Abstract
Aims
To estimate the magnitude of genetic and environmental influences on timing of first alcohol use and alcohol dependence (AD) and to quantify the overlap in these influences across the two alcohol-related outcomes.
Participants
The sample consisted of 5,382 twins (2,691 complete pairs), aged 24 to 36 years, from the Australian Twin Registry.
Measurements
History of alcohol use and DSM-IV alcohol dependence were assessed by structured telephone interview.
Findings
In both sexes, the relationship between age at first alcohol use and risk for AD followed a linear trend, such that the highest rates of AD were observed in individuals who began drinking at an earlier than average age (14 years or younger). Heritability estimates for timing of first alcohol use and AD were 36% and 53%, respectively. Shared environmental factors accounted for 15% of variance in initiation. There was no evidence of shared environmental influences on AD. The genetic correlation between timing of first alcohol use and AD was 0.59.
Conclusions
Findings highlight the substantial role of genetics in the development of AD and the early manifestation of that genetic risk in the timing of alcohol use initiation, which, unlike AD, is also influenced to a modest degree by shared environmental factors. The considerable overlap in heritable influences – and the virtual absence of overlap in individual-specific environmental influences – on initiation of alcohol use and AD indicates that the association between age at first drink and AD is attributable in large part to common genetic sources of variance.
Keywords: initiation of alcohol use, alcohol dependence, twins
INTRODUCTION
Early age at first drink has consistently been associated with elevated rates of alcohol dependence (AD)1-5. Using data from the National Epidemiological Survey on Alcohol and Related Conditions (NESARC), Hingson et al.3 found that the lifetime prevalence of AD was 47% among individuals who had initiated alcohol use at age 14 years or younger compared with only 9% among those who began drinking at 21 years or older. Results confirmed findings that had been reported nearly 10 years earlier by Grant and Dawson2 using data from the National Longitudinal Alcohol Epidemiologic Survey. Over 40% of those who initiated alcohol use before age 15 met criteria for AD vs. 10% of those who started drinking at ages 21 or 22. Elevated rates of AD in early initiators have led some to conclude that the association is a causal one and that delaying first alcohol use will largely reduce risk conferred by early drinking6-7. In contrast, it has been argued that, rather than causing AD, early age at first drink is a marker for familial liability to the disorder8-10. McGue et al. 9, for example, found that age at first drink was associated with a psychophysiological marker of AD risk (reduced P3 amplitude). A recent study by King and Chassin11 examining the association between age at first drink and AD also supports this hypothesis: after accounting for family history of alcohol-related problems (in combination with other shared risk factors) the association was reduced to non-significance.
The most notable (and widely cited) contribution to this literature comes from Prescott and Kendler10, who addressed the issue in a study conducted with 8,746 adult twins. Their investigation revealed that the strong association between age at first drink and AD was attributable in full to familial sources of influence – both shared environment and genetics – and thus did not reflect a causal relationship. This study built on an extensive literature documenting the substantial genetic contributions to AD and a growing body of research addressing the relative genetic and environmental influences on the initiation of alcohol use. Heritability of AD has been estimated at about 50-60%12-16, but, in contrast, genetic factors have been reported to account for between 0 and 39% of the variance in alcohol initiation17-19, which is explained to a greater degree by shared environmental factors20-23. The majority of studies addressing heritability of alcohol use have defined initiation in terms of “ever use” without reference to the timing of first drink, which, given the variability in prevalence of AD by age at first drink, is a key aspect of initiation to consider. The few known studies to do so found that, like “ever use,” shared environmental factors accounted for the most variance in age at initiation, but additive genetic factors played a modest role as well23,24. Importantly, although initiation is influenced to a lesser degree by genetics than AD, evidence from the small number of investigations in this area indicates that the genetic factors that contribute to the timing of first drink overlap significantly with those that influence onset of AD10,24, suggesting that at least some component of the observed association is non-causal.
