Abstract
Work from our laboratory has shown that orexin (ORX; or hypocretin) neurons in the lateral hypothalamus are involved in preference for morphine, cocaine, and food. Other groups have demonstrated a connection between the ORX system and ethanol-related behaviors. Here we extended those results to investigate, in outbred Sprague-Dawley rats, the relationship between ethanol preference and the ORX system. In Experiment 1, rats were trained to drink 10% ethanol using the intermittent access (IA) technique. In Experiment 2, different groups of rats were trained to drink 10% ethanol using either IA or the sucrose fade (SF) technique. Following ethanol drinking acquisition, ethanol preference was assessed using 2-bottle-choice tests. Rats were then tested for changes in preference with additional 2-bottle-choice tests following administration of the orexin-1 receptor antagonist SB-334867 (SB; 30 mg/kg, ip). Differences in ethanol preference were observed across individuals, with a significantly higher ethanol preference observed in rats trained to drink using IA compared to SF. In both Experiments 1 and 2, SB reduced ethanol preference selectively in rats with high ethanol preference. These results demonstrate a strong, causal relationship between the ORX system and ethanol preference in outbred rats. These findings provide additional evidence that the orexin system provides opportunities to develop novel treatments for alcohol abuse.
Keywords: Orexin, Lateral Hypothalamus, Alcohol, Reward, Preference
Introduction
The orexins (also known as hypocretins) are neuropeptides expressed within a subset of neurons located exclusively within the lateral, perifornical and dorsomedial hypothalamus (de Lecea and Sutcliffe, 1999; Sakurai et al., 1998). These neurons project widely across the neuraxis and exhibit potent and diverse influences over behaviors such as feeding, arousal, and sleep/wake regulation (Chemelli et al., 1999; Nishino, 2007; Nishino et al., 2000; Peyron et al., 1998; Sakurai et al., 1998; Sutcliffe and de Lecea, 2002; Willie et al., 2001). In recent years, it has become clear that the orexin (ORX) system is also critically involved in controlling general reward-associated behaviors including responses both to natural rewards as well as to drugs of abuse (Aston-Jones et al., 2009; DiLeone et al., 2003; Georgescu et al., 2003; Harris and Aston-Jones, 2006; Narita et al., 2006). In particular, work from our laboratory has demonstrated a strong and direct correlation between preference for rewards and activation of ORX neurons of the lateral hypothalamus (Harris et al., 2005; Harris et al., 2007).
Work from a number of laboratories in the last few years has demonstrated an important relationship between the ORX system and ethanol consumption. Lawrence and colleagues reported, in an alcohol-preferring strain of rat, that ethanol consumption upregulated ORX mRNA in the lateral hypothalamus (LH), and that the orexin-1 receptor (OX1R) antagonist SB-334867 (SB) reduced operant responding for ethanol or for cues related to ethanol (Lawrence et al., 2006). Richards and colleagues also described decreased operant responding for ethanol as well as decreased yohimbine-induced reinstatement of ethanol-seeking following SB pretreatment (Richards et al., 2008). Dayas and colleagues reported increased Fos activation of ORX neurons following contextual reinstatement to ethanol-seeking (Dayas et al., 2008). Similarly, Hamlin and colleagues found increased Fos activation in ORX neurons during renewal (contextual reinstatement) for alcoholic beer (Hamlin et al., 2009). Finally, Schneider and colleagues described increased ethanol consumption following ORX infusion into the paraventricular nucleus (PVN) of the hypothalamus or in the lateral hypothalamus itself (Schneider et al., 2007).
The goals of the present study were twofold. First, we sought to determine the influence of the ORX system on preference for ethanol, since we have previously shown a direct relationship between ORX neuron activation and preference for other drugs of abuse. Second, we wanted to explore ethanol preference in outbred (Sprague Dawley) rats because our previous work demonstrated the ORX-preference relationship in this strain. Outbred strains are interesting due to their genetic heterogeneity (which may approximate differences in human populations) as well as due to their inconsistent ethanol drinking which can be strongly influenced by the method of drinking acquisition (Schneider et al., 2007; Simms et al., 2008). To address the influence of the ORX system on ethanol preference in Sprague Dawley rats, we employed a 2-bottle-choice test following training to drink ethanol by either the sucrose-fade or intermittent exposure method, and then tested the influence of OX1R antagonism on the different levels of preference expressed.
