Abstract
Hydrogen peroxide (H2O2) functions as a second messenger that can activate cell proliferation through chemoselective oxidation of cysteine residues in signaling proteins. The connection between H2O2 signaling, thiol oxidation, and activation of growth pathways has emerged as fertile ground for the development of strategies for cancer treatment. Central to achieving this goal is the development of tools and assays that facilitate characterization of the molecular events associated with tumorigenesis and evaluation of patient response to therapy. Here we report on the development of an immunochemical method for detecting sulfenic acid, the initial oxidation product that results when a thiolate reacts with H2O2. For this approach, the sulfenic acid is derivatized with a chemical tag to generate a unique epitope for recognition. The elicited antibody is exquisitely specific, context-independent, and capable of visualizing sulfenic acid formation in cells. Applying this approach to several systems, including cancer cell lines, shows it can be used to monitor differences in thiol redox status and reveals a diverse pattern of sulfenic acid modifications across different subtypes of breast tumors. These studies demonstrate a general strategy for producing antibodies against a specific oxidation state of cysteine and show the utility of these reagents for profiling thiol oxidation associated with pathological conditions such as breast cancer.
Keywords: cancer, chemical biology, redox signaling, thiol modification
Reactive oxygen species (ROS) play important roles in normal biological functions and pathological processes (1). Cancer cells are frequently under persistent oxidative stress due to oncogenic stimulation, increased metabolic activity, and mitochondrial malfunction (2, 3). Elevated ROS generation in cancer cells serves as an endogenous source of DNA-damaging agents that promote genetic instability (4). Mounting evidence also supports a physiological role for ROS as second messengers in intracellular signaling cascades that control cell growth, proliferation, migration, and apoptosis (5–7). In these pathways, stimulation of various cell surface receptors activate the NADPH oxidase complex to generate a burst of hydrogen peroxide (H2O2). H2O2 modulates signal transduction through chemoselective oxidation of cysteine residues in proteins, thereby altering their cellular function (8–11). In cancer cells, increased ROS may generate inappropriate proliferation signals and thus, contribute to tumor growth and other biological events that promote malignancy (12).
The connection between H2O2 signaling, thiol oxidation, and enhanced signaling through growth factor pathways provides a framework to develop therapeutics that selectively target cancer cells based on their redox status (2, 12–14). Central to achieving this goal is the development of tools and assays that facilitate characterization of the molecular events associated with tumorigenesis and evaluation of patient response to therapeutic treatment. Toward this end, immunochemical methods offer a potentially powerful and facile approach for monitoring changes in thiol oxidation state, as an indicator of normal biological function and pathological processes. Antibodies are available to detect S-glutathionylation (Cys-SSG) (15), S-nitrosylation (Cys-SNO) (16), and sulfonated cysteine (Cys-SO3−) (17). However, these reagents have limited utility in monitoring protein oxidation that occurs in H2O2 signaling because (i) disulfide bonds undergo thiol-disulfide exchange and can easily be reduced, leading to a loss signal, (ii) S-nitrosylation is not mediated by H2O2, and (iii) α-sulfonylated cysteine antibodies only recognize specific peptide sequences. Here we present the development and application of a method for detecting cysteine sulfenic acid, the initial oxidation product that results when a thiolate reacts with H2O2. We show that this antibody exhibits high specificity and sensitivity for sulfenic acids in proteins, independently of sequence content. Application of this immunochemical approach, to protein lysate arrays and cancer cell lines, allowed us to monitor changes in thiol redox status and revealed diversity in sulfenic acid modifications among different subtypes of breast tumors.
Results and Discussion
Generation of 2-Thiodimedone-Specific IgG.
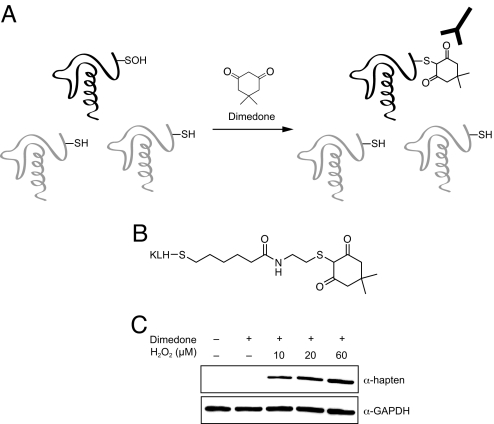
To detect H2O2-mediated thiol oxidation, we envisioned an antibody that could specifically recognize cysteine sulfenic acid. However, the structures of related oxoforms, such as sulfinic or sulfonic acid, are very similar, and thus, isolation of an antibody devoid of cross-reactivity might be difficult. In addition, the inherent reactivity of sulfenic acids could make it challenging to generate a homogenous hapten-protein conjugate. To address these issues, we have taken a chemical approach to convert the sulfenic acid into an epitope that enables antibody recognition (Fig. 1). Dimedone is a cell-permeable, nucleophilic reagent that reacts selectively with the electrophilic sulfur atom in sulfenic acid to form a stable thioether product (Fig. 1A) (18, 19). Since other functional groups in proteins do not react with dimedone, this chemical tag provides a unique starting point from which to differentiate sulfenic acids from all other cysteine oxoforms. Based on this strategy, we designed and synthesized the hapten depicted in Fig. 1B, which consists of the dimedone moiety on a cysteamine backbone with a five-carbon linker to keyhole limpet hemocyanin (KLH). The resulting hapten-protein conjugate was then used to elicit rabbit α-hapten-Ig gamma (IgG). In vitro experiments show that the antibodies only recognized the oxidized form of glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Fig. 1C) and papain (Fig. S1A) when modified by dimedone, verifying that the antibodies discriminate the adduct from other protein functional groups and that recognition is independent of protein sequence. In addition, the α-hapten antibody displays greater sensitivity in Western blot analysis compared to other chemical methods of sulfenic acid detection such as dimedone-biotin (20, 21) and an azide-tagged analog of dimedone (22, 23) (Fig. S1B).
