Abstract
Although animal models of Alzheimer’s disease (AD) recapitulate β-amyloid-dependent hippocampal synaptic and cognitive dysfunctions, it is poorly understood how cortex-dependent remote memory stabilization following initial hippocampal coding is affected. Here, we systematically analyzed biophysical and behavioral phenotypes, including remote memory functions, of 5XFAD APP/PS1 transgenic mice containing five familial AD mutations. We found that 5XFAD mice show hippocampal dysfunctions as observed by reduced levels of baseline transmission and long-term potentiation at Schaffer collateral-CA1 synapses. Hippocampus-dependent memory tested 1 day after contextual fear conditioning was also impaired age-dependently in 5XFAD mice, as correlated with the onset of hippocampal synaptic failures. Importantly, remote memory stabilization during 30 days after training significantly declined in 5XFAD mice at time well before the onset of hippocampal dysfunctions. Our results indicate that 5XFAD mice provide a useful model system to investigate the mechanisms and therapeutic interventions for multiple synaptic and memory dysfunctions associated with AD.
Keywords: Alzheimer’s disease, amyloid, synaptic plasticity, LTP, hippocampus, cognition, remote memory, fear conditioning, transgenic, 5XFAD mice
Introduction
During the past decade, animal models of Alzheimer’s disease (AD) have made considerable contribution to our understanding of molecular and pathophysiological mechanisms of AD leading to memory deficits (Ashe, 2001; Eriksen and Janus, 2007; Gotz and Ittner, 2008; Kobayashi and Chen, 2005; LaFerla and Oddo, 2005; McGowan et al., 2006), which are difficult to address in human studies and impossible in cultured cells. Although to date no perfect model of AD has emerged, transgenic mice that overexpress the mutated human amyloid precursor protein (APP), presenilin (PS) and tau genes or combine more than one of these mutations successfully recapitulate many aspects of AD such as β-amyloid (Aβ) plaques, neurofibrillary tangles, gliosis, synaptic degeneration, and neuronal loss to some extent. Furthermore, extensive behavioral studies have demonstrated progressive Aβ-dependent memory impairments in a series of AD transgenic mouse models, using a broad battery of hippocampus-dependent memory assays such as Morris water maze, Y-maze, fear conditioning and object or social recognition tasks. However, these studies predominantly tested the acquisition of learning or memories shortly (~24 h) after training in APP mice, thus mainly evaluating defects in hippocampal coding of various types of memories (Ashe, 2001; Eriksen and Janus, 2007; Kobayashi and Chen, 2005; Ohno, 2006). On the other hand, evidence is accumulating that memories of daily life may depend initially on the hippocampus, but are not formed instantaneously and undergo a subsequent prolonged period of reorganization (Dudai, 2004; Frankland and Bontempi, 2005; McClelland et al., 1995; Squire and Bayley, 2007). In particular, as memories mature, they become increasingly independent of the hippocampus and memory traces are gradually stabilized and eventually transformed into remote memories in cortical networks (Bontempi et al., 1999; Frankland et al., 2004; Maviel et al., 2004). Importantly, significant Aβ accumulation occurs not only in the hippocampus but also in the cerebral cortex of AD patients and APP mice (Gotz and Ittner, 2008; McGowan et al., 2006) and almost every learning and memory system including long- as well as short-term memories is impaired in AD (Carlesimo and Oscar-Berman, 1992; Hom, 1992; Pepin and Eslinger, 1989). Nevertheless, it remains unclear how the prolonged processing of remote memory stabilization is affected in AD mouse models, as compared to the initial hippocampal memory formation.
Among APP transgenic mice that have been developed so far, we previously reported that 5XFAD mice (Tg6799 line) that co-overexpress human APP and presenilin 1 (PS1) harboring five familial AD (FAD) mutations represent one of the most early-onset and aggressive amyloid mouse models (Oakley et al., 2006; Ohno et al., 2006; Ohno et al., 2007). While the majority of AD transgenic mice take ~6–12 months, or longer, to form amyloid plaques (Eriksen and Janus, 2007), 5XFAD mice start to develop visible amyloid deposits as early as ~2 months of age consistent with their dramatically accelerated Aβ42 production. Aβ deposition first emerges in the subiculum of hippocampal area and the layer 5 of the cortex and increases rapidly with age, spreading to fill much of the hippocampus and cortex in 5XFAD mice by 6 months of age (Oakley et al., 2006). In this study, we tested <4- and 6-month-old 5XFAD mice that showed moderate and massive amyloid deposition in both hippocampal and cortical areas at these ages, respectively, and characterized their electrophysiological and behavioral phenotypes including changes in remote memory stabilization. Here we report that 5XFAD mice not only have hippocampal dysfunctions, as evidenced by reduced basal synaptic transmission, deficient long-term potentiation (LTP: a cellular basis of learning and memory) at Schaffer collateral-CA1 synapses and impaired contextual fear memory formation, but also exhibit significant remote memory dysfunction, which precedes the onset of hippocampal synaptic and cognitive failures.
