Abstract
Background
Proteinuria development and decrease in glomerular filtration rate (GFR) has been observed after successful islet transplantation. The aim of this study was to determine clinical, laboratory and immunosuppressant-related factors associated with kidney dysfunction in islet transplant recipients.
Methods
A retrospective cohort study was conducted in 35 subjects submitted to pancreatic islet transplantation as treatment for unstable type 1 diabetes mellitus. Demographic, anthropometrical and laboratory data, as well as immunosuppressive and anti-hypertensive therapy were recorded. Kidney function was assessed by albuminuria and estimated GFR (eGFR), calculated by Modification of Diet in Renal Disease formula.
Results
Age was the only independent risk factor for low eGFR (<60 ml/min/1.73 m2) [OR=1.78 (1.22–2.61)]. LDL-cholesterol [OR=2.90 (1.37–6.12)] and previous microalbuminuria [OR=6.42 (1.42–29.11)] were risk factors for transient macroalbuminuria. Interestingly, tacrolimus was a protective factor for macroalbuminuria [OR=0.12 (0.06–0.26)]. Six-out-of-thirty (20%) normoalbuminuric subjects at baseline progressed to microalbuminuria. No subject developed sustained macroalbuminuria. Surprisingly, overall eGFR remained stable during follow-up (before transplant: 74.0±2.0, during immunosuppressive therapy: 75.4±2.8, after withdrawal: 76.3±5.3 ml/min/1.73 m2; P>0.05). Even subjects with low eGFR and/or microalbuminuria at baseline (n=10) maintained stable values post-transplantation (61.13±3.25 vs. 63.32±4.36 ml/min/1.73 m2, P=0.500).
Conclusions
Kidney function remained stable after islet transplantation alone. The unchanged kidney function found in this sample may be attributed to healthier kidney status at baseline and possibly to prompt treatment of modifiable risk factors. Aggressive treatment of risk factors for nephropathy, such as blood pressure, LDL-cholesterol and careful tacrolimus levels monitorization, should be part of islet transplant recipient care.
Keywords: islet transplantation, kidney function, albuminuria, lipids, blood pressure
INTRODUCTION
Intensive treatment of type 1 diabetes mellitus (T1DM) results in lower hyperglycemia-related micro- and macrovascular chronic complications (1, 2). However, a better metabolic control can only be achieved at the expense of an increase in the number and severity of hypoglycemic episodes (1). There is a subset of subjects with unstable T1DM which are especially prone to develop severe hypoglycemic episodes due to unawareness of its alert symptoms (3). In these cases, transplantation of allogeneic islets of Langerhans results in stabilization of blood glucose levels with resolution of severe hypoglycemia, subsequent improvement in their metabolic control and quality of life (4, 5).
Emerging evidence of proteinuria development (6), decrease in glomerular filtration rate (GFR) (7–9) and, in some cases, progression to end-stage renal disease (ESRD) (8) have been observed after successful islet transplantation. Stable kidney function after islet transplantation has also been recently described under a sirolimus-sparing immunosuppressive protocol (10). Being diabetic nephropathy (DN) a major cause of morbidity and mortality in T1DM patients (11), concerns about patient’s selection based on pre-transplant kidney function status (9), as well as immunosuppressive agents combinations (10) have been raised.
The aim of this study was to determine the clinical, laboratory and immunosuppressant-related factors associated with renal dysfunction in subjects with T1DM after allogeneic islet transplantation alone (ITA).
MATERIAL AND METHODS
Subjects
A retrospective cohort study was conducted on 36 subjects with T1DM and hypoglycemia unawareness that have been followed in a single center, before [median of 20 months (minimum-maximum: 0–111)] and after [49 months (7–72)] allogeneic ITA between 2000 and 2007. One subject was excluded due to early withdrawal (102 days after transplant) of immunosuppressive therapy due to an adverse event (aspiration pneumonia) and subsequent graft failure. Follow-up included 33 subjects at 12 months, 29 at 18 months, 23 at 24 months, 19 at 30 months, 17 at 36 months, 16 at 42 months and 15 after 48 months. Subjects with baseline renal dysfunction (serum creatinine >1.6 mg/dl or albuminuria >300 mg/24 h) were considered not eligible for islet transplantation.
Transplant related procedures
The pancreatic islet isolation, infusion and immediate post-transplant management were performed as previously described (4). The mean number of infusions per patient was 2.0±0.9 (single n=12; two n=13; three n=8 and four infusions n=2, respectively). The maintenance immunosuppressive regimen was based on tacrolimus (Prograf®, Astellas-Pharma US, Inc., Deerfield, IL, USA; target trough level 4–6 ng/mL) and sirolimus (Rapamune®, Wyeth Pharmaceuticals, Inc., Madison, NJ, USA; target trough level 10–15 ng/mL for 3 months, 8–12 ng/mL thereafter).
Twelve subjects were converted from tacrolimus or sirolimus to Mycophenolate Mofetil (MMF; CellCept®, Roche, Nutley, NJ, USA) or Mycophenolate sodium (MS; Myfortic®, Novartis, East Hanover, NJ, USA), targeting maximum tolerable dosage (maximum of 2000 mg and 1440 mg, respectively). The reason for conversion in 5 subjects was tacrolimus-related side-effects [nephrotoxicity (n=2), eczema (n=1), depression (n=1) and neurotoxicity (n=1)], and in 2 was due to sirolimus side-effects [mouth ulcer (n=1) and migraine (n=1)]. The other 5 subjects received alemtuzumab (Campath-1H®, Genzyme, Cambridge, MA, USA) induction and were converted 3 months after islet infusion from tacrolimus to MMF or MS as per protocol, except for one subject in whom sirolimus was substituted for tacrolimus due to gastrointestinal intolerance.
