Abstract
Background
Actinobacillus pleuropneumoniae is the causative agent of porcine contagious pleuropneumonia, a highly contagious respiratory infection in pigs, and all the 15 serotypes are able to cause disease. Current vaccines including subunit vaccines could not provide satisfactory protection against A. pleuropneumoniae. In this study, the immunoproteomic approach was applied to the analysis of extracellular and outer membrane proteins of A. pleuropneumoniae JL03 serotype 3 for the identification of novel immunogenic proteins for A. pleuropneumoniae.
Results
A total of 30 immunogenic proteins were identified from outer membrane and extracellular proteins of JL03 serotype 3, of which 6 were known antigens and 24 were novel immunogenic proteins for A. pleuropneumoniae.
Conclusion
These data provide information about novel immunogenic proteins for A. pleuropneumoniae serotype 3, and are expected to aid in development of novel vaccines against A. pleuropneumoniae.
Background
Actinobacillus pleuropneumoniae, a gram negative capsulated rod bacterium, is the etiologic agent of a severe, highly infectious and often fatal pleuropneumonia in swine, which is distributed world wide and results in severe losses in the swine industry. Based on capsular antigens, 15 serotypes of A. pleuropneumoniae to date have been documented, and all serotypes are capable of causing disease though differences in virulence have been described [1]. Among these serotypes, serotype 3 is one of the predominant serotypes in China [2].
So far, satisfactory protection has not been achieved in the A. pleuropneumoniae vaccination field in spite of intensive attempts made on inactivated whole-cell vaccines, live avirulent vaccines, which showed partial protection against challenges with homologous or heterologous serotypes[3]. Although currently available subunit vaccines contain important antigens, such as ApxI, ApxII and ApxIII, produced in various combinations by the different serotypes of A. pleuropneumoniae[4], they could not provide complete protection against A. pleuropneumoniae[3]. Thus identifying more conserved antigens is necessary for the development of novel vaccines, and in this study the immunogenic proteins of JL03 serotype 3 will be investigated to provide data for novel vaccine development. Extracellular proteins (ECPs) and OMPs in pathogens are involved in colonization, adhesion to and invasion of host cells. They interact directly with the host immune systems while playing crucial roles in the course of infections. Thus it is feasible to identify the important vaccine candidates from these sub-fractions. Currently, the immunoproteomic approach is a powerful tool to systematically identify immunogenic proteins from pathogens, and novel antigens have been successfully discovered from S. streptococcus [5], B. anthrax [6] and S. flexneri [7] by this approach from bacterial subfractions, such as outer membrane proteins.
Recently, Chung et al. performed systematically proteomic analysis on OMPs of A. pleuropneumoniae serotype 5b, and 47 OMPs were identified[8], and there have been attempts – but they are not recent and, therefore, could not use a proteomics approach. And no attempt has been reported so far in analysis of the ECPs of A. pleuropneumonae. The complete genome sequence of A. pleuropneumonia JL03 provided an essential database for applying immunoproteomic approach to JL03. In the present study, we report this approach to JL03 for the first time which involved the identification of immunogenic proteins from its OMPs and ECPs.
Results and Discussion
2-DE profile of the ECPs and OMPs, immunoblotting analysis and identification of immunogenic proteins
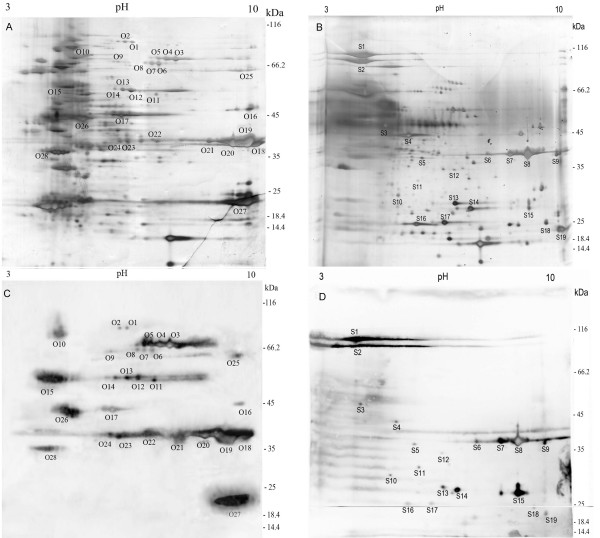
In the present study, linear immobilized pH gradient strips (3–10 L IPG 13 cm) and 10% SDS-PAGE gels were used for the prepared samples separation. Figure 1A and 1B show the 2-DE profile of OMPs and ECPs of A. pleuropneumoniae JL03. The 2-DE and immunoblotting were repeated three times and the results were reproducible. A total of 110 spots and 98 spots were detected on the silver-stained gels of OMPs and ECPs respectively by the software ImageMaster v 6.01. After immunoblotting analysis with convalescent sera, 28 immunoreactive spots from OMPs (Figure 1A and 1C) were identified, and they represented 17 proteins. Chung et al. recently identified 47 OM proteins from A. pleuropneumoniae 5b with an optimized extraction protocol based on the sucrose-density gradient which yielded preparations highly enriched for OM proteins and lipoproteins[8], and 10 of the 47 OM proteins were identified as immunogenic proteins in this study. In addition, Rhonda et al. recently demonstrated the sucrose-density gradient extraction of outer membranes in Campylobacter jejuni produced purer sample than carbonate extraction [9] that was applied in this study. So further study needs to be tried on immunoproteomic analysis of other serotypes of A. pleuropneumoniae with the optimized OMP extraction protocol of Chung et al. for search of more immunogenic OMPs. All the 19 immunoreactive spots from ECPs (Figure 1B and 1D) that represented 16 proteins were identified whereas no specific immunoreactive protein spot was observed from OMPs and ECPs using control sera. The detailed Peptide Mass Fingerprinting (PMF) results of the immunoreactive proteins are listed in supplemental table S1 [see additional file 1]. Overall, values of gel estimated pI and MW are matched well with their theoretical ones but some discrepancies still exist. Similar migration for several proteins has been observed in proteomic analysis of other pathogens previously[10,11]. This might be due to the presence of natural isoforms, posttranslational processing, and/or modification, or an artifact caused by sample preparation.