Materials and methods
Mouse lines
We used 5XFAD APP/PS1 doubly transgenic mice that co-express and co-inherit FAD mutant forms of human APP (the Swedish mutation: K670N, M671L; the Florida mutation: I716V; the London mutation: V717I) and PS1 (M146L; L286V) transgenes under transcriptional control of the neuron-specific mouse Thy-1 promoter (Tg6799 line) (Oakley et al., 2006; Ohno et al., 2006). In 5XFAD mice, the Swedish mutation increases the production of total Aβ while the other four mutations specifically increase the production of Aβ42. Consequently, five FAD mutations act together to additively increase levels of cerebral Aβ peptides, especially neurotoxic Aβ42. 5XFAD lines (B6/SJL genetic background) were maintained by crossing heterozygous transgenic mice with B6/SJL F1 breeders (Taconic, Hudson, NY). All 5XFAD transgenic mice used were heterozygotes with respect to the transgene, and non-transgenic wild-type littermate mice served as controls. All experiments were done at <4 (3.5–4) months or 6 (5.5–6.5) months of age, blind with respect to the genotype of the mice, and were conducted with the approval of the Nathan Kline Institute Animal Care and Use Committee.
Electrophysiology
After brains were removed from mice following decapitation under deep isoflurane anesthesia, transverse hippocampal slices (400 μm thick) were prepared using a vibratome (VT1200, Leica) and were maintained in an artificial cerebral spinal fluid (aCSF)-filled holding chamber at room temperature for at least 1 h. The aCSF contained (in mM) 124 NaCl, 3 KCl, 2.4 CaCl2, 2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3 and 10 D-glucose, and was equilibrated with 95% O2 and 5% CO2. Slices were individually transferred to the submerged glass-bottom recording chamber, which was constantly perfused with oxygenated aCSF (2 ml/min) at 30°C.
Field excitatory postsynaptic potential (fEPSP) was recorded with a metallic (Pt/Ir) electrode (FHC, Bowdoin, ME) from the stratum radiatum layer of CA1 area, and the Schaffer collateral afferents were stimulated with 100-μs test pulses via a bipolar cluster electrode (FHC) (Chen et al., 2006; Ohno et al., 2001; Ohno et al., 2002). To evaluate basal synaptic transmission, we applied different stimulation strengths (20 μA to 100 μA in steps of 10 μA) and plotted fEPSP slopes versus the amplitudes of presynaptic fiber volleys to compare the slope of input/output (I/O) curves of fEPSP. In the following experiments, stimulus current was adjusted so that fEPSP stabilized at 40–50% of maximum. To test paired-pulse facilitation, we measured the percentage increase in the slope of fEPSP relative to the first one with different interpulse intervals (20–500 ms). LTP was induced by 3- and 10-theta-burst stimulation (TBS) protocols (each burst consisted of 4 pulses at 100 Hz with a 200-ms interburst interval). Before TBS application, the responses were monitored for at least 20 min to ensure a stable baseline of fEPSP. To determine whether the magnitude of LTP differed significantly between groups, average responses during the last 10-min block of recordings (30–40 min after TBS) were compared.
Contextual fear conditioning
Contextual fear conditioning was tested as described previously (Ohno et al., 2001; Ohno et al., 2005). The experiments were performed using four standard conditioning chambers, each of which was housed in a soundproof isolation cubicle and equipped with a stainless-steel grid floor connected to a solid-state shock scrambler. Each scrambler was connected to an electronic constant-current shock source that was controlled via an interface connected to a Windows XP computer running FreezeFrame software (Coulbourn Instruments, Allentown, PA). A digital camera was mounted on the steel ceiling of each chamber, and video signals were sent to the same computer for analysis. During training, mice were placed in the conditioning chamber for 3 min and then received one unsignaled footshock (1.0 mA, 2 s). When mice at 6 months of age received more intensive training to ensure initial hippocampal memory formation, they were given 3 or 5 footshocks at 1 min intervals. After the last shock delivery, mice were left in the chamber for another 30 s.
Hippocampus-dependent contextual fear memory formation and the subsequent remote memory stabilization were evaluated by scoring freezing behavior (the absence of all but respiratory movement) for 3 min when the mice were placed back into the same conditioning chamber 1 and 30 days after training, respectively. To avoid the confounding effects of extinction due to repeated exposures to the chamber, separate groups of mice were tested at each retention delay. The automated FreezeFrame system (Coulbourn) digitizes the video signal at 4 Hz and compares movement frame by frame to score the amount of freezing. To investigate memory extinction, different groups of mice were given a single 30-min re-exposure to the training chamber in the absence of footshock 1 day after contextual conditioning. Twenty-four hours later, we placed back the mice into the chamber again for 3 min and tested whether contextual fear memory was extinguished.
Statistical analysis
The significance of differences between the groups was determined by a one-way ANOVA or two-way ANOVA with repeated measures, and post-hoc Fisher’s PLSD tests were performed when appropriate. Paired t-test was used to compare levels of freezing between the first 6 min of extinction session and 24 h post-extinction. Data were presented as mean ± SEM and the level of significance was set for P value less than 0.05.
