Abstract
Background
Neuregulin-1 (NRG-1) is a paracrine factor released by microvascular endothelial cells that has cardioprotective effects in animal models of heart failure. However, circulating NRG-1 has not been studied in human heart disease. We used a novel immunoassay to test whether circulating neuregulin-1β (NRG-1β) is associated with disease severity and clinical outcome in chronic heart failure.
Methods and Results
Serum NRG-1β was quantified in 899 outpatients in the Penn Heart Failure Study, a referral cohort representing a broad spectrum of systolic heart failure. Circulating NRG-1β was significantly elevated in patients with worse disease severity (NYHA Class IV median 6.2 versus 4.4ng/ml for Class I, p=0.002). In adjusted models, NRG-1β was independently associated with an increased risk of death or cardiac transplantation over a median follow-up of 2.4 years (adjusted HR 1.58 [95% CI 1.04–2.39, p=0.03] comparing 4th versus 1st NRG-1β quartile). Associations with outcome differed by heart failure etiology and symptom severity, with the strongest associations observed in patients with ischemic cardiomyopathy (interaction p=0.008) and NYHA Class III/IV symptoms (interaction p=0.01). These findings were all independent of BNP, and assessment of NRG-1β and BNP jointly provided better risk stratification than each biomarker individually in patients with ischemic or NYHA Class III/IV heart failure.
Conclusions
Circulating NRG-1β is independently associated with heart failure severity and risk of death or cardiac transplantation. These findings support a role for NRG-1/ErbB signaling in human heart failure and identify serum NRG-1β as a novel biomarker that may have clinical applications.
Keywords: Neuregulin, Heart Failure, Cardiomyopathy
Introduction
Virtually all forms of heart failure are characterized at the cellular level by abnormalities in growth and survival of cardiac myocytes. Animal studies have shown that the epidermal growth factor Neuregulin-1 (NRG-1) is a critical regulator of these processes. NRG-1 is released from microvascular endothelial cells and acts as a paracrine factor via the ErbB family of tyrosine kinase receptors expressed in cardiac myocytes to regulate myocyte differentiation and stress response.1–4 Transgenic mice with impaired NRG-1/ErbB signaling die from severe cardiac defects including a lack of ventricular trabeculation.5–7 In the adult animal, cardiac-specific mutations in NRG-1, ErbB4, or ErbB2 result in ventricular dilatation, reduced function, and premature death, worsened by increased afterload and anthracycline exposure.8–10 Conversely, enhancement of NRG-1 signaling via peripheral infusion of recombinant NRG-1β causes dramatic improvements in cardiac function and survival in animal models of cardiomyopathy.11 These data provide compelling evidence for a cardioprotective role of NRG-1/ErbB signaling, and suggest that circulating NRG-1β in particular may exert beneficial effects in the setting of heart failure.
Whether these findings translate to humans is unknown. Important insights come from the cardiotoxic side effects of the chemotherapeutic agent trastuzumab (Herceptin ®), a monoclonal antibody directed against ErbB2 (Her2) that blocks NRG-1/ErbB signaling and is used to treat receptor-positive breast carcinoma. Exposure to trastuzumab can induce a clinically significant cardiomyopathy that often reverses after discontinuation of therapy.12 This observation suggests that, as in animals, NRG-1 signaling has a protective function in the human heart, and loss of this pathway may contribute to heart failure. Despite these observations, the role of circulating NRG-1 in human heart disease has not been studied.
The objective of our study was to determine the relevance of circulating NRG-1 in human heart failure. We chose to study the NRG-1β isoform given its potent in vitro and in vivo effects on the cardiovascular system11, 13 and used a novel immunoassay to quantify NRG-1β levels in a large referral cohort of patients with a broad spectrum of systolic heart failure. We hypothesized that circulating NRG-1β would be independently associated with heart failure severity and transplant-free survival.
Methods
Study Population
The Penn Heart Failure Study (PHFS) is an ongoing prospective cohort study of outpatients with chronic heart failure recruited from the Penn Heart Failure and Transplantation program.14 The primary inclusion criterion is a clinical diagnosis of heart failure, and the cohort is comprised of patients with a broad spectrum of disease. Participants are excluded if they have a noncardiac condition resulting in an expected mortality of less than 6 months, as judged by the treating physician.
At time of study entry, detailed clinical data were obtained using questionnaires administered to the patient and treating physician, with verification via medical records. Variables such as NYHA Class and cardiomyopathy etiology (ischemic versus nonischemic) were determined by the physician based upon all available clinical data and according to standard heart failure clinical practice guidelines.15 Venous blood samples were obtained at enrollment and stored at −80°C.
Two-dimensional transthoracic echocardiography with Doppler color flow imaging was performed in all patients at an ICAEL-accredited laboratory within 30 days of blood sampling. Echocardiograms were performed by an experienced sonographer and clinical interpretation performed by a Level III-certified echocardiographer, with all personnel blinded to NRG-1β levels. Ejection fraction (EF) was visually estimated by the expert reader, according to standard clinical protocol.
Follow-up events including all-cause mortality and cardiac transplantation were prospectively ascertained every 6 months via direct patient contact and verified through death certificates, medical records, and contact with patients' family members. Of the 899 patients assessed, all but 2 patients had complete outcomes data available for analysis.