The aim of the current study is to characterize the relative contributions of genetic and environmental influences on timing of first alcohol use and AD, adding to a limited but important literature for the development of etiological and prevention models. Heritability estimates for AD are consistent across sexes12,13,25,26, but there is some evidence that shared environmental factors play a larger role in the initiation of alcohol use in females than males18,27, so we test for potential distinctions by sex in sources of variance. Most importantly, we test the hypothesis that the link between early initiation and AD can be explained by common genetic risk. Under this model, we would expect covariance between the two phenotypes to be attributed to genetic sources, with no significant overlap in individual-specific environmental influences on the two phenotypes.
METHODS
Participants
Twins born in Australia between 1964 and 1971 were recruited for the Australian Twin Registry through school systems and mass media appeals and registered by their parents from 1980 to 1982. Members of the twin registry were contacted as adults in 1989 by means of mailed questionnaires. They were subsequently administered diagnostic assessments via telephone between 1996 and 2000, when they were 24-36 years of age. (Participation in the telephone interview was not contingent on response to questionnaires mailed in 1989.) Interviews were conducted with 6,257 individuals (2,761 complete pairs and 735 singletons), 73% of the targeted sample. Informed consent was obtained prior to the start of interviews, as approved by the Human Research Protection Offices of Washington University and the Queensland Institute of Medical Research.
The sample for the current study consists of 5,382 twins (2,691 pairs), representing all twin pairs in which both twins endorsed consumption of at least one alcoholic drink over their lifetimes (97.5% of all complete pairs). Breakdown by sex and zygosity are as follows: 681 female monozygotic (MZ), 480 MZ male, 502 dizygotic (DZ) female, 387 DZ male, and 641 DZ opposite sex twin pairs. Mean age at the time of interview was 29.9 years.
Assessment
Psychiatric data was collected with a modified Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA-OZ)28,29, adapted for administration via telephone and updated for DSM-IV diagnostic criteria. In addition to assessing substance use, mood, conduct, and anxiety disorders, SSAGA-based interviews gathered detailed histories of alcohol and other substance use.
Operationalization of Alcohol Use Variables
Age at initiation of alcohol use was assessed by asking, “How old were you the first time you had more than just a sip of beer, wine, or spirits?” Mean age at first drink was 16.2 years for females and 15.4 years for males. In order to account for the potential skewness in its continuous form, a categorical variable representing age at first drink was created based on the distribution of age at initiation for males and females in combination. Those falling within the lowest 20% in the age range, 14 years and younger, (15.2% of females and 28.3% of males) were labeled “early” and those in the highest 20%, 18 years or older, (25.8% of females and 15.0% of males) were labeled “late.” Average age at first drink was defined as 15-17 years (representing 59.0% of females and 56.8% of males). Lifetime alcohol dependence (AD) was defined as endorsing full DSM-IV diagnostic criteria for the disorder (i.e., 3 or more of the 7 possible symptoms clustering in a 12 month period of time). A total of 15.0% of females and 30.4% of males met AD criteria.
Data Analysis
Twin Modeling
Data from monozygotic (MZ) and dizygotic (DZ) twins can be utilized to parse out the relative contribution of additive genetic (A), shared environmental (C) and non-shared environmental (E) influences on population variation in behaviors of interest (in this case, timing of first alcohol use and AD). Non-additive genetic influences (including dominance, and thus denoted as D) can be estimated in place of shared environmental influences when the correlation between members of DZ twin pairs is less than half the correlation between their MZ counterparts for a given behavior, but C and D cannot be jointly estimated when data from twins alone are used. In our sample, the DZ correlations for age at initiation of alcohol use and AD were greater than half the MZ correlations, indicating that a model incorporating C rather D (i.e., an ACE model) would be most appropriate for these data.
Univariate Models
Univariate twin models were fit to raw categorical data, with timing of first alcohol use represented by ‘0’, ‘1’, and ‘2’ values (late, average, and early, respectively) and AD by a dichotomous variable. A test of multivariate normality was conducted for the variable representing timing of first use and the assumption was met. Genetic models were fitted by the method of maximum likelihood, using the structural equation modeling program Mx30. The thresholds (assessed as z-scores on the underlying standard normal distribution) were adjusted for age at the time of interview to minimize the potential influence of forward telescoping (i.e., reporting events closer to the time of interview than they actually were). The age distribution was divided into thirds (24-28, 29-31, and 32 years and older) and represented in the models by two dummy variables, with the 29-31 year-olds as the reference group. A series of sub-models examining the statistical significance of A, C and E were compared to the full model to derive the best-fitting univariate models. Sub-models were tested by calculating the difference between the -2 log likelihood fit of the full model and nested sub-model, which is distributed as chi-square for the given degrees of freedom.