Results
Hippocampal synaptic failures in 5XFAD mice
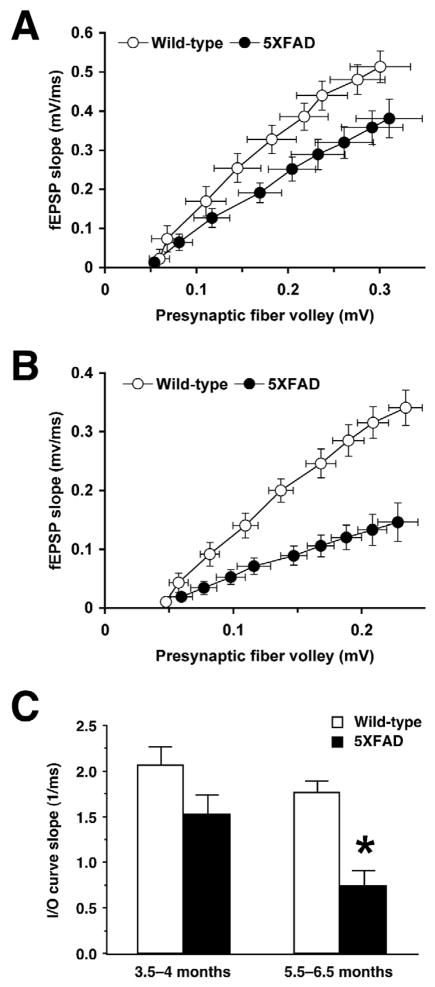
Neurophysiological consequences of Aβ accumulation that may underlie deficits in hippocampal memory formation are principally reductions in baseline excitatory transmission and/or deficient LTP at hippocampal CA1 glutamatergic synapses in a series of AD transgenic mouse models such as APPSwe (Tg2576) (Chapman et al., 1999; Fitzjohn et al., 2001; Jacobsen et al., 2006), APPInd (H6) (Hsia et al., 1999), APPSwe·Ind (J20) (Palop et al., 2007), PDAPP (Larson et al., 1999), APP23 (Roder et al., 2003), APP[V717I] (Dewachter et al., 2008) and APP/PS1 (Gureviciene et al., 2004; Trinchese et al., 2004) transgenic mice (for review, see Rowan et al., 2003). We conducted a systematic evaluation of changes in hippocampal synaptic functions in 5XFAD mice at different stages of disease progression. In hippocampal slices from <4-month-old 5XFAD mice, I/O curves of fEPSPs in response to different stimulation strengths were not different from those of wild-type controls (Fig. 1A). In contrast, the I/O responses at Schaffer collateral-CA1 synapses in 5XFAD mice at 6 months of age showed deficits when compared with those of their wild-type littermate controls (Fig. 1B). Consequently, basal synaptic transmission as assessed by the average slope of I/O curves was significantly reduced in 5XFAD mice at 6 months of age [F(1,19)=24.86, P<0.05] but not at <4 months of age [F(1,16)=3.45, P>0.05] (Fig. 1C).
Fig. 1.
Age-dependent impairments of basal synaptic transmission in hippocampal slices from 5XFAD mice. A and B, Input/output (I/O) curves relating the amplitude of the presynaptic fiber volley to the slope of fEPSP at various stimulus intensities (20–100 μA) in the CA1 region of 5XFAD mice at <4 months (A) and 6 months (B) of ages. C, The summary bar graph shows significant reductions in basal synaptic transmission as measured by the average slope of I/O curves in 5XFAD mice at 6 months of age but not at <4 months of age (n = 9–11 mice per group). * P<0.05 versus wild-type controls. All data are presented as mean ± SEM.
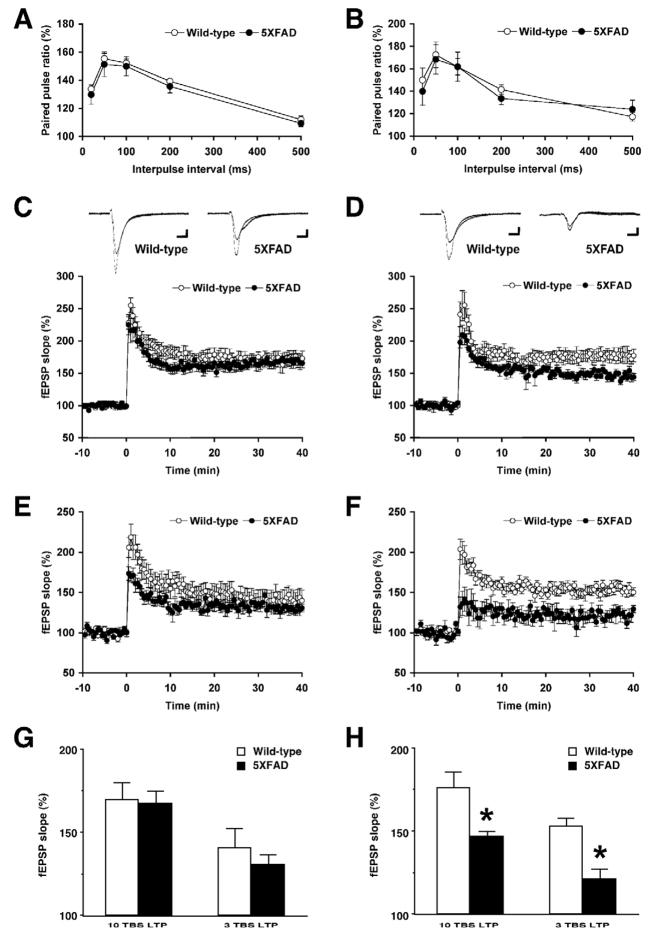
We next examined plasticity at hippocampal Schaffer collateral-CA1 synapses of 5XFAD mice (Fig. 2). First, we tested paired-pulse facilitation, which represents a short-term form of synaptic plasticity reflecting presynaptic release probability. Paired-pulse facilitation was indistinguishable between 5XFAD mice and wild-type controls at both <4 months of age (Fig. 2A) and 6 months of age (Fig. 2B). Therefore, short-lived presynaptic plasticity was normal in 5XFAD mice, as reported in other APP transgenic mouse models (Chapman et al., 1999; Fitzjohn et al., 2001; Palop et al., 2007). In contrast, LTP at Schaffer collateral-CA1 synapses was significantly affected in 5XFAD mice in an age-dependent manner. While no difference was found between 5XFAD mice and wild-type controls at <4 months of age in LTP induced by 10-TBS (Fig. 2C) or 3-TBS (Fig. 2E), 6-month-old 5XFAD mice showed impairments of LTP following the application of 10-TBS (Fig. 2D) and 3-TBS (Fig. 2F). A one-way ANOVA comparing the average magnitude of LTP during 30–40 min after induction revealed that LTP was significantly reduced in 5XFAD mice at 6 months of age in 10-TBS [F(1,11)=5.61, P<0.05] and 3-TBS protocols [F(1,12)=18.85, P<0.05] (Fig. 2H), whereas neither LTP was affected in 5XFAD mice at <4 months of age [10-TBS, F(1,13)=0.03, P>0.05; 3-TBS, F(1,12)=0.59, P>0.05] (Fig. 2G).