All participants provided written, informed consent, and the PHFS protocol was approved by our Institutional Review Board.
NRG-1β Assay
NRG-1β was measured from frozen, previously unthawed serum samples that had been stored at −80°C until time of assay. An indirect sandwich enzyme-linked immunosorbent assay (ELISA) was used (NRG1-β1 Duoset ELISA development system from R&D Systems, Minneapolis, MN). Assay technology was adapted for human samples, and all measurements were performed at Vanderbilt University (Nashville, TN). Serum was applied to plates that had been coated with mouse anti-human NRG1-β1 capture antibody. Following incubation, a biotinylated goat anti-human NRG1-β1 detection antibody was added to each well, and a streptavidin-HRP system was used for detection. The monoclonal antibody used in this assay detects a biologically active NRG-1β peptide fragment that is highly expressed in the cardiovascular system, and that activates the ErbB receptor and downstream signaling pathways in ventricular myocytes in vitro.13 Extensive validation was performed and there was no cross-reactivity with NRG-1α isoform or epidermal growth factor.
All samples were measured in duplicate, and the average of two values was used for analysis. The average intra-assay coefficient of variation (CV) was 5.6%, and the average inter-assay CV was 13%. The detection limits of the assay were 0.30 to 30ng/ml. Samples below the detectable limit (n=13) were assigned a value halfway between 0 and the lowest detectable limit of 0.30ng/ml (0.15ng/ml).16 Samples above the detectable limit at a 1:3 dilution (n=18) were assigned a value of 30ng/ml.17 In sensitivity analyses, we used an alternative approach of assigning samples below the lower limit of detection to a value equal to 0.30ng/ml and extrapolated those samples above the upper limit of detection. This had negligible impact on our results.
BNP Assay
The Architect™ BNP immunoassay from Abbott Diagnostics (Abbott Park, IL) was used to quantify BNP in peripheral plasma in the first 744 patients enrolled.14 The intra- and inter-assay CVs were 0.9 to 5.6% and 1.7 to 6.7%, respectively. The lower limit of detection of BNP was 10 pg/ml.
Statistical Analysis
The distribution of NRG-1β was skewed (Supplementary Figure 1), and the natural log- transformation of NRG-1β [Ln(NRG-1β] approximated a normal distribution. To determine cross-sectional associations between NRG-1β and clinical variables at time of study entry, linear regression was used with Ln(NRG-1β) modeled as the continuous, dependent variable. To determine the association between baseline NRG-1β level and risk of death or transplantation, Kaplan-Meier analysis and Cox proportional hazards models were used. Univariate models were constructed with NRG-1β as the predictor variable and time to all-cause death or cardiac transplantation as the combined outcome. We determined hazard ratios (HR) for the 4th versus 1st quartile of NRG-1β and for each unit increase in Ln(NRG-1β) modeled continuously. Assuming an α of 0.05 and event rate of 15%, 900 patients provided 80% power to detect a hazard ratio (HR) of 1.22 for each unit increment in Ln(NRG-1β).
For multivariable models, confounders were selected using clinical judgment, cross-sectional associations with NRG-1β, and statistical evidence of potential confounding. Statistical evidence included a univariate association with death/transplant at a p<0.20 or a change in the HR between NRG-1β and death/transplant by at least 10% after inclusion of the covariate in the model.18 Models were built in a sequential fashion with the addition of covariates by 1) demographics; 2) cross-sectional associations with NRG-1β (tobacco use, atrial fibrillation, bundle branch block, cardiomyopathy etiology); and 3) both statistical evidence and clinical judgment (body mass index, medication use, device therapy, and plasma BNP). The backwards elimination method was used to assess for additional confounding.19 We a priori decided not to adjust for echocardiographic parameters of ventricular function given that, based upon biologic data, changes in ventricular function may mediate the association between NRG-1β and risk of adverse outcomes.10, 11, 20
We hypothesized that the role of NRG-1β might differ according to heart failure etiology and severity of disease. To test these hypotheses, we added interaction terms between Ln(NRG-1β) and heart failure etiology (ischemic versus nonischemic) and between Ln(NRG-1β) and NYHA Class (I/II versus III/IV) into our models. To explore the joint effects of NRG-1β and BNP, we divided patients into subgroups according to their median NRG-1β and BNP levels. Cox proportional hazards ratios were computed for each of the four groups, with the reference group being NRG-1β and BNP less than the median. These analyses were stratified by heart failure etiology and NYHA Class. All analyses were performed using STATA 10.0 (Statacorp, TX).
All authors had full access to the data and take full responsibility for their integrity. All authors have read and agree to the manuscript as written.
Results
Study Population
Between December 2003 and October 2007, 899 subjects were enrolled with a frozen blood sample available for analysis. The mean±sd age was 56±14 years, 68% were male, and 81% were Caucasian (Table 1). Patients suffered primarily from systolic heart failure, with a mean EF of 31±17 percent. The full spectrum of NYHA Class was represented, with the majority being NYHA Class II or III. One-third of patients had ischemic cardiomyopathy and the remaining two-thirds had nonischemic cardiomyopathy. The mean±sd NRG-1β was 7.0±5.9 ng/ml and the median (IQR) was 5.2 (3.4, 8.6) ng/ml.
Table 1.