Materials and Methods
Subjects
Male Sprague-Dawley rats (initial weight approximately 200–250 g (Experiment 2, n = 24) or 300–350 g (Experiment 1, n = 8); Charles River, Wilmington, MA) were single- or pair-housed under a reversed 12-hr light/dark cycle (lights off 6 a.m.) and had ad libitum access to food and water. Animals were housed in a temperature- and humidity-controlled animal facility at MUSC (AAALAC-accredited; NCRR C06 grant RR015455). All experiments were approved by the IACUC at MUSC and conducted according to specifications of the NIH as outlined in the Guide for the Care and Use of Laboratory Animals.
Drug Treatments
The OX1R antagonist SB-334867 (SB: 1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea hydrochloride, generously donated by Eli Lilly, Indianapolis, IN), was suspended in 2% DMSO and 10% 2-hydroxypropyl-β-cyclodextrin (Sigma, St. Louis, MO) in sterile water; 30 mg/kg was given in a volume of 4 ml/kg (i.p.) 30 min prior to the test session. Vehicle was delivered at the same volume as the SB solution. Ethanol solutions (10% or 20%, v/v) were prepared using 95% ethanol (AAPER, Shelbyville, KY) and filtered water. Sucrose solutions (10% sucrose w/v) were prepared using filtered water.
Procedures and Statistical Analyses
Animals were trained to drink ethanol using one of two methods: sucrose fade (SF) (Samson, 1986) or intermittent access (IA) (Simms et al., 2008; Wise, 1975). In SF, animals were initially given access to a 10% sucrose solution for three hours/day. Over the course of two weeks, sucrose was faded out and ethanol was faded in in 2% stages. Upon reaching 10% ethanol : 0% sucrose, animals received one more week of 10% ethanol exposure. In IA, rats received either 20% ethanol or water for 24 hours on alternating days for two weeks. For eight SF rats and all IA rats, solutions were given in home cages with ad lib access to food and water. Eight other SF rats (which had been pair-housed) were separated each day for ethanol exposure. There was no influence of housing on ethanol or water consumption or preference (all p values > 0.05), so the data were combined. After acquisition, all rats were given one week of 2-bottle-choice tests (10% ethanol and water, three hours/day) to establish baseline preference. The next day, rats were treated with either SB or vehicle 30 minutes prior to 2-bottle-choice preference testing. Two days later, the opposite treatment was given and animals were tested again. Vehicle and SB injections were counterbalanced across days. Volumes of ethanol and water were measured each day, either at the end of three hours (in the case of SF and 2-bottle-choice testing) or upon bottle-switching (IA). In Experiment 1, volumes were measured after two hours. Measurement times were increased to three hours in Experiment 2 to allow for greater possible differences in preference across experimental groups. During all testing, “leak test bottles” were mounted in separate cages and the amount of leak was measured. Body weights were measured for calculation of ethanol consumption in g/kg. Preference scores in 2-bottle-choice testing were calculated as (vol. ethanol/(vol. ethanol + vol. water)) consumed over the three hour test session. All statistical comparisons were made using mixed model two-factor ANOVAs (preferrer/non-preferrer vs. days or preferrer/non-preferrer vs. treatment) or else paired or unpaired t-tests for within or between group comparisons respectively.