Figure 1.
2-DE profile of ECPs and OMPs and immunoblot. 2-DE profile of OMPs (A) and ECPs (B) from A. pleuropneumoniae JL03 strain. Preparative gel stained with Silver Nitrate. Immunoblot of OMPs and ECPs from convalescent sera (C) and (D). The gel spots were encoded using a letter followed by the protein number, which was assigned based on their similar locations on different gels/membranes.
All identified proteins were predicted by PSORTb 2.0, 10 proteins are annotated as periplasmic proteins, 7 are OMPs, 2 are extracellular proteins, 2 are cytoplasmic proteins, 1 is cytoplasmic membrane protein, and 8 are unknown. The detailed functions of the identified immunoreactive proteins are shown in supplemental table S1 [see additional file 1] according to the results predicted by COGnitor.
Interestingly, 3 immunogenic proteins, MomP1, MomP2 and elongation factor Tu were identified from OMPs and ECPs simultaneously, which might be due to outer membrane vesicles released in the milieu [12], from which outer membrane proteins have been identified successfully from E. coli and A. pleuropneumoniae[8,13], and to dual localization of elongation factor Tu [14].
Characterization of identified immunogenic proteins
Our immunogenic approach led to the identification of 6 known antigens of A. pleuropneumoniae, namely MomP1, MomP2, ApxIIA, ApxIIIA, Na+-translocating NADH-ubiquinone oxidoreductase subunit A (NqrA) and outer membrane ferric hydroxamate receptor (FhuA)[15,16]. And other well-known antigens, like ApxI, ApxIV, outer membrane lipoprotein A (OmlA), outer membrane protein precursor (PalA) and Transferrin binding proteins (Tbp) proteins could not be detected in the present study. ApxIV is only induced in vivo and JL03, serotype 3 strain, can not produce ApxI, and therefore we could not detect ApxI and ApxIV. Tbp proteins are expressed under iron limited conditions and the cells we collected were not prepared under such conditions. So Tbp proteins did not appear in our results. The highly hydrophobic nature of OmlA and PalA might cause their loss during extraction procedure. PalA has been proved to be detrimental when used in vaccines[17], and thus we should be cautious about similar immunogenic proteins while applying them to vaccine development.
In addition, we found 16 immunogenic proteins that had an significant sequence similarity to known proteins, and they have already been shown immunogenic in certain pathogenic bacteria, but not in A. pleuropneumoniae before, namely D15/OmpD, LppB, FrdA, MDH, FepA, FrpB, TufB, PotD, GapA, ZnuA, TIG, DegP, TufB, PsaA, FkpA and PTA. The homolog D15/Omp85 is an essential component for outer membrane biogenesis and OMP assembly [18,19]. The immunogenicity of D15 and its homolog Omp87 has been demonstrated in Haemophilus ducreyi [20] and Pasteurella multocida [21] respectively. Furthermore, antibodies against the COOH-terminal "surface antigen" domain of D15 are protective against Haemophilus influenzae infection in animal models [22]. The immunoreactive spot O16 was homologous to LppB and shared 49% sequence identity with LppB of H. somni that has been shown as an immunodominant protein [23], and the gene lppB of A. pleuropneumoniae is important for survival during infection[24]. Fumarate reductase (FrdA) was found to be involved in biosynthesis of flagella and cell motility, which might contribute to an significantly attenuated A. pleuropneumoniae [25] and loss of the ability in colonizing in the gastric mucosa in Helicobacter pylori[26] after frdA genes were inactivated. Furthermore, Joseph et al. described FrdA as an antigen in Brucella abortus [27]. FepA, FrpB and HbpA are important components in several ABC transport pathways for obtaining iron or regulating iron utilization in vivo or vitro. The immunogenic activity of FepA and FrpB was shown in Klebsiella pneumoniae [28] and Neisseria meningitides [29] respectively, and HbpA was widely conserved and served as an antigen in Leptospira interrogans[30]. Moreover, homologous analysis of these proteins at NCBI revealed a high level identity (>98%) with the sequenced serotype 1, 5 and 7 strains respectively. These suggest that they might be new common antigens for A. pleuropneumoniae. High-affinity zinc uptake system protein ZnuA precursor, was essential of B. abortus for intracellular survival and virulence in mice[31] and shown immunogenic in Streptococcus suis[5]. PsaA is needed for the adherence of pneumococcal cells and antibodies to PsaA contributed to reduce the nasopharyngeal colonization of challenged pneumococcal cells [32,33]. DegPs, a member of the widely conserved HtrA family of serine proteases, were frequently identified as antigens in other pathogens, such as B. abortus [34] and Chlamydia trachomatis [35]. Besides, trigger factor (TIG) has been demonstrated to be an excellent candidate for vaccination against Brucella melitensis [36] and a virulence-related protein in