Fig. 2.
Age-dependent impairments of LTP but not paired-pulse facilitation in hippocampal slices from 5XFAD mice. A and B, Paired-pulse facilitation across different inter-stimulus intervals (20–500 ms) is normal in the CA1 region of 5XFAD mice at <4 months (A) and 6 months (B) of ages (n = 6–12 mice per group). C–F, LTP induced by 10-TBS (C: <4 months of age, D: 6 months of age) and 3-TBS (E: <4 months of age, F: 6 months of age) in the CA1 region of 5XFAD mice. Each point indicates the fEPSP slope normalized to the average baseline response before TBS applied at time 0. Traces are the average of fEPSPs recorded during baseline and 30–40 min after TBS. Calibration: 0.1 mV, 10 ms. G and H, The summary bar graphs show significant reductions in LTP as measured by the average normalized fEPSP slopes during 30–40 min after TBS in 5XFAD mice at 6 months of age (H) but not at <4 months of age (G) (n = 5–8 mice per group). * P<0.05 versus wild-type controls. All data are presented as mean ± SEM.
Impairments of hippocampal memory formation in 5XFAD mice
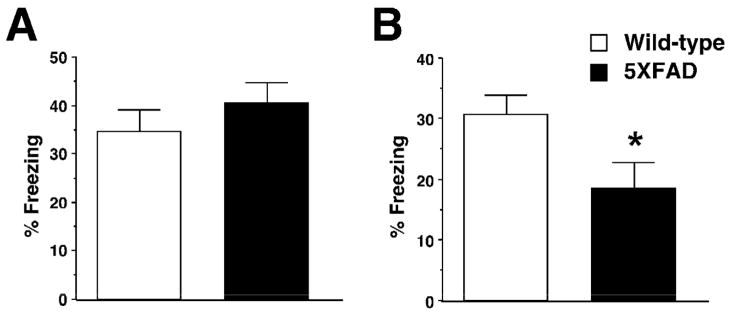
We tested 5XFAD mice with hippocampus-dependent contextual fear conditioning, in which mice learn to associate a distinct context (CS: conditioned stimuli) with an aversive footshock (US: unconditioned stimuli) (Fanselow, 2000). Wild-type mice exhibited a robust conditioned fear response as assessed by freezing (the absence of all but respiratory movements) when placed back into the conditioning chamber 1 day after training with a single CS-US paring (Fig. 3). While 5XFAD mice at <4 months of age showed freezing that was indistinguishable from that of wild-type controls [F(1,54)=0.90, P>0.05] (Fig. 3A), 5XFAD mice at 6 months of age exhibited significantly lower levels of freezing as compared to wild-type littermates [F(1,25)=5.04, P<0.05] (Fig. 3B). Therefore, 5XFAD mice showed age-dependent deficits in hippocampus-dependent formation of contextual fear memory consistent with the onset of hippocampal CA1 synaptic failures (Figs. 1 and 2).
Fig. 3.
Age-dependent impairments of contextual fear conditioning in 5XFAD mice. A and B, 5XFAD mice at <4 months (A) or 6 months (B) of age and their wild-type littermate mice were trained with a single CS-US pairing for contextual fear conditioning. 5XFAD mice at 6 months of age but not at <4 months of age show significantly lower levels of contextual freezing than wild-type mice when tested 1 day after training (n = 13–30 mice per group). * P<0.05 versus wild-type controls. All data are presented as mean ± SEM.