Results
Experiment 1
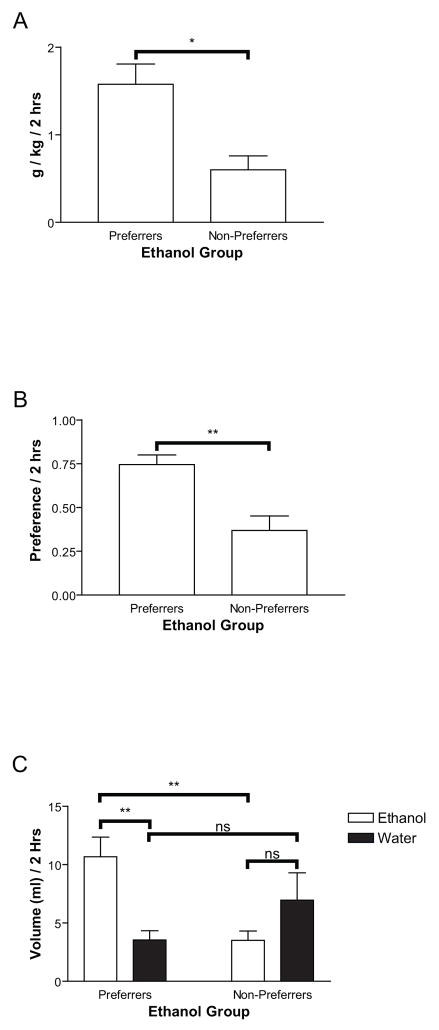
In a preliminary study, eight rats were trained to drink 10% ethanol using intermittent access (IA, see Methods). Following ethanol drinking acquisition, ethanol preference was tested on a daily basis using 2-bottle-choice tests (see Methods). Rats were characterized as ethanol preferrers or non-preferrers based on the last three days of preference testing. A median split by preference scores demonstrated a differentiation between ethanol preferrers and non-preferrers that was reliable and significant with respect to g/kg consumed (Figure 1A, F(1,6)=12.24, p<0.05), preference (Figure 1B, F(1,6)=14.08, p<0.01), or volume of ethanol (F(1,6)=14.71, p<0.01) consumed (Figure 1C). Accordingly, there was a significant difference between ethanol and water consumed in ethanol preferring rats (Figure 1C, F(1,6)=14.61, p<0.01), but not in non-preferring rats (Figure 1C, p>0.05). There was no significant difference in volume of water consumed across preferring and non-preferring rats (Figure 1C). There was also no influence of day on ethanol or water consumption or preference (p>0.05).
Figure 1.
Rats in Experiment 1 (n=8) were sorted into two groups (preferrers and non-preferrers) based on a median split for preference for ethanol vs. water. These groups exhibited a significant difference in ethanol consumption in g/kg (1A), ethanol preference (1B), and ethanol vs. water consumption (1C). In this and all other figures *p<0.05, **p<0.01, ***p<0.005, ****p<0.001, ns = not significant
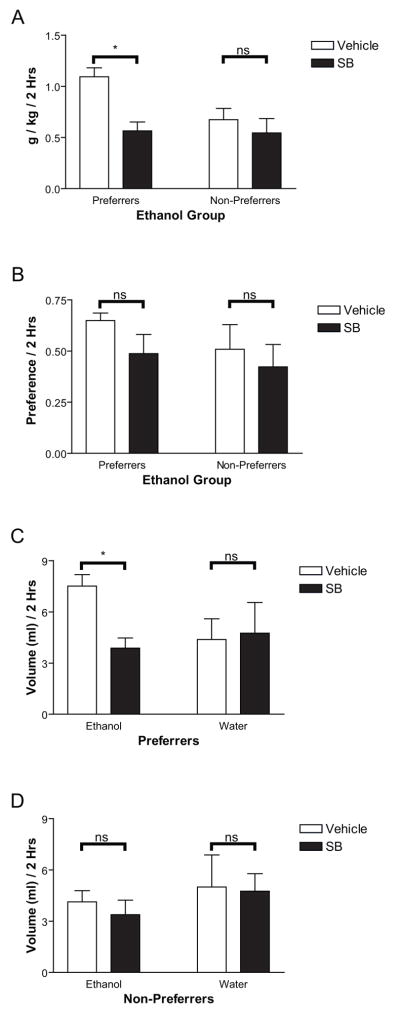
We then tested the OX1R antagonist SB-334867 (SB; 30 mg/kg, ip) or vehicle given 30 min before 2-bottle-choice testing on ethanol preference in these animals. As shown in Figure 2A, following SB administration preferrers showed significantly decreased ethanol consumption as measured in g/kg as demonstrated by a main effect of treatment (vehicle/SB: F(1,6)=20.73, p<0.01) and an interaction effect for treatment (vehicle/SB X preferrer/non-preferrer, F(1,6)=7.67, p<0.05), which was selective for ethanol preferrers only (t(3)=4.63, p<0.05). Treatment with SB selectively decreased ethanol volume consumption (Figure 2C, F(1,6)=20.08, p<0.005), and this decrease was selective for ethanol-preferring rats, as demonstrated by a significant interaction effect (vehicle/SB X preferrer/non-preferrer, F(1,6)=8.67, p<0.05) that was selective for ethanol preferring rats only (t(3)=4.39, p<0.05). There was no effect of SB on water consumption as shown in Figures 2C and 2D (p>0.05). There was a tendency for preferrers to exhibit a greater decrease in preference following SB administration than non-preferrers (Figure 2B). This difference, however, was not significant, presumably due to the low number of subjects (n = 8) and the high variability among animals. To refine these results, we carried out a more thorough study, described in Experiment 2.