Characteristics of the study population*
| Characteristic | Entire cohort (n=899) |
|---|---|
| Age, years | 56±14 |
| Male gender | 612 (68) |
| Race | |
| Caucasian | 725 (81) |
| African American | 127 (14) |
| Other | 32 (4) |
| Tobacco use | |
| Never | 348 (39) |
| Former | 492 (55) |
| Current | 59 (6) |
| Hypertension | 320 (36) |
| Diabetes mellitus | 209 (23) |
| Serum creatinine, mg/dL | 1.3±0.7 |
| Body mass index, kg/m2 | 29±6.6 |
| Systolic blood pressure, mmHg | 113±18 |
| Cardiomyopathy etiology | |
| Ischemic | 293 (33) |
| Nonischemic | 606 (67) |
| NYHA Class | |
| I | 134 (15) |
| II | 388 (43) |
| III | 267 (30) |
| IV | 110 (12) |
| Ejection Fraction, percent | 32±17 |
| Bundle Branch Block | |
| Neither | 742 (82) |
| Left BBB | 98 (11) |
| Right BBB | 59 (7) |
| Atrial Fibrillation/Flutter | 307 (35) |
| Ventricular Tachycardia | 254 (28) |
| Cardiac resynchronization | 249 (28) |
| Ace-inhibitor or ARB | 768 (85) |
| Beta blocker | 749 (83) |
| Digoxin | 417 (46) |
| Diuretic | 637 (71) |
| Serum NRG-1β, ng/ml | |
| Median (IQR) | 5.2 (3.4, 8.6) |
| Mean±sd | 7.0±5.9 |
| Plasma BNP, pg/ml (n=744) | |
| Median (IQR) | 120 (35, 384) |
| Mean±sd | 322±507 |
Data are displayed as mean±sd or frequency (percent), unless otherwise indicated
Cross-sectional Associations between Neuregulin-1β and Clinical Parameters
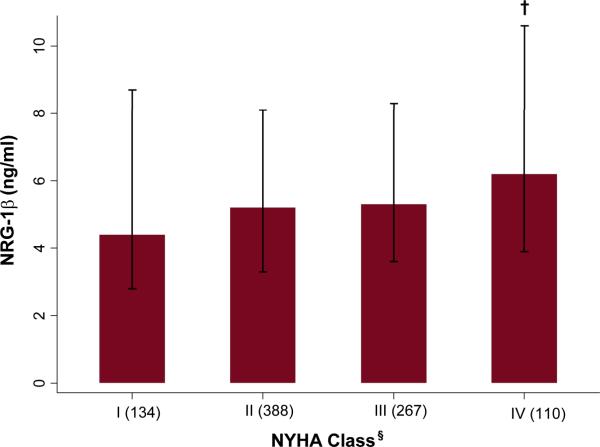
As shown in Figure 1, NYHA Class was significantly associated with NRG-1β (overall p=0.01), with higher NRG-1β levels associated with more advanced symptom severity (NYHA Class IV median [IQR] 6.2ng/ml [3.9, 10.6] versus Class I median 4.4ng/ml [2.8, 8.7], p=0.002). NRG-1β was also associated with a history of atrial fibrillation/flutter, presence of left bundle branch block, cardiomyopathy etiology, and tobacco use. These covariates were treated as potential confounders in multivariable models.
Figure 1.
Median NRG-1β according to NYHA class. Bars represent 25th and 75th percentiles.
†p<0.01 for NYHA Class IV compared to I (p=0.01 overall). §Numbers in each class displayed in parentheses.
Association between NRG-1β and Risk of All-Cause Death or Cardiac Transplant
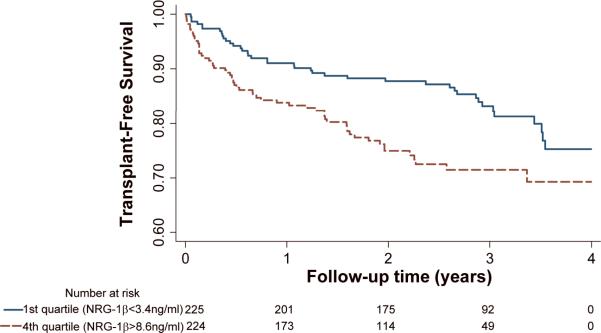
There were 192 outcomes (103 deaths and 89 transplants) over a median follow-up of 2.4 years. Patients in the highest quartile of NRG-1β (NRG-1β>8.6ng/ml, n=224) compared to those in the lowest quartile (NRG-1β<3.4ng/ml, n=225) had a significantly increased risk of death or transplant (Figure 2).
Figure 2.
Transplant-free survival by NRG-1β quartiles (4th versus 1st). p=0.03 by the log-rank test.
In Cox models (Table 2), the unadjusted hazard ratio in the highest versus lowest NRG-1β quartile was 1.75 (95% CI 1.17–2.63, p=0.007). After adjusting for demographics, this association between NRG-1β and risk of death or transplant remained significant (Table 2, Model 1). With the addition of covariates associated with NRG-1β from our cross-sectional analyses (Model 2), there were minimal changes in the relative hazard (HR 1.63, 1.08–2.47, p=0.02). Furthermore, in fully adjusted models (Models 3 and 4) that included additional potential confounders (medications, cardiac resynchronization therapy, and BNP), significance was retained. No single covariate changed the univariate HR between NRG-1β and death/transplant by more than 10%, and the backwards elimination method did not reveal further evidence of confounding. Thus, circulating NRG-1β was an independent predictor of adverse clinical outcomes in our study population.
