Abstract
Environmental enrichment produces functional changes in mesolimbic dopamine transmission and alters sensitivity to psychomotor stimulants. These manipulations also alter the control rate of many behaviors that are sensitive to stimulant administration, which can make comparison of drug effects between isolated and enriched subjects difficult. The purpose of this study was to examine the effects of environmental enrichment on control rates of behavior and on sensitivity to cocaine in tests of locomotor activity, drug self-administration, conditioned place preference, and toxicity. In the locomotor activity test, isolated rats exhibited greater activity after the administration of cocaine, but also had higher control rates of activity. When locomotor activity was expressed as a percentage of saline control values, enriched rats exhibited a greater increase relative to their own control than isolated rats. In the drug self-administration procedure, isolated rats had higher breakpoints on a progressive-ratio schedule of reinforcement when responding was maintained by cocaine; however, isolated rats also had higher breakpoints in saline substitution tests and higher rates of inactive lever responding. When the self-administration data were expressed as a percentage of these control values, enriched rats exhibited a greater increase in responding relative to their own control rates than isolated rats. No differences were observed between isolated and enriched rats under control conditions in the place preference and toxicity studies. In both of these procedures, enriched rats were more sensitive than isolated rats to all the doses of cocaine tested. These data emphasize the importance of considering control rates of behavior in studies examining environmental enrichment and drug sensitivity, and suggest that environmental enrichment increases sensitivity to cocaine across a range of dependent measures whether differences in control rates of behavior are taken into account.
Keywords: cocaine, conditioned place preference, enrichment, environment, female, locomotor, rat, self-administration, social, toxicity
Introduction
Rearing animals in enriched environments (i.e. environments in which animals are housed socially in groups and provided with novel objects to explore and manipulate) during critical periods in development produces a number of neuroanatomical effects that are associated with functional changes in behavior. For instance, rats reared under conditions of environmental enrichment have greater cortical mass (Rosenzweig et al., 1962; Bennett et al., 1969), have greater dendritic branching (Davies and Katz, 1983; Johansson and Belichenko, 2002), and perform better on learning and memory tasks (Kobayashi et al., 2002; Dhanushkodi et al., 2007) than rats reared in isolation. There is also a growing body of evidence that environmental enrichment influences an organism's sensitivity to centrally acting drugs. In perhaps the most compelling series of studies, Bardo et al. (1995) have reported that environmental enrichment produces functional changes in mesolimbic dopamine transmission and alters sensitivity to the effects of the indirect dopamine agonist amphetamine. These investigators have shown, for example, that enriched male rats are more sensitive than isolated male rats to the effects of amphetamine in the conditioned place preference procedure (Bowling and Bardo, 1994; Bardo et al., 1995), and exhibit greater amphetamine-induced dopamine synthesis and metabolism in the striatum and nucleus accumbens, respectively (Bowling et al., 1993). Enriched male rats also exhibit greater amphetamine-induced increases in locomotor activity than isolated rats (Bowling et al., 1993; Bowling and Bardo, 1994; Bardo et al., 1995), and enriched rats of both the sexes self-administer less amphetamine on fixed-ratio (FR) and progressive-ratio (PR) schedules of reinforcement (Bardo et al., 2001; Green et al., 2002).
Environmental enrichment also influences a number of emitted and elicited behaviors that are subject to manipulation by drugs. It is well established, for instance, that enrichment reduces exploratory behavior and increases habituation to a novel environment (Zimmermann et al., 2001; Schrijver et al., 2002). The consequences of these effects are lower control rates of activity in enriched subjects relative to isolated subjects. In the studies described above, for example, significant differences in baseline rates of locomotor activity were observed between isolated and enriched subjects after saline administration (Bowling et al., 1993; Bardo et al., 1995). There is also evidence that environmental enrichment decreases rates of emitted behavior in free operant procedures. For instance, in the absence of drug administration, enriched rats respond less than isolated rats when responding is maintained by a conditioned stimulus on an FR schedule and by food on a PR schedule (Smith et al., 1997). Similarly, enriched rats respond less than isolated rats during extinction (Stairs et al., 2006), and emit fewer responses than isolated rats on an inactive lever in the absence of any explicit operant training (Cain et al., 2006). Interestingly, in at least one study examining the effects of environmental enrichment on drug self-administration, significant differences in operant responding were observed between isolated and enriched subjects during saline control sessions (Green et al., 2002).
Differences in control rates of behavior owing to environmental enrichment can make interpretation of drug effects difficult. For instance, in studies measuring locomotor activity, the dependent measure is often depicted as a percentage of a subject's individual control values (e.g., Boyle et al., 1991; Bowling and Bardo, 1994; Smith et al., 1997); however, in studies examining schedule-controlled behavior, such as drug self-administration, absolute values are more often depicted (e.g. Bardo et al., 2001; Green et al., 2002). Few studies have specifically compared these two methods of data presentation, and thus it is unclear whether qualitatively different conclusions might be reached based on the method employed. It is conceivable, for example, that drug administration may lead to higher absolute rates of behavior in one subject population, but the percentage increase in the rate of that behavior relative to control values would be less than that of a second population, if control rates of behavior were indeed different between the two groups.
The purpose of this study was to examine the effects of environmental enrichment on sensitivity to cocaine and to determine whether these effects are influenced by differences in control rates of behavior. To this end, the effects of environmental enrichment were examined on measures of locomotor activity and drug self-administration, two procedures that are capable of detecting differences between isolated and enriched subjects under control conditions. Data generated in these procedures were depicted as both absolute rates of behavior and as a percentage of control values, and all statistical analyses were performed on both sets of data. These data were then compared with data obtained in tests of cocaine-induced toxicity and conditioned place preference, two procedures that do not engender between-group differences under control conditions.