Figure 2.
Administration of SB prior to 2-bottle-choice testing in Experiment 1 significantly decreased ethanol consumption in g/kg (2A), and volume in preferrers (relative to non-preferrers (2C, 2D). Preference (2B) was also reduced, but not significantly.
Experiment 2
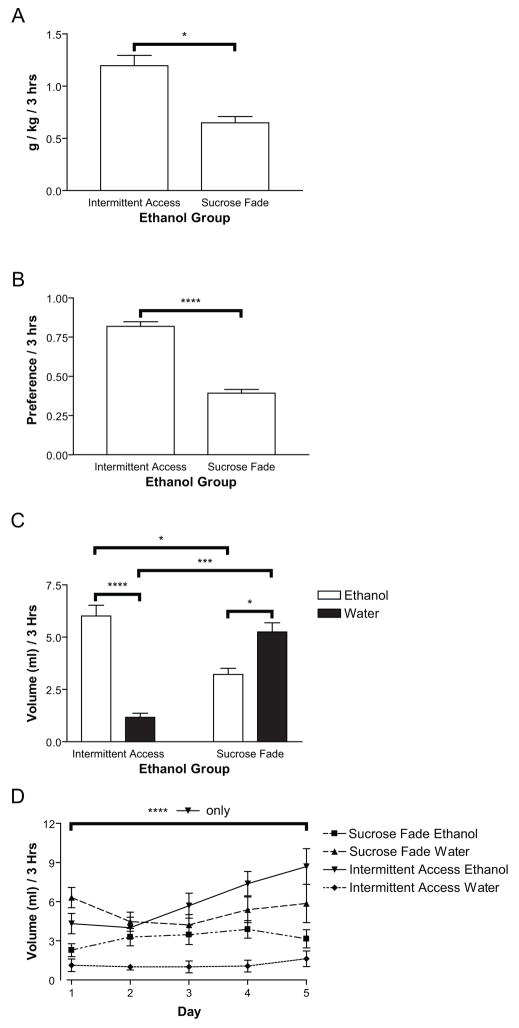
In a second study, 16 rats were trained to drink 10% ethanol using SF and eight rats were trained to drink 10% ethanol using IA. A third group of eight rats served as a water control, and were faded from 10% sucrose to water in steps equivalent to SF for ethanol. Following ethanol drinking acquisition, ethanol preference was characterized using 2-bottle-choice testing. Preference was assessed based on the last five days of 2-bottle-choice testing. As can be seen in Figure 3B, rats that acquired ethanol drinking via IA exhibited a significantly greater preference for ethanol than did rats that acquired ethanol drinking via SF (F(1,22)=42.80, p<0.001). This preference was driven by a significantly higher amount of ethanol consumed, both in g/kg (Figure 3A, F(1,22)=7.21, p<0.05) and volume (Figure 3C, F(1,22)=7.99, p<0.05), as well as by a lower volume of water consumed (Figure 3C, F(1,22)=13.20, p<0.005) during the testing period. Differences between water consumption and ethanol consumption within the acquisition technique were also significant (Figure 3C, IA: F(1,14)=31.72, p<0.001, SF: F(1,30)=4.74, p<0.05). We also observed a significant effect of 2-bottle-choice day (F(4,56)=8.73, p<0.001) and ethanol/water vs. day interaction (F(4,56)=5.95, p<0.005) in IA rats that was specific to significant increases in ethanol drinking across 2-bottle-choice days (Figure 3D, ethanol: F(4,28)=9.00, p<0.001, water: p>0.05) and was not present for SF rats (p>0.05).
Figure 3.