Six-out-of-eight subjects who received concomitant bone marrow cell (CD34+ enriched) infusion from the same islet donor discontinued immunosuppressive drugs per protocol at one year after the transplant (12). Other variations within protocols were related to the induction agents: five-dose course of daclizumab (1 mg/kg biweekly; Zenapax®, Roche, Nutley, NJ, USA; n=30); or alemtuzumab (20 mg IV, 2 doses before the transplant n=5); and infliximab (5–10 mg/kg 2 h prior to islet infusion, single dose; Remicade®, Centocor, Malvera, PA, USA; n=11) or etanercept (50 mg IV 1 h prior to islet infusion and 25 mg twice a week for 2 weeks; Enbrel®, Amgen, Thousand Oaks, CA, USA;, n=11). All subjects received prophylaxis for cytomegalovirus (13) and P. carinii pneumonia.
In addition to the intervention directly related to transplant, all recipients received aggressive management of risk factors for nephropathy, which included treatment of hypertension and dyslipidemia, as well as tight control of tacrolimus through levels, since this immunosuppressive agent is known to cause nephrotoxicity. The aggressive management was defined according to the American Diabetes Association (ADA) guidelines for preventing/treating DM chronic complications (14) and included treating hypertension with a target blood pressure (BP) <130/80 mmHg, using preferentially angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) and treating dyslpidemia with a target LDL-cholesterol level <100 mg/dl, using statins. Monitoring of tacrolimus levels was also performed with an intensive approach (two to three times a week in the first month, once a week in the second month, twice a month in the third month and every three months thereafter, guaranteeing that the subjects really receive a low-dose tacrolimus protocol (target trough level 4–6 ng/mL).
All protocol procedures were approved by the University of Miami health research ethics board (IRB) and appropriate informed consent was obtained from each subject.
Clinical data assessment
Clinical variables [age, gender, ethnicity, diabetes duration, body mass index (BMI), systolic and diastolic BP], anti-hypertensive therapy (ACEi and ARB) and immunosuppressive medication data were recorded. Diabetic retinopathy (DR) was classified as absent, nonproliferative, or proliferative based on ophthalmologic report. Peripheral neuropathy was diagnosed by clinical symptoms and compatible physical examination and cardiovascular disease by history and stress test.
Laboratorial analysis
Kidney function was evaluated by serum creatinine (Jaffé method, Roche Diagnostics, Roche Cobas-Mira, inter- and intra-assay variation: 1.4% and 2.1%; 4 (1–15) and 17 (4–39) measurements/patient available pre- and post-transplant, respectively) and urinary albumin excretion rate [UAER; immunoturbidimetry, Beckman-Synchron/CX9, Ramsey, Minnesota, USA; 3 (1–10) and 15 (3–31) measurements/patient available pre- and post-transplant, respectively] was measured in 24 h urine collections. The subjects were classified as normoalbuminurics (UAER <30 mg/24 h), microalbuminurics (30–299 mg/24 h) or macroalbuminurics (≥300 mg/24 h) based on 2-out-of-3 pre-transplant measurements and the same criterion was employed for kidney status definition during follow-up. Albuminuria progression was considered when the elevation, based in this criterion, has persisted until the end of the study. If the increment in albuminuria was followed by a regression to the previous stage, the elevation was considered transient. The estimated GFR (eGFR) was calculated by the Modification of Diet in Renal Disease (MDRD) formula (15): 186*[serum creatinine−1.154*age−0.203*(0.742 if female)*(1.210 if afro-American)]. Subjects were classified following the National Kidney Foundation (NKF) guidelines for chronic kidney disease (CKD) (stage 1: ≥90, stage 2: 60 – 89, stage 3: 30 – 59, stage 4: 15–29 and stage 5: <15 ml/min/1.73 m2) (16). Glycemic profiles were evaluated by fasting plasma glucose (hexokinase method) and A1c [high performance liquid chromatography (HPLC), Variant II Hemoglobin Testing System, BioRad, Richmond, CA, inter- and intra-assay variation: 1.7% and <2.0%, normal values 4.2–6.1%]. Fasting lipids (total cholesterol, HDL-cholesterol and triglycerides) were measure by the enzymatic method and LDL-cholesterol was determined by the Friedewald equation (17).
Statistical analysis
Statistical analysis and graphics were done using Excel® for Windows®, SAS 9.1 (SAS Institute Inc., Cary, NC, USA) and SSPS 15.0 software. Results of continuous variables were expressed as means ± SEM (SD in Table 1) except for albuminuria and triglycerides [median (minimum-maximum)] and categorical as number of cases (%). To assess changes in binary outcomes [abnormal eGFR (CKD stage 3) or albuminuria (UAER ≥300 mg/24 h) values at anytime post-transplant], multiple logistic regressions employing Generalized Estimating Equations were utilized, in a total of 734 observations from the 35 subjects. Stepwise model building techniques were employed initially considering as covariates the factors found to be associated with each variable in bivariate analysis. Covariates considered in these models as potential explanatory or confounding factors include: time interval (pre- and post-transplant time-points), age, diabetes duration, gender, BMI, systolic and diastolic BP, A1c, lipids (LDL, HDL and triglycerides levels), use of ACEi, ARB, sirolimus and tacrolimus at each time point and pre-transplant kidney function (CKD stage 1 or 2 and microalbuminuria). To verify if kidney function parameters (eGFR and albuminuria) have varied post-transplantation, we utilized the pre-transplant values as controls. The comparison between post- vs. pre-transplant time-points was performed by repeated measures analysis. For continuous outcomes where normality assumptions were appropriate, linear mixed models regression were used. Appropriate interaction terms were included in repeated measures regression models and were assessed for statistical significance to compare the slopes between regression lines. P values of <0.05 (2-tailed) were considered to be statistically significant.