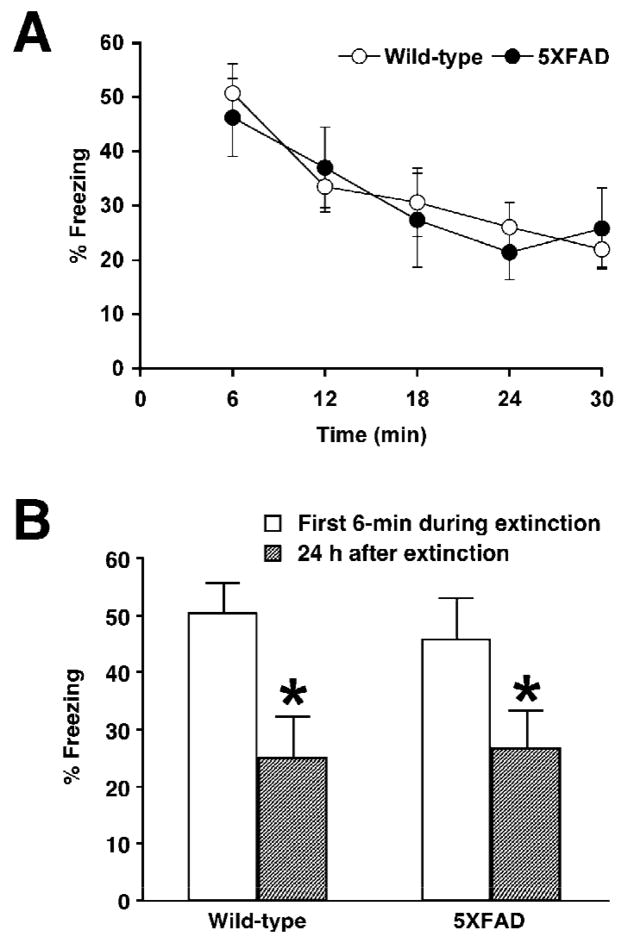
To further explore hippocampal processing of contextually conditioned fear in 5XFAD mice, we next tested memory extinction following acquisition (Fig. 4). If animals are given repeated or prolonged re-exposure to CS without US after fear conditioning, they exhibit memory extinction as measured by a decline in freezing (Isiegas et al., 2006; Suzuki et al., 2004), which is thought to be an active learning process resulting in new memories that compete with and suppress the expression of original fear memory (Myers and Davis, 2002; Myers and Davis, 2007). When mice received a single 30-min re-exposure to the chamber in the absence of footshock 1 day after training, freezing levels gradually decreased in both 5XFAD mice at <4 months of age and wild-type littermate controls with the time course of extinction session [F(4,56)=10.72, P<0.05] (Fig. 4A). There was no difference in extinction rates between both groups, as indicated by no significant interaction between genotype and session [F(4,56)=0.46, P>0.05]. Furthermore, significantly lower levels of freezing during memory testing 1 day post-extinction relative to the first 6 min period of extinction session were observed in 5XFAD mice [paired t-test, t(6)=2.72, P<0.05] as well as in wild-type control mice [paired t-test, t(8)=3.75, P<0.05], representing similar retention of extinction in both groups (Fig. 4B). Collectively, 5XFAD mice at <4 months of age were normal not only in hippocampus-dependent contextual learning (Fig. 3) but also in extinguishing these fear memories after acquisition (Fig. 4).
Fig. 4.
Normal memory extinction following contextual fear conditioning in 5XFAD mice at <4 months of age. A, 5XFAD mice and their wild-type littermate mice were trained with one CS-US pairing for contextual fear conditioning. Mice were subjected to a single 30-min re-exposure to the conditioning context 1 day after training. Levels of freezing are plotted every 6 min through the 30-min extinction session. 5XFAD mice as well as wild-type control mice exhibit significant reductions in contextual freezing within the extinction session. B, Both wild-type and 5XFAD mice show significantly lower levels of freezing during the test session given 1 day after the 30-min re-exposure, as compared with the first 6-min of extinction session (n = 7–9 mice per group). * P < 0.05 versus the first 6-min of extinction. All data are presented as mean ± SEM.
Impairments of remote memory stabilization in 5XFAD mice
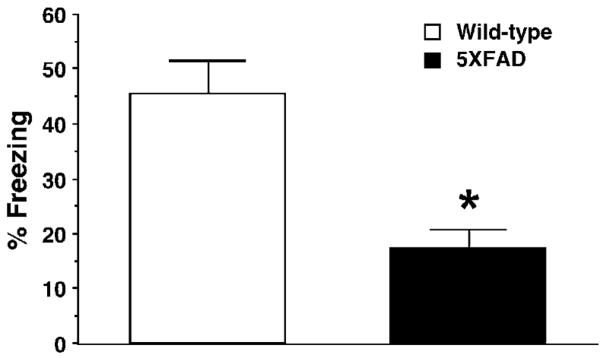
Previous studies with brain inactivation and imaging techniques demonstrate the temporary role of the hippocampus in the formation and maintenance of memory, and provide convergent evidence for the time shift from hippocampus-dependent recent memories (1 day post-training) to hippocampus-independent (but cortex-dependent) remote memories (~30 days post-training) in a variety of learning tasks including contextual fear conditioning (Bontempi et al., 1999; Frankland and Bontempi, 2005; Frankland et al., 2004; Maviel et al., 2004; Squire and Bayley, 2007). Therefore, we tested remote memory function by re-exposing separate groups of mice to the training chamber 30 days after contextual fear conditioning (Fig. 5). Remarkably, 5XFAD mice at <4 months of age showed significantly lower levels of contextual freezing as compared to wild-type control mice 30 days after training with one CS-US pairing [F(1,21)=11.22, P<0.05] (Fig. 5) in contrast to their normal 24-h memory at the same age (Fig. 3A). Therefore, 5XFAD mice at <4 months of age were specifically impaired in remote memory stabilization without changes in hippocampus-dependent memory formation.
Fig. 5.
Impairments of remote memory stabilization following contextual fear conditioning in 5XFAD mice at <4 months of age. 5XFAD mice and their wild-type littermate mice were trained with one CS-US pairing for contextual fear conditioning. 5XFAD mice show significantly lower levels of contextual freezing than wild-type mice when tested 30 days after training (n = 8–15 mice per group). * P<0.05 versus wild-type controls. All data are presented as mean ± SEM.