Method
Subjects
Female Long-Evans rats (Charles River Laboratories, Raleigh, North Carolina, USA) were obtained at weaning (approximately 21 days) and immediately divided into two groups. Isolated rats were housed individually in opaque laboratory cages (interior dimensions: 43×21×20λcm) that permitted no visual or tactile contact with other rats. Enriched rats were housed in groups of four in large cages (interior dimensions: 92×38×40λcm) that permitted extensive social interactions between cagemates. Rats in this group received supplemental enrichment from a variety of objects (e.g. cardboard and polycarbonate tubes, ladders, ping-pong balls, animal toys) that were changed daily. All rats were housed under their respective conditions throughout the duration of the study. Except during behavioral testing, both groups were maintained in a large colony room maintained on a 12-h light/dark cycle with food and drinking water freely available in the home cages. Throughout the study, estrous phase was allowed to cycle normally and was not monitored. All the procedures were approved by the Davidson College Animal Care and Use Committee and animals were maintained in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources, 1996). Behavioral testing commenced 6–8 weeks after arrival.
Locomotor activity
Locomotor activity was measured in an open-field test chamber (interior dimensions: 43×43×30λcm). Two circuit boards were located on opposite sidewalls mounted 2.5λcm above the base of the apparatus. One board contained 16 infrared photocells spaced 2.5λcm apart, whereas the other contained 16 infrared detectors with the same spacing. All photocells and detectors were interfaced to a microprocessor that continuously recorded photobeam interruptions throughout the session using software supplied by Med Associates, Inc. (St. Albans, Vermont, USA).
Approximately 8 weeks after arrival, rats (nλ=λ16; eight isolated, eight enriched) were habituated to the locomotor activity chamber for 5λmin a day for 3 consecutive days. Drug and saline (control) tests were conducted over the next 2 consecutive days, with the order of drug and saline testing counterbalanced across subjects. During drug tests, each rat was initially injected with the lowest dose of cocaine and then immediately returned to its home cage. After a 15-min interval, the rat was placed in the activity chamber for 120λs and locomotor activity was recorded. After 120λs, the rat was removed from the chamber, injected with the next dose of cocaine, and returned to its home cage. After another 15-min interval, the rat was again placed in the chamber, and locomotor activity was recorded. The session continued in this fashion until cumulative doses of 3.0, 10, and 30λmg/kg were tested in all rats. Saline control tests were conducted after a similar protocol with the exception that saline (1.0λml/kg) was administered at the beginning of each component in lieu of cocaine.
Drug self-administration
Self-administration sessions were conducted in polycarbonate and aluminum operant conditioning chambers (interior dimensions: 31×24×21λcm) using software and interfacing from Med Associates, Inc. Each chamber was equipped with two response levers located 10λcm above the chamber floor and a single house light located on the rear wall beneath the ceiling. A white stimulus light located above the response lever signaled the availability of a drug infusion from a pump mounted outside the conditioning chamber. All infusions were delivered through a tygon tube attached to a steel swivel at the top of the chamber. The left lever was designated as the active lever for all rats. Responses on the right (inactive) lever were recorded but had no programmed consequences.
Six weeks after arrival, rats were anesthetized with a combination of ketamine HCl (100λmg/kg, intraperitoneal) and xylazine HCl (8.0λmg/kg, intraperitoneal), and surgically implanted with indwelling venous catheters (CamCaths, Cambridge, UK). A silastic tube was inserted into the right jugular vein and exited the body on the dorsal surface of the scapulae. Butorphanol HCl (1.0λmg/kg, subcutaneous) was given immediately after the surgery as an analgesic. A solution of heparinized saline and ticarcillin (20λmg/kg) was infused through the catheter daily over the next 7 days to maintain patency and prevent infection. After 7 days, ticarcillin administration was discontinued but infusions of heparinized saline continued daily until the end of the study. The catheter tip was capped with tygon tubing and fishing line between sessions, and a brass wood nut was screwed onto the base of the catheter to protect the plastic threading.
Behavioral training commenced 3 days after the surgery. In these sessions, rats were removed from their home cages, placed in the operant conditioning chambers, and connected to the infusion pump through tygon tubing. In the initial sessions, lever pressing was reinforced on a FR1 schedule of reinforcement. Under these conditions, each lever press activated the infusion pump and delivered 1.0λmg/kg cocaine over a 4–5λs duration (based on body weight). Concurrent with the start of each infusion, a tone was sounded for the duration of the infusion, and the stimulus light above the lever was turned off for 20λs to signal a time-out period in which cocaine was not available and responses had no programmed consequences. In this and all subsequent training sessions, the session was terminated after 3λh or once 10 infusions were obtained, whichever occurred first. Once 10 infusions were obtained on the FR1 schedule during any 2 training days, contingencies were changed on the following day and responding was reinforced on an FR2 schedule of reinforcement. Once 10 infusions were obtained on the FR2 schedule during any one session, behavioral training was terminated and behavioral testing commenced on the following day. Thus, all rats completed a minimum of three training sessions before advancing to behavioral testing.