Table 2.
Associations between NRG-1β and risk of death or cardiac transplantation
| Covariates | HR (95% CI)* 4th versus 1st quartile of NRG-1β | P-value | |
|---|---|---|---|
| Unadjusted (n=897) | None | 1.75 (1.17–2.63) | 0.007 |
| Model 1 (n=881) | Age, gender, race | 1.73 (1.15–2.61) | 0.008 |
| Model 2 (n=864) | Model 1 & cardiomyopathy etiology, history of atrial fibrillation/flutter, bundle branch block, tobacco use | 1.63 (1.08–2.47) | 0.02 |
| Model 3 (n=864) | Model 2 & body mass index, ace-inhibitor or angiotensin-receptor blocker use, beta-blocker use, and cardiac resynchronization therapy | 1.58 (1.04–2.39) | 0.03 |
| Model4 (n=721) | Model 3 & BNP | 1.57 (1.01–2.44) | 0.04 |
HR = hazard ratio; 1st quartile NRG-1β defined as NRG-1β<3.4ng/ml; 4th quartile NRG-1β>8.6ng/ml; CI = confidence interval
Interaction by Cardiomyopathy Etiology and Heart Failure Severity
Given the underlying biological differences between ischemic and nonischemic heart failure and the changes in compensatory mechanisms as heart failure progresses,21–23 we postulated that the role of NRG-1β might differ according to heart failure etiology and severity of disease. We tested these hypotheses by introducing interaction terms between NRG-1β and heart failure etiology (ischemic versus nonischemic) and NRG-1β and NYHA Class (I/II versus III/IV) in our Cox models.
The relationship between NRG-1β and risk of death or transplantation differed significantly according to cardiomyopathy etiology in both unadjusted and multivariable adjusted analyses (adjusted interaction p=0.008, Table 3). In patients with ischemic heart failure, there was an elevated risk of death or transplant with higher circulating NRG-1β, with an adjusted HR of 1.67(1.24–2.26, p=0.001) per Ln(NRG-1β). By contrast, in patients with nonischemic heart failure, there was no significant association (HR 0.98, 0.76–1.27, p=0.88). Similarly, the relationship between NRG-1β and risk of death or transplantation differed by heart failure severity (adjusted interaction p=0.01, Table 3). Patients with NYHA III/IV symptoms demonstrated a HR of 1.40 (1.11–1.76, p=0.005), whereas patients with NYHA I/II showed no significant association (HR 0.84, 0.61–1.16, p=0.28). Thus, the associations between NRG-1β and adverse outcomes were most evident in patients with ischemic heart failure and in patients with more advanced disease.
Table 3.
Associations between NRG-1β and risk of death or cardiac transplantation according to heart failure etiology and severity
| Model | Adjusted HR (95% CI) per 1-unit increment in Ln(NRG-1β)* | Interaction P-value |
|---|---|---|
| Entire cohort (n=721) | 1.25 (1.02–1.53) | |
| Stratified by Etiology | ||
| Nonischemic (n=482) | 0.98 (0.76–1.27) | 0.008 |
| Ischemic (n=239) | 1.67 (1.24–2.26) | |
| Stratified by NYHA Class | ||
| Class I/II (n=421) | 0.84 (0.61–1.12) | 0.01 |
| Class III/IV (n=300) | 1.40 (1.11–1.76) |
Adjusted for covariates listed in Table 2, Model 4; HR = hazard ratio; CI = confidence interval
NRG-1β and Circulating BNP
Higher BNP levels were associated with a significant risk of death or transplant, with a HR per Ln(BNP) of 1.64 (1.47–1.84, p<0.001). However, the Spearman correlation coefficient between BNP and NRG-1β was only 0.09 (p=0.02), suggesting a weak relationship between these two biomarkers. In addition, the association between NRG-1β and risk of death or transplantation was independent of BNP (Table 2, Model 4).
Because baseline levels of NRG-1β and BNP may measure different aspects of heart failure pathophysiology, we tested the combined influence of NRG-1β and BNP on risk of transplant-free survival, with the objective of exploring the potential additive effects of both markers. Based on results from our previous tests for interaction, these analyses were stratified according to heart failure etiology and severity.
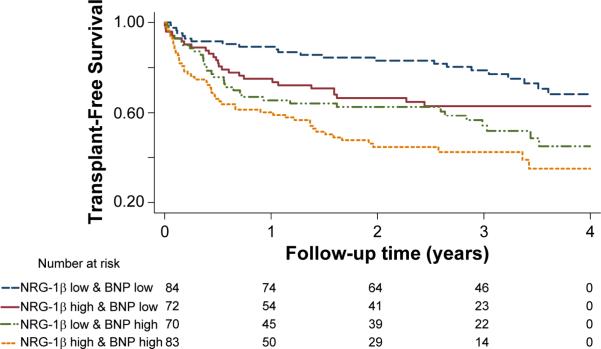
The ischemic and nonischemic groups were each divided into four sub-groups based on median cutpoints of NRG-1β and BNP. In ischemic heart failure, NRG-1β provided an additive effect to BNP in defining risk of adverse outcomes in both Kaplan-Meier (Figure 3) and multivariable adjusted models (Table 4). The adjusted HR for patients with high NRG-1β and BNP levels was 3.88 (1.88–8.07, p<0.001) compared to patients with low levels of both biomarkers, while the risk was 1.53 (0.68–3.46, p=0.30) in those with low NRG-1β and high BNP. There was no clear additive effect of NRG-1β and BNP assessment in nonischemic heart failure.
