Abstract
Background
Ageing is associated with changes in the immune system with substantial alterations in T-lymphocyte subsets. Cytomegalovirus (CMV) is one of the factors that affect functionality of T cells and the differentiation and large expansions of CMV pp65-specific T cells have been associated with impaired responses to other immune challenges. Moreover, the presence of clonal expansions of CMV-specific T cells may shrink the available repertoire for other antigens and contribute to the increased incidence of infectious diseases in the elderly. In this study, we analyse the effect of ageing on the phenotype and frequency of CMV pp65-specific CD8 T cell subsets according to the expression of CCR7, CD45RA, CD27, CD28, CD244 and CD85j.
Results
Peripheral blood from HLA-A2 healthy young, middle-aged and elderly donors was analysed by multiparametric flow cytometry using the HLA-A*0201/CMV pp65495–504 (NLVPMVATV) pentamer and mAbs specific for the molecules analysed. The frequency of CMV pp65-specific CD8 T cells was increased in the elderly compared with young and middle-aged donors. The proportion of naïve cells was reduced in the elderly, whereas an age-associated increase of the CCR7null effector-memory subset, in particular those with a CD45RAdim phenotype, was observed, both in the pentamer-positive and pentamer-negative CD8 T cells. The results also showed that most CMV pp65-specific CD8 T cells in elderly individuals were CD27/CD28 negative and expressed CD85j and CD244.
Conclusion
The finding that the phenotype of CMV pp65-specific CD8 T cells in elderly individuals is similar to the predominant phenotype of CD8 T cells as a whole, suggests that CMV persistent infections contributes to the age-related changes observed in the CD8 T cell compartment, and that chronic stimulation by other persistent antigens also play a role in T cell immunosenescence. Differences in subset distribution in elderly individuals showing a decrease in naive and an increase in effector-memory CD8 T cells may be relevant in the age-associated defective immune response.
Background
Human cytomegalovirus (CMV) infection in immunocompetent individuals is normally asymptomatic, but can be a major cause of morbility in immunosuppressed individuals. After primary infection the virus persists throughout life in a latent form in a variety of tissues, particularly in precursor cells of the monocytic lineage [1]. Host defence against infection by CMV is ensured in great part by cytotoxic CD8 T lymphocytes directed against the tegument protein pp65 [2]. In immunosuppressed, and occasionally immunocompetent persons, CMV can be reactivated and, in these situations, the presence of CMV-specific CD8 T cells which are not producing IFNγ, and therefore potentially anergic or in vivo exhausted is frequent [3].
Ageing is associated with changes in the immune system with substantial alterations in T-lymphocyte subsets. CMV infection substantially modulates the peripheral lymphoid pool in healthy donors [4,5]. CMV also affects functionality of T cells and the differentiation and large expansion of CMV-specific T cells have been associated with impaired responses to other immune challenges [6]. Moreover, clonal expansions of CMV-specific T cells may shrink the available repertoire for other antigens and contribute to the increased incidence of infectious disease in the elderly [7-10]. In aged people, the CMV phosphoprotein pp65 (UL83) is the major antigen recognised by T lymphocytes targeting functionally efficient T cell effector responses with massive production of Th1 cytokines and exhibition of CD107a degranulation marker [11]. The percentage of these cells are strikingly expanded and the great majority are CD28-[12,13]. In addition the CD8 T cell subset shows a significant increase of the CD45RA+ CD27- subset, likely as a consequence of acute CMV infection [14].
The pool of memory T cells functions as a dynamic repository of antigen-experienced T lymphocytes that accumulate over the individual's lifetime. Several subpopulations of human CD8 T cells are defined according to the expression of CCR7, CD45RA and cytolytic effector molecules [15-17]. The expression of CCR7 divides human memory T cells into two functionally distinct subsets with distinct homing capacity and effector function [15,16]: those expressing CCR7 are termed central memory (CM), whereas effector memory (EM) cells are characterised by the lack of CCR7. Whereas CM CD8 T cells are phenotypically homogeneous, different EM subpopulations have been defined within the CCR7null subset according to the expression of different markers including the level of expression of CD45RA or the expression of the co-stimulatory molecules CD27 and CD28 [18-20].
In vitro senescence models and cross-sectional ex vivo studies have consistently demonstrated that senescent T cells and T cells from aged individuals express unusually high densities of receptors that are normally found on natural killer (NK) cells [21,22]. On CD8 T cells, the expression of NK associated receptors increases with age whereas expression of CCR7 decreases [23]. CD85j (also called LIR-1/ILT2/LILRB1), is an inhibitory cell surface receptor expressed on NK cells, B lymphocytes, monocytes, dendritic cells, and T cell subsets. CD85j recognises a broad range of classical and non-classical MHC class I molecules and CD85j triggering inhibits T cell function [24,25]. In contrast, CD85j interaction with UL18, an MHC class I homologue encoded by human cytomegalovirus, leads to activation of T cells, resulting in lysis of CMV-infected cells [26,27]. The expression of CD85j has been correlated with CD8 T-cell differentiation into effector cells and it is increased on virus-specific CD8 T cells in chronic infection [25]. CD244 (2B4) is a transmembrane receptor of the Ig superfamily primarily expressed by NK cells and antigen-experienced CD8 T cell subsets [28,29]. It is required for optimal activation of CD8 T cells and NK cells [30] playing an important role in activating cytotoxicity through its interaction with CD48 on some target cells [28,29,31].