Tests were conducted 7 days per week at approximately the same time each day. Throughout behavioral testing, lever pressing was reinforced on a PR schedule of reinforcement. Under these conditions, the number of responses required for a drug infusion progressively increased within a session through the following ratio values: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, 268, 346, 445, and 573 (for complete algorithm, see Ref. Suto et al., 2002). Each session continued until a breakpoint was reached, with breakpoint defined as the number of infusions obtained before 1λhr elapsed with no infusions. Test sessions continued at a given dose until breakpoints were stable (i.e. 3 consecutive days during which the breakpoint varied by no more than three increments with no increasing or decreasing trends). Breakpoints were determined for 0.0 (saline), 0.1, 0.3, and 1.0λmg/kg/infusion cocaine in a pseudo random order. Isolated rats that lost catheter patency were removed from the study and their data were not used in the statistical analysis. Enriched rats that lost catheter patency were maintained in their home cage to provide social enrichment for their cagemates; however, they were not placed back into the chambers and their data were not used for the statistical analysis. A total of 11 isolated rats and 10 enriched rats completed all phases of the study.
Conditioned place preference
Conditioned place preference was assessed with a three-compartment place preference chamber obtained from Med Associates, Inc. The chamber consisted of two choice compartments (25×20×20λcm) separated by a smaller center compartment (13×20×20λcm). One choice compartment was painted black and had a steel-rod floor covering corncob bedding. The other choice compartment was painted white and had a wire-screen floor covering pine bedding. The center compartment was painted a neutral gray and had a solid PVC floor with no underlying bedding. Each choice compartment was separated from the center compartment by a manually operated guillotine door. Behavior was monitored by a video camera mounted 1.5λm above the chamber.
Six weeks after arrival and one day before the first conditioning trial, each rat was given 15λmin to habituate to the conditioning chamber. During this habituation session, rats were placed in the center (neutral) compartment and given free access to the entire chamber by opening the guillotine doors separating the two choice compartments from the center compartment. The amount of time spent in each of the three compartments was recorded and summated over the entire 15-min session.
Over the next 8 consecutive days, rats received daily conditioning trials in which they were injected with either a selected dose of cocaine or saline and placed into one of the two choice compartments for 30λmin. Both guillotine doors were closed during these conditioning trials, and rats were confined to the appropriate compartment for the duration of the trial. Drug and saline administration alternated daily such that each rat received four conditioning trials with both the drug-paired and saline-paired compartments. The drug-paired and saline-paired compartments were assigned randomly, such that the black compartment served as the drug-paired compartment for half of the subjects, and the white compartment served as the drug-paired compartment for the other half of the subjects. To determine whether a compartmental bias would develop in the absence of drug administration, and to establish a control condition to which the cocaine data could be compared, a subset of rats was conditioned with saline in both compartments. For these rats, the white compartment was delineated as the ‘drug-paired’ compartment for half of the rats, whereas the black compartment was delineated as the ‘drug-paired’ compartment for the other half.
On the day immediately following the last conditioning trial, place preference was assessed in each rat. During this test session, rats were placed in the center compartment and both guillotine doors were opened. Rats had free access to the entire chamber for 15λmin, and the amount of time spent in each of the three compartments was recorded. Tests were conducted with 5.0 and 10λmg/kg cocaine, as well as with saline. Five to nine rats from each group were used for each dose condition.
Cocaine-induced toxicity
Six weeks after arrival, rats were anesthetized and surgically implanted with venous catheters as described above (see Methods: Drug self-administration). One week after the surgery, each rat was connected intravenously to a drug syringe containing 1.0λml pentobarbital (100λmg/ml) that would later be used as a euthanasia agent (see below). At the beginning of the test, each rat was administered with either 50λmg/kg (nλ=λ16; eight isolated, eight enriched) or 75λmg/kg (nλ=λ16; eight isolated, eight enriched) cocaine through intraperitoneal injection. Immediately after the injection, each rat was placed individually into a transparent polycarbonate cage and cocaine-induced convulsive activity was measured over a 30-min observation period. Convulsive activity was scored through a point system and classified using well-established criteria (see Refs Racine, 1972, Barat and Abdel-Rahman, 1996; Potschka et al., 1998; Duarte et al., 2008): stage 0: no change in behavior; stage 1: immobility, eye closure, twitching of vibrissae, sniffling, facial clonus; stage 2: head nodding associated with more severe facial clonus; stage 3: clonus of one forelimb; stage 4: rearing accompanied by bilateral forelimb clonus; stage 5: rearing with loss of balance and falling, accompanied by generalized clonic seizures.
All rats were euthanized immediately upon reaching stage 5 (i.e. when a loss of balance was observed indicating the onset of a generalized clonic seizure) with an overdose of pentobarbital (approximately 250λmg/kg, intravenous). Any rat that failed to reach level 5 was euthanized promptly at the end of the 30-min observation period. Convulsive activity was scored after saline administration in 16 additional rats (nλ=λ8 sedentary, nλ=λ8 enriched), but these rats were not implanted with venous catheters or euthanized after testing, as convulsive activity was not expected or observed in this group. Cocaine-induced lethality was not an endpoint in this study and duration of seizure activity was not measured.
Data analysis
In the locomotor activity test, total distance traveled (cm) was computed automatically for each rat during each of the 120-s test periods. Locomotor activity was expressed as distance traveled in both the saline control test and in the test conducted with cocaine. Locomotor activity after cocaine administration was also expressed as a percentage of saline control values by dividing the distance traveled during each component of the test session by that obtained during the corresponding component of the saline control session, and then multiplying by 100. Locomotor activity data were analyzed through mixed-factor analysis of variance (ANOVA), with group serving as a between-subjects factor and dose (or component) serving as the repeated measure.