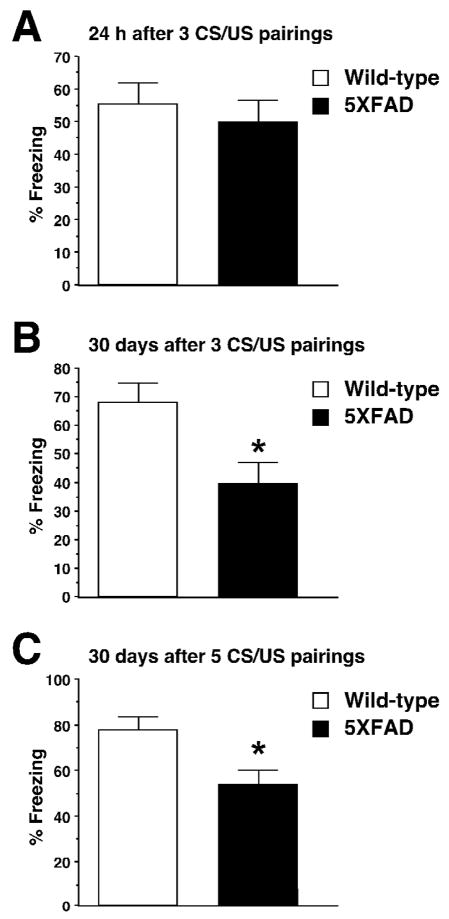
We further tested whether remote memory function remained impaired in 5XFAD mice of advanced age (6-month-old) (Fig. 6). To this end, we trained the mice using stronger training protocols with three to five CS-US pairings, since the deficient hippocampal memory formation following milder training with one CS-US pairing (Fig. 3B) would preclude the analyses of subsequent remote memory stabilization. 5XFAD mice at 6 months of age exhibited robust contextual freezing that was comparable to wild-type controls 24 h after training with 3 footshocks [F(1,30)=0.38, P>0.05] (Fig. 6A). Notably, we found that while 5XFAD mice at this advanced age successfully overcame 24-h memory deficits with the more intensive training, they showed impairments in remote memory stabilization as tested 30 days following 3 CS-US pairings [F(1,24)=8.48, P<0.05] (Fig. 6B). Remote memory defects in contextual conditioning were also observed in 6-month-old 5XFAD mice, when they were trained with further stronger protocols with 5 shocks and were tested for memory 30 days later [F(1,26)=8.26, P<0.05] (Fig. 6C). Collectively, remote memory failures were consistently observed in 5XFAD mice independently of ages or training protocols, and preceded the onset of hippocampal dysfunctions.
Fig. 6.
Impairments of remote memory stabilization following contextual fear conditioning in 5XFAD mice at 6 months of age. A, 5XFAD mice show levels of contextual freezing equivalent to those of wild-type mice when tested 1 day after training with three CS-US pairings (n = 15–17 mice per group). B, 5XFAD mice show significantly lower levels of freezing than wild-type mice when tested 30 day after training with three CS-US pairings (n = 13 mice per group). C, 5XFAD mice show significantly lower levels of freezing than wild-type mice when tested 30 day after training with five CS-US pairings (n = 14 mice per group). * P<0.05 versus wild-type controls. All data are presented as mean ± SEM.
Discussion
5XFAD APP/PS1 doubly transgenic mice recapitulate many features of AD-related pathological changes in an accelerated fashion (Oakley et al., 2006; Ohno et al., 2006; Ohno et al., 2007). Those include astrogliosis and microgliosis that are proportional to Aβ deposition in hippocampal and cortical areas and begin at 2 months of age, degeneration of synapses by 9 months of age as evidenced by reductions in synaptic marker proteins (synaptophysin, syntaxin and PSD-95), and pronounced loss of large pyramidal neurons by 9 months of age in the cortical layer 5 and subiculum (the same regions with the greatest amyloid burden), a feature that is absent in the majority of APP mouse models. In line with our previous findings that Aβ levels and amyloid burden dramatically increase in 5XFAD brains between 4 and 6 months of ages (Oakley et al., 2006), this study demonstrates that hippocampal synaptic dysfunctions, as assessed by reductions in both basal transmission (AMPA/kainate receptor-mediated synaptic responses) and LTP (a measure of NMDA receptor-dependent synaptic plasticity) at Schaffer collateral-CA1 synapses, emerge in 5XFAD mice at 6 months of age but are not found at <4 months of age. Our results are consistent with the observations that both NMDA and AMPA receptor-mediated currents were decreased and NMDA-dependent LTP was impaired in hippocampal CA1 region of other APP transgenic mice (Dewachter et al., 2008; Jacobsen et al., 2006; Larson et al., 1999; Priller et al., 2008; Trinchese et al., 2004). Reduced synaptic levels of the AMPA receptor subunit GluR1 (Almeida et al., 2005) or the NMDA receptor subunits NR1 and NR2B (Dewachter et al., 2008; Snyder et al., 2005) in APP mouse neurons provide a molecular basis for these physiological changes, although precise mechanisms by which Aβ accumulation alters synapses remain to be determined.
Importantly, we found that concomitant with the onset of hippocampal synaptic failures, 5XFAD mice begin to exhibit impairments in hippocampus-dependent memory formation at 6 months of age, as tested 1 day after contextual fear conditioning. Therefore, it is conceivable that Aβ-dependent reductions in basal synaptic transmission, deficits in LTP or both may contribute to the hippocampal memory decline in 5XFAD mice. Notably, our recent findings indicated that while genetic reductions of BACE1 (BACE1+/−), the major β-secretase enzyme that initiates APP cleavage to produce Aβ, rescued contextual memory deficits found in 5XFAD mice at 6 months of age, only LTP deficits were restored to normal consistent with memory improvements and basal synaptic transmission remained significantly reduced in BACE1+/−· 5XFAD mice (Kimura and Ohno, unpublished results). Collectively, it seems reasonable to suppose that deficient LTP rather than reduced levels of baseline transmission at CA1 synapses may be more closely related with hippocampus-dependent memory dysfunction in 5XFAD mice, although further investigation is required.
