouse strains were purchased from the Jackson Laboratories (Bar Harbor, ME): C57BL/6 (WT B6) [16], and αβ T cell KO (strain B6.129P2-Tcrbtm1Mom/J) [17]. The characteristics of these mice, as well as the strain abbreviations used in this study, are shown in Suppl. Table 1. Mice were housed for 3–7 days until immunized. Immunization procedures and VEEV challenge was performed in the UTMB ABSL2 and —3 facility, respectively. Telemetric transponders were used to identify animals and to measure body temperature as follows. Animals were anaesthetized with isoflurane (5%) and implanted subcutaneously with BMDS IPTT-300 transponders (BMDS, Seaford, Delaware), using a trocar needle assembly and monitored for signs of infection or migration of transponder. Transponders were then scanned using a DAS-6007 transponder reader (BMDS) and digital data was downloaded in accordance with the manufacturer’s protocol.
2.2. Viruses
All work with the virulent VEEV ZPC738 strain and the attenuated recombinant alphavirus, SIN/ZPC strain was approved by institutional (UTMB, Galveston, Texas) and federal agencies (US Centers for Disease Control and Prevention/US Department of Agriculture/Department of Defense). SIN/ZPC: The chimeric live-attenuated virus, SIN/ZPC, is a vaccine candidate that has been evaluated for safety and efficacy in mice and hamsters [4, 10, 11]. SIN/ZPC encodes the replicative machinery from Sindbis (SIN) virus, and the structural genes from the virulent VEEV strains, ZPC738. Work with SIN/ZPC was performed in UTMB’s A/BSL-2 facility; VEEV: The challenge VEEV strain used in this study, ZPC738, is a 1997 sentinel hamster isolate from Venezuela [18]. It is an enzootic subtype ID strain that is virulent in mice and in hamsters and has been used extensively in VEE pathogenesis studies (Genbank Accession No. AF100566) [18, 19]. Work with ZPC738 was performed in UTMB’s ABSL-3 facility. Virus stock for both SIN/ZPC and ZPC738 were obtained as previously described [4, 10, 11]. Briefly, infectious virus was prepared by electroporation of BHK-21 cells with viral RNA obtained from SP6-promoter driven in vitro transcription of the respective infectious clone (Invitrogen, Carlesbad, NJ). Virus was then harvested at 24 h following electroporation [20].
2.3. Passive transfer of VEEV HIAF
Three independent trials were performed (HIAF Trial 1–3). HIAF treatment time points are defined relative to VEEV challenge, as day 0. Procedures were performed as follows. Female WT or αβ TCR KO (αβ TCR KO) mice were inoculated via i.p. route with 50–100 μl of VEEV (TC83) mouse HIAF (kindly provided by Dr. Robert Tesh, UTMB Arboviral Collection ID# T-35490) 9 days prior to VEEV challenge (day -9). As controls, the corresponding age- and sex-matched mouse strains were left untreated; “WT/challenge only” or “αβ TCR KO/challenge only”. All mice were challenged with virulent VEEV (ZPC738) via i.n. route with a dose of 3.5 × 102 – 8 × 103 PFU per animal. For each independent trial, the delivered dose, based on back-titration of the viral inoculum, as well as the number of animals per group (N) and age of mice are indicated in figure legends. In the first WT trial, WT/HIAF treated/challenged (N=6) and WT/challenge only (N=1) animals that were paralyzed were euthanized and organs were collected for evaluation of tissue viral load via plaque assay. In the second WT trial, the viral load of tissues obtained on day +4 from randomly preselected WT/HIAF treated/challenged (N=4) and WT/challenge only (N=4) was assessed. In the αβ TCR KO trial, the viral load of tissues obtained on day +5 from randomly preselected αβ TCR KO/HIAF treated/challenged (N=2) and αβ TCR KO/challenge only (N=1) was assessed.
2.4. Quantitation of infectious virus in organs
Organs were collected, homogenized in serum-free minimal essential medium (MEM) with antibiotics in a 10% suspension, and maintained at −80°C. Viral replication in the brains and peripheral organs was assessed by standard plaque assay [21]. Titrations were performed in duplicate and the average is reported.
2.5. VEEV neutralizing antibody
VEEV neutralizing antibody was assessed via PRNT using serum samples obtained at time points indicated in figure legends. Prediluted (1:20) individual mouse serum was heat-inactivated at 56°C for 30 min. Two-fold dilutions of each serum sample in MEM/1% FBS were mixed in duplicate with an equal volume with VEEV TC83 virus (25 PFU) and incubated (5% CO2/37oC/1 h). Confluent Vero monolayers were then incubated with 100 μl of each mix as above. Inocula were aspirated, 0.5% agarose overlay added, and incubated for 48 h. At the end of the incubation period, cells were fixed (30 min/room temperature) with 10% formalin/PBS and stained with 0.5% crystal violet [21]. The titer was recorded as the reciprocal of the serum dilution corresponding to an endpoint of 50% plaque reduction.
2.6. T cell transfer studies
Four independent trials were performed (T Cell Transfer Trial 1–4). Donor mice immunization time points are defined relative to T cell isolation day. Recipient mice immunization time points are defined relative to VEEV challenge, as day 0. The age of recipient mice is provided in the respective figure legend.
2.6.1. Immunization of T cell donor mice
WT mice were inoculated subcutaneously (s.c.) with SIN/ZPC (5 × 105 PFU) [4, 9, 10, 22], according to the following schedules: A) two vaccinations (trial 1, 2, and 3): 35 and 14 days prior to isolation or B) three vaccinations (trial 4): 98, 84 and 70 days prior to isolation. For all four trials, CD4+ and CD8+ T cells were isolated from immunized donor mice 14 days after delivery of the last SIN/ZPC dose [23]. Briefly, 30 donor spleens were removed and single cells suspensions generated by mechanical disruption. Splenocytes were fractionated over Lympholyte-M (Accurate Chemicals, Westbury, NY). The cells were washed three times in MACS buffer (PBS, pH 7.4 supplemented with 0.5% bovine serum albumin (BSA), 2 mM EDTA) and CD4+/CD8+ T cells were separated by magnetic sorting-based negative selection AutoMacs, Miltenyi Biotec; Auburn, CA). Purity was confirmed by immunostaining and flow cytometric analysis prior to adoptive transfer, which was performed on the same or subsequent day. Immunostaining of T cell subpopulations isolated via AutoMacs was performed as previously described [4, 9, 10, 22].