In the self-administration sessions, breakpoint was defined as the total number of infusions, rather than the total number of responses, to insure that the data were distributed normally for all statistical tests (i.e. to insure a Gaussian distribution). Breakpoints maintained by cocaine were also expressed as a percentage of control values, by dividing breakpoints obtained at each dose of cocaine to those obtained with saline, and then multiplying by 100. The number of active and inactive lever presses was also recorded during each session. Active lever responding at each dose condition was also expressed as a percentage of control responding, by dividing the number of active lever presses by the number of inactive lever presses, and then multiplying by 100. All self-administration data were analyzed through mixed-factor ANOVA, with group serving as a between-subjects factor and dose serving as the repeated measure. Data on acquisition and from saline substitution tests were analyzed through independent-samples t-tests using group as a factor.
In the conditioned place preference study, difference scores were obtained for each rat by subtracting the amount of time spent in the drug-paired compartment before conditioning (i.e. during the free-access habituation session) from the amount of time spent in the drug-paired compartment after conditioning (i.e. during the free-access place preference test). These scores were then analyzed through two-way ANOVA, with dose and group serving as between-subjects factors.
Cocaine-induced toxicity was scored through a 5-point system, with greater numbers indicating greater convulsive activity. These data were averaged across rats and analyzed through two-way ANOVA, with dose and group serving as between-subjects factors.
Results
Locomotor activity
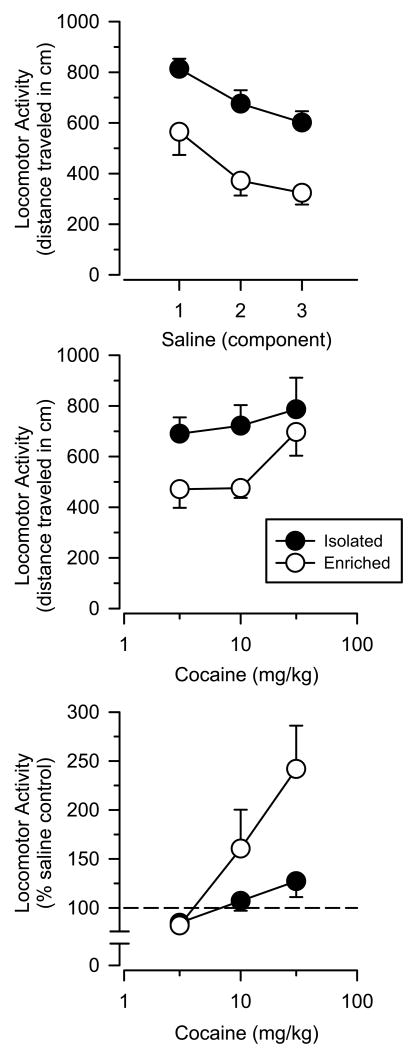
As expected, rats in both groups exhibited significant within-session habituation to the locomotor activity chamber during the saline control session (Fig. 1, top panel). Distance traveled (as expressed in cm) systematically decreased across each component of the session in both groups [main effect of component: F(2.28)λ=λ30.49; P<0.001]. Throughout the session, isolated rats were significantly more active than enriched rats [main effect of group: F(1.14)λ=λ15.57; Pλ=λ0.001], with isolated rats traveling approximately 300λcm further than enriched rats during each component.
Fig. 1.
Locomotor activity in isolated and enriched rats (mean ±SEM). Top panel: locomotor activity expressed as distance traveled (cm) during three consecutive components of the saline control session. Middle panel: locomotor activity expressed as distance traveled (cm) after cumulative doses of cocaine. Bottom panel: locomotor activity expressed as a percentage of saline control values after cumulative doses of cocaine.
When expressed as distance traveled, cocaine produced dose-dependent increases in locomotor activity in both the groups (Fig. 1, middle panel), with this effect approaching but failing to reach statistical significance [main effect of dose: F(2.28)λ=λ2.97; Pλ=λ0.068]. Locomotor activity was significantly greater in isolated rats than enriched rats after cocaine administration [main effect of group: F(1.14)λ=λ4.94; P<0.05]. Isolated rats traveled approximately 200λcm further than enriched rats at the two lower doses of cocaine, and approximately 50λcm further than enriched rats at the highest dose of cocaine.
Relative to baseline (i.e. saline control values), enriched rats were significantly more sensitive than isolated rats to the locomotor effects of cocaine, and this effect was most pronounced at the highest dose (Fig. 1, bottom panel). At this dose, locomotor activity averaged 127 and 242% of saline control values in isolated and enriched rats, respectively. Consistent with these observations, a mixed-factor ANOVA revealed a significant main effect of dose [F(2.28)λ=λ10.83; P<0.001] and a significant dose × group interaction [F(2.28)λ=λ3.64; P<0.05]. The main effect of group approached but failed to reach statistical significance [F(1.14)λ=λ4.33; Pλ=λ0.056].
Drug self-administration
Isolated and enriched rats advanced to the PR schedule in 7.18 (1.91) and 5.50 (1.09) days, respectively; a difference that was not statistically significant (P>0.05).