Bivariate Model
To assess the degree of overlap in genetic and environmental influences between timing of first alcohol use and AD, a bivariate triangular decomposition (also known as a Cholesky decomposition) was fitted. The proportion of variance attributable to common vs. phenotype-specific sources of influence on initiation of alcohol use and AD were estimated under this model. Models were fitted in Mx using Full Information Maximum Likelihood estimation with raw categorical data. As in the univariate cases, thresholds were adjusted for age at the time of interview. The final model was derived by testing sub-models against the full model, as described above.
RESULTS
Timing of First Alcohol Use and AD
Age at initiation of alcohol use (early, average, or late) and the corresponding prevalence of AD for each age group are shown separately by sex in Table 1. Risk for AD was significantly higher among women who began drinking at an early (OR=4.16; CI: 2.93-5.89) or average age (OR=2.26; CI: 1.68-3.04) when compared with those who initiated alcohol use at a later than average age. The prevalence of AD in women who began drinking at 14 years of age or younger was 25.3%, compared with 7.5% for those who were 18 or older when they first drank. Similar results were found for males: 41.1% of early vs. 14.6% of late onset drinkers met AD criteria and when compared with late initiators, significant elevations in risk for AD were observed for men who began drinking at an early (OR=4.09; CI: 2.90-5.76) or average age (OR=2.42; CI: 1.74-3.37). The interaction between age at onset and sex was non-significant (OR=0.97; CI: 0.77-1.22), indicating that although the prevalence of AD was higher for males than females (χ2 (1)=181.79; p<0.001), the association between age at initiation of alcohol use and risk for AD was consistent across sexes. The correlation between age at first drink (categorized as a three-level variable) and AD for the full sample was 0.31 (p = 0.02).
Table 1.
Alcohol Dependence by Sex and Age at First Drink
| Alcohol Dependence | ||||
|---|---|---|---|---|
| Age at First Drink | Females | Males | ||
| Prevalence | Odds Ratio* (95% CI) | Prevalence | Odds Ratio* (95% CI) | |
| Early (<=14 years) | 25.3% | 4.16 (2.93-5.89) | 41.1% | 4.09 (2.90-5.76) |
| Average (15-17 years) | 15.5% | 2.26 (1.68-3.04) | 29.2% | 2.42 (1.74-3.37) |
| Late (>=18 years) | 7.5% | ------ | 14.6% | ------ |
OR relative to late age at first drink group
Univariate Models
The univariate models for the timing of first alcohol use and AD are shown in Table 2. For each phenotype, the full model, which included separate estimates for females and males, is shown above the final model. For the timing of first alcohol use, equating A, C, and E across females and males did not result in a significant difference in model fit (Δχ2 (1)=0.87; p=0.83). A deterioration in model fit was observed both when the A pathway was dropped (Δχ2 (1)= 23.61; p<0.001) and when the C pathway was dropped (Δχ2 (1)= 7.14; p<0.01). Thus the best-fitting model was an ACE model that equated A, C, and E values in females and males. Under this model, genetic influences accounted for 37% and shared environmental influences accounted for 14% of the variance in the initiation of alcohol use. Individual-specific factors (E) accounted for the remaining 49%. In the univariate alcohol dependence model, A, C, and E could be equated across sexes (Δχ2 (1)= 0.11; p=0.99). Dropping the C pathway did not result in a significant change in model fit (Δχ2 (1)= 0.03; p=0.87), indicating that an AE model provided the best fit for the data. In the final model, just over half (53%) of the variance in AD was attributable to genetic (A) factors, 47% to unique environmental sources of variance.
Table 2.