2.6.2. Immunization, T cell transfer and challenge of T cell recipient mice
Recipient WT or αβ TCR KO mice (four to six week old females) were immunized with SIN/ZPC at a dose of 5 × 105 PFU per animal (each vaccination) at the following time points: trial 1) single dose: day -14; trial 2) two doses: day -35 and -14; trial 3) three doses: day -49, -35 and -14; trial 4) three doses: day -44 (or -35 for WT control), -30, and -16. On day 0, mice were challenged via i.n. route with 4 × 105 PFU of VEEV ZPC738 per animal in 40 μl of PBS. Mice were observed daily up to 28 days for clinical illness (anorexia, encephalitis, and/or paralysis) and/or death. Body temperature measurements were recorded via telemetry (see “Mice”). The group size (N) is indicated in the figure or figure legend.
In T cell transfer trial 4, euthanasia and organ collection was performed on randomly preselected animals (day +5) in two of the groups: 1) αβ TCR KO recipients receiving WT CD4+ T cells (N=3), and 2) WT recipients that did not receive T cells (N=3). In addition, organs were collected from mice that died or developed severe disease (clinical encephalitis or paralysis) on day +5. Viral titration or tissue examination (histopathological analysis and immunofluorescent analysis) was performed. Adoptive transfer was performed 2–5 days prior to the first vaccination using i.p. route to deliver 5 × 107 CD4+ or CD8+ T cells per animal. As controls, age- and sex-matched mice were inoculated in parallel with an equivalent volume of diluent (PBS) alone.
2.7. Histopathology and immunofluorescence
Brains were fixed in 10% buffered formalin for 48 h, stored in 70% ethanol, and embedded in paraffin, sectioned (5 μm), and mounted on slides for hematoxylin and eosin (H&E) staining. Immunoflourescence staining for T or B cell and astrocyte markers was performed. All procedures were performed at room temperature. Briefly, brain sections (4–5 μm) were deparaffinized and incubated with 2.5% normal goat serum in PBS for 15 min to block non-specific binding. Sections were incubated with antibody to glial fibrillary acidic protein (GFAP, 1:200, rabbit anti-human IgG, Sigma), CD3 (T cell marker; 1:40, rabbit anti-human CD3, DakoCytomation) or B220 (B cell marker; 1:25, rat anti-mouse CD45R, BD Pharmingen) for 1 h. After washing (PBS/0.1% Tween 20), positively labeled cells were detected by Alexa 594 conjugated GFAP or CD3 (both at 1:200). For B220, biotinylated goat anti-rat secondary antibody (1:20, Invitrogen) was used, followed by detection with Alexa 594 conjugated streptavidin (1:200, Invitrogen). All sections were counter stained with DAPI (Invitrogen) and images collected using an Olympus XL71/DP70 fluorescence microscope/digital camera system.
2.8. Statistical analysis
Statistical analysis of survival for all groups over the entire monitoring period was performed using logrank test at a significance level of α<0.05 in GraphPad® Prism (San Diego, CA). Pairwise comparison of the survival proportions of recipients of CD4+ or CD8+ T cells with recipients that received PBS (mock transfer) was performed using Fisher’s Exact Test at a significance level of α<0.05 in GraphPad® Prism.
3. Results
3.1. Passive immunization with high concentrations of VEEV HIAF prolongs survival but does not protect mice against lethal VEE
3.1.1. Experimental design
Prior studies of live-attenuated virus-based protection from lethal i.n. challenge with VEEV in mice with selective immunodeficiencies in the T and B cell compartments suggested that the contribution of VEEV-specific antibody to protection from lethal VEEV challenge may not be as profound in this model as previously reported for peripheral challenge models [10]. To further assess this possibility, a series of studies was performed to evaluate the survival benefit provided by passive transfer of VEEV HIAF to WT mice 9 days prior to VEEV challenge (day -9). As a positive control for disease development, we used age- and sex-matched mice that were mock (PBS) treated, and challenged in parallel. Initially, in a small pilot study, an arbitrary HIAF dose of 50 μl per mouse was tested (HIAF Trial 1, Fig. 1A). First, to evaluate the level of protection from lethal VEEV, daily monitoring of survival and disease development, including telemetric temperature and weight measurements, was carried out. Secondly, to assess the level of VEEV neutralizing antibody, serum was collected from mice 24 h (day -1) prior to VEEV challenge (8 days following transfer) and PRNT was performed (data not shown). Finally, to evaluate the effect of HIAF treatment on the level of infectious virus in the brain and peripheral organs, standard plaque assay was performed on tissues collected from animals that developed severe clinical disease, e.g., paralysis.
Fig. 1.
Survival of WT mice following passive transfer of VEEV HIAF and subsequent challenge with virulent VEEV. A) WT mice were treated nine days prior to challenge (day -9) with 50 μl (HIAF Trial 1, panel A) or 100 μl (HIAF Trial 2 and 3, panel B and C) of VEEV HIAF via i.p. route, or, in parallel, as controls, age- and sex- matched WT mice were left untreated. On day 0, all mice were challenged with virulent VEEV (ZPC738) via i.n. route with a dose of 8 × 103 PFU per animal. Survival and disease were monitored daily and mice that developed paralysis were euthanized and recorded as “dead” on the subsequent day. The following outcomes are presented: survival (i), and organ titers of paralyzed mice (ii). Organ titrations: Aii) WT/HIAF treated (day -9)/challenged (N=6) animals were euthanized and organs were collected for evaluation of tissue viral load via plaque assay. Bii) At 4 days post infection (day +4), randomly preselected animals (N=4 per group) were euthanized and organs were collected for evaluation of tissue viral load via plaque assay. Dots (●) represent titer values for individual animals.