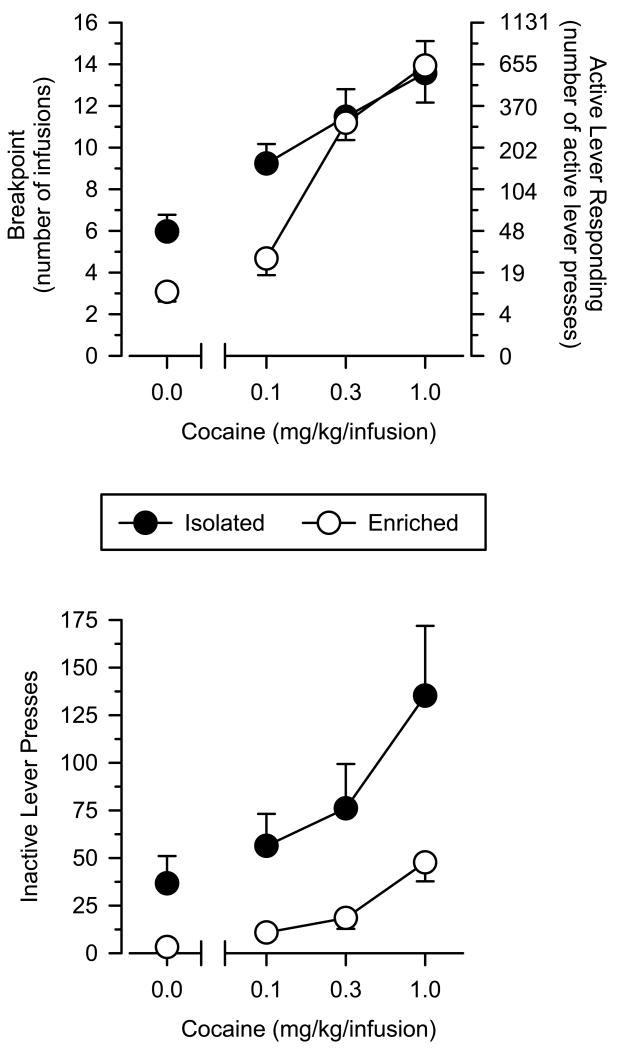
Based on number of infusions, isolated rats had significantly higher breakpoints than enriched rats during saline substitution tests [t(19)λ=λ3.05; P<0.01]. Under these conditions, breakpoints in isolated rats were approximately twice that observed in enriched rats (Fig. 2, upper panel). When responding was maintained by cocaine, isolated rats had significantly greater breakpoints than enriched rats at the lowest dose of cocaine (Pλ=λ0.002), but no differences were observed at the higher doses (P>0.05). A mixed-factor ANOVA revealed a significant main effect of dose [F(2.38)λ=λ43.99; P<0.001] and a significant dose × group interaction [F(2.38)λ=λ38.74; P<0.005].
Fig. 2.
Drug self-administration in isolated and enriched rats (mean ±SEM). Upper panel: breakpoints expressed as number of infusions (left axis) and number of active lever presses (right axis) on a progressive-ratio schedule of reinforcement. Lower panel: number of inactive lever presses under each dose condition.
Both isolated and enriched rats allocated a number of responses to the inactive lever under all dose conditions (Fig. 2, lower panel). Isolated rats emitted a significantly greater number of inactive lever presses than enriched rats, and this effect was apparent at all doses. A mixed-factor ANOVA revealed significant main effects of both dose [F(3.57)λ=λ12.66; P<0.001] and group [F(1.19)λ=λ6.94; P<0.02].
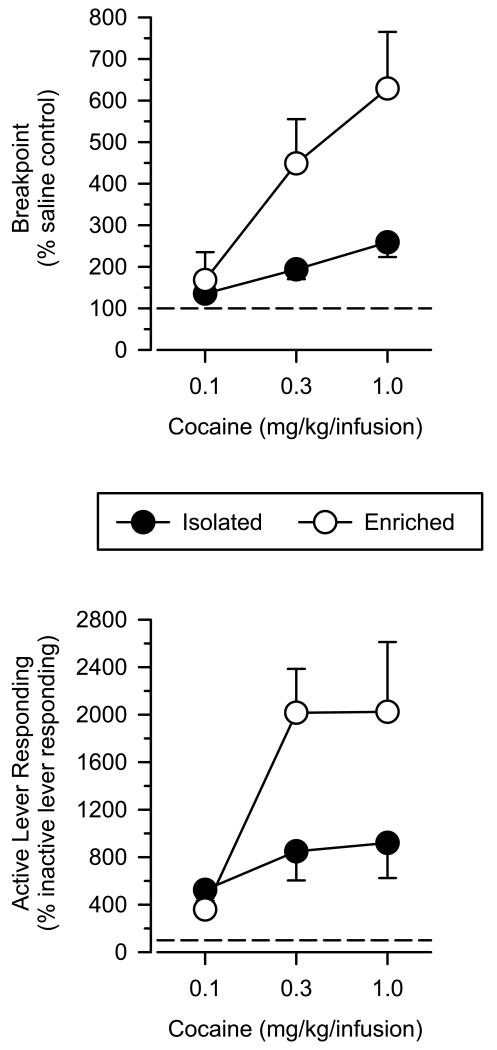
Relative to baseline (i.e. saline control values and inactive lever responding), enriched rats were significantly more sensitive than isolated rats to the positive-reinforcing effects of cocaine, and this effect was particularly apparent at higher doses. For instance, at the highest dose of cocaine tested (1.0λmg/kg/infusion), breakpoints were 259 and 629% of saline control values in isolated and enriched rats, respectively (Fig. 3, upper panel). Consistent with these observations, a mixed-factor ANOVA revealed significant main effects of dose [F(2.38)λ=λ20.16; P<0.001] and group [F(1.19)λ=λ5.91; P<0.025] and a significant dose × group interaction [F(2.38)λ=λ11.40; P<0.001]. Similarly, active lever responding was significantly greater in enriched rats than isolated rats when the data were expressed as a percentage of control values (Fig. 3, lower panel). At the highest dose of cocaine, active lever responding was 920 and 2023% of inactive lever responding in isolated and enriched rats, respectively. Once again, a mixed-factor AVOVA revealed significant main effects of dose [F(2.38)λ=λ9.12; P<0.001] and group [F(1.19)λ=λ5.91; P<0.05] and a significant dose × group interaction [F(2.38)λ=λ3.77; P<0.05].