Listeria monocytogenes [37], and similar findings were described about malate dehydrogenase (MDH) of Candida albicans [38] and spermidine/putrescine-binding periplasmic protein (PotD) of Streptococcus pneumoniae [39]. Glyceraldehyde 3-phosphate dehydrogenase (GapA) has been proven to be antigenically conserved proteins, suggesting potential for vaccines in several microorganisms [40]. Homologous protein of translation elongation factor EF-Tu (TufB), a very abundant protein, had been detected in immunological researches of other bacteria, such as C. trachomatis[41] and Shigella flexneri[7]. The periplasm of gram-negative bacteria is well equipped with ATP-independent chaperones and folding catalysts, including peptidyl-prolyl isomerases (FkpA). It is reported recently that FkpA was found to be immunogenic in Bordetella pertussis[42]. Phosphate acetyltransferase (PTA), an enzyme that catalyzes the reversible transfer of the acetyl group from acetyl phosphate to coenzyme A plays a major role in the energy-yielding metabolism[43] and recently has been reported to be immunogenic in S. suis[5].
It is notable that CbiK, IlvG, FepB, AfuC, FatB, GGBP, CysG and Ttg2D, are reported to be immunogenic proteins for the first time in this study whose functions have been biologically demonstrated in some bacteria. Putative periplasmic binding protein CbiK is involved in the uptake of Ni2+, a cofactor required for urease activity that is important in pathogenesis of pleuropneumonia [44]. The ilv gene of Brucella suis has been identified as a virulence gene[45], and its product, acetohydroxyacid synthase, catalyzes the first common step in the biosynthetic pathway of the branched-amino acids such as leucine, isoleucine, and valine. Iron is essential for bacterial growth, especially for A. pleuropneumoniae in invading and reproducing in porcine respiratory tract where iron is limited. Iron-restriction is an important signal that regulates expression of many genes including some coding for virulence factors[46]. FepB, AfuC and FatB are components of known iron transport pathways, and the immunogenic reactivity of these proteins in this study indicates that these iron-uptake proteins might be potential candidates for development of subunit vaccines. D-Galactose/D-glucose binding protein (GGBP) is a bacterial periplasmic protein, an initial component for both chemotaxis towards galactose and glucose and active transport of the two sugars in Escherichia coli[47]. The crystal structure of uroporphyrinogen-III methylase (CysG) from Thermus thermophilus has been reported[48] and the cysG gene of Salmonella typhimurium is involved in synthesis of both cobalamin (B12) and siroheme[49]. The ttg2D gene encodes a periplasmic component of an ABC-type transport system related to resistance to organic solvents, and Ttg proteins of Pseudomonas putida and N. meningitidis were verified to participate in the uptake of L-glutamate[50].
Novel vaccine candidates need to be highly conserved between strains and so that they induce cross-protection against A. pleuropneumoniae. Recently Goure et al. have identified A. pleuropneumoniae genes that are conserved among all 15 serotypes by comparative genomic hybridization[51]. Of these conserved genes, the genes encoding proteins MomP1 (OMP P5), MomP2 (OMP P5), D15 (OmpD), LppB, PotD, FkbP and FrpB were observed in our results. Besides, NqrA has been demonstrated to be common to all serotypes[15]. Thus these conserved proteins could potentially induce protection against a wide variety of strains and are attractive vaccine candidates.
Conclusion
In conclusion, the 2DE in combination with Western blot is a specific and powerful method to discover novel antigens from bacterial pathogens. In this study, the identified immunogenic proteins from ECPs and OMPs may be significant for the development of new efficient vaccine against A. pleuropneumoniae. The protective efficacy of the identified immunogenic proteins either by alone or in different combinations remains to be evaluated in further studies. The data of this study are expected to aid in development of novel vaccines against A. pleuropneumoniae. The present study has focused on 2DE analysis coupled with Western blotting.
Methods
Bacterial strains and culture conditions
A. pleuropneumoniae JL03 [52], a Chinese field isolate strain of serotype 3, was used for this study. Bacteria were routinely maintained on tryptic soy agar (Difco Laboratories, Detroit) containing 10% bovine calf serum and 0.01% nicotinamide adenine dinucleotide (NAD) and cultured in brain heart infusion (BHI, Difco Laboratories, Detroit) media containing 0.01% NAD in a rotary incubation shaker running at 180 rev min. For protein extractions, overnight bacterial cultures were diluted 1:100 in 1 L BHI broth and grown aerobically at 37°C at 180 rev min. Three independent cultures at different days were prepared for ECPs and OMPs preparation respectively.