3.1.2. HIAF Trial 1
Passive transfer of this HIAF dose into immunocompetent mice lead to a statistically significant increase in survival time from a median of 8 days for the WT/HIAF treated group in comparison to 5.5 days for the placebo (PBS-treated) control (logrank test, p=0.0009, Fig. 1Ai). Nevertheless, all HIAF treated animals succumbed to infection within 12 days (0% survival, 0/8 mice), indicating that additional factors are of importance for the development of acute lethal encephalitis. The body temperature and weight trends of animals in the treated versus mock-treated groups mimicked the survival pattern and no significant differences in the average percent change in temperature or weight (relative to baseline) were detected between groups (data not shown). Despite the failure of this HIAF treatment to provide a long term survival benefit, all mice were PRNT positive 24 hrs prior to challenge, with PRNT50 titers >640 (100%, 8/8). Virus levels were higher in the brain than in the peripheral organs (Fig. 1Aii) but overall, were not largely diminished relative to what is observed in WT untreated animals [10].
3.1.3. HIAF Trial 2
A second trial was performed using a similar experimental format, but at a higher HIAF treatment dose of 100 μl total volume (HIAF Trial 2, Fig. 1B). In addition to the daily monitoring of death (Fig. 1Bi), disease development, telemetric monitoring (data not shown), and PRNT (Table 1), randomly pre-selected animals (N=4 per group) were euthanized at an early time point (day +4) for evaluation of VEEV titers in the organs (Fig. 1Bii). This higher dosage of HIAF provided a statistically significant increase in survival time following VEEV challenge (Fig. 1Bi), with median survival of 9.5 days in comparison to 5 days for the WT untreated (logrank, α=0.05; p=0.0082), as for the first trial performed at a lower dose (Fig. 1Ai). Although 25% (1/4) of the WT treated mice survived challenge, this proportion was not significantly different from that of WT untreated (0%, 0/4; Fisher’s exact test, p=1.00). On day -6 and day 0, the geometric mean PRNT50 titer was 2560 in WT/HIAF treated mice; 100% (8/8 on each day) were positive (Table 1). On both days, PRNT titers for individual WT/HIAF treated mice were ≥1280. As expected, none of the WT untreated mice were PRNT-positive; titers were <20 in 100% (8/8) of these mice. The presence of a relatively high serum neutralizing antibody titer was not correlated with protection. This is surprising, given that these titers were relatively higher than the titers for wild type (C57BL/6) SIN/ZPC vaccinated mice, which are in the range of 20–80, as 100% of vaccinated mice survive subsequent challenge (Paessler, unpublished data). Overall, the largest difference in viral titer on day +4 between WT HIAF treated and WT untreated mice was evident in the periphery (Fig. 1Bii). In the brain, WT/HIAF treated mice, on average, had a minimal decrease in the viral titer (<1 log10 PFU/g), whereas in the spleen, lung and liver had 2.8, 3.5, and 4.5 log10 decrease, respectively, relative to the WT untreated group.
Table 1.
VEEV neutralizing antibody levels for pre-challenge sera in HIAF Trial 2.
| PRNT50 (Day -6) |
PRNT50 (Day 0) |
|||||
|---|---|---|---|---|---|---|
| Group description | No. positive/total tested | Percent positive (titer≥20) |
Geometric mean titer | No. positive/total tested | Percent positive (titer≥20) |
Geometric mean titer |
| WT/HIAF treated/ (challenged)a |
8/8 | 100% | 2560 | 8/8 | 100% | 2560 |
| WT/ (challenged only)a |
N.D. | N.D. | N.D. | 0/8 | 0% | 1 |
VEEV-specific antibody response was assessed by PRNT of individual sera samples in HIAF Trial 2 (described in Fig. 1B). For PRNT values that were below the limit of detection (<20), an arbitrary value of 1 was used for calculation of the geometric mean titer, presented by group. The titer is reported as the reciprocal of the serum dilution corresponding to an endpoint of 50% plaque reduction.
(), procedure to be performed;
N.D., not done.
3.1.4. HIAF Trial 3
To further examine the potential contribution of the inflammatory response in diminishing the protective capacity of antibody-mediated protection and to test the antibody effect in absence of the majority of T cells, a passive antibody transfer study was performed in αβ T cell KO mice, as described for the second trial in WT mice (HIAF Trial 3, Fig. 1C). Daily monitoring of death (Fig. 1Ci), disease development, telemetric monitoring, and PRNT (data not shown) was performed. In addition, the level of infectious virus in the organs of randomly preselected animals (day +5) was determined (data not shown). Survival outcome in HIAF treated αβ T cell KO mice (Fig. 1Ci) was identical to that of WT mice when treated at the same dose (Fig. 1Bi); 25% (Fisher’s exact, p=1; not significant); median survival time of 9.5 days (logrank test, α=0.05; p=0.0159, statistically significant) in comparison to WT untreated group, among which none survived and median survival time following challenge was 6 days. All HIAF treated αβ T cell KO mice were PRNT positive (100%, 6/6), with geometric mean titers of 2560 and 1810 on day -6 and 0, respectively; as expected none of the untreated (challenge only) αβ T cell KO mice had detectable antibody levels prior to VEEV infection (data not shown). In the brains, there was little difference in the organ titers between the αβ TCR KO treated mice and the αβ TCR KO untreated mice; in the periphery, a marked difference in the lung, spleen and liver titers was observed (data not shown). This trend in organ viral load was similar to that of the second WT mouse trial (shown in Fig. 1Bii).