Table 1.
Clinical and laboratory characteristics of the subjects (n=35) at baseline.
| Age (years) | 42.5 ± 8.6 |
| Diabetes duration (years) | 26.5 ± 13.4 |
| Male gender - n (%) | 13 (37) |
| White – n (%) | 35 (100) |
| Body mass index (kg/m2) | 24.0 ± 2.3 |
| Hypertension – n (%) | 9 (26) |
| Systolic blood pressure (mmHg) | 119.5 ± 12.5 |
| Diastolic blood pressure (mmHg) | 69.3 ± 6.8 |
| ACEi/ARB – n (%) | 13 (37) |
| A1c (%) | 7.4 ± 1.1 |
| Total Cholesterol (mg/dl) | 181.9 ± 43.5 |
| LDL cholesterol (mg/dl) | 102.6 ± 35.4 |
| HDL cholesterol (mg/dl) | 66.6 ± 16.8 |
| Triglycerides (mg/dl) | 55.58 (32.0–116.0) |
| Creatinine (mg/dl) | 1.0 ± 0.2 |
| eGFR (ml/min/1.73 m2) | 76.2 ± 22.5 |
| Albuminuria (mg/24 h) | 6.9 (0 – 104.0) |
| Nephropathy (normo-/microalbuminuria)-–n (%) | 30 (86)/5 (14) |
| Retinopathy (normal/non-proliferative/prolifarative) –n (%) | 14 (40)/10 (29)/11 (31) |
| Peripheral neuropathy – n (%) | 9 (26) |
| Cardiovascular disease – n (%) | 0 (0) |
Data expressed as the mean ± SD, median (minimum - maximum) or number of subjects (%). ACEi = angiotensin-converting enzyme inhibitors, ARB = angiotensin receptor blockers and eGFR = estimated glomerular filtration rate measured by Modification of Diet in Renal Disease study (MDRD) equation.
RESULTS
Main clinical and laboratory characteristics of the 35 subjects enrolled are shown in Table 1. Patients’ age at transplant was 42.5±8.6 years and the diabetes duration was 26.5±13.4 years. All subjects were white and 13 (37%) were males. BP means were 119.5±12.5/69.3±6.8 mmHg, 9 subjects (26%) had history of hypertension and 13 (37%) were on ACEi or ARB at baseline. Chronic diabetes complications at baseline included DR in 60% of the subjects (n=21; 11 proliferative). Nine subjects (26%) had peripheral neuropathy and none had cardiovascular disease. Thirty subjects (86%) were normoalbuminuric [UAER: 5.6 (0.0–26.3) mg/24 h] and 5 (14%) microalbuminuric [UAER: 50.6 (31.7–104.0) mg/24 h]. Baseline eGFR was 76.2±22.5 ml/min/1.73 m2, 6 subjects (17%) had moderate decrease in eGFR (CKD stage 3) since baseline and the other 29 had preserved kidney function (CKD stage 1: n=9; stage 2: n=20).
A1c was normal in all patients after transplant (pre: 7.45±0.11 vs. post: 6.09±0.09%, P<0.001). A mild but statistically significant increment in LDL-cholesterol levels after transplantation was found (pre: 93.3±3.3 vs. post: 101.6±2.3 mg/dl, P=0.008), in spite of higher statins use (20 vs. 83%, P<0.001). The BP remained stable during the follow-up (pre: 121.7±2.3/71.9±1.6 vs. post: 119.3±1.6/71.5±1.0 mmHg, P>0.05), mainly at the expense of increasing the number of anti-hypertensive medications per patient (0.34±0.48 vs. 0.71±0.86, P=0.002) and ACEi/ARBs use (37 vs. 66%, P=0.029).
Variables associated with kidney function after islet transplantation
Variables associated with decreased eGFR or macroalbuminuria at any time-point during post-transplant follow-up are summarized in Table 2 (bivariate analysis). Age and pre-transplant microalbuminuria were associated with lower eGFR. In contrast, better pre-transplant kidney function was found to be a protective factor (OR=0.22, 95%CI=0.05–0.97, P=0.050). An association trend between low eGFR and use of ARBs (OR=2.80, 95%CI=0.99–7.94, P=0.052) and/or ACEi (OR=2.45, 95%, CI=0.98–6.14, P=0.056) was observed, as expected due to their mechanism of action. No other clinical or laboratory variables, neither immunosuppressive agent in use (Table 2) or combination (tacrolimus/sirolimus: 74.4±2.7 vs. MMF/tacrolimus or sirolimus: 69.0±3.3 ml/min/m2, P=0.070), showed statistically significant association with low eGFR. Multiple regression analysis indicated that only age remained significantly associated with low eGFR (OR=1.78, 95%CI=1.22–2.61, P=0.002) and all other variables, including pre-transplant kidney function markers, were not statistically significant.
Table 2.