Extracellular proteins sample preparation
Growth was stopped at the early exponentional phase at an OD600 of ~0.4. Supernatant containing protease inhibitor cocktail (Roche, Mannheim, Germany) was collected by centrifugation for 10 min at 7200 × g (Sigma 3K12, Nr. 12150) at 4°C and filtered with 0.22 μm membrane (Millipore, USA). The supernatant was then treated with 15% TCA (Sigma Chemical) in ice for 30 min to precipitate protein. The precipitate was collected by centrifugation at 10 000 × g for 20 min at 4°C. Then the precipitated protein was washed three times with ice cold acetone containing 0.1% DTT to remove traces of TCA, and acetone was finally removed by speed vacuum treatment. Extracellular proteins were solubilized with 7 M urea, 2 M thiourea, 4% CHAPS, and 65 mM DTT. Protein concentration was determined by the GE Healthcare 2-D Quant Kit (Piscataway, NJ, USA).
OMPs sample Preparation
OMPs were prepared according to Molloy et al [53], with minor modifications. Briefly, cells were harvested in the middle exponential phase (OD600, ~1.0) by centrifugation for 10 min at 7200 × g (Sigma 3K12, Nr. 12150) at 4°C, and then the pellets were washed twice with cold 50 mM Tris-EDTA (pH 7.5) containing protease inhibitor cocktail (Roche, Germany) and subsequently the cells were suspended in 10 ml 50 mM Tris-EDTA (pH 7.5) and ruptured by sonication at 50 watts; pulse on, 3 sec.; pulse off, 3 sec. (Sonorex Digital 10 P Sonicator Bandelin, Berlin, Germany), till the value of OD600 decreased to 1/10 of its origin. Unbroken cells and cellular debris were removed by centrifugation at 6000 × g for 10 min. The supernatant was diluted 10-fold with ice cold 0.1 M Na2CO3 (pH 11), and stirred slowly on ice for 1 h. The OMPs were pelleted by ultracentrifugation in a Beckman Optima MAX ultracentrifuge (Palo Alto, CA, USA) at 100 000 × g for 1 h at 4°C. The supernatant was then discarded, and the pellet was resuspended and washed in 50 mM Tris-EDTA (pH 7.5) twice. The pellet was collected by centrifugation at 100 000 × g for 1 h at 4°C and subsequently solubilized in a lysis buffer (7 M urea, 2 M thiourea, 4% CHAPS, 1% Triton X-100 and 65 mM DTT). Protein concentration was determined by the GE Healthcare 2-D Quant Kit.
Preparation of convalescent sera
Bacteria JL03 were prepared as described above and the cells were washed once with PBS before dilution. Five pigs free of A. pleuropneumoniae were inoculated intratracheally at dose of 1.0 × 107CFU/pig in PBS to prepare the convalescent sera, and three pigs survived. Twenty days after the first infection, the survivors were rechallenged with another identical dose of JL03. Sera were collected a week after the second inoculation and evaluated. Antibody titer of mixed sera from survivors 1:512 was measured by IHA kit (Lanzhou Bioproducts Factory, Lanzhou, China). Sera were collected before inoculation as control sera. All animals were housed and maintained in isolation facilities in accordance with the Animal Care and Use Committee guidelines of Huazhong Agricultural University.
2-DE and immunoblotting analysis
IEF was performed with the IPGphor II™ system (GE Healthcare, USA) and the Immobiline DryStrip™ IPGstrips of 13 cm (linear 3–10 pH gradient) according to Gorg et al[54]. The prepared ECPs and OMPs (150 μg/strip) was mixed with rehydration buffer (7 M urea, 2 M thiourea, 2% w/v CHAPS, 1%w/v DTT, 0.5%v/v IPG buffer, 0.002% w/v bromophenol blue). The ECPs and OMPs samples were focused for 50 kVh and 75 kVh respectively. After IEF, three gels were run as follows. The IPGstrips were respectively equilibrated for 15 min with 10 mg/ml DTT and 40 mg/ml iodoacetamide in equilibration buffer (6 M urea, 2% w/v SDS, 30% v/v glycerol, 0.002% w/v bromophenol blue, 50 mM Tris-HCl, pH 8.8). After equilibration, the second dimension electrophoresis was performed on a 10% SDS polyacrylamide gel using Hoefer SE600 Ruby (Amersham Biosciences). Proteins of one gel were visualized by staining with silver nitrate (Bio Basic Inc). And gel evaluation and data analysis were carried out using the ImageMaster v 6.01 program (GE Healthcare, USA).
Immunoblot was performed according to Mansfield [55]. Gels were blotted onto PVDF transfer membranes (Hybond-P, 0.45 mm; Amersham Biosciences). The membranes were blocked in 5% BSA in TBS +0.05%(v/v) Tween 20 for 1 h at room temperature and probed with the convalescent swine sera and control sera (1:1000), for 1 h at room temperature, and then were washed and incubated with goat anti-porcine IgG (H+L) -HRP (1:5,000) (Southern Biotech, Birmingham, AL, USA) for 1 h at room temperature, followed by development with Supersignal west pico chemiluminescent substrate (Pierce, Rockford, IL, USA) and imaged on the Image Station 2000 MM (Kodak, Rochester, NY, USA). All experiments were done in triplicate.