3.1.4. Summary of HIAF results
The results from these studies clearly indicate the importance of neutralizing antibody in controlling the infection of visceral organs while it has a minimal effect on the outcome of established brain infection. Our data also indicates that the host benefits from this antibody treatment in the presence or absence of naïve αβ T cells, as shown by prolonged survival time and reduced peripheral infection in αβ T cell KO mice.
3.2. CD4+ but not CD8+ T cells are responsible for mediating protection from lethal encephalitis elicited by vaccination with a live-attenuated vaccine
3.2.1. Experimental design
In order to further define the effector and memory cell population(s) responsible for the protection induced by vaccination, splenic CD4+ and CD8+ T cells from vaccinated WT mice were sorted to relatively high purity (>95%) and transferred into αβ TCR KO mice (data not shown). To elicit further expansion of VEEV-specific T cells, recipient mice were vaccinated one, two or three times following transfer of the respective T cell subset (CD4+ or CD8+). Subsequently, mice were challenged with virulent VEEV (ZPC738) and survival was monitored daily. The results from T cell transfer experiments are shown in Fig. 2, representing the survival curves of mice receiving T cells from recipients receiving one (trial 1, Fig. 2A), two (trial 2, Fig. 2B) or three (trial 3, Fig. 2C) vaccinations.
Fig. 2.
Survival of α β TCR KO mice following T cell transfer from vaccinated WT donor, vaccination with SIN/ZPC, and subsequent challenge with VEEV. Naïve αβ TCR KO mice were injected via i.p. route with 5 × 107 T cells isolated by negative selection from spleens of vaccinated WT mice, and the purity assessed by immunostaining/flow cytometry (FACS), as described in Materials and Methods. FACS results are described in the text. As controls, mock transfer was performed in age-matched mice in parallel using PBS in identical volume. Two to five days after adoptive transfer of T cells (or mock transfer), recipient mice were immunized once (day -14, panel A), twice (day -35 and -14, panel B) or three times (day -49, -35 and -14, panel C & D) with SIN/ZPC (s.c., 5 × 105 PFU per mouse). Four weeks following the final vaccination, all animals were challenged i.n. with VEEV ZPC738 (4 × 105 PFU/animal). Survival curves were evaluated via logrank test (α=0.05) and the p-values are indicated on the bottom left of each graph. Asterisk (*) indicates a statistically significant result. Survival proportions of CD4+ or CD8+ transfer group was compared to the mock-transfer group via Fisher’s exact test and the p-values are indicated to the right of each curve. Double asterisk (**) indicates statistically significant results. For comparisons that were not significant (“ns”), no p-value is shown.
3.2.2. T cell transfer trial 1–3
In the first trial (Fig. 2A), transfer of CD8+ T cells followed by a single vaccination dose only provided minimal protection (20% survival, 1/5 mice), which was not significantly different from control group (logrank test, α=0.05: p= 0.6221; Fisher’s exact test,α =0.05: not significant). No protection was provided for mice receiving CD4+ T cells (0/10 mice). The time course of death for mice receiving either CD4+ or CD8+ T cell subset was similar (8 and 7 days, respectively) to that of the vaccinated αβ TCR KO mice receiving PBS (mock transfer, median survival of 7 days), and is similar to that of unvaccinated WT mice, as previously reported [10]. In the second trial (Fig. 2B), no protection was provided to recipients receiving either T cell subset when followed by two vaccination doses and the time course of death was identical to that of mock (PBS)-transferred mice. In contrast, three vaccinations (trial 3, Fig. 2C) augmented the protection to 80% (8/10) for mice receiving CD4+ T cells (statistically significant; logrank, α=0.05: p=0.0163; Fisher’s exact test, α=0.05: p=0.007), while only 20% (2/10) of those receiving the donor CD8+ T cells (Fisher’s exact test, α=0.05: p=0.5238) survived. Thus, the time course of death for mice receiving CD8+ T cells was similar to that of mock-transferred mice.
3.2.3. T cell transfer trial 4
A fourth independent trial was aimed at confirming our previous results (T Cell Transfer Trial 4, Fig. 2D), CD4+ cells were isolated by negative selection from WT mice and were transferred into susceptible αβ T cell KO mice, followed by delivery of three doses of the chimeric vaccine. A statistically significant survival benefit of 83% (10/12) was observed for αβ T cell KO mice receiving CD4+ T cells from WT mice (logrank, α=0.05: p<0.0001; Fisher’s exact test, α=0.05: p=0.0002). This survival benefit was consistent with the results of the third T cell trial (shown in Fig. 2C) and confirms the reproducibility of the effect provided by this cell population in our model.
For this fourth T cell trial, VEEV-specific antibody response 24 h prior to VEEV challenge was evaluated by PRNT performed on individual mouse serum samples (Table 2). Eighty percent (12/15) of the vaccinated αβ T cell KO mice in the group designated to receive CD4+ T cells from WT donors were PRNT-positive 24 hours prior to challenge (titer >1:20); the geometric mean serum titer was 17 (titers of positive samples ranging from 20 to 160). As expected, none (0/9) of the mock transferred αβ TCR mice were PRNT-positive.
Table 2.
VEEV neutralizing antibody levels for pre-challenge sera samples in T cell transfer trial 4
| Group description | PRNT50 (Day -1) |
||||
|---|---|---|---|---|---|
| Recipient strain | T cell donor strain | No. positive/total tested | Percent positive (titer≥20) |
Titer range | Geometric mean titer |
| αβ TCR KO | WT | 12/15 | 80% | 20 – 160 | 17 |
| αβ TCR KO | None | 0/9 | 0% | - | 1 |
VEEV-specific antibody response was assessed by PRNT of individual sera samples in T Cell Transfer Trial 4 (described in Fig. 2D) obtained 24 hours (day -1) prior to VEEV challenge. The titer is reported as the reciprocal of the serum dilution corresponding to an endpoint of 50% plaque reduction. For PRNT values that were below the limit of detection (<20), an arbitrary value of 1 was used for calculation of the geometric mean titer, presented by group. a(), procedure to be performed; bN.D., not done.