Variables associated with CKD stage 3 and/or macroalbuminuria in any time-point after islet transplant
| CKD stage 3 OR (95% CI) | P | Macroalbuminuria OR (95% CI) | P | |
|---|---|---|---|---|
| Age (each 10 years) | 2.18 (1.19 – 3.98) | 0.011 | 1.20 (0.59 – 2.45) | 0.617 |
| Diabetes duration (years) | 1.04 (0.99 – 1.09) | 0.088 | 1.03 (0.98 – 1.08) | 0.222 |
| Gender (male) | 0.44 (0.09 – 2.24) | 0.324 | 0.30 (0.04 – 2.10) | 0.225 |
| BMI (kg/m2) | 1.00 (0.93 – 1.08) | 0.982 | 1.28 (1.00 – 1.64) | 0.046 |
| Systolic BP ≥ 130 mmHg | 1.74 (0.83 – 3.65) | 0.144 | 1.37 (0.31 – 6. 08) | 0.679 |
| Diastolic BP ≥ 80 mmHg | 0.82 (0.50 – 1.34) | 0.437 | 0.63 (0.17 – 2.28) | 0.477 |
| A1c ≥6% | 0.76 (0.46 – 1.26) | 0.294 | 0.51 (0.14 – 1.86) | 0.304 |
| LDL-cholesterol ≥ 100 mg/dl | 0.91 (0.66 – 1.24) | 0.546 | 2.63 (1.11 – 6.25) | 0.028 |
| Low HDL cholesterol a | 0.96 (0.40 – 2.32) | 0.936 | 0.70 (0.22 – 2.17) | 0.535 |
| Triglycerides >150 mg/dl | 1.52 (0.96 – 2.45) | 0.073 | 0.24 (0.04 – 1.69) | 0.153 |
| CKD stage 1 or 2 b | 0.22 (0.05 – 0.97) | 0.050 | 0.70 (0.12 – 3.94) | 0.681 |
| Microalbuminuria b | 13.12 (3.53 – 48.80) | <0.001 | 5.44 (1.58 – 18.70) | 0.007 |
| ACEi | 2.45 (0.98 – 6.14) | 0.056 | 2.38 (0.58 – 9.82) | 0.231 |
| ARB | 2.80 (0.99 – 7.94) | 0.052 | 3.00 (0.79 – 11.40) | 0.107 |
| Statins | 1.76 (0.96 – 3.21) | 0.067 | 3.25 (0.90 – 11.75) | 0.071 |
| 3 or 4 islets transplants | 2.2 (0.83 – 6.24) | 0.110 | 3.87 (0.99 – 15.12) | 0.051 |
| Tacrolimus | 0.69 (0.43 – 1.09) | 0.111 | 0.20 (0.09 – 0.41) | <0.001 |
| Sirolimus | 1.13 (0.61 – 2.11) | 0.704 | 3.09 (0.37 – 24.35) | 0.289 |
Data expressed as odds ratio (OR) and 95% confidential interval (CI). CKD = chronic kidney disease based on National Kidney Foundation (NKF) guidelines, BMI = body mass index, BP = blood pressure, ACEi = angiotensin-converting enzyme inhibitors, ARB = angiotensin receptor blockers.
HDL cholesterol <40 mg/dl for males or <50 mg/dl for females
based on pre-transplant classification.
In the case of albuminuria, a higher BMI, LDL-cholesterol and pre-transplant microalbuminuria resulted as risk factors for macroalbuminuria. Interestingly, current use of tacrolimus was associated with an 80% reduction in macroalbuminuria occurrence risk (OR=0.20, 95%CI=0.09–0.41, P<0.001). A borderline association between macroalbuminuria and higher number of islet transplants (3 or 4 infusions) was found (OR=3.87, 95%CI=0.99–15.12, P=0.051). However, after adjustments in multiple regression, only LDL-cholesterol (OR=2.90, 95%CI=1.37–6.12, P=0.005) and previous microalbuminuria (OR=6.42, 95%CI=1.42–29.11, P=0.001) remained as risk factors for macroalbuminuria. The protective effect of tacrolimus maintained statistical significance in multiple regression analysis (OR=0.12, 95%CI=0.06–0.26, P<0.001). A deleterious effect of sirolimus on kidney function was demonstrated in the multiple regression analysis for microalbuminuria (OR=3.68, 95%CI=1.17–10.70, P=0.020) but not for macroalbuminuria.