It is well known that pharmacological inactivation or lesions of the hippocampus produces temporally graded effects on the memories, with recent memories more profoundly disrupted in the absence of changes in remote memories (Anagnostaras et al., 1999; Frankland and Bontempi, 2005; Frankland et al., 2004; Maviel et al., 2004; Squire et al., 2001; Takehara et al., 2003). In contrast, inactivating or lesioning cortical areas (e.g., the medial prefrontal or anterior cingulate cortex) at various intervals after the establishment of hippocampal learning showed specific impairments in memories at remote (28–36 days) but not recent (1 day) time points in a battery of behavioral paradigms including contextual fear conditioning (Frankland et al., 2004), water maze (Teixeira et al., 2006), five-arm discrimination (Maviel et al., 2004) and trace eyeblink conditioning (Takehara et al., 2003). The present study revealed that remote memory stabilization is consistently impaired in 5XFAD mice at different ages when tested 30 days after contextual fear conditioning with multiple training protocols. To our knowledge, this is the first demonstration of deficient remote memory stabilization in AD transgenic model mice, reflecting cortical dysfunction probably due to the significant Aβ accumulation in this brain structure. Interestingly, while hippocampal processing of memories, including their formation and extinction 1 day after contextual fear training, are normal in 5XFAD mice at <4 months of age, cortical remote memory processing is specifically impaired at this age with moderate levels of Aβ deposition in the cortex and hippocampus of 5XFAD mice (Oakley et al., 2006). Therefore, the emergence of the remote memory deficits precedes the onset of hippocampal cognitive as well as synaptic dysfunctions in 5XFAD mice. Therefore, it is possible that memory traces cannot be successfully transferred and stabilized as remote memories into cortical neuron networks, even if they may be temporarily encoded within hippocampal circuits during earlier stages of AD progression. It should be noted that NMDA receptor-dependent forms of LTP, but not basal synaptic transmission, are significantly impaired in the cerebral cortex (including the medial prefrontal cortex) of APP/PS1 transgenic mice and that neocortical LTP-like plasticity declines in AD patients with mild-to-moderate AD as compared to age-matched normal controls (Battaglia et al., 2007). Taken collectively, it is reasonable to speculate that the early-onset cortical plasticity deficits may account for the AD-associated impairments of remote memory stabilization, although further studies are needed to fully elucidate the mechanisms underlying cortical dysfunctions in AD.
AD transgenic mouse models have enabled dramatic advances in our understanding not only of the pathogenic mechanism in AD but also of therapeutic interventions for this disease (Ashe, 2001; Eriksen and Janus, 2007; Gotz and Ittner, 2008; Kobayashi and Chen, 2005; LaFerla and Oddo, 2005; McGowan et al., 2006). In particular, the use of behavioral tests to study the cognitive asset of AD animal models is essential for evaluating possible therapeutic strategies, but previous studies have focused mainly on the hippocampus-dependent mechanisms of memory encoding (Kobayashi and Chen, 2005; Ohno, 2006; Van Dam and De Deyn, 2006). Only recently, a couple of papers started to reveal that APP and APP/PS1 transgenic mice are also impaired in a hippocampus-independent associative implicit memory as tested by the conditioned taste aversion, consistent with clinical observations that implicit as well as hippocampal explicit memories are affected in AD (Janus et al., 2004; Pistell et al., 2008). Furthermore, our present results demonstrate that cortical remote memory stabilization, an important process that follows hippocampal memory formation, is profoundly impaired in 5XFAD APP/PS1 transgenic model mice. As such, it will be important to pay more attention to the hippocampus-independent processing of memories in future experiments with AD mouse models and more comprehensively test the efficacies of potential therapeutic approaches in treating a broad spectrum of multiple cognitive dysfunctions associated with AD.
Acknowledgments
This work was supported by grants from National Institute of Mental Health (R01 MH067251) and Alzheimer’s Association (IIRG-08-91231) to M.O.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe KH. Learning and memory in transgenic mice modeling Alzheimer’s disease. Learn Mem. 2001;8:301–308. doi: 10.1101/lm.43701. [DOI] [PubMed] [Google Scholar]
- Battaglia F, Wang HY, Ghilardi MF, Gashi E, Quartarone A, Friedman E, Nixon RA. Cortical plasticity in Alzheimer’s disease in humans and rodents. Biol Psychiatry. 2007;62:1405–1412. doi: 10.1016/j.biopsych.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Oscar-Berman M. Memory deficits in Alzheimer’s patients: a comprehensive review. Neuropsychol Rev. 1992;3:119–169. doi: 10.1007/BF01108841. [DOI] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TVP, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Chen AP, Ohno M, Giese KP, Kuhn R, Chen RL, Silva AJ. Forebrain-specific knockout of B-raf kinase leads to deficits in hippocampal long-term potentiation, learning, and memory. J Neurosci Res. 2006;83:28–38. doi: 10.1002/jnr.20703. [DOI] [PubMed] [Google Scholar]