These results confirm that VEEV-specific CD4+ T cells are the primary cell population responsible for protection from lethal encephalitis.
3.2.4. Virus level in organs
To measure the antiviral effect in the periphery as well as in the CNS, we have compared the levels of infectious virus in the brain and peripheral organs (liver, lung and spleen) on day +5 for randomly pre-selected mice that had received CD4+ T cells from WT mice (αβ TCR recipients/WT CD4+, N=3), among which none of the animals had clinically apparent disease (Fig. 3). As controls, we evaluated the virus titers from WT vaccinated mice that did not receive any T cells (WT/no transfer, N=3). For comparison, the VEEV titers in the organs of mice that developed severe disease (clinical encephalitis and/or paralysis) and died on day +5 were evaluated for αβ TCR KO mice that received PBS (αβ TCR recipients/mock, N=1, used as a control for virus titration, data not shown).
Fig. 3.
Infectious virus in organs of α β TCR KO mice following T cell transfer from vaccinated WT donors, vaccination with SIN/ZPC, and subsequent challenge with VEEV. The experimental design is described in Fig. 2, T cell transfer trial 3. Randomly preselected animals were euthanized on day +5 for organ titration of two of the groups: 1) αβ TCR KO recipients receiving WT CD4+ T cells, and 2) WT (B6) that did not receive T cells, but were vaccinated (day -44, -35 and -16) and challenged as described for the T cell transfer experiment (“No transfer/WT”). For comparison, the VEEV titer in the organs of one αβ TCR KO mouse that received PBS only (mock/αβ TCR recipients) and that developed severe disease and died on day +5 is presented (upper right, grey box). Virus levels were determined by standard plaque assay [9]. The group size, N, is indicated along the x-axis. Histopathological and immunofluorescent analysis was also performed, and is shown in Fig. 4.
Overall, the VEEV levels in the brains of αβ TCR KO mice were not substantially reduced by CD4+ transfer and reached levels of 7.5–8.5 log10 PFU/g (Fig. 3C). Thus, high titers were observed irrespective of whether these two groups of mice received CD4+ T cells or not. On average, the titer for the αβ TCR KO/WT CD4+ and the WT/no transfer groups were identical (7.6 log10 PFU/g). In the peripheral organs, the trend among the groups was comparable. In the lungs, the VEEV levels among αβ TCR KO mice that received WT CD4+ cells were similar to levels of the WT mice (vaccinated) that did not receive CD4+, e.g., in the range of 2.6–4.3 log10 PFU/g; on average, 3.6–3.8 log. In the former group, virus was undetectable in 1/3 mice. In contrast, lung titers were higher for the αβ TCR KO/mock mouse; in the range of 5.2–6.7 log10 PFU/g; on average 6 log. In the spleen, the average titer was 2.9 and 1.7 log, respectively for the αβ TCR KO/WT CD4+ and the WT/no transfer groups, with no detectable titer in 2/3 (67%) of the mice in either of these groups. Again, the titers were ~3–5 log higher (in the range of 5.7–7.6 log) for the αβ TCR KO/mock mouse. In the liver, the difference in titer between groups was less striking. The average titer was 3.5 log (2/3, 67% undetectable) and 2.3 log (1/3, 33% undetectable), respectively for the αβ TCR KO/WT CD4+ and the WT/no transfer groups. Titers were ~1–3 log higher (in the range of 4.8–6.8 log) for the αβ TCR KO/mock mouse.
These results (Fig. 3C), in combination with the group survival patterns (Fig. 3A), suggest that CD4+ T cells from WT mice have strongly (up to 10,000 fold) reduced virus titers in the peripheral organs, but not in the brain. These results strongly suggest that VEEV-specific CD4+ T cells are the primary cell population responsible for protection from lethal encephalitis elicited by vaccination with this chimeric live-attenuated vaccine.
3.3. Early influx of CD3+ T cells into infected brains correlates with protection
To evaluate potential qualitative differences that could account for the alternative survival outcomes among the adoptive transfer groups from T cell transfer trial 4 shown in Fig. 3, we performed H&E and immunostaining for T cell (CD3) and B cell (B220) markers on tissues that were obtained from randomly preselected animals euthanized on day +5 for organ titration (Fig. 4) and from sick animals euthanized on day +11 (data not shown).
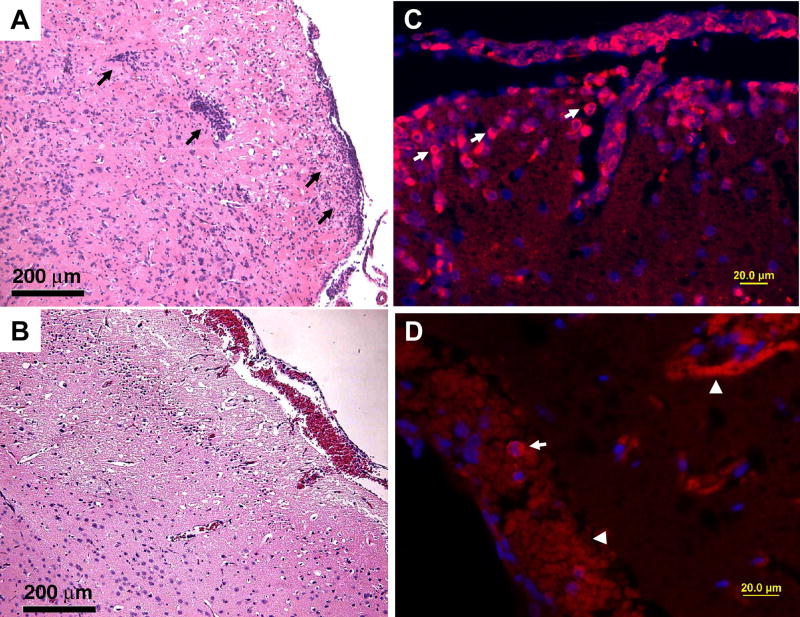
Fig. 4.