In-gel digestion of proteins[5]
Protein spots of interest were excised from gels and detained with 100 μl 30 mM potassium ferricyanide and 100 μl 100 mM sodium thiosulfate (at a ratio of 1:1). And the gel pieces were shrunken with 50 μl acetonitrile and then re-swollen with 5 μl of 25 mM ammonium bicarbonate containing 10 ng of trypsin at 4°C for 30 min. In-gel tryptic degradation was performed overnight at 37°C, followed by three subsequent extractions. The pooled extracts were lyophilized and reconstituted in 2 μl 0.1% TFA v/v prior to MALDI-TOF MS analysis.
MALDI-TOF MS analysis and database searchs
The sample solution with equivalent matrix solution was applied onto the MALDI-TOF target and prepared for MALDI-TOF-MS analysis according to a previously described procedure [56]. CHCA was used as the matrix. MALDI-TOF spectra were calibrated using trypsin autodigestive peptide signals and matrix ion signals. MALDI analysis was performed by a fuzzy logic feedback control system (Ultraflex αMALDI TOF/TOF system Bruker, Karlsruhe, Germany) equipped with delayed ion extraction. PMF data were searched against the database of JL03 by MASCOT licensed in-house and the NCBInr database using the MASCOT program http://www.matrixscience.com.
Bioinformatics tools
COGnitor http://www.ncbi.nlm.nih.gov/COG/old/xognitor was applied to sort the identified proteins of A. pleuropneumoniae JL03 into functional categories. PSORTb v.2.0 is accessible at http://www.psort.org/psortb/index.html and applied to predict the subcellular location of the identified proteins.
Authors' contributions
YL and MJ carried out the molecular genetic studies, participated in the sequence alignment and drafted the manuscript. AZ, JD, YH, YL and MZ carried out the immunoassays. MZ and JD participated in the sequence alignment. MJ and YL participated in the design of the study and performed the statistical analysis. HC conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Supplementary Material
Supplementary table S1. List of immunoreactive proteins of OMPs and ECPs
Contributor Information
Yonghong Liao, Email: ebrain04@163.com.
Junhua Deng, Email: dengbetter@gmail.com.
Anding Zhang, Email: andye8019@webmail.hzau.edu.cn.
Mingguang Zhou, Email: zhoumingguangt@foxmail.com.
Yong Hu, Email: huyong1980@126.com.
Huanchun Chen, Email: chenhch@mail.hzau.edu.cn.
Meilin Jin, Email: jml8328@126.com.
Acknowledgements
This work was supported by 973 program (2006CB504404), the National Natural Science Foundation of China (30530590), 863 program (2006AA10A206) and National Key Technology R&D Program (2006BAD06A11). The work was performed in collaboration with Hubei University. We thank Yanxiu Liu for her suggestions and careful revision of the language of this manuscript.
References
- Jacobsen MJ, Nielsen JP, Nielsen R. Comparison of virulence of different Actinobacillus pleuropneumoniae serotypes and biotypes using an aerosol infection model. Vet Microbiol. 1996;49(3–4):159–168. doi: 10.1016/0378-1135(95)00184-0. [DOI] [PubMed] [Google Scholar]
- Lu Z, Zhao P, Shao Y, Liu J, Lu B. Study on the inactivated trivalent vaccine against swine infectious pleuropneumoniae: selection of the seed strain, preparation and safety trials of the vaccine. Chinese Journal of Veterinary Science and Technology. 2002;37:33–35. [Google Scholar]
- Ramjeet M, Deslandes V, Gouré J, Jacques M. Actinobacillus pleuropneumoniae vaccines: from bacterins to new insights into vaccination strategies. Animal Health Research Reviews. 2008;9(01):25–45. doi: 10.1017/S1466252307001338. [DOI] [PubMed] [Google Scholar]
- Frey J, Bosse JT, Chang YF, Cullen JM, Fenwick B, Gerlach GF, Gygi D, Haesebrouck F, Inzana TJ, Jansen R. Actinobacillus pleuropneumoniae RTX-toxins: uniform designation of haemolysins, cytolysins, pleurotoxin and their genes. J Gen Microbiol. 1993;139(8):1723–1728. doi: 10.1099/00221287-139-8-1723. [DOI] [PubMed] [Google Scholar]
- Zhang A, Xie C, Chen H, Jin M. Identification of immunogenic cell wall-associated proteins of Streptococcus suis serotype 2. Proteomics. 2008;8(17):3506–3515. doi: 10.1002/pmic.200800007. [DOI] [PubMed] [Google Scholar]
- Ariel N, Zvi A, Makarova K, Chitlaru T, Elhanany E, Velan B, Cohen S, Friedlander A, Shafferman A. Genome-based bioinformatic selection of chromosomal Bacillus anthracis putative vaccine candidates coupled with proteomic identification of surface-associated antigens. Infect Immun. 2003;71(8):4563–4579. doi: 10.1128/IAI.71.8.4563-4579.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying T, Wang H, Li M, Wang J, Wang J, Shi Z, Feng E, Liu X, Su G, Wei K. Immunoproteomics of outer membrane proteins and extracellular proteins of Shigella flexneri 2a 2457T. Proteomics. 2005;5(18):4777–4793. doi: 10.1002/pmic.200401326. [DOI] [PubMed] [Google Scholar]