In Experiment 2, rats that underwent intermittent access training for ethanol drinking (IA, n=8) consumed significantly more ethanol (3A and 3C) and exhibited a greater preference for ethanol over water (3B and 3C) than did rats that underwent sucrose-fade training (SF, n=16). The difference in preference was driven by a greater preference for water over ethanol in SF rats, as well as a greater preference for ethanol over water in IA rats. IA rats also demonstrated a significant increase in ethanol (and not water) drinking over days of 2-bottle-choice testing that was not seen in SF rats (3D).
These results confirm previous studies demonstrating that IA produces higher levels of ethanol drinking than SF (Simms et al., 2008; Wise, 1975), and extend these findings to Sprague Dawley rats. Of note, among the 16 SF rats, only two exhibited high levels of ethanol preference and consumption (preference scores > 0.5 and g/kg ethanol > 1.0). One additional rat with high preference (0.61) exhibited low g/kg ethanol (0.34), suggesting that it consumed little fluid overall. Among the eight IA rats, only one demonstrated a relatively low preference and g/kg ethanol scores (preference = 0.63, g/kg ethanol = 0.25). One other rat showed relatively low preference (0.64) but high g/kg ethanol (1.06) scores. This degree of consistency within acquisition method suggests that although individual differences in preference exists (as seen in Experiment 1), the ethanol acquisition method strongly influences the degree of individual preference (e.g., SF produces low and IA produces high ethanol preference).
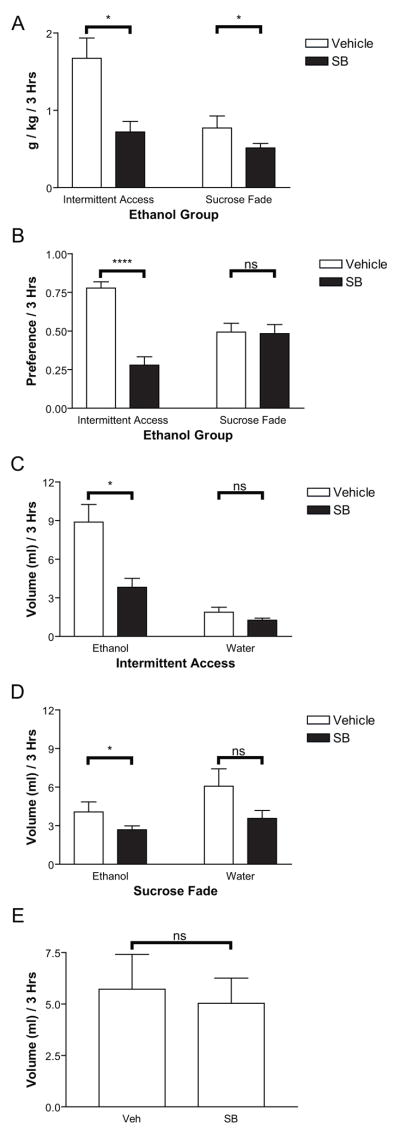
Following ethanol preference characterization in the 2-bottle-choice test, we assessed the influence of OX1R antagonism in both the SF and IA groups. As in Experiment 1, SB (30 mg/kg, ip) or vehicle was administered 30 min prior to 2-bottle-choice testing. Administration of SB produced a significant decrease in ethanol preference (Figure 4B, F(1,22)=9.15, p<0.01) selectively in IA rats (IA: t(7)=7.82, p<0.001, SF: p>0.05). This decreased preference was exemplified by a drop in g/kg ethanol consumed (Figure 4A, F(1,22)=21.92, p<0.001). This decrease was more than three times larger in IA rats than SF rats (mean decrease IA/preferrers: 0.95 g/kg, mean decrease SF/non-preferrers: 0.26 g/kg). The effect of SB administration was significant in both cases (SF: t(15)=2.14, p=0.049, IA: t(7)=3.42, p=0.011), although we also observed an interaction effect (vehicle/SB X preferrer/non-preferrer) suggesting a stronger decrease in IA/preferrers than SF/non-preferrers (F(1,22)=7.19, p<0.05). The effect of SB appeared to be most strongly influenced by preference and g/kg of ethanol consumed. Of the two preferrers in the SF group, one showed a large decrease in preference and amount of ethanol consumed following SB administration (veh. pref.: 0.87, veh. g/kg: 2.63; SB pref.: 0.33, SB g/kg: 0.79). The second rat was displayed abnormally high water consumption (> mean+2SD of all tested rats) on vehicle test day which obscured preference measurements, although SB had no effect on ethanol consumption in g/kg in this animal (veh: 0.78, SB: 0.81). The one non-preferrer in the IA group showed a minimal effect of SB (veh. pref.: 0.60, veh. g/kg: 0.54; SB pref.: 0.50, SB g/kg: 0.36). Overall, these results support the hypothesis that the effect of OX1R antagonism on ethanol preference and consumption is strongly related to baseline levels of ethanol preference and consumption. Although training regimen clearly influenced preference, even those animals exhibiting opposite preference from the majority of the population within a training regimen were influenced by SB in a preference-specific manner. This conclusion for preference-dependent effects of SB is also consistent with results of Experiment 1, where high-preferrers within a group trained on IA showed a greater decrease in preference following SB than low-preferring rats.