WT CD4+ T cells restore the inflammatory process in αβ TCR KO mice at day 5 post infection. Naïve αβ TCR KO mice were injected via i.p. route with 5 × 107 CD4+ T cells isolated by negative selection from spleens of vaccinated WT mice (T cell Trial 4). As controls, mock transfer was performed in age-matched mice in parallel using PBS in identical volume. Five days after adoptive transfer of T cells (or mock transfer with PBS alone), recipient mice were immunized with three doses of SIN/ZPC on day -44, -30, and -16. At 4 weeks following the final vaccination, all animals were challenged i.n. with VEEV ZPC738 (4 × 105 PFU/animal) and monitored daily for disease development and survival. The corresponding survival data is shown in Fig. 3A. Tissues were obtained from randomly preselected animals euthanized on day +5 for organ titration (shown in Fig. 3C), histopathology and immunofluorescence analysis. H&E and immunofluorescent staining of brain sections was performed as described in Materials & Methods. For the latter, sections were incubated with normal goat serum followed by antibody to glial fibrillary acidic protein (rabbit anti-human GFAP, Sigma), or CD3 (T cell marker, rabbit anti-human CD3, DakoCytomation). Alexa 594 conjugated secondary antibody was used for detection. All sections were counter stained with DAPI (Invitrogen) prior to fluorescence microscopy and capture of photographic images (Olympus XL71; DP70 digital camera). VEEV infection results at day 5 post infection are illustrated in H&E images (A to B) and in immunofluorescence (C to D). Three groups of mice were used in this experiment: αβ TCR KO mice transferred with CD4+ T cells from WT mice (A and C), and without transfer (B and D). Mice in C did not show a high level of mononuclear infiltration and karyorrhexis as seen in A, in which mononuclear cells defused into the brain parenchyma from vessels of cortex and meninge (arrows). The majority of infiltrated cells are identified as CD3 positive T cells in mice transferred with WT CD4+ T cells (arrows in C), and the ring-like stain is a typical feature in staining membrane molecules on T cells which have limited cytoplasm. Mice from the other group only showed few cells with CD3 positivity (arrows in D). Arrowheads in D point to autofluorescence on red blood cells which can be verified by their lack of nucleus.
3.3.1. Histopathology
The common finding in both groups of animals (αβ TCR KO mice transferred with CD4+ T cells from WT mice, or mice that did not receive cell transfer) involves the injury to neurons at the fronto-basolateral cortex at day 5 post infection, as shown in Fig. 4B. Mononuclear cell infiltration is mainly observed in the animals transferred with CD4+ T cells from WT mice and these cells infiltrated from both meninges and cortex blood vessels to the brain parenchyma (Fig. 4A). There is only minimal infiltration observed in mice without transfer (Fig. 4B). In addition, high levels of karyorrhexis are seen in the first group and the pattern is similar to that in brains of VEEV infected WT mice. Focal karyorrhexis is only localized in the fronto-basolateral cortex in the latter of the two groups, indicating minimal inflammation in the brain despite the development of more severe disease. For animals that survived for a longer time period (11 days post infection, the first group), macrophages, neutrophils and hypertrophic astrocytes were found in response to necrosis, suggesting that WT CD4+ T cells were able to restore inflammation development in these KO mice (data not shown).
3.3.2. Immunohistochemistry
To further characterize the types of mononuclear cells that infiltrated into the brain, we performed immunofluorescence using antibody to CD3 molecule, which is expressed in all T cells (Fig. 4), or antibody to CD45R (B220) molecule to detect B cells. The majority of infiltrating cells in the first group and very few cells around the meningeal blood vessels in the later two groups were labeled by CD3 antibody (Fig. 4C–D). However, in both groups there were only a few cells labeled by B220 antibody with no obvious differences between groups (data not shown). The limitations of using B220 and CD3 antibodies are that the B220 antibody is not able to detect plasma blasts and plasma cells [24], and that CD3 can not be used to differentiate CD4+ from CD8+ T cells; however, due to biosafety considerations, formalin fixation followed by paraffin embedding must be performed on our tissue sections. Antibodies to CD4/CD8 molecules were developed to be used in frozen sections, and do not work on these embedded samples.
4. Discussion
Protection from natural alphavirus infection is thought to rely partially on the induction of virus binding and neutralizing antibodies which may also require T-dependent helper activities conferred by CD4+ T cells [25, 26]. While virus neutralizing antibody is important to host survival and protection from natural (peripheral) challenge, our studies clearly demonstrate that even relatively high serum titers of neutralizing antibody achieved via passive transfer (not achievable with any vaccination known to authors) do not protect mice from intranasal (i.n.) VEEV challenge. We believe that virus neutralizing antibody plays a significant role in preventing the penetration of the CNS after subcutaneous or mosquito-borne challenge with VEEV, while it is ineffective in controlling the rapid onset of CNS disease following i.n. infection. In this case, the virus most likely immediately penetrates the brain via an olfactory route and the brain infection is detectable relatively early post exposure (<24 hours). Prior studies utilizing antibody treatment approach against i.n. VEEV challenge were also reported by Kinney et al. [27]. However, in contrast to the Kinney et al. study, we show a benefit for the WT host when the anti-VEEV polyclonal antibody is provided prior to challenge. The studies reported here show that polyclonal antiserum treatment reduces the viral titers in the visceral organs and significantly prolongs survival time, parameters that were not shown in the Kinney study. It is possible that the difference in outcome of these two different studies can be attributed to one or more of the following factors: different virus strain, different immune status and the strain of mice used, usage of polyclonal versus monoclonal antibody and the schedule of dosing. We further demonstrate, in experiments with animals lacking this T cell population, that the effect is independent of the presence of alpha/beta T cells.