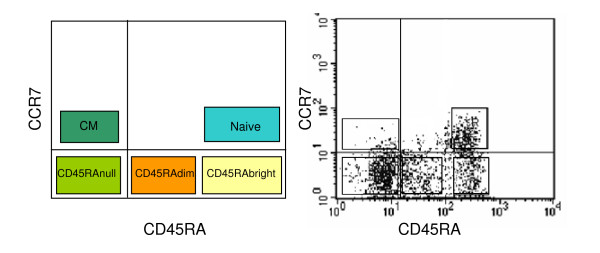
In this study, we analyse the effect of ageing on the frequency of total and CMV-specific CD8 T cell subsets defined by the expression of CCR7 and CD45RA. Using these markers we have defined naïve (CCR7+ CD45RAbright), CM (CCR7+ CD45RAnulls) and three subpopulations of EM CD8 T cells, characterised by the absence of CCR7, and defined according to the level of expression of CD45RA as EMRAnull, EMRAdim and EMRAbright (Figure 1). The expression of CD27, CD28 and the NK cell-associated markers CD85j and CD244 on these subsets is also studied.
Figure 1.
CD8 T cell-subset distribution according to the expression of CCR7 and CD45RA. Schematic representation (left) and a representative experiment (right) of the five CD8 T cell subsets that can be defined by the analysis of CCR7 and CD45RA markers.
Results
The frequency of CMV pp65-specific CD8 T cells is increased in healthy elderly when compared with young and middle aged individuals
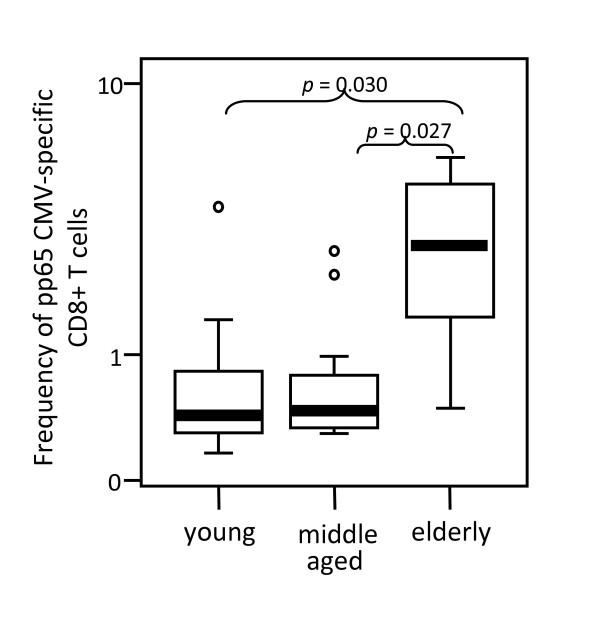
The analysis of the frequencies of CMV pp65-specific CD8 T cells in HLA-A2, CMV-seropositive donors using A2/CMV-pp65 pentamers is shown in Figure 2. Pentamer-positive CD8 T cells were increased in the elderly compared with young (p = 0.03) and middle aged (p = 0.027) donors. No significant differences were observed when values from young and middle aged donors were compared.
Figure 2.
Elderly individuals show an increased frequency of CMVpp65-specific CD8 T cells. The frequency of A2/CMVpp65 pentamer-positive CD8 T cells was analysed in young, middle aged and elderly donors. Data are presented as diagrams of boxes; the lower boundary of the box indicates the 25th percentile and the upper boundary the 75th percentile. Error bars above and below the box indicate the 90th and 10th percentiles. A line within the box marks the median. Outliers are represented as individual points. P values lower than 0.05 were considered statistically significant.
Age-associated expansion of effector-memory CMVpp65-specific CD8 T cells
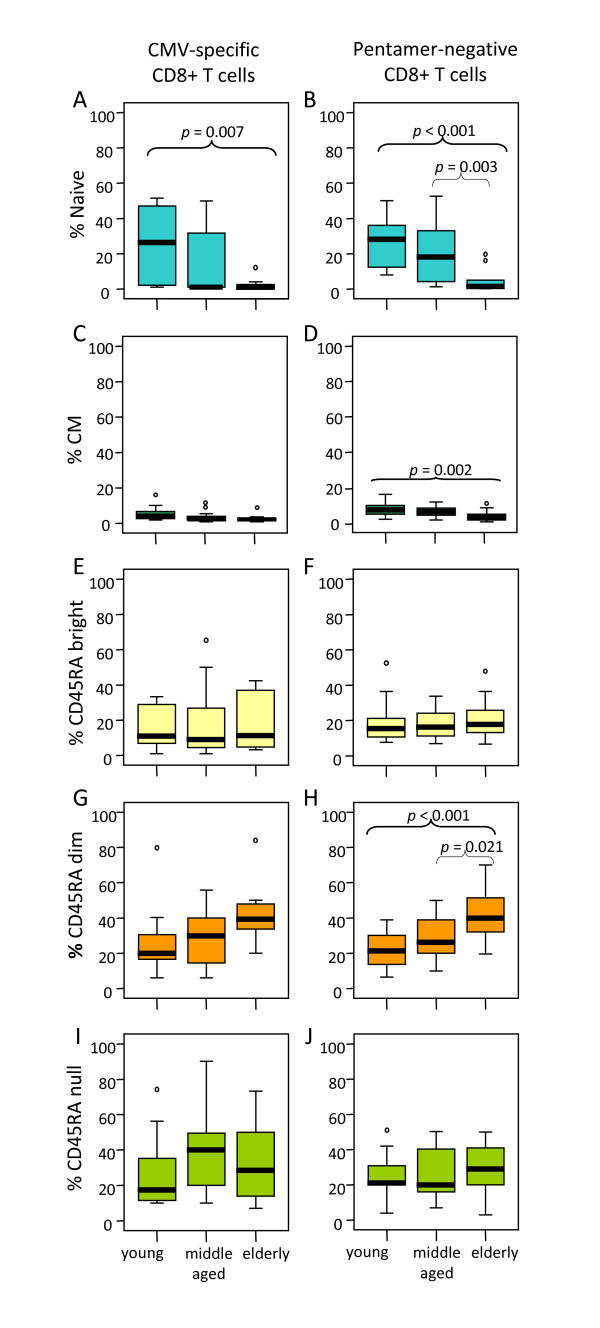
Age-associated changes in naive, CM and the three subsets of EM (EMRAnull, EMRAdim and EMRAbright) in pentamer-positive and pentamer-negative CD8 T cells are shown in Figure 3. The results demonstrated that the proportion of naïve A2/CMV-pp65 pentamer-positive CD8 T cells were significantly reduced in elderly individuals compared with young donors (p = 0.007) (Figure 3A). No significant changes on CM pentamer-positive CD8 T cells were found (Figure 3C). When pentamer-negative CD8 T cells were analysed an age-associated decrease in the proportion of naïve cells (elderly vs young p < 0.001 and elderly vs middle aged p = 0.003) and CM (elderly vs young p = 0.002), (Figure 3B and 3D) were found, corroborating that the proportions of these CD8 T cell subsets are decreased with age.