Kidney function after islet transplant
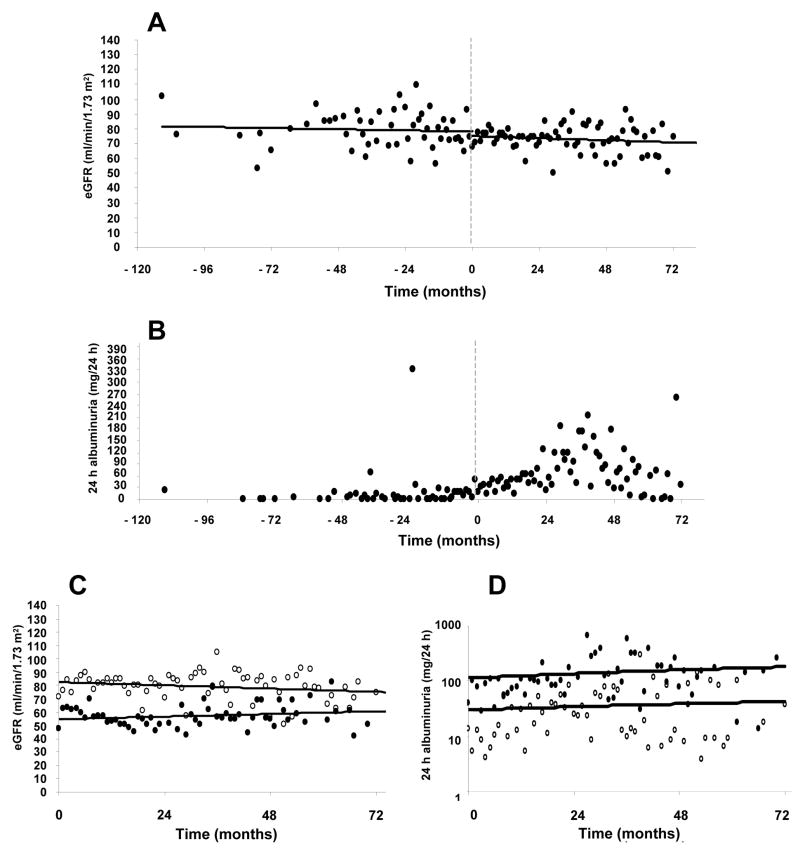
Unexpectedly, the overall eGFR remained stable during all pre- and post-transplant follow-up (line equations pre-: y=76.512± −0.034x vs. post-transplant: y=76.109± −0.015x, P=0.400 for slopes comparisons) (Figure 1A). To analyze the eGFR in detail and evaluate possible acute nephrotoxic effects of immunosuppressive drugs in the early post-transplant period (when sirolimus target levels are higher), pre-transplant eGFR means were compared to those of post-transplant time-points (3-month intervals) and no significant differences were found (pre: 70.5±6.3; 0–3: 72.0±3.3; 3–6: 78.0±3.3; 6–12: 77.2±3.1; 12–18: 74.2±3.3; 18–24: 72.9±3.5; 24–30: 76.5±3.8; 30–36: 82.1±3.8; 36–42: 80.3±4.1; 42–48: 77.1±3.9 and >48 months: 76.4±3.5 ml/min/1.73 m2; P>0.05 for all comparisons).
Figure 1.
(A) Pre- and post-transplant eGFR (line equations pre-: y=76.512± −0.034x vs. post-transplant: y=76.109± −0.015x, P=0.400 for slopes comparisons), (B) pre- and post-transplant albuminuria [0–24 months: 11.7 (0–632.5), 24–48 months: 25.6 (0–1112.3), >48 months: 13.4 (0–387.4) mg/24 h, P=0.065], (C) post-transplant eGFR and (D) albuminuria in patients with baseline normoalbuminuria and CKD stage 1 or 2 (white circles) vs. baseline microalbuminuria and/or CKD stage 3 (black circles). P<0.05 for differences between groups (white circles vs. black circles) and P>0.05 for differences between pre- and post-transplant values for both groups. eGFR = estimated glomerular filtration rate, CKD = chronic kidney disease
To verify the specific effect of immunosuppressive therapy on eGFR, we performed a separate analyzes only with subjects off immunosuppressive drugs at the most recent follow-up (due to graft failure, n=3; adverse events, n=1; per protocol, n=5 or patient option, n=1; total n=10, 25±17 months after first transplant) and compared the eGFR means before immunosuppressive treatment initiation (pre-transplant values), during and after its withdrawal. There were no statistically significant differences among the three periods (before: 74.0±2.0, during: 75.4±2.8 and after withdrawal: 76.3±5.3 ml/min/1.73 m2, P>0.05).
Since pre-transplant kidney function could influence the outcomes post-transplantation, subjects were stratified based on pre-transplant kidney status. Those with either low eGFR (<60 ml/min/1.73 m2) and/or microalbuminuria (n=10) showed lower but stable eGFR, when compared to patients with normal kidney function pre-transplant (pre: 61.13±3.25 vs. 78.81±2.46; and post: 63.32±4.36 vs. 81.34±2.73 ml/min/1.73 m2, respectively, P<0.05 for comparisons between normal and abnormal baseline kidney function). Both groups maintained stable eGFR after transplantation (P>0.05) (Figure 1C). The frequency of CKD stage 3 in the final evaluation was not statically higher than at baseline [9 (26%) vs. 6 (17%), P=0.532]. None of the patients progressed to CKD stage 4 or 5 in this sample.
Urinary albumin excretion values are depicted in Figure 1B. A transitory increment in albuminuria seemed to occur in the period between 24 and 48 months after first transplant [0–24 months: 11.7 (0–632.5); 24–48: 25.6 (0–1112.3); >48 months: 13.4 (0–387.4) mg/24 h, P=0.065]. When the subjects were analyzed based on the baseline DN classification, from the 30 normoalbuminuric subjects, 24 (80%) remained in normal range at the most recent evaluation, 21 (70%) within normoalbuminuric range during all period and 3 (10%) with a transient increase in albuminuria, reaching the microalbuminuric range and then regressing to normal levels. Six subjects (20%) experienced sustained increase in albuminuria (2 subjects reaching transient macroalbuminuria), and have progressed to microalbuminuria. One-out-of-five patients with microalbuminia at baseline showed transient macroalbuminuria, although all of them were in the microalbuminuria range at final evaluation and sustained macroalbuminuria was not detected. Higher post-transplant albuminuria levels, although not statistically significant, were observed in subjects with poorer baseline kidney function (eGFR <60 ml/min/1.73 m2 and/or microalbuminuria) [31.1 (0–121.0) vs. 74.4 (0–1112.3) mg/24 h, P=0.080], whereas it remained stable in the others [0 (0–996) vs. 7.9 (0–632.5) mg/24 h, P=0.510] (Figure 1D).