Figure 3.
Combined effects of NRG-1β and BNP on transplant-free survival in ischemic heart failure (n=245). p<0.001 by the log-rank test.
Table 4.
Combined effects of NRG-1β and BNP on risk of death or cardiac transplantation according to cardiomyopathy etiology
| Group* | Nonischemic |
Ischemic |
||
|---|---|---|---|---|
| N | Adjusted HR (95% CI)† | N | Adjusted HR (95% CI)† | |
| Low NRG-1β & Low BNP | 124 | 1 (reference) | 67 | 1(reference) |
| High NRG-1β & Low BNP | 117 | 1.08 (0.36–3.24) | 54 | 1.63 (0.73–3.65) |
| Low NRG-1β & High BNP | 116 | 5.37 (2.34–12.32) | 54 | 1.53 (0.68–3.46) |
| High NRG-1β & High BNP | 125 | 4.78 (2.06–11.10) | 64 | 3.88 (1.86–8.07) |
Low/High NRG-1β defined as below/above median (5.2ng/ml in nonischemics; 4.9ng/ml in ischemics). Low/High BNP defined as below/above median (82.7 pg/ml in nonischemics; BNP= 246.8pg/ml in ischemics).
Adjusted for covariates in Table 2, Model 3; HR=hazard ratio; CI=confidence interval
Similarly, the NYHA III/IV and NYHA I/II groups were each divided into four sub-groups based upon their median cutpoints of NRG-1β and BNP. In NYHA III/IV heart failure, NRG-1β provided an additive effect to BNP in defining risk of adverse outcomes in both Kaplan-Meier (Figure 4) and multivariable adjusted models (Table 5). The adjusted HR for patients with high NRG-1β and BNP was 2.79 (1.62–4.79, p<0.001) compared to patients with low levels of each marker, while the risk was 1.79 (0.99–3.22, p=0.06) in those with low NRG-1β and high BNP. There was no clear additive value to NRG-1β to BNP assessment in NYHA I/II heart failure. Thus, NRG-1β and BNP in combination were superior to either biomarker alone in describing the risk of adverse outcomes in patients with ischemic heart failure or NYHA III/IV symptoms.
Figure 4.
Combined Effects of NRG-1β and BNP on transplant-free survival in NYHA III/IV heart failure (n=309). p<0.001 by the log-rank test.
Table 5.
Combined effects of NRG-1β and BNP on risk of death or cardiac transplantation according to heart failure severity
| Group* | NYHA I/II |
NYHA III/IV |
||
|---|---|---|---|---|
| N | Adjusted HR (95% CI)† | N | Adjusted HR (95% CI) | |
| Low NRG-1β & Low BNP | 106 | 1(reference) | 82 | 1(reference) |
| High NRG-1β & Low BNP | 104 | 0.23 (0.03–2.02) | 69 | 1.69 (0.92–3.12) |
| Low NRG-1β & High BNP | 103 | 1.89 (0.63–5.67) | 70 | 1.79 (0.99–3.22) |
| High NRG-1β & High BNP | 108 | 1.79 (0.56–5.74) | 79 | 2.79 (1.62–4.79) |
Low/High NRG-1β defined as below/above median (4.8ng/ml in NYHA I/II; 5.4ng/ml in NYHA III/IV); Low/High BNP defined as below/above median (70.2pg/ml in NYHA I/II; 294.8pg/ml in NYHA III/IV).
Adjusted for covariates in Table 2, Model 3; HR=hazard ratio; CI=confidence interval
Discussion
We report that increased levels of NRG-1β were independently associated with disease severity and adverse outcomes in a referral cohort of 899 patients with a broad range of chronic heart failure. These are the first data investigating circulating NRG-1β in human heart disease. Our findings suggest a role for NRG-1/ErbB signaling in common forms of human heart failure and identify NRG-1β as a novel biomarker with potential clinical applications.
Considered by itself, a link between elevated NRG-1β and adverse outcomes can be interpreted in different ways. It may be that NRG-1β is pathogenic and contributes to detrimental effects in heart failure, similar to catecholamines that contribute to deterioration of cardiac function and poor survival.24 Alternatively, it may be that heart failure induces an elevation in NRG-1β as a favorable compensatory response, similar to natriuretic peptides, and that elevated NRG-1β reflects the underlying disease severity.25, 26 Discerning which of these interpretations is correct in humans will require longitudinal studies with serial assessments of NRG-1β and clinical trials in which NRG-1β signaling is pharmacologically altered.