Endogenous production of antibody may be required by resident plasma cells in the brain in order to neutralize virus, given the potential for lack of transport of antibody across the blood-brain barrier, even at peak points in viral infection, as shown with Sindbis infection [28, 29]. However, contrary to that reported for Sindbis infection, we have not observed any difference in influx of B cells, as detected by antibody to B220, a pan-B cell marker into the brains of various recipients (data not shown). Further, the antiviral effect in the first 5 days is almost completely absent, indicating the low level of virus neutralization in the brain. Future studies will be aimed at examining the functional effects of CD4+ T cells in brains of infected mice.
The role of CD4+ T cells appears to differ in our VEEV model, in contrast to studies of experimental autoimmune encephalomyelitis [30]. In our study, we demonstrated in two independent trials that the adoptive transfer of CD4+ T cells to αβ TCR KO mice conferred protection. It is intriguing to note that the cell transfer has no impact on virus growth in the brain and encephalitic and non-encephalitic mice have similar virus titers by day 5 post infection (time of encephalitic disease development), however, the animals that survive the challenge reduce the infection below the detection limit by day 28. Expansion of T cells is required, i.e. single immunization is not sufficient, to promote survival, although we can only speculate about the direct role of CD4 T cells using this experimental approach. Further studies in other immunodeficient strains are ongoing to assess the role of varying levels of antigen presentation and/or reduction in pathology in delaying death.
We believe that our results have direct implications for vaccine design aimed at protecting against lethal VEEV upon i.n. delivery or, potentially, aerosol exposure and have a bearing on historical approaches to defining efficacy. Our previous assumption that the induction of neutralizing antibody is sufficient to protect against VEEV should be reevaluated based on the findings presented here and additional studies are needed to better understand the role of CD4+ T cells in the pattern of neuroinflammation in VEEV infected brains. From these studies, we cannot conclude if CD4 T cells play a direct or indirect role (e.g., via provision of B cell help) in altering the progression of VEE in this model. This first step in dissecting the effector mechanisms in SIN/ZPC based immunity to and pathogenesis following VEEV challenge has allowed us to develop additional mechanistic hypotheses related to the role of CD4, which are suitable for in vitro and in vivo experimental approaches. Additional studies designed to evaluate the importance of ligation of CD40-CD40 ligand in VEEV challenge outcomes, as well as examination of the role of further distinct T cell subpopulation, i.e., regulatory T cells, are currently in progress. This model opens up the opportunity to study the reversal of the response to acute VEE that leads to a lethal outcome. Consequently, this enables us to study in mechanistic detail the repair process in the murine brain, which is of potential interest in a variety of infectious and non-infectious pathogenic processes.
Supplementary Material
Acknowledgments
We thank Dr. Gerald Campbell for providing his expertise and consultation on histopathology studies, Dr. Ilya Frolov for his critical review of the manuscript, Seth Linde for technical assistance with the animal studies and Jenna Linde for data entry and preparation of the manuscript figures. This work was supported by National Institutes of Health K08 Grant AI-059491 (to SP); and faculty start-up funding provided by the Institute for Human Infections and Immunity at UTMB.
Abbreviations
- αβ
alpha-beta (TCR)
- B6
C57BL/6
- BSL
biosafety level
- DC
dendritic cells
- GFAP
glial fibrillary acidic protein
- HIAF
hyperimmune ascitic fluid
- i.n
intranasal
- KO (−/−)
knockout
- N
sample (group) size
- PRNT
plaque reduction neutralization test
- TCR
T cell receptor
- VEEV
Venezuelan equine encephalitis virus
- WT
wild type
- UTMB
University of Texas Medical Branch
- min
minutes
- FACS
fluorescence activated cell sorting
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weaver SC. Host range, amplification and arboviral disease emergence. Arch Virol Suppl. 2005;19:33–44. doi: 10.1007/3-211-29981-5_4. [DOI] [PubMed] [Google Scholar]
- 2.Weaver SC, Salas R, Rico-Hesse R, Ludwig GV, Oberste MS, Boshell J, et al. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. Lancet. 1996;348:436–40. doi: 10.1016/s0140-6736(96)02275-1. [DOI] [PubMed] [Google Scholar]
- 3.Weaver SC, Ferro C, Barrera R, Boshell J, Navarro JC. Venezuelan equine encephalitis*. Annu Rev Entomol. 2004;49:141–74. doi: 10.1146/annurev.ento.49.061802.123422. [DOI] [PubMed] [Google Scholar]
- 4.Paessler S, Ni H, Petrakova O, Fayzulin RZ, Yun N, Anishchenko M, et al. Replication and clearance of Venezuelan equine encephalitis virus from the brains of animals vaccinated with chimeric SIN/VEE viruses. J Virol. 2006 Mar;80(6):2784–96. doi: 10.1128/JVI.80.6.2784-2796.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis NL, Brown KW, Greenwald GF, Zajac AJ, Zacny VL, Smith JF, et al. Attenuated mutants of Venezuelan equine encephalitis virus containing lethal mutations in the PE2 cleavage signal combined with a second-site suppressor mutation in E1. Virology. 1995;212(1):102–10. doi: 10.1006/viro.1995.1458. [DOI] [PubMed] [Google Scholar]
- 6.Charles PC, Brown KW, Davis NL, Hart MK, Johnston RE. Mucosal immunity induced by parenteral immunization with a live attenuated Venezuelan equine encephalitis virus vaccine candidate. Virology. 1997;228(2):153–60. doi: 10.1006/viro.1996.8381. [DOI] [PubMed] [Google Scholar]