Proportion of Variance Attributable to Additive Genetic (A), Shared Environmental (C) and Non-shared Environmental (E) Influences on Timing of First Alcohol Use and AD: Univariate Models*
| Model | A | C | E | ||
|---|---|---|---|---|---|
| Timing of First Alcohol Use | Full | Females | 0.21 [95% CI: 0.00 – 0.46] | 0.29 [95% CI: 0.13 – 0.49] | 0.50 [95% CI: 0.42 – 0.57] |
| Males | 0.39 [95% CI: 0.10 – 0.59] | 0.13 [95% CI: 0.00 – 0.38] | 0.48 [95% CI: 0.40 – 0.57] | ||
| Final | (F & M set equal) | 0.37 [95% CI: 0.22 – 0.54] | 0.14 [95% CI: 0.01 – 0.26] | 0.49 [95% CI: 0.43 – 0.54] | |
| Alcohol Dependence | Full | Females | 0.46 [95% CI: 0.03 – 0.65] | 0.07 [95% CI: 0.00 – 0.42] | 0.47 [95% CI: 0.35 – 0.61] |
| Males | 0.55 [95% CI: 0.17 – 0.65] | 0.00 [95% CI: 0.00 – 0.31] | 0.45 [95% CI: 0.35 – 0.58] | ||
| Final | (F & M set equal) | 0.53 [95% CI: 0.44 – 0.61] | ------- | 0.47 [95% CI: 0.39 – 0.56] |
All models adjusted for age at time of interview
Bivariate Model
The bivariate model is shown with unstandardized path coefficients in Figure 1. The proportion of total variance attributable to genetic and environmental sources is reported with 95% confidence limits in Table 3. Equating A, C, and E across females and males did not produce a significant change in model fit for timing of first use (Δχ2 (3)=0.64; p=0.89) or for AD (Δχ2 (3)=0.27; p=0.97). Covariance in timing of first use and AD could also be equated for females and males without a deterioration in model fit (Δχ2 (2)=0.28; p=0.87). Dropping the C pathway for AD also did not result in a significant change in model fit (Δχ2 (2)=1.03; p=0.60), but C could not be dropped for timing of first use (Δχ2 (2)=6.98; p=0.03). Dropping the E pathway between the two phenotypes did not significantly impact model fit (Δχ2 (2)=3.88; p=0.14), indicating that although a portion of covariance was attributable to individual-specific factors common to the two phenotypes (16.5%), the contribution of common E was not statistically significant. By contrast, the A pathway representing the genetic covariance between timing of first alcohol use and AD could not be dropped without a deterioration in model fit (Δχ2 (2)=6.57;p=0.04).
Figure 1.
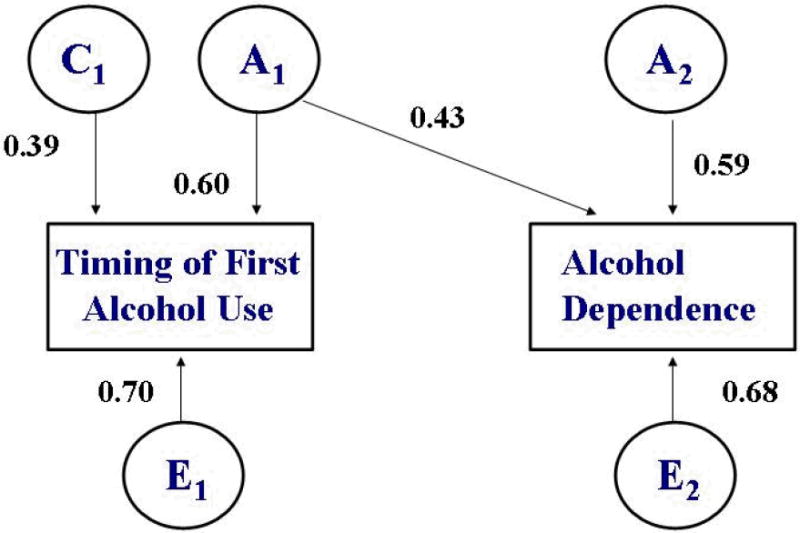
Bivariate Cholesky Decomposition: Timing of First Alcohol Use and Alcohol Dependence*
* Adjusted for age at time of interview
Table 3.