Figure 4.
Administration of SB in Experiment 2 produced significant reductions in ethanol consumed in g/kg (4A) and volume (4C, 4D) as well as significant reductions in ethanol preference (4B). Decreases in preference (4B) were seen selectively for IA rats. Decreases in ethanol consumed (4A, 4C, 4D) were seen in both groups, though the decrease was dramatically greater for IA compared to SF rats (see text). Water consumption was not significantly affected in either group (4C, 4D), indicating a selective effect of SB on ethanol drinking. This finding was confirmed in a separate group of rats with no ethanol experience (n=8) which demonstrated no effect of SB vs. vehicle on water consumption (4E).
The difference in the effect of SB on preference was further characterized by assessing the influence of SB on ethanol and water consumption in the two groups. Although ethanol drinking levels were statistically different under vehicle conditions (mean ethanol IA vehicle: 8.9 ml/1.67 g/kg; mean ethanol SF vehicle: 4.1 ml/0.77 g/kg), SB administration decreased ethanol drinking in IA/preferrers to a level statistically equivalent to levels of ethanol drinking in SF/non-preferrers under vehicle conditions (mean ethanol IA SB: 3.8 ml/0.72 g/kg, p>0.05). As noted above, SF/non-preferrers were demonstrated a small (though significant) decrease in g/kg ethanol consumed following SB treatment. This can also be seen in volume of ethanol consumed as shown in figure 4D (preferrers: t(7)=3.31, p<0.05, non-preferrers: t(15)=2.27, p<0.05). Again, however, the SB-related decrease in ethanol consumption in IA rats was more than three times that of SF rats (Figure 4D, mean decrease preferrers: 5.06 ml, mean decrease non-preferrers: 1.375 ml). This observation was also supported by a significant interaction effect (vehicle/SB X preferrer/non-preferrer; F(1,22)=7.29, p<0.05). Water consumption was unaffected (p>0.05) in both groups. These results suggest that the inhibitory effects of OX1R antagonism were selective for ethanol preference/consumption and not for fluid consumption per se. A further demonstration of this specificity can be seen in Figure 4E in which data are shown from 8 control animals that were trained on sucrose fade-to-water demonstrating almost no change in water consumption following SB vs. vehicle administration (mean volume water vehicle: 5.72 ml, mean volume water SB: 5.03 ml, p>0.05).