However, in light of the extensive work in animal models that demonstrates a cardioprotective effect of NRG-1, we favor the interpretation that NRG-1β in human heart failure is beneficial. NRG-1 is an epidermal growth factor signaling protein that is essential for fetal cardiac growth.5–7, 27–29 NRG-1 is expressed in endocardial and myocardial microvascular endothelial cells and its receptors, ErbB2 and ErbB4, are expressed in cardiomyocytes. NRG-1 acts through ErbB2 and ErbB4 in a paracrine fashion to stimulate Akt/PI3-kinase, MEK/ERK, Src/FAK, and NO synthase, which together promote myocyte function and survival in the setting cardiac stress.1, 20, 27, 30–32 In particular, the β isoform of NRG-1 is highly expressed in the heart and dramatically improves cardiac function and survival when administered peripherally in animal models of heart failure.11, 13 Considered in this context, we propose that elevated NRG-1β in human failure is compensatory, not pathogenic.
Previous studies have explored changes in NRG-1/ErbB signaling in human myocardium. Rohrbach et al. found that ventricular tissue from 32 patients with advanced heart failure was characterized by increased expression of NRG-1 mRNA, but decreased expression of ErbB2/ErbB4 mRNA in comparison to normal hearts.33 With ventricular assist device unloading, there was reversal of these trends, with a subsequent decrease in NRG-1 mRNA but increase in ErbB2/ErbB4 mRNA. Uray et al. also demonstrated induction of myocardial ErbB2/ErbB4 mRNA expression with LVAD implantation, with more profound increases in ischemic versus nonischemic heart failure patients.34 A small case-control study of chronic heart failure patients found increased levels of circulating ErbB2 in the serum of heart failure patients (n=50) versus age and gender-matched controls (n=15), possibly indicative of increased shedding of ErbB2 into the circulation in heart failure.35 These findings support alterations of NRG-1/ErbB signaling in heart failure.
We found a stronger relationship between NRG-1β and outcomes in patients with ischemic heart failure, which is also consistent with previous studies. Hearts exposed to ischemia-reperfusion injury demonstrate an increase in NRG-1β activity, with detection of NRG-1β in the coronary effluent and increased ErbB receptor phosphorylation.27 A major downstream mediator of NRG-1β's action is PI3-kinase, which is important in ischemic preconditioning.20, 36 In human myocardium, ErbB receptor expression is induced to a greater extent in patients with ischemic versus nonischemic heart failure.34 Based on these and our own findings, further research is necessary to define role of NRG-1/ErbB signaling in ischemic heart disease.
In light of its association with adverse outcomes, NRG-1β may have applications in the management of heart failure patients by offering improved risk stratification. Currently, natriuretic peptides such as BNP are the dominant biomarkers for this purpose.14, 26, 37–39 However, heart failure is a complex clinical syndrome with derangements in multiple biologic pathways, suggesting that a multi-marker strategy might be more useful for establishing diagnosis, prognosis, and monitoring response to therapy.25 Consistent with this paradigm, we found that the association of NRG-1β with adverse outcomes was independent of BNP, and that assessment NRG-1β and BNP jointly improves risk stratification beyond assessment of each biomarker individually in patients with ischemic heart failure and in patients with NYHA Class III/IV symptoms. Of note, the lack of additional prognostic value of NRG-1β to BNP in NYHA I/II heart failure may be due to small sample size and limited power in this subgroup, or it may indicate that the compensatory activation of NRG-1β is more prominent in advanced disease. With further study, NRG-1β may be useful as part of a panel of biomarkers to assess prognosis in chronic heart failure.
Although our study is large and involves a broad spectrum of heart failure patients, several limitations warrant discussion. First, since this an observational study, we cannot prove a causal link between NRG-1β and heart failure pathogenesis, and there may be confounding factors that we failed account for in our analysis. Pharmacologic administration of NRG-1β and longitudinal assessment of NRG-1β levels with serial cardiac imaging will help define these relationships and changes in NRG-1β over time as heart failure progresses.20 Second, we only quantified the β isoform of NRG-1, and other isoforms could be important in human heart failure. Circulating NRG-1β in periphery also may not entirely reflect activity within the myocardium or may derive from extracardiac sources.1, 40 However, peripheral administration of the NRG-1β improves cardiac function and survival in animal models of heart failure, providing a strong rationale to study circulating NRG-1β in heart failure patients.11 Third, our subjects were recruited from an outpatient, tertiary referral center, and many patients had advanced disease. As such, our results may not be generalizable to all subjects with heart failure or to healthy subjects in the community who may be at risk for future development of heart failure. Assessment of NRG-1β in community-based cohorts or cohorts with less advanced heart failure should help define these relationships.
In summary, we observed important, novel associations between circulating NRG-1β, heart failure severity, and risk of adverse outcomes in chronic heart failure. These findings help translate basic insights regarding the biology of NRG-1 signaling by providing human data supporting a role for NRG-1 in human heart disease. Future studies are needed to test potential clinical applications for measuring endogenous NRG-1β and the therapeutic potential of pharmacologic NRG1/ErbB agonists in heart failure patients.
Clinical Perspective.