Proportion of Variance Attributable to Additive Genetic (A), Shared Environmental (C) and Non-shared Environmental (E) Influences on Timing of First Alcohol Use and AD: Bivariate Model*
| A | C | E | |
|---|---|---|---|
| Timing of First Alcohol Use | 0.36 [95% CI: 0.21 – 0.52] | 0.15 [95% CI: 0.02 – 0.27] | 0.49 [95% CI: 0.44 – 0.49] |
| Alcohol Dependence | 0.53 [95% CI: 0.45 – 0.61] | ------- | 0.47 [95% CI: 0.39 – 0.55] |
Adjusted for age at time of interview
Under the best-fitting model, heritable influences accounted for a substantial proportion of the variance in the timing of first alcohol use (36%) and an even larger proportion of the variance in AD status (53%). There was also a considerable degree of overlap in these genetic factors (rA1-A2 = 0.59; CI: 0.45-0.80). The timing of alcohol use initiation was attributable in small part to shared environmental factors (15%), but there was no evidence that shared environment played a role in the development of AD.
DISCUSSION
In the current study we examined age at first drink and AD in a genetically-informative framework, toward the end of estimating the magnitude of heritable and environmental influences on the two alcohol-related outcomes and quantifying the overlap in these influences. Results provide additional support for the strong association between early onset of alcohol use and AD and, by producing evidence that common genetic risk accounts in large part for this association, address the much-debated issue of whether the relationship is causal in nature. Consistent with prior studies1-3,5, initiation of alcohol use at a younger than average age was associated with elevated rates of subsequent AD. By operationalizing timing of first use as a three-level variable (as opposed to using a dichotomous indicator of early onset) we were able to detect the higher prevalence in the average vs. late onset group as well, which suggests a linear effect of age at first drink on AD. Also consistent with the existing literature, rates of AD were higher among men than women in our sample31-34, but, importantly, the relationship between early initiation and AD did not vary by sex.
Results indicated that the timing of first alcohol use was attributable in part (36%) to heritable factors but that shared environment also played a role, accounting for 15% of individual differences in timing of first alcohol use. Earlier investigations have also found that initiation of alcohol use (both “ever use” and timing of first use) is influenced by a combination of genetic and shared environmental factors, but in contrast to the current study, the relative contributions of shared environmental factors have been somewhat greater than those from heritable factors20,23,24. Distinctions between our findings, based on 24 to 36 year-olds, and earlier work in this area, which has been conducted primarily with adolescent samples, may reflect the tendency of heritability estimates for substance use initiation to increase with age of the sample35,36. Koopmans and Boomsma21, for example, reported that shared environment accounted for 58-88% of variance in initiation in a sample of 15-16 year olds, whereas in their sample of 17-22 year-olds, 43% was attributable to genetic factors and only 37% to shared environmental influences. (A possible explanation for this phenomenon is the decrease in the relative influence of the family environment and the corresponding increase in the degree of influence from genetic factors with increasing age35,36.)
Just over half of the variance in AD (53%) was accounted for by genetic factors, with the remainder explained by unique environmental factors. There was no evidence for shared environmental influences on AD. Our heritability estimate falls within the 50-60% range reported in the larger literature, which, like the current study, supports a model of alcohol dependence as a disorder shaped about equally by genetic and individual-specific environmental factors12,14-16.
The best-fitting genetic models both for initiation and alcohol dependence equated A, C, and E across males and females, indicating that the nature and strength of the influences on timing of first alcohol use and AD did not vary by sex - an interesting parallel to our finding that the relationship between the two outcomes was the same for men and women. The absence of distinctions by sex in the relative contributions of genetic and environmental influences on AD is consistent with the highly similar heritability estimates for males and females reported in the larger literature12,13,25,26. In studies of alcohol use initiation, comparisons between sex-specific and non sex-specific genetic models have only rarely been made and findings across the few that have reported on these comparisons are inconsistent. Higher heritability estimates for males, non-significant trends for a greater influence of shared environment for females, and the absence of significant differences between females and males4,18,19 have all been reported. Thus, our findings contribute to an as-yet inconclusive literature on an aspect of alcohol use that, given its important implications for understanding the etiology of drinking behaviors, merits further investigation.