Figure 3.
Influence of age on CD8 T cell subset distribution. According to the subset model depicted in Figure 1, CD8 T cells were subdivided into naive, CM, EMRAbright, EMRAdim, EMRAnull cells and analysed in young, middle aged and elderly individuals. Results for CMV pentamer-positive (left) and pentamer-negative (right) CD8 T cells are shown. Data are presented as diagrams of boxes as indicated in Figure 2 legend.
In relation with the EM subsets, the results showed, both in the pentamer-positive and pentamer-negative CD8 T cells, an age-associated increase of the percentage of EMRAdim cells (Figure 3G and 3H), whereas no significant differences were found in the EMRAbright (Figure 3E and 3F) or in the EMRAnull (Figure 3I and 3J) subsets. No significant differences in the distribution of naïve/memory CD8 T cell subsets have been found when comparing pentamer-positive and pentamer-negative CD8 T cells in each age group.
Decreased expression of CD27 and CD28 on the EM CD8 T cell subsets in the elderly
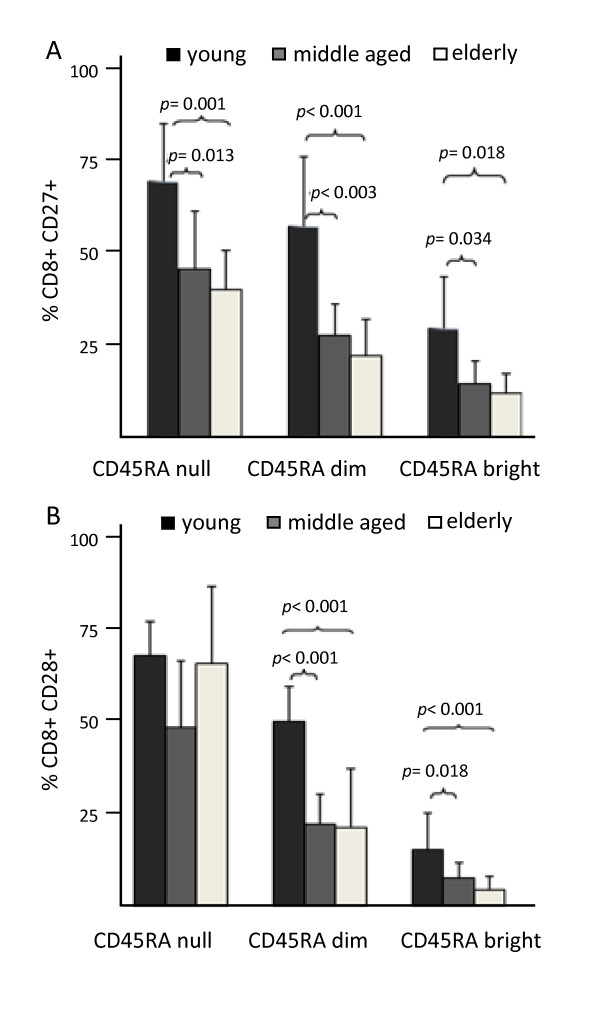
In order to further characterise age-associated changes in the CCR7null EM T cell subsets, the expression of CD27 and CD28 was analysed. The results showed that the EMRAnull subset had a higher expression of both CD27 and CD28 markers than the EMRAdim and EMRAbright subsets in the groups of young, middle age and elderly donors. Furthermore, a decreased expression of CD27 was observed in the middle age and elderly group compared with young individuals in the three EM subsets considered (Figure 4A). A similar age-associated decrease was observed in the expression of CD28 in the EMRAdim and EMRAbright subsets, whereas no significant age-associated differences were found in the expression of CD28 within the EMRAnull subset (Figure 4B).
Figure 4.
Expression of CD27 and CD28 on the effector-memory CD8 T cell subsets. EM CD8 T cells defined as CCR7null were subdivided into further subsets according to the model in Figure 1 and analysed in young, middle aged and elderly individuals. Columns and bars represent the mean and SD.