- Chung J, Ng-Thow-Hing C, Budman L, Gibbs B, Nash J, Jacques M, Coulton J. Outer membrane proteome of Actinobacillus pleuropneumoniae : LC-MS/MS analyses validate in silico predictions. Proteomics. 2007;7(11) doi: 10.1002/pmic.200600979. [DOI] [PubMed] [Google Scholar]
- Hobb RI, Fields JA, Burns CM, Thompson SA. Evaluation of procedures for outer membrane isolation from Campylobacter jejuni. Microbiology. 2009;155(Pt 3):979–988. doi: 10.1099/mic.0.024539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy MP, Herbert BR, Slade MB, Rabilloud T, Nouwens AS, Williams KL, Gooley AA. Proteomic analysis of the Escherichia coli outer membrane. Eur J Biochem. 2000;267(10):2871–2881. doi: 10.1046/j.1432-1327.2000.01296.x. [DOI] [PubMed] [Google Scholar]
- Walz A, Mujer CV, Connolly JP, Alefantis T, Chafin R, Dake C, Whittington J, Kumar SP, Khan AS, DelVecchio VG. Bacillus anthracis secretome time course under host-simulated conditions and identification of immunogenic proteins. Proteome Sci. 2007;5:11. doi: 10.1186/1477-5956-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete-Abascal E, Garcia RM, Reyes ME, Godinez D, de la Garza M. Membrane vesicles released by Actinobacillus pleuropneumoniae contain proteases and Apx toxins. FEMS Microbiol Lett. 2000;191(1):109–113. doi: 10.1111/j.1574-6968.2000.tb09326.x. [DOI] [PubMed] [Google Scholar]
- Lee E, Bang J, Park G, Choi D, Kang J, Kim H, Park K, Lee J, Kim Y, Kwon K. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics. 2007;7(17) doi: 10.1002/pmic.200700196. [DOI] [PubMed] [Google Scholar]
- Sanderova H, Hulkova M, Malon P, Kepkova M, Jonak J. Thermostability of multidomain proteins: elongation factors EF-Tu from Escherichia coli and Bacillus stearothermophilus and their chimeric forms. Protein Sci. 2004;13(1):89–99. doi: 10.1110/ps.03272504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz W, Nedialkov Y, Thacker B, Mulks M. Molecular characterization of a common 48-kilodalton outer membrane protein of Actinobacillus pleuropneumoniae. Infect Immun. 1996;64(1):83–90. doi: 10.1128/iai.64.1.83-90.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesebrouck F, Chiers K, Van Overbeke I, Ducatelle R. Actinobacillus pleuropneumoniae infections in pigs: the role of virulence factors in pathogenesis and protection. Vet Microbiol. 1997;58(2–4):239–249. doi: 10.1016/S0378-1135(97)00162-4. [DOI] [PubMed] [Google Scholar]
- Bosch H van den, Frey J. Interference of outer membrane protein PalA with protective immunity against Actinobacillus pleuropneumoniae infections in vaccinated pigs. Vaccine. 2003;21(25–26):3601–3607. doi: 10.1016/s0264-410x(03)00410-9. [DOI] [PubMed] [Google Scholar]
- Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299(5604):262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- Gentle I, Gabriel K, Beech P, Waller R, Lithgow T. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J Cell Biol. 2004;164(1):19–24. doi: 10.1083/jcb.200310092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D, Thomas K, Leduc I, Olsen B, Thomas C, Cameron D, Elkins C. Cloning, overexpression, purification, and immunobiology of an 85-kilodalton outer membrane protein from Haemophilus ducreyi. Infect Immun. 2001;69(7):4438–4446. doi: 10.1128/IAI.69.7.4438-4446.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri P, Goswami PP. Cloning of 87 kDa outer membrane protein gene of Pasteurella multocida P52. Res Vet Sci. 2001;70(3):255–256. doi: 10.1053/rvsc.2001.0470. [DOI] [PubMed] [Google Scholar]
- Loosmore S, Yang Y, Coleman D, Shortreed J, England D, Klein M. Outer membrane protein D15 is conserved among Haemophilus influenzae species and may represent a universal protective antigen against invasive disease. Infect Immun. 1997;65(9):3701. doi: 10.1128/iai.65.9.3701-3707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M, Rioux CR, Potter AA. Molecular cloning, nucleotide sequence, and characterization of lppB, encoding an antigenic 40-kilodalton lipoprotein of Haemophilus somnus. Infect Immun. 1993;61(5):1793–1798. doi: 10.1128/iai.61.5.1793-1798.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan BJ, Bosse JT, Beddek AJ, Rycroft AN, Kroll JS, Langford PR. Identification of Actinobacillus pleuropneumoniae genes important for survival during infection in its natural host. Infect Immun. 2003;71(7):3960–3970. doi: 10.1128/IAI.71.7.3960-3970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner F, Bendallah I, Bosse J, Dreckmann K, Nash J, Langford P, Gerlach G. Analysis of the Actinobacillus pleuropneumoniae ArcA Regulon Identifies Fumarate Reductase as a Determinant of Virulence. Infect Immun. 2008;76(6):2284–2295. doi: 10.1128/IAI.01540-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Feng Y, Dangler C, Xu S, Taylor N, Fox J. Fumarate reductase is essential for Helicobacter pylori colonization of the mouse stomach. Microb Pathog. 2000;29(5):279–287. doi: 10.1006/mpat.2000.0391. [DOI] [PubMed] [Google Scholar]