Regarding the nature of the link between timing of first alcohol use and AD, our findings indicate that common genetic risk plays a major role. The moderate to high (0.59) genetic correlation is indicative of substantial overlap in the heritable factors that influence timing of first use and those that influence AD development. Environmental influences, by contrast, did not overlap substantially across the two alcohol outcomes. Shared environment contributed to timing of first use but not to AD and the individual-specific environmental influences on timing of first alcohol use and AD were not significantly correlated. Thus, our results are consistent with the interpretation of early alcohol use initiation as a marker for familial liability to AD rather than a direct cause of the disorder9-11. However, given that a small (albeit non statistically significant) portion of covariance (16.5%) was attributable to individual-specific factors, we cannot conclude, as Prescott and Kendler10 did, that the association is explained in full by familial sources. Overall, our findings highlight the prominent role of genetics in the development of AD and the early manifestation of that genetic risk in the timing of alcohol use initiation, which, unlike AD, is also influenced to a modest degree by shared environmental factors.
Limitations and Future Directions
In examining the possible mechanisms underlying the association between early initiation of alcohol use and AD, the current study raised questions that are critical to address in future research efforts. First, the generalizability of our findings to populations with different attitudes toward alcohol use is not known. To what extent might cross-cultural differences in norms for drinking behaviors (including distinctions by gender in those norms) translate into differences in heritability and common genetic risk in other populations? Second, to what degree do genetic factors account for variance in other stages of alcohol use, such as cessation of problem drinking, and do they overlap with those that influence timing of initiation and AD? Furthermore, are these associations explained by inherited vulnerability to disinhibitory behaviors more generally9 rather than - or perhaps in addition to - genetic liability specific to alcohol-related behaviors? Another important consideration for future work in this area is the potential bias posed by retrospective reporting, most notably, forward telescoping. We attempted to minimize the influence of this bias by adjusting for age at which participants were interviewed, but studying drinking behaviors prospectively would provide a higher degree of accuracy in the assessment of changes over the course of alcohol use. Finally, which shared environmental factors are most influential in the timing of first drink and what specific genes contribute to initiation and AD? The integration of findings from these lines of research would contribute significantly to the development of a comprehensive etiological model of the course of alcohol use and dependence. It would also provide a framework for devising interventions that target biological and psychosocial risk factors most relevant to a given stage of alcohol use.
Acknowledgments
Funding for this study was provided by grants AA010247, AA007728, AA011998, and AA017010 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and grants DA012854 and DA018660 from the National Institute on Drug Abuse (NIDA).
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.DeWit D, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: A risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–50. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- 2.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the Longitudinal Alcohol Epidemiological Survey. Journal of Adolescent Substance Abuse. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 3.Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: Age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–46. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- 4.McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001;25:1166–73. [PubMed] [Google Scholar]
- 5.Nelson CB, Wittchen H-U. DSM-IV alcohol disorders in a general population sample of adolescents and young adults. Addiction. 1998;93:1065–77. doi: 10.1046/j.1360-0443.1998.937106511.x. [DOI] [PubMed] [Google Scholar]
- 6.Chou P, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. Br J Addict. 1992;87:1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen W, Skrondal A. Alcohol consumption debut: predictors and consequences. J Stud Alcohol. 1998;59:32–42. doi: 10.15288/jsa.1998.59.32. [DOI] [PubMed] [Google Scholar]
- 8.Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, et al. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol Med. 2005;35:1–10. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- 9.McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25:1156–65. [PubMed] [Google Scholar]