As discussed earlier, clinically important deterioration of kidney function, requiring immunosuppressive regimen modification was seen in two subjects (5.7%). In both cases, a history of microalbuminuria and CKD stage 2 was present at baseline. In these cases, intermittent increases in creatinine, albuminuria and proteinuria, starting approximately one year after transplant, were observed. The immunosuppressive therapy was modified when a clinical significant increase in creatinine was observed. These subjects were maintained on sirolimus while tacrolimus was replaced with MMF. Both subjects returned to microalbuminuria and showed mild decrease in eGFR (pre-transplant: 64 and 66; most recent evaluation: 51 and 59 ml/min/1.73 m2, with 54 and 51 months of follow-up, respectively).
In order to better understand if BP and lipid levels, as well as their respective treatments, could play a role in transient increment of albuminuria, we compared means before, during and after the higher albuminuria period (24–48 months). A progressive decrease in LDL-cholesterol levels (105.38±2.84, 94.03±4.10 and 87.72±4.32 mg/dl; P=0.004 for comparison between 0–24 and 24–48 months) along with higher frequency in statins use per patient (0.45±0.07, 0.75±0.08, 0.68±0.11; P=0.003) was observed. Additionally, BP levels remained stable while an increment in the number of anti-hypertensive drugs/patient was found in the late period (0.57±0.12, 0.65±0.13, 1.07±0.21; P=0.007 for comparison between 24–48 months and >48 months).
DISCUSSION
In this sample of ITA recipients, abnormal values of eGFR and/or albuminuria at baseline were predictors of less favorable kidney outcomes during the follow-up. Moreover, LDL-cholesterol was found to be an important modifiable risk factor and immunosuppressive maintenance therapy with tacrolimus protected from transient macroalbuminuria. Notably, there was no significant deterioration in overall kidney function in the post-transplant period and none of the subjects progressed to sustained macroalbuminuria or ESRD.
Intensive insulin therapy and whole-organ pancreatic transplantation can prevent chronic complications of T1DM (1, 18). Since clinical islet transplantation restores blood glucose homeostasis (4, 7, 19, 20), it would be expected to reduce microvascular diabetes chronic complications, at least in the same way as seen in intensive insulin therapy trials. In earlier reports, progression of both DR and DN after islet transplantation was attributed to natural history of the disease (20). Later on, proteinuria was described in three patients from Edmonton cohort (6) and its resolution following sirolimus discontinuation and increase in tacrolimus dosage called attention for immunosuppressive-related nephrotoxicity, already known in the kidney transplantation setting (21). These concerns were amplified due to the description of decrease in eGFR (9) and the unexpected evolution to ESRD in 2 patients (10% of the sample) observed in another cohort (8). Recently, the Vancouver group reported no GFR decline in islet transplant recipients (10), under a sirolimus-sparing immunosuppressive protocol. Notably, the lack of worsening in GFR in their study was based on a borderline statistical difference (P=0.07) between baseline and post-transplant GFR levels instead of comparison between groups (10).
It is conceivable that the impairment in renal function observed in recent islet transplantation trials be related to the combination of sirolimus and tacrolimus. Tacrolimus is a calcineurin-inhibitor with well-established propensity to cause CKD of both native and transplanted kidneys (22, 23). Sirolimus was thought to be a renal-safe drug, but lately it has been associated with kidney damage trough glomerular (24) and tubular (25) mechanisms (26). The combination of both drugs might synergize enhancing renal damage (27).
A systematic evaluation of risk factors for kidney dysfunction after ITA had not been conducted so far. An association between baseline GFR and the eGFR delta in the first year post-transplant has been suggested, but clear correlations with immunosuppressive drugs levels could not be established (9). In our sample, classical risk factors for DN (namely, age, previous microalbuminuria and higher LDL cholesterol) (28) were associated with decrease in eGFR or macroalbuminuria post-transplantation. Additionally, another variable of interest, even though with weak association not sustained after adjustments in multiple regression, was recipient’s BMI, formerly associated with islet graft failure (29) and potentially with DN, through its relation with metabolic syndrome (30).
The protective role of tacrolimus observed in our series was an unexpected finding. An improvement in albuminuria and proteinuria after sirolimus withdrawal and increase in tacrolimus dose has been previously described, even though a cause-effect relationship could not be established since both medications were modified simultaneously (6). Interestingly, tacrolimus has recently been reported to be effective as a steroid-sparing agent drug for minimal changes nephrotic syndrome (31). Furthermore, the known hemodynamic effects of tacrolimus (32) might have contributed, at least in part, to the observed positive effects on albuminuria in our series.
Progression to microalbuminuria was higher (20%) in our sample than in the Diabetes Control and Complications Trial (DCCT; <10% in a similar period of observation) (1). However, patients from the DCCT cannot be directly compared to those included in islet transplantation trials, since unstable T1DM control was an exclusion criterion and also duration of the disease was shorter than in islet transplant recipients (1). Our results are in fact between classical DN progression studies (17% in 5–10 years) (33) and those from the International Multicenter Trial (36%) (7) and the Edmonton cohorts (26%) (9).