- Dewachter I, Filipkowski RK, Priller C, Ris L, Neyton J, Croes S, Terwel D, Gysemans M, Devijver H, Borghgraef P, Godaux E, Kaczmarek L, Herms J, Van Leuven F. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.06.011. in press. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Janus CG. Plaques, tangles, and memory loss in mouse models of neurodegeneration. Behav Genet. 2007;37:79–100. doi: 10.1007/s10519-006-9118-z. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Morton RA, Kuenzi F, Rosahl TW, Shearman M, Lewis H, Smith D, Reynolds DS, Davies CH, Collingridge GL, Seabrook GR. Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. J Neurosci. 2001;21:4691–4698. doi: 10.1523/JNEUROSCI.21-13-04691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Gureviciene I, Ikonen S, Gurevicius K, Sarkaki A, van Groen T, Pussinen R, Ylinen A, Tanila H. Normal induction but accelerated decay of LTP in APP + PS1 transgenic mice. Neurobiol Dis. 2004;15:188–195. doi: 10.1016/j.nbd.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Hom J. General and specific cognitive dysfunctions in patients with Alzheimer’s disease. Arch Clin Neuropsychol. 1992;7:121–133. [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci U S A. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isiegas C, Park A, Kandel ER, Abel T, Lattal KM. Transgenic inhibition of neuronal protein kinase A activity facilitates fear extinction. J Neurosci. 2006;26:12700–12707. doi: 10.1523/JNEUROSCI.2743-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janus C, Welzl H, Hanna A, Lovasic L, Lane N, St George-Hyslop P, Westaway D. Impaired conditioned taste aversion learning in APP transgenic mice. Neurobiol Aging. 2004;25:1213–1219. doi: 10.1016/j.neurobiolaging.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kobayashi DT, Chen KS. Behavioral phenotypes of amyloid-based genetically modified mouse models of Alzheimer’s disease. Genes Brain Behav. 2005;4:173–196. doi: 10.1111/j.1601-183X.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Oddo S. Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol Med. 2005;11:170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G, Games D, Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 1999;840:23–35. doi: 10.1016/s0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McGowan E, Eriksen J, Hutton M. A decade of modeling Alzheimer’s disease in transgenic mice. Trends Genet. 2006;22:281–289. doi: 10.1016/j.tig.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M. Genetic and pharmacological basis for therapeutic inhibition of β- and □-secretases in mouse models of Alzheimer’s memory deficits. Rev Neurosci. 2006;17:429–454. doi: 10.1515/revneuro.2006.17.4.429. [DOI] [PubMed] [Google Scholar]
- Ohno M, Chang L, Tseng W, Oakley H, Citron M, Klein WL, Vassar R, Disterhoft JF. Temporal memory deficits in Alzheimer’s mouse models: rescue by genetic deletion of BACE1. Eur J Neurosci. 2006;23:251–260. doi: 10.1111/j.1460-9568.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- Ohno M, Cole SL, Yasvoina M, Zhao J, Citron M, Berry R, Disterhoft JF, Vassar R. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol Dis. 2007;26:134–145. doi: 10.1016/j.nbd.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Frankland PW, Chen AP, Costa RM, Silva AJ. Inducible, pharmacogenetic approaches to the study of learning and memory. Nat Neurosci. 2001;4:1238–1243. doi: 10.1038/nn771. [DOI] [PubMed] [Google Scholar]
- Ohno M, Frankland PW, Silva AJ. A pharmacogenetic inducible approach to the study of NMDA/αCaMKII signaling in synaptic plasticity. Curr Biol. 2002;12:654–656. doi: 10.1016/s0960-9822(02)00767-4. [DOI] [PubMed] [Google Scholar]
- Ohno M, Tseng W, Silva AJ, Disterhoft JF. Trace eyeblink conditioning requires the hippocampus but not autophosphorylation of αCaMKII in mice. Learn Mem. 2005;12:211–215. doi: 10.1101/lm.90205. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin EP, Eslinger PJ. Verbal memory decline in Alzheimer’s disease: a multiple-processes deficit. Neurology. 1989;39:1477–1482. doi: 10.1212/wnl.39.11.1477. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Zhu M, Ingram DK. Acquisition of conditioned taste aversion is impaired in the amyloid precursor protein/presenilin 1 mouse model of Alzheimer’s disease. Neuroscience. 2008;152:594–600. doi: 10.1016/j.neuroscience.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priller C, Mitteregger G, Paluch S, Vassallo N, Staufenbiel M, Kretzschmar HA, Jucker M, Herms J. Excitatory synaptic transmission is depressed in cultured hippocampal neurons of APP/PS1 mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.10.016. in press. [DOI] [PubMed] [Google Scholar]
- Roder S, Danober L, Pozza M, Lingenhoehl K, Wiederhold K, Olpe H. Electrophysiological studies on the hippocampus and prefrontal cortex assessing the effects of amyloidosis in amyloid precursor protein 23 transgenic mice. Neuroscience. 2003;120:705–720. doi: 10.1016/s0306-4522(03)00381-6. [DOI] [PubMed] [Google Scholar]
- Rowan M, Klyubin I, Cullen W, Anwy lR. Synaptic plasticity in animal models of early Alzheimer’s disease. Philos Trans R Soc Lond B Biol Sci. 2003;358:821–828. doi: 10.1098/rstb.2002.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Squire LR, Bayley PJ. The neuroscience of remote memory. Curr Opin Neurobiol. 2007;17:185–196. doi: 10.1016/j.conb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Clark RE, Knowlton BJ. Retrograde amnesia. Hippocampus. 2001;11:50–55. doi: 10.1002/1098-1063(2001)11:1<50::AID-HIPO1019>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci. 2006;26:7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol. 2004;55:801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- Van Dam D, De Deyn PP. Drug discovery in dementia: the role of rodent models. Nat Rev Drug Discov. 2006;5:956–970. doi: 10.1038/nrd2075. [DOI] [PubMed] [Google Scholar]