- 10.Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: A noncausal association. Alcohol Clin Exp Res. 1999;23:101–07. [PubMed] [Google Scholar]
- 11.King KM, Chassin L. A prospective study of the effects of age of initiation of alcohol and drug use on young adult substance dependence. J Stud Alcohol Drugs. 2007;68:256–65. doi: 10.15288/jsad.2007.68.256. [DOI] [PubMed] [Google Scholar]
- 12.Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut RJ, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–96. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- 13.Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, et al. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol Med. 2004;34:1519–30. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- 14.Reed T, Page WF, Viken RJ, Christian JC. Genetic predisposition to organ-specific endpoints of alcoholism. Alcohol Clin Exp Res. 1996;23:1528–33. doi: 10.1111/j.1530-0277.1996.tb01695.x. [DOI] [PubMed] [Google Scholar]
- 15.True WR, Heath AC, Bucholz KK, Slutske W, Romeis JC, Scherrer JF, et al. Models of treatment seeking for alcoholism: the role of genes and environment. Alcohol Clin Exp Res. 1996;20:1577–81. doi: 10.1111/j.1530-0277.1996.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 16.van den Bree MBM, Johnson EO, Neale MC, Svikis DS, McGue M, Pickens RW. Genetic analysis of diagnostic systems of alcoholism in males. Biol Psychiatry. 1998;43:139–45. doi: 10.1016/S0006-3223(97)00225-4. [DOI] [PubMed] [Google Scholar]
- 17.Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, et al. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;101:413–22. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–93. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- 19.Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–64. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- 20.Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, et al. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2006;101:413–22. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koopmans JR, Boomsma DI. Familial resemblance in alcohol use: genetic or cultural transmission? J Stud Alcohol. 1996;57:19–28. doi: 10.15288/jsa.1996.57.19. [DOI] [PubMed] [Google Scholar]
- 22.Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcohol Clin Exp Res. 2001;25:1594–1604. [PubMed] [Google Scholar]
- 23.Stallings MC, Hewitt JK, Beresford T, Heath AC, Eaves LJ. A twin study of drinking and smoking onset and latencies from first to regular use. Behav Genet. 1999;6:409–21. doi: 10.1023/a:1021622820644. [DOI] [PubMed] [Google Scholar]
- 24.Sartor CE, Agrawal A, Lynskey MT, Bucholz KK, Heath AC. Genetic and environmental influences on the rate of progression to alcohol dependence in young women. Alcohol Clin Exp Res. 2008;32:632–8. doi: 10.1111/j.1530-0277.2008.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LC. A twin-family study of alcoholism in women. Am J Psychiatry. 1994;151:707–15. doi: 10.1176/ajp.151.5.707. [DOI] [PubMed] [Google Scholar]
- 26.Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcohol Clin Exp Res. 1999;23:1136–44. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- 27.Hopfer CJ, Stallings MC, Hewitt JK. Common genetic and environmental vulnerability for alcohol and tobacco use in a volunteer sample of older female twins. J Stud Alcohol. 2001;62:717–23. doi: 10.15288/jsa.2001.62.717. [DOI] [PubMed] [Google Scholar]
- 28.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report of the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 29.Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA - A comparison with the SCAN. Addiction. 1999;94:1361–70. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- 30.Neale MC, Boker SM, Xie G, Maes HH, editors. Mx: Statistical Modeling. 6. Richmond, VA: Department of Psychiatry, Medical College of Virginia; 2003. [Google Scholar]
- 31.Grant BF. Prevalence and correlates of drug use and DSM-IV drug dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;8:195–210. doi: 10.1016/s0899-3289(96)90249-7. [DOI] [PubMed] [Google Scholar]
- 32.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiological Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 33.Holdcraft LC, Iacono WG. Cohort effects on gender differences in alcohol dependence. Addiction. 2002;97:1025–36. doi: 10.1046/j.1360-0443.2002.00142.x. [DOI] [PubMed] [Google Scholar]
- 34.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 35.Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J Am Acad Child Adolesc Psychiatry. 2003;42:710–9. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- 36.Viken RJ, Kaprio J, Kiskenvuo M, Rose RJ. Longitudinal analyses of the determinants of drinking and of drinking to intoxication in adolescent twins. Behav Genet. 1999;29:455–61. doi: 10.1023/a:1021631122461. [DOI] [PubMed] [Google Scholar]