Discussion
Work from our laboratory has shown a direct relationship between the activation of ORX neurons and preference for both natural and drug rewards, such that animals with high preference show strong activation in ORX neurons (Harris et al., 2005). Although the relationship between the ORX system and ethanol-seeking has been demonstrated in a number of instances (Dayas et al., 2008; Hamlin et al., 2009; Lawrence et al., 2006; Richards et al., 2008; Schneider et al., 2007), it is not known if ORX neurotransmission relates to preference for ethanol. In this study we addressed the issue of preference using a simple behavioral measure: ethanol-preference in a 2-bottle-choice test. We determined that Sprague Dawley rats could be separated into groups of ethanol preferrers and non-preferrers based on either individual differences (as seen in Experiment 1) or the influence of ethanol acquisition method on preference (as seen in Experiment 2). Furthermore, we determined that interference with the ORX system via administration of an OX1R antagonist decreased ethanol drinking and preference strongly in ethanol preferrers but only weakly in ethanol non-preferrers. Critical to interpretation of these results was the finding that SB administration produced minimal effects on water consumption, both in ethanol-experienced and non-experienced animals. Although some research indicates that the ORX system may influence water drinking (Kunii et al., 1999), other groups have failed to find a reliable influence of ORX on water consumption while simultaneously showing a potent influence of ORX on ethanol drinking (Schneider et al., 2007). Although we observed an overall decrease in fluid consumption in SB-treated ethanol-drinking rats, this effect was driven primarily by decreases in ethanol consumption, as evidenced by significant decreases in ethanol volume consumption and no significant decreases in water consumption, a finding that is bolstered by the observation that SB had no influence on water consumption in ethanol-naïve rats (figure 4E). These findings rule out alternate explanations of the effects of the OX1R antagonist, such as reduction in arousal or overall drinking behavior. Our results also support previous research related to the relationship of ORX to ethanol-seeking as well as to preference for other rewards, including other drugs of abuse (Aston-Jones et al., 2009; Harris and Aston-Jones, 2006; Harris et al., 2005).
One basic, but important implication from this investigation is that behavioral heterogeneity in outbred strains for alcohol preference, whether through training or individual differences, is an informative and valuable characteristic of a population. In the instance of the current study, ethanol preference was seen to vary across subjects, due to differences in training (Experiment 2) or across individuals (Experiment 1). In both cases, the effect of OX1R antagonism on ethanol drinking and preference was dependent on the initial preference of the individual. In many studies using rat models of ethanol consumption, it is preferable to use strains of rats that produce reliable levels of drinking, high or low. However, when addressing issues such as substance abuse, it is often valuable to have a genetically or experientially heterogeneous population to better model the diversity seen in human drug users.
Our results are well-aligned with previous studies investigating the influence of the OX1R on ethanol-seeking and consumption. In the first study of this type, Lawrence and colleagues demonstrated, in the alcohol-preferring (iP) rat strain, that administration of SB decreased operant responding for ethanol and for ethanol-associated cues, without affecting operant responding for water or water-associated cues (Lawrence et al., 2006). This result was replicated by Richards and colleagues in Long Evans rats, who additionally showed decreases in yohimbine-induced reinstatement of ethanol seeking with SB pre-administration (Richards et al., 2008). Our study supports these results while extending them in a number of ways. First, we show that SB blocks ethanol drinking in the absence of an operant response. Although this is a subtle point, it confirms that SB is decreasing ethanol-seeking per se, as opposed to inhibiting the ability to perform a learned arbitrary operant response. Second, we show that the influence of SB is dependent on the subject’s prior preference for ethanol. In prior reports no variability in rat ethanol preference was noted, presumably because high levels of preference were established either genetically (in the case of Lawrence and colleagues, 2006) or through training (in the case of Richards and colleagues, 2008) so as to support operant responding for ethanol. Because our tasks required no a priori ethanol preference, we were able to 1) observe striking differences in preference across individuals and 2) use these differences to better characterize the influence of the ORX system on ethanol seeking.
The volumes consumed in our alcohol preferring groups were high (mean: 1.20 g/kg, with individual values as high as 2.88 g/kg) and significantly greater than those consumed by the non-preferring groups. Indeed, similar to results reported by Simms and colleagues (Simms et al., 2008), we observed reliable escalation in consumption in IA rats that continued through vehicle testing days (i.e., the average ethanol consumed by IA rats under vehicle in figure 4C is more similar to the final data point in figure 3D than the average data shown in figure 3C). However, it is likely that individual amounts of ethanol consumed infrequently corresponded to blood ethanol levels resembling that achieved by binge-like drinking (≥ 0.08 g%, NIAAA National Advisory Council, 2004), particularly given that these values were measured after three hours of drinking. In a number of IA animals we measured the amount of ethanol consumed in 30 minutes. Although these values were lower than that measured at three hours as expected, they were still relatively high (mean = 0.88 g/kg, max = 1.37 g/kg) but still only approached values reported to produce blood ethanol levels at ≥ 0.08 g% in recent studies of Wistar (Ji et al., 2008) and Long-Evans rats (Simms et al., 2008). Establishing high blood ethanol levels purely through drinking is a common difficulty with rat models of ethanol consumption (though see (Ji et al., 2008)), and is something that we will address in future studies using ethanol dependence induction methods (e.g., inhalation chambers or use of a ‘supersac’ (3% glucose + 0.125% saccharin) solution) as well as measurements of blood ethanol levels. Inclusion of these techniques will allow us to better characterize the influence of the ORX system in excessive ethanol consumption and dependence. The present experiments clearly demonstrated a relationship between ethanol preference and the ORX system, and based on these data we hypothesize that this relationship, or a stronger one, will be present in bingeing and/or dependent animal models.