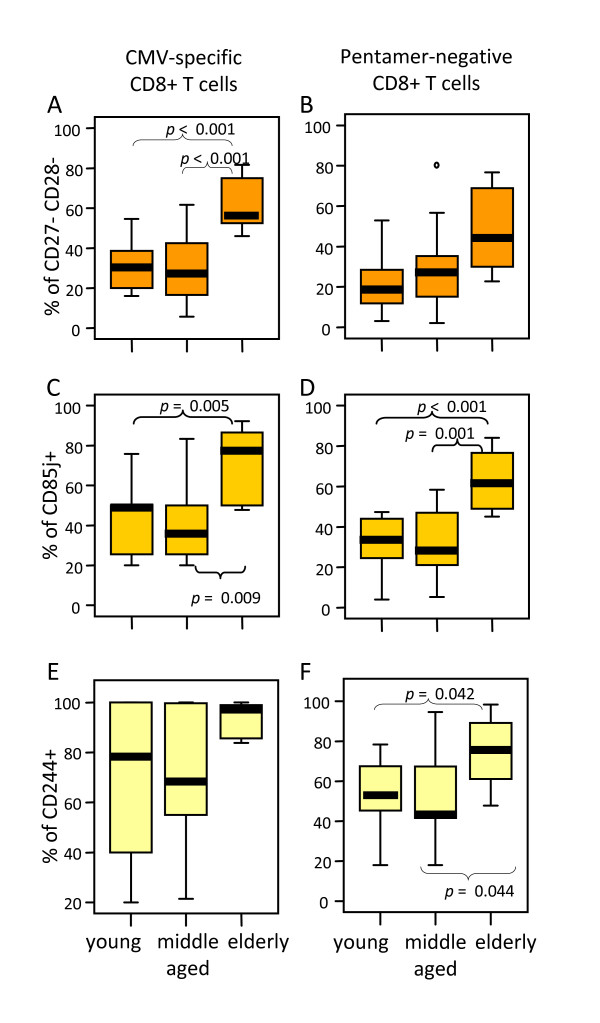
Most A2/CMV-pp65 pentamer-positive CD8 T cells from elderly individuals are CD27 and CD28 negative cells expressing CD85j and CD244
The analysis of CD27 and CD28 expression in CMV pp65-specific CD8 T cells demonstrated that elderly individuals had an increased proportion of CD27-CD28- cells compared with young (p < 0.001) and middle aged (p < 0.001) donors (Figure 5A). Although, an age-associated increase of CD27-CD28- cells was also found in A2/CMV-pp65 pentamer-negative CD8 T cells, no significant statistical differences were found (Figure 5B).
Figure 5.
The majority of A2/CMV-pp65 pentamer-positive CD8 T cells from elderly individuals display a CD27- CD28- phenotype and express CD85j and CD244. The percentage of CD27-CD28- (A), CD85j+ (B) and CD244+ (C) CD8 T cells was analysed on PBMC gated on CMV pentamer-positive (left) and pentamer-negative (right) CD8 T cells in young, middle aged and elderly donors. Data are presented as diagrams of boxes as indicated in Figure 2 legend.
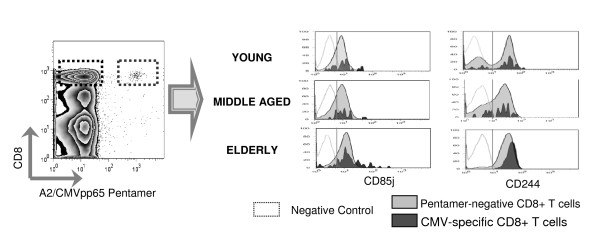
To further characterise the phenotype of CMV pp65-specific CD8 T cells we have analysed the expression of CD85j, a receptor for the human CMV MHC class I homologue UL18. Our results showed an increased expression of CD85j both in A2/CMV-pp65 pentamer-positive and pentamer-negative CD8 T cells from elderly individuals when compared with young and middle aged donors (Figure 5C and 5D). An age-associated increase in the expression of CD244 on CD8 T cells was also found in pentamer-negative CD8 T cells. Statistically significant differences were observed when elderly donors were compared with young (p = 0.044) and middle aged (p = 0.042) individuals whereas no statistical significant differences were found in the expression of CD244 when CMV pp65-specific T cells were considered (Figure 5E and 5F). Representative flow cytometry analyses of the expression of CD85j and CD244 in pentamer-positive and negative CD8 T cells from young, middle-aged and elderly individuals are shown in Figure 6.
Figure 6.
Analysis of CD85j and CD244 expression on CD8 T cells. Dot-plot chart illustrates how pentamer-positive and pentamer-negative CD8 T cells were selected. Histograms show the expression of CD85j and CD244 in A2/CMVpp65 pentamer-positive (dark grey) and pentamer-negative (light grey) CD8 T cells from representative young, middle-aged and elderly individuals. The empty histograms represent the negative isotype control for each experiment.
Discussion
In this work the effect of ageing on the frequency and phenotype of CMV-specific CD8 T cell subsets has been studied. Increasing evidences support that many of the alterations observed in the T cell compartment in the elderly can be the consequence of chronic activation of the immune system by latent virus such as CMV [9,10,13,32-40]. Thus remodelling of the CD8 T cell compartment in the elderly, characterised by a decrease of naive cells and increasing numbers of cells with a memory/activated phenotype [13,41-48], could be a consequence not only of thymic involution (changes related to age) but also due to CMV chronic antigenic stimulation as recently indicated [4,5,10,13,49].
An increased frequency of CMV-specific CD8 T cells was observed in the elderly compared to young and middle aged individuals. Our results on the analysis of the effect of ageing on the phenotype of CMV-specific CD8 T cells show that elderly individuals have a decrease of A2/CMVpp65 pentamer-positive CD8 T cells with a naive phenotype and an increase of those with an EM phenotype, compared with young individuals [10,13]. The increase in the EM subset is mainly due to the expansion of those A2/CMVpp65 pentamer-positive CD8 T cells displaying the EMRAdim phenotype, whereas the percentage values of EMRAbright and EMRAnull CD8 T cells were similar in young, middle aged and elderly donors.
The CD8 T cell compartment as a whole showed a decreased frequency of naive and CM cells and an increase of the EMRAdim CD8 T cell subset. These results confirm previous studies showing a dramatic decrease in the percentage of naive and the expansion of EM CD8 T cells with age [10,48,50]. The decreased percentage of naive CD8 T cells (defined as CCR7+ CD45RA+ CD28+ CD27+) and the expansion of EM CD8 T cells, showing a wide range of phenotypes [19], have been used in several studies as biomarkers of immunosenescence [38,40,48]. A recent study that analyses age-associated changes in CD8 cells in CMV seronegative and seropositive individuals strongly support that CMV is the predominant stimulus for the generation of CD45RA+ EM CD8 cells [4]. However, our results showing that there are not remarkable differences in the naïve/and different memory T cells between A2/CMV-pp65 -specific and non specific CD8 T cells, suggest that this is not the unique chronic stimulus as previously demonstrated in the northern European population and in CMV-negative elderly donors [4,5,7,51,52].