Fig. 3.
Cocaine self-administration in isolated and enriched rats (mean ±SEM). Upper panel: breakpoints expressed as a percentage of saline control values when responding was maintained by various doses of cocaine. Lower panel: active lever responding expressed as a percentage of inactive lever presses when responding was maintained by various doses of cocaine.
Conditioned place preference
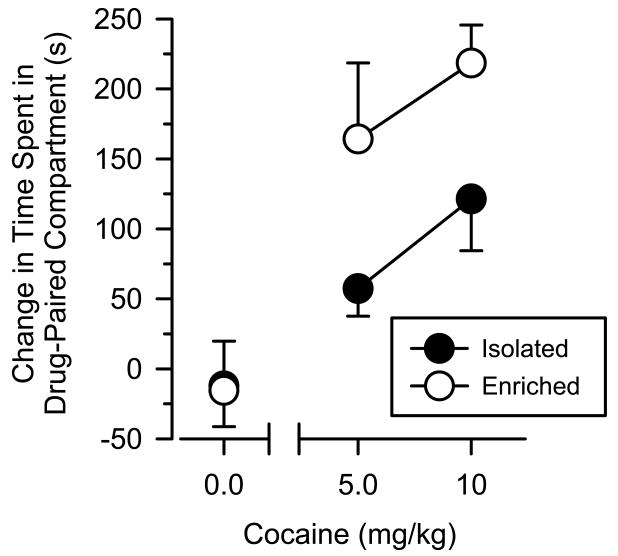
Saline did not produce a conditioned place preference in either isolated or enriched rats (Fig. 4). Difference scores for saline approximated zero and were similar between the two groups. Cocaine produced a dose-dependent conditioned place preference in both the groups [main effect of dose: F(2.41)λ=λ10.09; P<0.001], and this effect was greater in enriched rats than isolated rats at both the doses of cocaine [main effect of group: F(1.41)λ=λ4.32; P<0.05].
Fig. 4.
Difference scores of isolated and enriched rats in the conditioned place preference procedure (mean ±SEM). All data reflect change in time (s) spent in the drug-paired compartment after conditioning.
Cocaine-induced toxicity
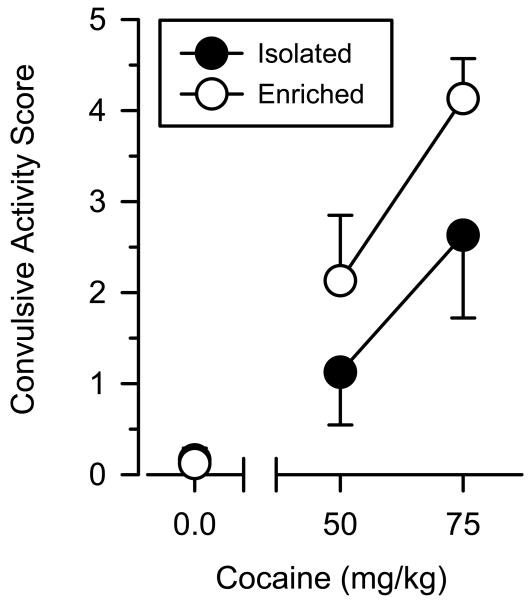
Saline administration did not produce convulsive activity in either group of rats (Fig. 5). Under these conditions, convulsive activity approximated zero in both the groups and no rat scored greater than 1 on the 5-point scale. Cocaine produced convulsive activity in both the groups in a dose-dependent manner. Similar to what was observed in the conditioned place preference test, enriched rats were more sensitive to these effects than isolated rats, and this effect was apparent at both the doses of cocaine. A two-way ANOVA revealed a significant main effect of dose [F(2.47)λ=λ16.734; P<0.001]. The main effect of group approached but failed to reach statistical significance [F(2.47)λ=λ3.29; Pλ=λ0.077].
Fig. 5.
Cocaine-induced toxicity in isolated and enriched rats (mean ±SEM). All data reflect convulsive activity based on a 5-point scale.
Discussion
The principal findings of this study are that (i) environmental enrichment produced differences in control rates of behavior in tests measuring locomotor activity and drug self-administration; (ii) environmental enrichment produced differences in sensitivity to the locomotor and positive-reinforcing effects of cocaine; (iii) the direction and magnitude of these effects depended on the way in which the data are expressed; and (iv) data obtained in these procedures are congruent with data obtained in tests of cocaine-induced toxicity and conditioned place preference if they are expressed as a percentage of control values, but not if they are expressed as absolute values.
In the locomotor activity test, enriched rats had lower baseline rates of activity and lower rates of activity after cocaine administration when the data were expressed as distance traveled. When the data were expressed as a percentage of saline control values, enriched rats were more sensitive than isolated rats to the effects of cocaine in this procedure. Previous studies examining environmental enrichment, or social enrichment in the absence of object enrichment (i.e. subjects are housed socially in groups but not given novel objects to explore and manipulate), have reported that isolated rats have greater control rates of locomotor activity than enriched rats (Boyle et al., 1991; Bowling et al., 1993; Smith et al., 1997; Hall et al., 2001; Zhu et al., 2004). Interestingly, studies examining locomotor activity after psychomotor stimulant administration have reported a different pattern of results, depending on whether the data were depicted as absolute values or as a percentage of control values. Studies that have used absolute values have reported that enriched rats are less sensitive than isolated rats to the locomotor effects of amphetamine and cocaine (Phillips et al., 1994; Smith et al., 1997; Hall et al., 2001). In contrast, studies that have depicted data as a percentage of control values have typically reported that enriched rats are more sensitive to the locomotor effects of these drugs (Boyle et al., 1991; Bowling et al., 1993; Bowling and Bardo, 1994). To our knowledge, only one study explicitly discussed locomotor activity in terms of both absolute values and percentage of control values. Consistent with this study, the authors reported that isolated rats exhibited greater locomotor activity after the administration of the dopamine reuptake inhibitor GBR 12935, but that enriched rats exhibited a greater percentage increase in locomotor activity relative to their own control (Zhu et al., 2004).