Mechanistically, how might ORX influence ethanol preference? One option is through modulation of dopamine (DA) neurons of the ventral tegmental area (VTA). It is known that ORX projections to VTA are involved in reward-associated behaviors (Harris et al., 2005; Narita et al., 2006). It is also known that ethanol and ethanol-seeking produces activation of DA neurons and/or DA release (Brodie et al., 1990; Gessa et al., 1985; Gonzales et al., 2004; Weiss et al., 1993). In addition to the potentially direct effect of ethanol on DA neurons (Brodie et al., 1999), ethanol could activate ORX neurons which, in turn, excite DAergic neurons (Borgland et al., 2006; Korotkova et al., 2006; Muschamp et al., 2007. This hypothesis is in line with the work of others showing that cues and contexts related to ethanol self-administration increase Fos activation of ORX neurons (Dayas et al., 2008; Hamlin et al., 2009) and that the OX1R antagonist SB reduces conditioned ethanol-seeking (Lawrence et al., 2006; Richards et al., 2008). Given the widespread distribution of ORX axons across the brain (Peyron et al., 1998), there are clearly multiple sites at which ORX could influence ethanol preference. As noted above, the effect of ORX in the PVN appears to facilitate ethanol drinking (Schneider et al., 2007) and this pathway also may be important for ethanol preference. Further work will be needed to identify specific circuits involved.
Notably, blocking OX1Rs in this study reduced ethanol in high-drinking/preferring animals, but only in very few cases did SB completely abolish ethanol drinking. Instead, SB administration to preferrers appeared to decrease the amount of alcohol consumed to a level equivalent to that of non-preferrers. This suggests that a major difference between high- and low-ethanol preference involves the orexin system. This difference could be genetic, as suggested by individual differences in ethanol preference, or based on experience, as suggested by differences in ethanol preference induced by different training regimens. One hypothesis suggested by these data is that the ORX system is activated in varying degrees in relation to alcohol preference. It is possible that dysregulation or hyper-activation of the ORX system could produce pathological alcohol craving, which may be restrained by dampening such activation. If this mechanism proved to be true, a treatment for alcohol abuse or alcoholism that involves the ORX system could reduce pathological or problematic craving and consumption but leave more modest (and natural) reward-seeking intact (via a normally functioning ORX system). Data from previous work (e.g., Lawrence et al., 2006; Richards et al., 2008) also supports this idea. Although the current studies address differences in individuals exhibiting high vs. low ethanol preference, future studies directly examining excessive ethanol consumption (as described above) will be needed to understand what role the ORX system specifically has in alcohol dependence and alcoholism.
Acknowledgments
We thank Dr. Howard Becker for helpful comments on this manuscript and Paul Knackstedt for excellent technical support. This work was supported by T32 AA007474, R37-DA06214 and R01-DA017289.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology . 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Sutcliffe JG. The hypocretins/orexins: novel hypothalamic neuropeptides involved in different physiological systems. Cell Mol Life Sci. 1999;56:473–480. doi: 10.1007/s000180050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA Newsletter. Bethesda MD: National Institute on Alcohol Abuse and Alcoholism; 2004. NIAAA Council approves definition of binge drinking. No 3 (NIH pub no 04–5346) [Google Scholar]
- Nishino S. Narcolepsy: pathophysiology and pharmacology. J Clin Psychiatry . 2007;68(Suppl 13):9–15. [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Wise RA. Maximization of ethanol intake in the rat. Adv Exp Med Biol. 1975;59:279–294. doi: 10.1007/978-1-4757-0632-1_19. [DOI] [PubMed] [Google Scholar]