The expression of the CD28- phenotype is a characteristic of replicative senescence [53,54]. Chronic antigenic stimulation has been associated with peripheral blood expansions of CD28- CD8 T cells also characterised by loss of CD27 and expression of NK cell-associated markers as CD57 [22,28,55-57]. We observed an increase of CMV-specific CD8 T cells displaying a CD27-CD28- phenotype in the elderly suggesting that clonal expansion of T cells in response to chronic antigenic stimulation results in the accumulation of senescent T cells. In the pentamer-negative CD8 T cells a similar tendency was observed in the expression of CD27 and CD28 although no significant differences were observed between young, middle aged and elderly individuals.
CD85j is an inhibitory cell surface receptor that has high affinity for UL18, an MHC class I homologue encoded by human cytomegalovirus. Despite the well defined inhibiting functions of CD85j, its interaction with UL18 leads to activation of T cells, resulting in lysis of CMV-infected cells [26,27]. CD85j expression has been correlated with CD8 T-cell differentiation into effector cells [25]. An age-associated increase of CD85j on CD8 T cells has been reported that correlates with CD28 down-regulation and CD57 up-regulation [23,58,59]. Here we show that elderly individuals have an increased expression of CD85j within both CMV-specific and pentamer-negative CD8 T cells. Thus, its expression could be used as an indicator of the encounter with CMV pp65 epitope presented by the APCs to CD8 T cells. In addition, CD85j expression could be a used as a marker of replicative senescence [23,59], in parallel with the CD27- CD28- phenotype or CD57 expression. CD85j+ CD27- CD28- CMV pp65-specific CD8 T cells were increased in the elderly compared with young and middle aged suggesting that CMV-specific CD8 T cells have undergone extensive rounds of CMV antigen-driven stimulation reaching replicative senescence. The expression of CD244, a marker of T cell differentiation into effector cells [60,61], was found elevated in CMV-specific CD8 T cells compared to whole CD8 T cells.
Similar frequencies of CMV-specific CD8 T cells and T cell subsets defined by CCR7 and CD45RA can be found in young and middle-age donors. The high variability observed in the middle aged population could be related to the time point of CMV infection or the different immune response from each individual to virus. It should be interesting to know the evolution of those individuals in this age-group. It can be speculated that chronic activation of CMV-specific CD8 T cells in young and middle aged donors might have detrimental effects on age-associated diseases in this population.
Conclusion
It has been demonstrated that naturally occurring CD4 and CD8 T cell responses to pp65 were detectable in many subjects [62]. Phenotypic characterization of CMV-specific CD8 T cells can benefit the development of adoptive therapies and vaccination protocols. The finding that the CMV-specific CD8 T cell phenotype in elderly individuals is similar to the predominant phenotype of CD8 T cells as a whole, suggest that latent infection with CMV can be considered as a major force contributing to the differentiation of CD8 T cells into CD27- CD28- cells. These data confirm that the chronic antigenic stimulation induced by persistent viral life-long infections like CMV may induce important changes in the CD8 T cell compartment.
Donors, materials and methods
Subjects
Peripheral blood from healthy donors was obtained after informed consent under the auspices of the appropriate Research and Ethics Committees. Eighteen healthy young individuals (age range, 21 to 40 years; mean ± standard deviation (SD), 29 ± 6 years), seventeen healthy middle aged donors (age range, 41 to 64 years; mean ± SD, 51 ± 6 years) and fifteen healthy elderly donors (age range, 65 to 101 years, mean ± SD, 78 ± 7 years) were selected on the basis of HLA-A*0201 expression and CMV seropositivity. Although elderly donors were not selected according to the SENIEUR criteria, all individuals studied were in good clinical condition. Peripheral blood mononuclear cells (PBMC) were obtained by centrifugation over Histopaque-1077 (Sigma, St Louis, MO, USA). After washing, PBMC were resuspended in phosphate-buffered saline (PBS) and used for flow cytometry analysis.
Reagents and Monoclonal Abs
For multiparametric flow cytometry the following monoclonal antibodies (mAbs) were used: peridinin chlorophyll protein (PerCP)-conjugated anti-CD8 (SKI); phycoerythrin (PE) conjugated anti-CCR7 (2H4); fluorescein isothiocyanate (FITC) conjugated anti-CD45RA (L48); FITC- and allophycocyanin (APC)-conjugated anti-CD27 (L128); PE- and APC-conjugated anti-CD28 (CD28.2); FITC-conjugated anti-CD244 (C1.7) and FITC-conjugated anti-CD85j (GHI/75). Isotype controls labeled with the different fluorochromes were used in all the experiments. All mAbs were purchased from BD Biosciences (San Jose, CA). The HLA-A*0201/CMV pp65495–504(NLVPMVATV) pentamer (A2/CMV-pp65), APC-conjugated, was purchased from Proimmune (Oxford, UK).
Cell surface staining and flow cytometry
For cell surface staining, 2–2.5 × 106 PBMC were incubated with A2/CMV-pp65 pentamer, labelled with APC. Staining was performed according to the specifications given by the manufacturer. Cells were then washed and stained with the appropriate combination of mAbs specific for CD8, CCR7, CD45RA, CD27, CD28, CD244 and CD85j for 30 min at 4°C. Subsequently, cells were washed twice with PBS and resuspended in FACS buffer. Flow cytometric analysis was performed on a FACScalibur and FACSCanto cytometers (BD Biosciences). Viable cells were selected using forward and side scatter characteristics. The frequency of A2/CMVpp65 pentamer-positive cells was referred to the CD8bright T cell population. The expression of the different markers was referred to the A2/CMV-pp65 pentamer-positive T cells or to the pentamer-negative CD8bright T cells as indicated. Resulting profiles were analysed using Cell Quest software (BD Biosciences).