- Connolly JP, Comerci D, Alefantis TG, Walz A, Quan M, Chafin R, Grewal P, Mujer CV, Ugalde RA, DelVecchio VG. Proteomic analysis of Brucella abortus cell envelope and identification of immunogenic candidate proteins for vaccine development. Proteomics. 2006;6(13):3767–3780. doi: 10.1002/pmic.200500730. [DOI] [PubMed] [Google Scholar]
- Kurupati P, Teh BK, Kumarasinghe G, Poh CL. Identification of vaccine candidate antigens of an ESBL producing Klebsiella pneumoniae clinical strain by immunoproteome analysis. Proteomics. 2006;6(3):836–844. doi: 10.1002/pmic.200500214. [DOI] [PubMed] [Google Scholar]
- Ala'Aldeen DA, Davies HA, Borriello SP. Vaccine potential of meningococcal FrpB: studies on surface exposure and functional attributes of common epitopes. Vaccine. 1994;12(6):535–541. doi: 10.1016/0264-410X(94)90314-X. [DOI] [PubMed] [Google Scholar]
- Sridhar V, Manjulata Devi S, Ahmed N, Sritharan M. Diagnostic potential of an iron-regulated hemin-binding protein HbpA that is widely conserved in Leptospira interrogans. Infection genetics and evolution. 2008;8(6):772–776. doi: 10.1016/j.meegid.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Suk K, Watanabe K, Shirahata T, Watarai M. Zinc uptake system (znuA locus) of Brucella abortus is essential for intracellular survival and virulence in mice. Journal of Veterinary Medical Science. 2004;66(9):1059–1063. doi: 10.1292/jvms.66.1059. [DOI] [PubMed] [Google Scholar]
- Berry A, Paton J. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun. 1996;64(12):5255. doi: 10.1128/iai.64.12.5255-5262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E, Briles D, Ades E, Paton J, Sampson J, Carlone G, Huebner R, Virolainen A, Swiatlo E, Hollingshead S. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect Immun. 2000;68(2):796–800. doi: 10.1128/IAI.68.2.796-800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatum FM, Cheville NF, Morfitt D. Cloning, characterization and construction of htrA and htrA-like mutants of Brucella abortus and their survival in BALB/c mice. Microb Pathog. 1994;17(1):23–36. doi: 10.1006/mpat.1994.1049. [DOI] [PubMed] [Google Scholar]
- Sanchez-Campillo M, Bini L, Comanducci M, Raggiaschi R, Marzocchi B, Pallini V, Ratti G. Identification of immunoreactive proteins of Chlamydia trachomatis by Western blot analysis of a two-dimensional electrophoresis map with patient sera. Electrophoresis. 1999;20(11) doi: 10.1002/(SICI)1522-2683(19990801)20:11<2269::AID-ELPS2269>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Yang X, Walters N, Robison A, Trunkle T, Pascual D. Nasal immunization with recombinant Brucella melitensis bp26 and trigger factor with cholera toxin reduces B. melitensis colonization. Vaccine. 2007;25(12):2261–2268. doi: 10.1016/j.vaccine.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Bigot A, Botton E, Dubail I, Charbit A. A homolog of Bacillus subtilis trigger factor in Listeria monocytogenes is involved in stress tolerance and bacterial virulence. Appl Environ Microbiol. 2006;72(10):6623–6631. doi: 10.1128/AEM.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylicki A, Ziolkowska G, Bolkun A, Siemieniuk M, Czerniecki J, Nowakiewicz A. Comparative study of the activity and kinetic properties of malate dehydrogenase and pyruvate decarboxylase from Candida albicans, Malassezia pachydermatis, and Saccharomyces cerevisiae. Can J Microbiol. 2008;54(9):734–741. doi: 10.1139/W08-062. [DOI] [PubMed] [Google Scholar]
- Shah P, Swiatlo E. Immunization with polyamine transport protein PotD protects mice against systemic infection with Streptococcus pneumoniae. Infect Immun. 2006;74(10):5888–5892. doi: 10.1128/IAI.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Casal J, Prysliak T. Detection of antibodies against the Mycoplasma bovis glyceraldehyde-3-phosphate dehydrogenase protein in beef cattle. Microb Pathog. 2007;43(5–6):189–197. doi: 10.1016/j.micpath.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bini L, Sanchez-Campillo M, Santucci A, Magi B, Marzocchi B, Comanducci M, Christiansen G, Birkelund S, Cevenini R, Vretou E. Mapping of Chlamydia trachomatis proteins by immobiline-polyacrylamide two-dimensional electrophoresis: spot identification by N-terminal sequencing and immunoblotting. Electrophoresis. 1996;17(1):185–190. doi: 10.1002/elps.1150170130. [DOI] [PubMed] [Google Scholar]