NRG-1 is a paracrine growth factor released from cardiac microvascular endothelial cells that acts via the ErbB family of tyrosine kinase receptors to regulate cardiomyocyte differentiation, growth, and stress responsiveness. Alteration of this pathway in animals induces heart failure, whereas peripheral administration of recombinant NRG-1β partially restores ventricular function and improves survival in animal models of cardiomyopathy. In humans, the chemotherapeutic agent Trastuzumab, which inhibits the ErbB2 receptor, can induce a reversible cardiomyopathy as a toxic side-effect, strongly suggesting that NRG-1/ErbB signaling is cardioprotective in the human heart. Here we provide the first study of endogenous circulating NRG-1β in human heart failure. We used a novel immunoassay to quantify circulating NRG-1β levels in 899 subjects in the Penn Heart Failure Study, a large referral cohort of patients with a broad spectrum of primarily systolic failure. We determined that increased levels of NRG-1β were independently associated with disease severity and risk of death or cardiac transplantation. Associations were independent of BNP and were strongest in patients with ischemic heart failure and in patients with NYHA Class III/IV symptoms. Moreover, assessment NRG-1β and BNP jointly improved risk stratification beyond assessment of each biomarker individually in these two subgroups. With further study, NRG-1β may be useful as part of a panel of biomarkers to assess prognosis in chronic heart failure.
Supplementary Material
Acknowledgments
Funding Sources Dr. Ky was supported through research funding from the National Institutes of Health T32 HL00789-09 and the Heart Failure Society of America Research Fellowship Award. This work was also supported by the National Heart, Lung, and Blood Institute grants HL088577 (Dr. Cappola) and HL068144 (Dr. Sawyer) and an Established Investigator Award from the American Heart Association (Dr. Sawyer).
Footnotes
Disclosures Dr. Sawyer received prior research support from Genentech, Inc. and Roche Diagnostics. He currently receives support from Acorda, Inc. Dr. Cappola received prior research support from Abbott Diagnostics and GlaxoSmithKline. The remaining authors have nothing to disclose.
References
- 1.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 2.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 3.Garratt AN. “To erb-B or not to erb-B…” Neuregulin-1/ErbB signaling in heart development and function. Journal of Molecular & Cellular Cardiology. 2006;41:215–218. doi: 10.1016/j.yjmcc.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Chien KR. Herceptin and the heart--a molecular modifier of cardiac failure. N Engl J Med. 2006;354:789–790. doi: 10.1056/NEJMp058315. [DOI] [PubMed] [Google Scholar]
- 5.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 6.Kramer R, Bucay N, Kane DJ, Martin LE, Tarpley JE, Theill LE. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc Natl Acad Sci U S A. 1996;93:4833–4838. doi: 10.1073/pnas.93.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Hwang H, Cao L, Buckland M, Cunningham A, Chen J, Chien KR, Graham RM, Zhou M. Domain-specific gene disruption reveals critical regulation of neuregulin signaling by its cytoplasmic tail. Proc Natl Acad Sci U S A. 1998;95:13024–13029. doi: 10.1073/pnas.95.22.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu FF, Stone JR, Schuldt AJ, Okoshi K, Okoshi MP, Nakayama M, Ho KK, Manning WJ, Marchionni MA, Lorell BH, Morgan JP, Yan X. Heterozygous knockout of neuregulin-1 gene in mice exacerbates doxorubicin-induced heart failure. American Journal of Physiology - Heart & Circulatory Physiology. 2005;289:H660–6. doi: 10.1152/ajpheart.00268.2005. [DOI] [PubMed] [Google Scholar]
- 9.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Gu X, Li Z, Li X, Li H, Chang J, Chen P, Jin J, Xi B, Chen D, Lai D, Graham RM, Zhou M. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48:1438–1447. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 12.Peng X, Chen B, Lim CC, Sawyer DB. The cardiotoxicology of anthracycline chemotherapeutics: translating molecular mechanism into preventative medicine. Molecular Interventions. 2005;5:163–171. doi: 10.1124/mi.5.3.6. [DOI] [PubMed] [Google Scholar]
- 13.Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res. 2005;311:135–146. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Vorovich E, Chuai S, Li M, Avena J, Marwin M, Wolfe D, Reilly MP, Cappola TP. Comparison of MMP-9 and BNP as clinical biomarkers in chronic heart failure. American Heart Journal. 2008;50:992–997. doi: 10.1016/j.ahj.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt SA, American College of Cardiology American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Astor BC, Yi S, Hiremath L, Corbin T, Pogue V, Wilkening B, Peterson G, Lewis J, Lash JP, Van Lente F, Gassman J, Wang X, Bakris G, Appel LJ, Contreras G. N-terminal prohormone brain natriuretic peptide as a predictor of cardiovascular disease and mortality in blacks with hypertensive kidney disease: the African American Study of Kidney Disease and Hypertension (AASK) Circulation. 2008;117:1685–1692. doi: 10.1161/CIRCULATIONAHA.107.724187. [DOI] [PubMed] [Google Scholar]