Unexpectedly, we did not observed a decrease in kidney function after islet transplantation in our cohort of recipients. The unchanged kidney function found in our series when compared to previous reports could be attributed to healthier kidney status at baseline. In our series, most patients were normoalbuminuric and no patient had macroalbuminuria, contrasting with pre-transplant values in macroalbuminuric range found on 5%, 7% and 29% of the Milan (8), Edmonton (9) and Vancouver (10) cohorts, respectively. Another aspect that may have contributed to the better results observed in our study was the prompt treatment of renal side effects, either by switching the immunosuppressive regimen (two cases) or by adding nephroprotective medications. Furthermore, in our experience aggressive management of conventional risk factors for kidney dysfunction, aiming a BP <130/80 mmHg, LDL-cholesterol <100 mg/dl and tacrolimus target levels <6 ng/mL, resulted in stability of BP and progressive decrease in LDL-cholesterol levels. This approach could have possibly contributed to the observed improvement in albuminuria levels at the late follow-up (>48 months).
Based on the results of the present study, a practical recommendation regarding patient selection and post-transplant clinical care can be done. Indication of islet transplant for a patient with previous kidney abnormalities should take into account fully risks and benefits and a post-transplant close surveillance with proactive management of possible complications should be planed, including kidney biopsies when clinically indicated (28). If a modification in the immunosuppressive therapy is required, maintenance of tacrolimus over sirolimus should be considered. Intensive treatment of conventional risk factors for nephropathy, such as tight BP and LDL-cholesterol control and careful tacrolimus target levels monitoring, might be part of clinical islet transplantation care in order to minimize other aggressors besides immunosuppressive therapy.
Limitations of our study included the relatively small sample size, the retrospective analyzes and the lack of a control group. The sample size restrictions were overcome by utilizing a large number of assessments per patient and employing statistics models specifically created for repeated measurements. The retrospective design prevented us from having a more comprehensive and prospective kidney function evaluation, either through direct function measurements (i.e., EDTA 51Chromium, iohexol and iodothalamate) or kidney biopsies. Although the use of indirect methods to estimate GFR (namely, the MDRD formula) may diminish the results accuracy by underestimating values when GFR is higher than 60 ml/min/m2, it is largely accepted as a kidney function estimator and is currently the formula of choice in the diabetic population (34). Furthermore, our aim was to analyze changes in kidney function during the follow-up and not to describe the frequency of low GFR in this population. Thus, a measurement bias, if present, would be applied to all values, and it would not affect the final observation. Finally, as the subjects were followed pre-transplant for a median of 20 months, we used pre-transplant eGFR values as recipient’s own controls.
In conclusion, kidney function remained stable after ITA, even with the combination of two nephrotoxic drugs. A mild increase in microalbuminuria incidence was seen, but no decline in eGFR could be detected. Possibly, treatment of modifiable risk factor, as pre-transplant microalbuminuria and LDL-cholesterol, as well as tight BP control, may minimize the deleterious effects of immunosuppressive drugs. These results could probably only be accomplished due to aggressive treatment of conventional risk factors for nephropathy.
Acknowledgments
The Authors are grateful to the members of the Clínical Islet Transplant Program, Human Cell Processing facility at the Cell Transplant Center, General Clinical Research Center and Administrative Offices at the Diabetes Research Institute, and Organ Procurement Organizations for the continuous enthusiasm and support to our Program. We thank Drs. Daniel H. Mintz (Diabetes Research Institute, Miami) and Jorge Luiz Gross (Endocrine Division of Hospital de Clinicas de Porto Alegre, RS, Brazil) for the careful review of the manuscript and valuable suggestions.
Abbreviations
- ACEi
angiotensin-converting enzyme inhibitors
- ARB
angiotensin receptor blockers
- CKD
chronic kidney disease
- DN
diabetic nephropathy
- DR
diabetic retinopathy
- ESRD
end-stage renal disease
- eGFR
estimated glomerular filtration rate
- IRB
health research ethics board
- HPLC
high performance liquid chromatography
- ITA
islet transplantation alone
- MDRD
Modification of Diet in Renal Disease
- MMF
Mycophenolate Mofetil
- MS
Mycophenolate sodium
- NKF
National Kidney Foundation
- T1DM
Type 1 Diabetes Mellitus
- UAER
urinary albumin excretion rate
Footnotes
Funding sources: National Institutes of Health/National Center for Research Resources (U42 RR016603, M01RR16587); Juvenile Diabetes Research Foundation International (#4-2000-946, 4-2004-361); National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (5 R01 DK55347, 5 R01 DK056953); State of Florida, and the Diabetes Research Institute Foundation (Hollywood, FL). CBL was the recipient of a scholarship from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq).