- Altindis E, Tefon BE, Yildirim V, Ozcengiz E, Becher D, Hecker M, Ozcengiz G. Immunoproteomic analysis of Bordetella pertussis and identification of new immunogenic proteins. Vaccine. 2009;27(4):542–548. doi: 10.1016/j.vaccine.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Xu QS, Shin DH, Pufan R, Yokota H, Kim R, Kim SH. Crystal structure of a phosphotransacetylase from Streptococcus pyogenes. Proteins. 2004;55(2):479–481. doi: 10.1002/prot.20039. [DOI] [PubMed] [Google Scholar]
- Bosse J, Gilmour H, MacInnes J. Novel genes affecting urease activity in Actinobacillus pleuropneumoniae. J Bacteriol. 2001;183(4):1242–1247. doi: 10.1128/JB.183.4.1242-1247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boigegrain RA, Liautard JP, Kohler S. Targeting of the virulence factor acetohydroxyacid synthase by sulfonylureas results in inhibition of intramacrophagic multiplication of Brucella suis. Antimicrob Agents Chemother. 2005;49(9):3922–3925. doi: 10.1128/AAC.49.9.3922-3925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes V, Nash JH, Harel J, Coulton JW, Jacques M. Transcriptional profiling of Actinobacillus pleuropneumoniae under iron-restricted conditions. BMC Genomics. 2007;8:72. doi: 10.1186/1471-2164-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Auria S, Ausili A, Marabotti A, Varriale A, Scognamiglio V, Staiano M, Bertoli E, Rossi M, Tanfani F. Binding of glucose to the D-galactose/D-glucose-binding protein from Escherichia coli restores the native protein secondary structure and thermostability that are lost upon calcium depletion. J Biochem. 2006;139(2):213. doi: 10.1093/jb/mvj027. [DOI] [PubMed] [Google Scholar]
- Rehse PH, Kitao T, Tahirov TH. Structure of a closed-form uroporphyrinogen-III C-methyltransferase from Thermus thermophilus. Acta Crystallogr D Biol Crystallogr. 2005;61(Pt 7):913–919. doi: 10.1107/S0907444905008838. [DOI] [PubMed] [Google Scholar]
- Fazzio T, Roth J. Evidence that the CysG protein catalyzes the first reaction specific to B12 synthesis in Salmonella typhimurium, insertion of cobalt. J Bacteriol. 1996;178(23):6952–6959. doi: 10.1128/jb.178.23.6952-6959.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco C, Tala A, Spinosa MR, Progida C, De Nitto E, Gaballo A, Bruni CB, Bucci C, Alifano P. Identification of a meningococcal L-glutamate ABC transporter operon essential for growth in low-sodium environments. Infect Immun. 2006;74(3):1725–1740. doi: 10.1128/IAI.74.3.1725-1740.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goure J, Findlay WA, Deslandes V, Bouevitch A, Foote SJ, MacInnes JI, Coulton JW, Nash JH, Jacques M. Microarray-based comparative genomic profiling of reference strains and selected Canadian field isolates of Actinobacillus pleuropneumoniae. BMC Genomics. 2009;10:88. doi: 10.1186/1471-2164-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Zhou Y, Li L, Zhou R, Xiao S, Wan Y, Zhang S, Wang K, Li W. Genome biology of Actinobacillus pleuropneumoniae JL03, an isolate of serotype 3 prevalent in China. PLoS ONE. 2008;3(1) doi: 10.1371/journal.pone.0001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy MP, Herbert BR, Walsh BJ, Tyler MI, Traini M, Sanchez JC, Hochstrasser DF, Williams KL, Gooley AA. Extraction of membrane proteins by differential solubilization for separation using two-dimensional gel electrophoresis. Electrophoresis. 1998;19(5):837–844. doi: 10.1002/elps.1150190539. [DOI] [PubMed] [Google Scholar]
- Gorg A, Obermaier C, Boguth G, Harder A, Scheibe B, Wildgruber R, Weiss W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000;21(6):1037–1053. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1037::AID-ELPS1037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Mansfield MA. Rapid immunodetection on polyvinylidene fluoride membrane blots without blocking. Anal Biochem. 1995;229(1):140–143. doi: 10.1006/abio.1995.1391. [DOI] [PubMed] [Google Scholar]
- Wyatt MF, Stein BK, Brenton AG. Characterization of various analytes using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and 2-[(2E)-3-(4-tert-butylphenyl)-2-methylprop-2-enylidene]malononitrile matrix. Anal Chem. 2006;78(1):199–206. doi: 10.1021/ac050732f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table S1. List of immunoreactive proteins of OMPs and ECPs