- 17.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–176. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 19.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 1996;49:907–916. doi: 10.1016/0895-4356(96)00025-x. [DOI] [PubMed] [Google Scholar]
- 20.Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007;116:954–960. doi: 10.1161/CIRCULATIONAHA.107.690487. [DOI] [PubMed] [Google Scholar]
- 21.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 22.Kaye DM, Hoshijima M, Chien KR. Reversing advanced heart failure by targeting Ca2+ cycling. Annu Rev Med. 2008;59:13–28. doi: 10.1146/annurev.med.59.052407.103237. [DOI] [PubMed] [Google Scholar]
- 23.Kittleson MM, Minhas KM, Irizarry RA, Ye SQ, Edness G, Breton E, Conte JV, Tomaselli G, Garcia JG, Hare JM. Gene expression analysis of ischemic and nonischemic cardiomyopathy: shared and distinct genes in the development of heart failure. Physiol Genomics. 2005;21:299–307. doi: 10.1152/physiolgenomics.00255.2004. [DOI] [PubMed] [Google Scholar]
- 24.Cohn JN. The management of chronic heart failure. N Engl J Med. 1996;335:490–498. doi: 10.1056/NEJM199608153350707. [DOI] [PubMed] [Google Scholar]
- 25.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 26.Lainchbury JG, Richards AM, Nicholls MG, Hunt PJ, Ikram H, Espiner EA, Yandle TG, Begg E. The effects of pathophysiological increments in brain natriuretic peptide in left ventricular systolic dysfunction. Hypertension. 1997;30:398–404. doi: 10.1161/01.hyp.30.3.398. [DOI] [PubMed] [Google Scholar]
- 27.Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, Sawyer DB. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem. 2004;279:51141–51147. doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- 28.Chen B, Peng X, Pentassuglia L, Lim CC, Sawyer DB. Molecular and cellular mechanisms of anthracycline cardiotoxicity. Cardiovascular Toxicology. 2007;7:114–121. doi: 10.1007/s12012-007-0005-5. [DOI] [PubMed] [Google Scholar]
- 29.Rentschler S, Morley GE, Fishman GI. Molecular and functional maturation of the murine cardiac conduction system. Cold Spring Harb Symp Quant Biol. 2002;67:353–361. doi: 10.1101/sqb.2002.67.353. [DOI] [PubMed] [Google Scholar]
- 30.Lemmens K, Fransen P, Sys SU, Brutsaert DL, De Keulenaer GW. Neuregulin-1 induces a negative inotropic effect in cardiac muscle: role of nitric oxide synthase. Circulation. 2004;109:324–326. doi: 10.1161/01.CIR.0000114521.88547.5E. [DOI] [PubMed] [Google Scholar]
- 31.Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. Journal of Molecular & Cellular Cardiology. 2006;41:228–235. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hertig CM, Kubalak SW, Wang Y, Chien KR. Synergistic roles of neuregulin-1 and insulin-like growth factor-I in activation of the phosphatidylinositol 3-kinase pathway and cardiac chamber morphogenesis. J Biol Chem. 1999;274:37362–37369. doi: 10.1074/jbc.274.52.37362. [DOI] [PubMed] [Google Scholar]
- 33.Rohrbach S, Niemann B, Silber RE, Holtz J. Neuregulin receptors erbB2 and erbB4 in failing human myocardium -- depressed expression and attenuated activation. Basic Res Cardiol. 2005;100:240–249. doi: 10.1007/s00395-005-0514-4. [DOI] [PubMed] [Google Scholar]
- 34.Uray IP, Connelly JH, Thomazy V, Shipley GL, Vaughn WK, Frazier OH, Taegtmeyer H, Davies PJ. Left ventricular unloading alters receptor tyrosine kinase expression in the failing human heart. J Heart Lung Transplant. 2002;21:771–782. doi: 10.1016/s1053-2498(02)00390-x. [DOI] [PubMed] [Google Scholar]
- 35.Perik PJ, de Vries EG, Gietema JA, van der Graaf WT, Smilde TD, Sleijfer DT, van Veldhuisen DJ. Serum HER2 levels are increased in patients with chronic heart failure. Eur J Heart Fail. 2007;9:173–177. doi: 10.1016/j.ejheart.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Ban K, Cooper AJ, Samuel S, Bhatti A, Patel M, Izumo S, Penninger JM, Backx PH, Oudit GY, Tsushima RG. Phosphatidylinositol 3-kinase gamma is a critical mediator of myocardial ischemic and adenosine-mediated preconditioning. Circ Res. 2008;103:643–653. doi: 10.1161/CIRCRESAHA.108.175018. [DOI] [PubMed] [Google Scholar]
- 37.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, Glazer RD, Tognoni G, Cohn JN, Val-HeFT Investigators Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 38.Masson S, Latini R, Anand IS, Vago T, Angelici L, Barlera S, Missov ED, Clerico A, Tognoni G, Cohn JN, Val-HeFT Investigators Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the Valsartan Heart Failure (Val-HeFT) data. Clin Chem. 2006;52:1528–1538. doi: 10.1373/clinchem.2006.069575. [DOI] [PubMed] [Google Scholar]
- 39.Tang WH, Girod JP, Lee MJ, Starling RC, Young JB, Van Lente F, Francis GS. Plasma B-type natriuretic peptide levels in ambulatory patients with established chronic symptomatic systolic heart failure. Circulation. 2003;108:2964–2966. doi: 10.1161/01.CIR.0000106903.98196.B6. [DOI] [PubMed] [Google Scholar]
- 40.LeBrasseur NK, Mizer KC, Parkington JD, Sawyer DB, Fielding RA. The expression of neuregulin and erbB receptors in human skeletal muscle: effects of progressive resistance training. Eur J Appl Physiol. 2005;94:371–375. doi: 10.1007/s00421-005-1333-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.