- 7.Anishchenko M, Bowen RA, Paessler S, Austgen L, Greene IP, Weaver SC. Venezuelan encephalitis emergence mediated by a phylogenetically predicted viral mutation. Proc Natl Acad Sci U S A. 2006 Mar 28;103(13):4994–9. doi: 10.1073/pnas.0509961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahrling PB, Scherer F. Histopathology and distribution of viral antigens in hamsters infected with virulent and benign Venezuelan encephalitis viruses. Am J Pathol. 1973;72(1):25–38. [PMC free article] [PubMed] [Google Scholar]
- 9.Paessler S, Fayzulin RZ, Anishchenko M, Greene IP, Weaver SC, Frolov I. Recombinant sindbis/Venezuelan equine encephalitis virus is highly attenuated and immunogenic. J Virol. 2003 Sep;77(17):9278–86. doi: 10.1128/JVI.77.17.9278-9286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paessler S, Yun NE, Judy BM, Dziuba N, Zacks MA, Grund AH, et al. Alpha-beta T cells provide protection against lethal encephalitis in the murine model of VEEV infection. Virology. 2007 Jul 2;367(2):307–23. doi: 10.1016/j.virol.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni H, Yun NE, Zacks MA, Weaver SC, Tesh RB, da Rosa AP, et al. Recombinant alphaviruses are safe and useful serological diagnostic tools. Am J Trop Med Hyg. 2007 Apr;76(4):774–81. [PubMed] [Google Scholar]
- 12.Charles PC, Walters E, Margolis F, Johnston RE. Mechanism of neuroinvasion of Venezuelan equine encephalitis virus in the mouse. Virology. 1995;208(2):662–71. doi: 10.1006/viro.1995.1197. [DOI] [PubMed] [Google Scholar]
- 13.Ryzhikov AB, Tkacheva NV, Sergeev AN, Ryabchikova EI. Venezuelan equine encephalitis virus propagation in the olfactory tract of normal and immunized mice. Biomed Sci. 1991;2(6):607–14. [PubMed] [Google Scholar]
- 14.Ryzhikov AB, Ryabchikova EI, Sergeev AN, Tkacheva NV. Spread of Venezuelan equine encephalitis virus in mice olfactory tract. Arch Virol. 1995;140(12):2243–54. doi: 10.1007/BF01323243. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–6. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 16.Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002 Dec 5;420(6915):520–62. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 17.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992 Nov 19;360(6401):225–31. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 18.Wang E, Barrera R, Boshell J, Ferro C, Freier JE, Navarro JC, et al. Genetic and phenotypic changes accompanying the emergence of epizootic subtype IC Venezuelan equine encephalitis viruses from an enzootic subtype ID progenitor. J Virol. 1999;73(5):4266–71. doi: 10.1128/jvi.73.5.4266-4271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roehrig JT, Bolin RA. Monoclonal antibodies capable of distinguishing epizootic from enzootic varieties of Subtype I Venezuelan equine encephalitis viruses in a rapid indirect immunofluorescence assay. J Clin Microbiol. 1997;35(7):1887–90. doi: 10.1128/jcm.35.7.1887-1890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bredenbeek PJ, Frolov I, Rice CM, Schlesinger S. Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J Virol. 1993 Nov;67(11):6439–46. doi: 10.1128/jvi.67.11.6439-6446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zacks M, Dziuba N, Ni H, Frolov I, Campbell G, Yun N, et al. Persistence of attenuated variants of Venezuelan equine encephalitis virus (VEEV) in the murine brain. American Society of Tropical Medicine and Hygiene 55th Annual Meeting; 2006 November 12–16; Atlanta, Georgia, USA. 2006. p. 197. [Google Scholar]
- 22.Zacks MA, Paessler S. Alphavirus virus-based chimeric vaccines against encephalitic alphaviruses. Croatian Journal of Infection. 2007;27(4):155–60. [Google Scholar]
- 23.Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, Estes DM. Estrogen Receptor {alpha} (ER{alpha}) Deficiency in Macrophages Results in Increased Stimulation of CD4+ T Cells while 17{beta}-Estradiol Acts through ER{alpha} to Increase IL-4 and GATA-3 Expression in CD4+ T Cells Independent of Antigen Presentation. J Immunol. 2005 Nov 1;175(9):5716–23. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003 Oct;19(4):607–20. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 25.Phillpotts RJ, O’Brien L, Appleton RE, Carr S, Bennett A. Intranasal immunisation with defective adenovirus serotype 5 expressing the Venezuelan equine encephalitis virus E2 glycoprotein protects against airborne challenge with virulent virus. Vaccine. 2005 Feb 18;23(13):1615–23. doi: 10.1016/j.vaccine.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 26.Phillpotts RJ, Jones LD, Howard SC. Monoclonal antibody protects mice against infection and disease when given either before or up to 24 h after airborne challenge with virulent Venezuelan equine encephalitis virus. Vaccine. 2002 Feb 22;20(11–12):1497–504. doi: 10.1016/s0264-410x(01)00505-9. [DOI] [PubMed] [Google Scholar]
- 27.Kinney RM, Esposito JJ, Mathews JH, Johnson BJ, Roehrig JT, Barrett AD, et al. Recombinant vaccinia virus/Venezuelan equine encephalitis (VEE) virus protects mice from peripheral VEE virus challenge. J Virol. 1988 Dec;62(12):4697–702. doi: 10.1128/jvi.62.12.4697-4702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin D, Levine B, Tyor W, Ubol S, Despres P. The role of antibody in recovery from alphavirus encephalitis. Immunol Rev. 1997;159:155–61. doi: 10.1111/j.1600-065x.1997.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 29.Byrnes AP, Durbin JE, Griffin DE. Control of Sindbis virus infection by antibody in interferon-deficient mice. J Virol. 2000;74(8):3905–8. doi: 10.1128/jvi.74.8.3905-3908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard LM, Dal Canto MC, Miller SD. Transient anti-CD154-mediated immunotherapy of ongoing relapsing experimental autoimmune encephalomyelitis induces long-term inhibition of disease relapses. J Neuroimmunol. 2002 Aug;129(1–2):58–65. doi: 10.1016/s0165-5728(02)00175-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.