A similar pattern of effects was observed in the drug self-administration study. Control rates of behavior, as measured by inactive lever responding and saline substitution tests, were significantly lower in enriched rats than isolated rats, and enriched rats had lower breakpoints than isolated rats when responding was maintained by a low dose of cocaine. When the data were expressed as a percentage of control values, enriched rats were more sensitive than isolated rats to the positive-reinforcing effects of cocaine, and this was particularly evident at higher doses. Studies examining the effects of enrichment (either social enrichment alone or social enrichment in conjunction with object enrichment) on cocaine self-administration have tended to depict the data as absolute response rates or their equivalent (e.g., number of infusions, total cocaine intake). These studies have reported that enriched rats are less sensitive than isolated rats to the positive-reinforcing effects of cocaine (Schenk et al., 1987; Howes et al., 2000), especially at lower doses (Boyle et al., 1991; but see Ref. Phillips et al., 1994 for opposite finding). In regard to amphetamine self-administration, enriched rats have lower response rates than isolated rats on both FR and PR schedules of reinforcement, but this effect is only apparent at low doses (Bardo et al., 2001; Green et al., 2002). In one of these studies, isolated rats had significantly higher breakpoints than enriched rats on a PR schedule when saline was substituted for amphetamine, but these differences were not taken into account in the analysis of the breakpoint data. We are aware of only one study reporting drug self-administration responding in terms of both absolute response rate (total number of lever presses) and relative response rate (ratio of number of infusions versus total lever presses). Consistent with the present findings, the total number of lever presses was greater in isolated rats than enriched rats, but the percentage of total lever presses that were reinforced was greater in enriched rats (Ding et al., 2005).
Data obtained in the conditioned place preference and toxicity studies were less ambiguous. Saline administration did not produce any obvious place preference or toxicity, and control values for both the procedures approximated zero in both the groups. In both the procedures, enriched rats were more sensitive to the effects of cocaine than isolated rats, and this effect was apparent at both low and high doses of cocaine. These data are consistent with previous reports that enriched rats are more sensitive to the effects of amphetamine in the conditioned place preference procedure (Bowling and Bardo, 1994; Bardo et al., 1995), and similar findings have been reported with drugs from other pharmacological classes (Smith et al., 2003, 2005). To our knowledge, this is the first study that examined the effects of environmental enrichment on cocaine-induced toxicity.
It is noteworthy that data from the locomotor and self-administration studies were congruent with data from the toxicity and place preference studies when the former were expressed as a percentage of saline control values, but not when they were expressed as absolute rates of behavior. Although arguments for both methods of data presentation are valid, the present findings suggest that locomotor activity and drug self-administration data might best be presented as relative to control values in situations where baseline rates of behavior differ across experimental conditions. When this premise is applied to the present findings, environmental enrichment produced a consistent pattern of effects across a number of behaviors that were sensitive to cocaine administration. Specifically, environmental enrichment increased sensitivity to the effects of cocaine, and these effects were consistent across a range of doses and dependent measures.
Although locomotor activity is often depicted in absolute values, it is not uncommon for it to be depicted as a percentage of control values when comparing across conditions that produce differences in baseline rates of behavior. In contrast, it is rare to see drug self-administration data represented in this fashion. One reason this may be the case is because differences in responding during saline substitution tests are rarely observed, or at least rarely reported or rarely determined. At least one previous study depicted drug self-administration data in terms of both absolute and relative rates of responding and reported qualitatively different effects based on the method employed (Ding et al., 2005). Very little discussion of this observation was provided, however, and descriptions of differences in control rates of responding remain an anomaly in the self-administration literature. Complicating matters further, there is no standardized method of presenting drug self-administration data when significant differences are observed under control conditions. Our decision to present the data as a percentage of saline control values and inactive lever responding was somewhat arbitrary, as we could have chosen other methods of data presentation, such as the ‘difference’ from saline control values or as a percentage of ‘total’ lever presses. Interestingly, when we calculated the self-administration data by these methods, we saw a similar pattern of results, which speaks to the generality of the present findings.
In summary, environmental enrichment influenced both control rates of behavior and sensitivity to cocaine in tests of locomotor activity and drug self-administration. The direction and magnitude of these effects on cocaine sensitivity depended on whether the data were presented as absolute values or as relative to control rates of behavior. When the data were depicted as a percentage of saline control values, they were consistent with those obtained in tests of cocaine-induced toxicity and conditioned place preference, two procedures that yielded comparable control values between the two groups. These findings reflect the importance of determining control rates of behavior in studies of locomotor activity and drug self-administration, and emphasize the need for a standardized method of depicting rate-dependent data in studies where control rates differ across experimental conditions. Perhaps of greater importance, the present data suggest that environmental enrichment increases sensitivity to the effects of cocaine across a range of behavioral endpoints if differences in control rates of behavior are taken into account. It is noteworthy that this conclusion could also be stated another way: cocaine increases low rates of behavior to a greater extent than high rates of behavior. We do not consider it happenstance that this conclusion supports a rate-dependency hypothesis.