The authors have no conflicts of interest to be reported
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329 (14):977. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353 (25):2643. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen-Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev. 2004;20 (6):479. doi: 10.1002/dmrr.482. [DOI] [PubMed] [Google Scholar]
- 4.Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5 (8):2037. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 5.Poggioli R, Faradji RN, Ponte G, et al. Quality of life after islet transplantation. Am J Transplant. 2006;6 (2):371. doi: 10.1111/j.1600-6143.2005.01174.x. [DOI] [PubMed] [Google Scholar]
- 6.Senior PA, Paty BW, Cockfield SM, Ryan EA, Shapiro AM. Proteinuria developing after clinical islet transplantation resolves with sirolimus withdrawal and increased tacrolimus dosing. Am J Transplant. 2005;5 (9):2318. doi: 10.1111/j.1600-6143.2005.01013.x. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355 (13):1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 8.Maffi P, Bertuzzi F, De Taddeo F, et al. Kidney function after islet transplant alone in type 1 diabetes: impact of immunosuppressive therapy on progression of diabetic nephropathy. Diabetes Care. 2007;30 (5):1150. doi: 10.2337/dc06-1794. [DOI] [PubMed] [Google Scholar]
- 9.Senior PA, Zeman M, Paty BW, Ryan EA, Shapiro AM. Changes in renal function after clinical islet transplantation: four-year observational study. Am J Transplant. 2007;7 (1):91. doi: 10.1111/j.1600-6143.2006.01573.x. [DOI] [PubMed] [Google Scholar]
- 10.Fung MA, Warnock GL, Ao Z, et al. The effect of medical therapy and islet cell transplantation on diabetic nephropathy: an interim report. Transplantation. 2007;84 (1):17. doi: 10.1097/01.tp.0000265502.92321.ab. [DOI] [PubMed] [Google Scholar]
- 11.Stadler M, Auinger M, Anderwald C, et al. Long-term mortality and incidence of renal dialysis and transplantation in type 1 diabetes mellitus. J Clin Endocrinol Metab. 2006;91 (10):3814. doi: 10.1210/jc.2006-1058. [DOI] [PubMed] [Google Scholar]
- 12.Pileggi A, Ricordi C, Kenyon NS, et al. Twenty years of clinical islet transplantation at the Diabetes Research Institute--University of Miami. Clin Transpl. 2004:177. [PubMed] [Google Scholar]
- 13.Cure P, Pileggi A, Faradji RN, et al. Cytomegalovirus infection in a recipient of solitary allogeneic islets. Am J Transplant. 2006;6:1089. doi: 10.1111/j.1600-6143.2006.01319.x. [DOI] [PubMed] [Google Scholar]
- 14.Standards of medical care in diabetes-2008. Diabetes Care. 2008;31(suppl 1):s12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130 (6):461. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139 (2):137. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18 (6):499. [PubMed] [Google Scholar]
- 18.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339 (2):69. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 19.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293 (7):830. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 20.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54 (7):2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 21.Morelon E, Kreis H. Sirolimus therapy without calcineurin inhibitors: Necker Hospital 8-year experience. Transplant Proc. 2003;35 (3 Suppl):52S. doi: 10.1016/s0041-1345(03)00244-6. [DOI] [PubMed] [Google Scholar]
- 22.Liptak P, Ivanyi B. Primer: Histopathology of calcineurin-inhibitor toxicity in renal allografts. Nat Clin Pract Nephrol. 2006;2 (7):398. doi: 10.1038/ncpneph0225. [DOI] [PubMed] [Google Scholar]
- 23.Creput C, Blandin F, Deroure B, et al. Long-term effects of calcineurin inhibitor conversion to mycophenolate mofetil on renal function after liver transplantation. Liver Transpl. 2007;13 (7):1004. doi: 10.1002/lt.21170. [DOI] [PubMed] [Google Scholar]
- 24.Saurina A, Campistol JM, Piera C, et al. Conversion from calcineurin inhibitors to sirolimus in chronic allograft dysfunction: changes in glomerular haemodynamics and proteinuria. Nephrol Dial Transplant. 2006;21 (2):488. doi: 10.1093/ndt/gfi266. [DOI] [PubMed] [Google Scholar]
- 25.Straathof-Galema L, Wetzels JF, Dijkman HB, Steenbergen EJ, Hilbrands LB. Sirolimus-associated heavy proteinuria in a renal transplant recipient: evidence for a tubular mechanism. Am J Transplant. 2006;6 (2):429. doi: 10.1111/j.1600-6143.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 26.Laugharne M, Cross S, Richards S, et al. Sirolimus toxicity and vascular endothelial growth factor release from islet and renal cell lines. Transplantation. 2007;83 (12):1635. doi: 10.1097/01.tp.0000266555.06635.bf. [DOI] [PubMed] [Google Scholar]
- 27.Podder H, Stepkowski SM, Napoli KL, et al. Pharmacokinetic interactions augment toxicities of sirolimus/cyclosporine combinations. J Am Soc Nephrol. 2001;12 (5):1059. doi: 10.1681/ASN.V1251059. [DOI] [PubMed] [Google Scholar]
- 28.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28 (1):164. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 29.Campbell PM, Salam A, Ryan EA, et al. Pretransplant HLA antibodies are associated with reduced graft survival after clinical islet transplantation. Am J Transplant. 2007;7 (5):1242. doi: 10.1111/j.1600-6143.2007.01777.x. [DOI] [PubMed] [Google Scholar]
- 30.Costa LA, Canani LH, Lisboa HR, Tres GS, Gross JL. Aggregation of features of the metabolic syndrome is associated with increased prevalence of chronic complications in Type 2 diabetes. Diabet Med. 2004;21 (3):252. doi: 10.1111/j.1464-5491.2004.01124.x. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Li H, Chen J, et al. Tacrolimus As a Steroid-Sparing Agent For Adults With Steroid-Dependent Minimal Change Nephrotic Syndrome. Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm637. [DOI] [PubMed] [Google Scholar]
- 32.Rostaing L, Tran-Van T, Cisterne JM, Tack I, Durand D, Ader JL. Influence of early FK 506 trough levels on glomerular hemodynamics at 3 months in kidney transplant recipients. Transplant Proc. 1998;30 (4):1282. doi: 10.1016/s0041-1345(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 33.Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes. 2000;49 (9):1399. doi: 10.2337/diabetes.49.9.1399. [DOI] [PubMed] [Google Scholar]
- 34.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49 (2 Suppl 2):S12. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]