Statistical analysis
SPSS for Windows version 11.5 (SPSS Inc., Chicago) was used for statistical analysis. Comparisons between young, middle aged, and elderly donors were done by analysis of sample normality and variance followed by post hoc multiple comparisons. The tests of Hochberg or Games-Howell were applied for samples with equal or unequal variances respectively. P values of less than 0.05 were considered statistically significant. Data are presented as diagrams of boxes; the lower boundary of the box indicates the 25th percentile and the upper boundary the 75th percentile. Error bars above and below the box indicate the 90th and 10th percentiles. A line within the box marks the median. Outliers are represented as individual points.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MLP helped design the study, carried out the flow cytometry studies and helped draft the manuscript, in contribution to work included in her PhD thesis. IG performed and analysed some of the flow cytometry. ODR participated in the design of the study and analysis of the flow cytometry data. EMG performed the statistical analysis. CA contributed to the selection of the donors and discussion. RT&JGC participate in the discussion of the results and in the writing of the manuscript. RS conceived the study, participated in its design and coordination, and draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported by grants FIS PI03/1383 and FIS PI06/1320 (to RS) from Spanish Ministry of Health, SAF06/03687 (to RT) from Spanish Ministry of Education and Science; 51/06 and 292/07 from Consejeria de Salud de la Junta de Andalucía and grants GRU08077 and GRU09156 from Junta de Extremadura, cofinanced by European Regional Development Fund (FEDER). This work was also supported in part by contract QLK6-CT2002-02283 (T cells in Ageing, T-CIA) from the 5th Framework Program of the European Union.
Contributor Information
María Luisa Pita-Lopez, Email: maria.pita@cusur.udg.mx.
Inmaculada Gayoso, Email: b62gacae@uco.es.
Olga DelaRosa, Email: orosa@cellerix.com.
Javier G Casado, Email: jgarcas@unex.es.
Corona Alonso, Email: mariac.alonso.sspa@juntadeandalucia.es.
Elisa Muñoz-Gomariz, Email: mugoe@yahoo.es.
Raquel Tarazona, Email: rtarazon@unex.es.
Rafael Solana, Email: rsolana@uco.es.
References
- Motta VN, Martins SL. Impairment of cytomegalovirus-specific cellular immune response as a risk factor for cytomegalovirus disease in transplant recipients. Braz J Med Biol Res. 2008;41:5–11. doi: 10.1590/S0100-879X2006005000193. [DOI] [PubMed] [Google Scholar]
- Allart S, Lule J, Serres B, Jones T, Davignon JL, Malecaze F, Davrinche C. Impaired killing of HCMV-infected retinal pigment epithelial cells by anti-pp65 CD8(+) cytotoxic T cells. Invest Ophthalmol Vis Sci. 2003;44:665–671. doi: 10.1167/iovs.02-0547. [DOI] [PubMed] [Google Scholar]
- Lang KS, Moris A, Gouttefangeas C, Walter S, Teichgraber V, Miller M, Wernet D, Hamprecht K, Rammensee HG, Stevanovic S. High frequency of human cytomegalovirus (HCMV)-specific CD8+ T cells detected in a healthy CMV-seropositive donor. Cell Mol Life Sci. 2002;59:1076–1080. doi: 10.1007/s00018-002-8488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidrawar S, Khan N, Wei W, McLarnon A, Smith N, Nayak L, Moss P. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol. 2009;155:423–432. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol. 2009;21:440–445. doi: 10.1016/j.coi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Miles DJ, Sanneh M, Holder B, Crozier S, Nyamweya S, Touray ES, Palmero MS, Zaman SM, Rowland-Jones S, Van Der SM, Whittle H. Cytomegalovirus infection induces T-cell differentiation without impairing antigen-specific responses in Gambian infants. Immunology. 2008;124:388–400. doi: 10.1111/j.1365-2567.2007.02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasto S, Colonna-Romano G, Larbi A, Wikby A, Caruso C, Pawelec G. Role of persistent CMV infection in configuring T cell immunity in the elderly. Immun Ageing. 2007;4:2. doi: 10.1186/1742-4933-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney RJ, Falsey A, Campbell D, Torres A, Kolassa J, Brower C, McCann R, Menegus M, McCormick K, Frampton M, Hall W, Abraham GN. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin Immunol. 1999;90:213–219. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- Schwanninger A, Weinberger B, Weiskopf D, Herndler-Brandstetter D, Reitinger S, Gassner C, Schennach H, Parson W, Wurzner R, Grubeck-Loebenstein B. Age-related appearance of a CMV-specific high-avidity CD8+ T cell clonotype which does not occur in young adults. Immun Ageing. 2008;5:14. doi: 10.1186/1742-4933-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Solana R, Dela RO, Pawelec G. Human cytomegalovirus infection and T cell immunosenescence: a mini review. Mech Ageing Dev. 2006;127:538–543. doi: 10.1016/j.mad.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Vescovini R, Biasini C, Fagnoni FF, Telera AR, Zanlari L, Pedrazzoni M, Bucci L, Monti D, Medici MC, Chezzi C, Franceschi C, Sansoni P. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J Immunol. 2007;179:4283–4291. doi: 10.4049/jimmunol.179.6.4283. [DOI] [PubMed] [Google Scholar]