Acknowledgments
This study was supported by the National Institutes of Health (Grant Number DA14255), the Howard Hughes Medical Institute (Grant Number 52006292), the National Science Foundation, the Duke Endowment, and Davidson College. All drugs used in this study were generously supplied by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC, USA). The authors wish to thank Amy Becton for expert animal care and technical assistance.
References
- Barat SA, Abdel-Rahman MS. Cocaine and lidocaine in combination are synergistic convulsants. Brain Res. 1996;742:157–162. doi: 10.1016/s0006-8993(96)01004-9. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology. 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Rosenzweig MR, Diamond MC. Rat brain: effects of environmental enrichment on wet and dry weights. Science. 1969;163:825–826. doi: 10.1126/science.163.3869.825. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48:459–464. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Gill K, Smith BR, Amit Z. Differential effects of an early housing manipulation on cocaine-induced activity and self-administration in laboratory rats. Pharmacol Biochem Behav. 1991;39:269–274. doi: 10.1016/0091-3057(91)90178-5. [DOI] [PubMed] [Google Scholar]
- Cain ME, Green TA, Bardo MT. Environmental enrichment decreases responding for visual novelty. Behav Processes. 2006;73:360–366. doi: 10.1016/j.beproc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CA, Katz HB. The comparative effects of early-life undernutrition and subsequent differential environments on the dendritic branching of pyramidal cells in rat visual cortex. J Comp Neurol. 1983;218:345–350. doi: 10.1002/cne.902180310. [DOI] [PubMed] [Google Scholar]
- Dhanushkodi A, Bindu B, Raju TR, Kutty BM. Exposure to enriched environment improves spatial learning performances and enhances cell density but not choline acetyltransferase activity in the hippocampus of ventral subicular-lesioned rats. Behav Neurosci. 2007;121:491–500. doi: 10.1037/0735-7044.121.3.491. [DOI] [PubMed] [Google Scholar]
- Ding Y, Kang L, Li B, Ma L. Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacol Biochem Behav. 2005;82:673–677. doi: 10.1016/j.pbb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Duarte FS, Marder M, Hoeller AA, Duzzioni M, Mendes BG, Pizzolatti MG, De Lima TC. Anticonvulsant and anxiolytic-like effects of compounds isolated from Polygala sabulosa (Polygalaceae) in rodents: in vitro and in vivo interactions with benzodiazepine binding sites. Psychopharmacology. 2008;197:351–360. doi: 10.1007/s00213-007-1037-z. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology. 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Hall FS, Fong GW, Ghaed S, Pert A. Locomotor-stimulating effects of indirect dopamine agonists are attenuated in Fawn hooded rats independent of postweaning social experience. Pharmacol Biochem Behav. 2001;69:519–526. doi: 10.1016/s0091-3057(01)00569-x. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology. 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ohashi Y, Ando S. Effects of enriched environments with different durations and starting times on learning capacity during aging in rats assessed by a refined procedure of the Hebb-Williams maze task. J Neurosci Res. 2002;70:340–346. doi: 10.1002/jnr.10442. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, Whitelaw RB, Wilkinson LS, Robbins TW, Everitt BJ. Isolation rearing enhances the locomotor response to cocaine and a novel environment, but impairs the intravenous self-administration of cocaine. Psychopharmacology. 1994;115:407–418. doi: 10.1007/BF02245084. [DOI] [PubMed] [Google Scholar]
- Potschka H, Löscher W, Wlaź P, Behl B, Hofmann HP, Treiber HJ, Szabo L. LU 73068, a new non-NMDA and glycine/NMDA receptor antagonist: pharmacological characterization and comparison with NBQX and L-701λ324 in the kindling model of epilepsy. Br J Pharmacol. 1998;125:1258–1266. doi: 10.1038/sj.bjp.0702172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL, Diamond MC. Effect of environmental complexity and training on brain chemistry and anatomy: a replication and extension. J Comp Physiol Psychol. 1962;55:429–437. doi: 10.1037/h0041137. [DOI] [PubMed] [Google Scholar]
- Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987;81:227–231. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Bahr NI, Weiss IC, Würbel H. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Biochem Behav. 2002;73:209–224. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology. 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- Smith MA, Bryant PA, McClean JM. Social and environmental enrichment enhances sensitivity to the effects of kappa opioids: studies on antinociception, diuresis and conditioned place preference. Pharmacol Biochem Behav. 2003;76:93–101. doi: 10.1016/s0091-3057(03)00189-8. [DOI] [PubMed] [Google Scholar]
- Smith MA, Chisholm KA, Bryant PA, Greene JL, McClean JM, Stoops WW, Yancey DL. Social and environmental influences on opioid sensitivity in rats: importance of an opioid's relative efficacy at the mu-receptor. Psychopharmacology. 2005;181:27–37. doi: 10.1007/s00213-005-2218-2. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Klein ED, Bardo MT. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol. 2006;17:597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- Suto N, Austin JD, Tanabe LM, Kramer MK, Wright DA, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner. Neuropsychopharmacology. 2002;27:970–979. doi: 10.1016/S0893-133X(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- Zimmermann A, Stauffacher M, Langhans W, Würbel H. Enrichment-dependent differences in novelty exploration in rats can be explained by habituation. Behav Brain Res. 2001;121:11–20. doi: 10.1016/s0166-4328(00)00377-6. [DOI] [PubMed] [Google Scholar]