- Vescovini R, Telera A, Fagnoni FF, Biasini C, Medici MC, Valcavi P, di PP, Lucchini G, Zanlari L, Passeri G, Zanni F, Chezzi C, Franceschi C, Sansoni P. Different contribution of EBV and CMV infections in very long-term carriers to age-related alterations of CD8+ T cells. Exp Gerontol. 2004;39:1233–1243. doi: 10.1016/j.exger.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Kuijpers TW, Vossen MT, Gent MR, Davin JC, Roos MT, Wertheim-van Dillen PM, Weel JF, Baars PA, Van Lier RA. Frequencies of circulating cytolytic, CD45RA+CD27-, CD8+ T lymphocytes depend on infection with CMV. J Immunol. 2003;170:4342–4348. doi: 10.4049/jimmunol.170.8.4342. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Takata H, Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006;177:4330–4340. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- Carrasco J, Godelaine D, Van PA, Boon T, van der BP. CD45RA on human CD8 T cells is sensitive to the time elapsed since the last antigenic stimulation. Blood. 2006;108:2897–2905. doi: 10.1182/blood-2005-11-007237. [DOI] [PubMed] [Google Scholar]
- Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- Casado JG, Delarosa O, Pawelec G, Peralbo E, Duran E, Barahona F, Solana R, Tarazona R. Correlation of effector function with phenotype and cell division after in vitro differentiation of naive MART-1-specific CD8+ T cells. Int Immunol. 2009;21:53–62. doi: 10.1093/intimm/dxn123. [DOI] [PubMed] [Google Scholar]
- Abedin S, Michel JJ, Lemster B, Vallejo AN. Diversity of NKR expression in aging T cells and in T cells of the aged: the new frontier into the exploration of protective immunity in the elderly. Exp Gerontol. 2005;40:537–548. doi: 10.1016/j.exger.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Tarazona R, Delarosa O, Alonso C, Ostos B, Espejo J, Pena J, Solana R. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121:77–88. doi: 10.1016/S0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- Northfield J, Lucas M, Jones H, Young NT, Klenerman P. Does memory improve with age? CD85j (ILT-2/LIR-1) expression on CD8 T cells correlates with 'memory inflation' in human cytomegalovirus infection. Immunol Cell Biol. 2005;83:182–188. doi: 10.1111/j.1440-1711.2005.01321.x. [DOI] [PubMed] [Google Scholar]
- Saverino D, Fabbi M, Ghiotto F, Merlo A, Bruno S, Zarcone D, Tenca C, Tiso M, Santoro G, Anastasi G, Cosman D, Grossi CE, Ciccone E. The CD85/LIR-1/ILT2 inhibitory receptor is expressed by all human T lymphocytes and down-regulates their functions. J Immunol. 2000;165:3742–3755. doi: 10.4049/jimmunol.165.7.3742. [DOI] [PubMed] [Google Scholar]
- Ince MN, Harnisch B, Xu Z, Lee SK, Lange C, Moretta L, Lederman M, Lieberman J. Increased expression of the natural killer cell inhibitory receptor CD85j/ILT2 on antigen-specific effector CD8 T cells and its impact on CD8 T-cell function. Immunology. 2004;112:531–542. doi: 10.1046/j.1365-2567.2004.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saverino D, Ghiotto F, Merlo A, Bruno S, Battini L, Occhino M, Maffei M, Tenca C, Pileri S, Baldi L, Fabbi M, Bachi A, De SA, Grossi CE, Ciccone E. Specific recognition of the viral protein UL18 by CD85j/LIR-1/ILT2 on CD8+ T cells mediates the non-MHC-restricted lysis of human cytomegalovirus-infected cells. J Immunol. 2004;172:5629–5637. doi: 10.4049/jimmunol.172.9.5629. [DOI] [PubMed] [Google Scholar]
- Wagner CS, Riise GC, Bergstrom T, Karre K, Carbone E, Berg L. Increased expression of leukocyte Ig-like receptor-1 and activating role of UL18 in the response to cytomegalovirus infection. J Immunol. 2007;178:3536–3543. doi: 10.4049/jimmunol.178.6.3536. [DOI] [PubMed] [Google Scholar]
- Casado JG, Soto R, Delarosa O, Peralbo E, Munoz-Villanueva MC, Solana R, Tarazona R. CD8 T cells expressing NK associated receptors are increased in melanoma patients and display an effector phenotype. Cancer Immunol Immunother. 2005;54:1162–1171. doi: 10.1007/s00262-005-0682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser DE, Migliaccio M, Pittet MJ, Valmori D, Lienard D, Lejeune F, Reichenbach P, Guillaume P, Luscher I, Cerottini JC, Romero P. Human CD8(+) T cells expressing HLA-DR and CD28 show telomerase activity and are distinct from cytolytic effector T cells. Eur J Immunol. 2001;31:459–466. doi: 10.1002/1521-4141(200102)31:2<459::AID-IMMU459>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McNerney ME, Lee KM, Kumar V. 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol Immunol. 2005;42:489–494. doi: 10.1016/j.molimm.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Ames JB, Vyas V, Lusin JD, Mariuzza R. NMR structure of the natural killer cell receptor 2B4 (CD244): implications for ligand recognition. Biochemistry. 2005;44:6416–6423. doi: 10.1021/bi050139s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Remarque E, Barnett Y, Solana R. T cells and aging. Front Biosci. 1998;3:d59–d99. doi: 10.2741/a266. [DOI] [PubMed] [Google Scholar]
- Ravkov EV, Myrick CM, Altman JD. Immediate early effector functions of virus-specific CD8+CCR7+ memory cells in humans defined by HLA and CC chemokine ligand 19 tetramers. J Immunol. 2003;170:2461–2468. doi: 10.4049/jimmunol.170.5.2461. [DOI] [PubMed] [Google Scholar]
- Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, Franceschi C, Passeri M. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- van Baarle D, Tsegaye A, Miedema F, Akbar A. Significance of senescence for virus-specific memory T cell responses: rapid ageing during chronic stimulation of the immune system. Immunol Lett. 2005;97:19–29. doi: 10.1016/j.imlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Wedderburn LR, Patel A, Varsani H, Woo P. The developing human immune system: T-cell receptor repertoire of children and young adults shows a wide discrepancy in the frequency of persistent oligoclonal T-cell expansions. Immunology. 2001;102:301–309. doi: 10.1046/j.1365-2567.2001.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19:47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Inui A, Sogo T, Fujisawa T, Nagasaka H, Nonoyama S, Sierro S, Northfield J, Lucas M, Vargas A, Klenerman P. Large scale analysis of pediatric antiviral CD8+ T cell populations reveals sustained, functional and mature responses. Immun Ageing. 2006;3:11. doi: 10.1186/1742-4933-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derhovanessian E, Solana R, Larbi A, Pawelec G. Immunity, ageing and cancer. Immun Ageing. 2008;5:11. doi: 10.1186/1742-4933-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JJ, Murphy KE, Kunkel EJ, Brightling CE, Soler D, Shen Z, Boisvert J, Greenberg HB, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Butcher EC, Wu L. CCR7 expression and memory T cell diversity in humans. J Immunol. 2001;166:877–884. doi: 10.4049/jimmunol.166.2.877. [DOI] [PubMed] [Google Scholar]
- Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit Rev Immunol. 2003;23:45–64. doi: 10.1615/CritRevImmunol.v23.i12.30. [DOI] [PubMed] [Google Scholar]
- Herndler-Brandstetter D, Schwanninger A, Grubeck-Loebenstein B. CD4+ CD8+ T cells in young and elderly humans. Comment on Macchia I, Gauduin MC, Kaur A, Johnson RP. Expression of CD8alpha identifies a distinct subset of effector memory CD4 T lymphocytes. Immunology 2006; 119:232-42. Immunology. 2007;120:292–294. doi: 10.1111/j.1365-2567.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Scandella E, Forster R, Krebs P, Krautwald S, Lipp M, Hengartner H, Ludewig B. Impact of CCR7 on priming and distribution of antiviral effector and memory CTL. J Immunol. 2004;173:6684–6693. doi: 10.4049/jimmunol.173.11.6684. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Solana R. Immunosenescence. Immunol Today. 1997;18:514–516. doi: 10.1016/S0167-5699(97)01145-6. [DOI] [PubMed] [Google Scholar]
- Unsoeld H, Pircher H. Complex memory T-cell phenotypes revealed by coexpression of CD62L and CCR7. J Virol. 2005;79:4510–4513. doi: 10.1128/JVI.79.7.4510-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J Immunol. 2005;175:5895–5903. doi: 10.4049/jimmunol.175.9.5895. [DOI] [PubMed] [Google Scholar]
- Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarosa O, Pawelec G, Peralbo E, Wikby A, Mariani E, Mocchegiani E, Tarazona R, Solana R. Immunological biomarkers of ageing in man: changes in both innate and adaptive immunity are associated with health and longevity. Biogerontology. 2006;7:471–481. doi: 10.1007/s10522-006-9062-6. [DOI] [PubMed] [Google Scholar]
- Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- van Baarle D, Tsegaye A, Miedema F, Akbar A. Significance of senescence for virus-specific memory T cell responses: rapid ageing during chronic stimulation of the immune system. Immunol Lett. 2005;97:19–29. doi: 10.1016/j.imlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Colonna-Romano G, Akbar AN, Aquino A, Bulati M, Candore G, Lio D, Ammatuna P, Fletcher JM, Caruso C, Pawelec G. Impact of CMV and EBV seropositivity on CD8 T lymphocytes in an old population from West-Sicily. Exp Gerontol. 2007;42:995–1002. doi: 10.1016/j.exger.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Effros RB. Loss of CD28 expression on T lymphocytes: a marker of replicative senescence. Dev Comp Immunol. 1997;21:471–478. doi: 10.1016/S0145-305X(97)00027-X. [DOI] [PubMed] [Google Scholar]
- Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Kourtis AP. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol. 2005;174:6088–6094. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- Cantisan S, Torre-Cisneros J, Lara R, Rodriguez-Benot A, Santos F, Gutierrez-Aroca J, Gayoso I, Gonzalez-Padilla M, Casal M, Rivero A, Solana R. Age-dependent association between low CD27/CD28 expression on pp65 CD8+ T cells and cytomegalovirus replication after transplantation. Clin Vaccine Immunol. 2009 doi: 10.1128/CVI.00214-09. DOI:10.1128/CVI.00214-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller EC, Day E, Sissons JG, Wills MR. Dynamics of T cell memory in human cytomegalovirus infection. Med Microbiol Immunol. 2008;197:83–96. doi: 10.1007/s00430-008-0082-5. [DOI] [PubMed] [Google Scholar]
- Speiser DE, Colonna M, Ayyoub M, Cella M, Pittet MJ, Batard P, Valmori D, Guillaume P, Lienard D, Cerottini JC, Romero P. The activatory receptor 2B4 is expressed in vivo by human CD8+ effector alpha beta T cells. J Immunol. 2001;167:6165–6170. doi: 10.4049/jimmunol.167.11.6165. [DOI] [PubMed] [Google Scholar]
- Dupre L, Andolfi G, Tangye SG, Clementi R, Locatelli F, Arico M, Aiuti A, Roncarolo MG. SAP controls the cytolytic activity of CD8+ T cells against EBV-infected cells. Blood. 2005;105:4383–4389. doi: 10.1182/blood-2004-08-3269. [DOI] [PubMed] [Google Scholar]
- Slezak SL, Bettinotti M, Selleri S, Adams S, Marincola FM, Stroncek DF. CMV pp65 and IE-1 T cell epitopes recognized by healthy subjects. J Transl Med. 2007;5:17. doi: 10.1186/1479-5876-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]