Fig. 1.
Strategy for tagging and antibody-based detection of protein sulfenic acids. (A) Chemoselective alkylation of sulfenic acids by dimedone yields the synthetic epitope, which is recognized by the α-hapten antibody. (B) Structure of the hapten conjugate used to elicit sulfenic acid-specific antibodies. (C) GAPDH was incubated with combinations of hydrogen peroxide and dimedone as indicated; reaction products were analyzed by Western blot with the α-hapten antibody.
Detecting Thiol Oxidation in Cells.
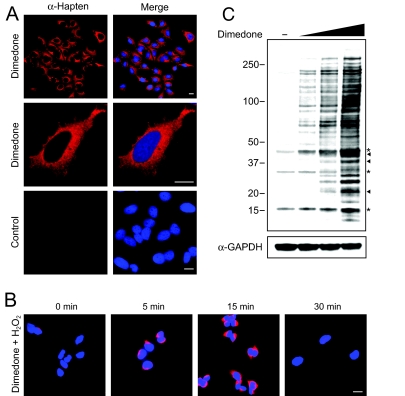
With in vitro results showing the specificity of the α-hapten antibody in hand, we next tested the ability of this reagent to image sulfenic acid in biological samples using immunofluorescence (IF) staining and fluorescence microscopy (Fig. 2A). Live human cervical cancer (HeLa) cells were incubated with dimedone to trap and tag protein sulfenic acids. In subsequent steps, cells were washed, fixed, permeabilized for antibody accessibility, and then probed with the α-hapten antibody. IF staining for sulfenic acids was diffuse throughout the cytoplasm of HeLa cells (Fig. 2A, top); perinuclear and peripheral staining of a lace-like network, reminiscent of the ER network, was also revealed (Fig. 2A, middle). Control experiments with cells excluding dimedone showed no background fluorescence (Fig. 2A, bottom), indicating that the observed IF staining was specific for dimedone-tagged sulfenic acids. Moreover, trypan blue exclusion after dimedone incubation confirms that cells remained viable throughout the treatment (Fig. S2A). Next, we investigated whether dynamic changes in sulfenic acid formation could be observed using the fluorescence microscopy and the α-hapten antibody. To simulate acute oxidative stress, HeLa cells were exposed to hydrogen peroxide. At various time points after oxidation, cells were incubated briefly with dimedone, and then processed for immunofluorescence staining as described above. Fig. 2B shows that IF staining for sulfenic acids increased in the first 15 min following stimulation with hydrogen peroxide and diminished to an undetectable level within 30 min.
Fig. 2.
Immunofluorescence microscopy and Western blot showing sulfenic acid-modified proteins in HeLa cells. (A) HeLa cells were incubated in media containing 5 mM dimedone (top and middle) or DMSO (2.5% vol/vol) (bottom) for 1 h at 37 °C and analyzed for protein sulfenic acids (red). Nuclei were counterstained with DAPI (blue). (Scale bars, 60 μm.) (B) HeLa cells were untreated or treated with 1 mM H2O2 for 5, 15, or 30 min at 37 °C. At the end of each time point, cells were washed and incubated in media containing 5 mM dimedone for 15 min at 37 °C and then analyzed for protein sulfenic acids (red). Nuclei were counterstained with DAPI (blue). (C) Protein was isolated from cells incubated in media containing 2.5, 5, or 10 mM dimedone or DMSO (2.5% vol/vol) for 2 h at 37 °C. Sulfenic acid-modified proteins were analyzed by Western blot with the α-hapten antibody. Arrowhead, protein bands corresponding to sulfenic acid-modified actin, GAPDH and PrxI (see also SI Materials and Methods); asterisk, immunoreactive background band.
To verify detection of protein sulfenic acid modifications, we performed Western blot analysis with lysates derived from cells incubated with dimedone (Fig. 2C). HeLa cells that were not exposed to dimedone showed some nonspecific immunoreactivity (Fig. 2C, lane 1, asterisks; see also Fig. 5). This background signal most likely arises from cross-reactivity of antibodies against the carrier protein, KLH, with a small number of unknown proteins present in human cells. Nonetheless, there was a clear, dose-dependent increase in the extent of protein labeling for dimedone-treated cells (Fig. 2C, lanes 2–4). Using protein-specific antibodies (see SI Materials and Methods), we identified three proteins in our Western blot analysis as actin, GAPDH, and peroxiredoxin I (PrxI), denoted by arrowheads in Fig. 2C. Actin and GAPDH are highly abundant proteins and known to contain reactive cysteines that are readily oxidized to sulfenic acid (24, 25). PrxI is an antioxidant protein that uses cysteine as the primary site of oxidation during the reduction of H2O2 (26). Positive identification of these sulfenic acid-modified proteins confirms that our methodology is capable of detecting H2O2-mediated thiol oxidation in cells. Furthermore, time and dose-dependent protein labeling was also observed in Jurkat T-cells, demonstrating the generality of the method (Fig. S2 B and C). Taken together, the IF and Western blot results establish that the α-hapten antibody is capable of detecting sulfenic acid-modified proteins, which have been trapped and tagged with dimedone in cells.
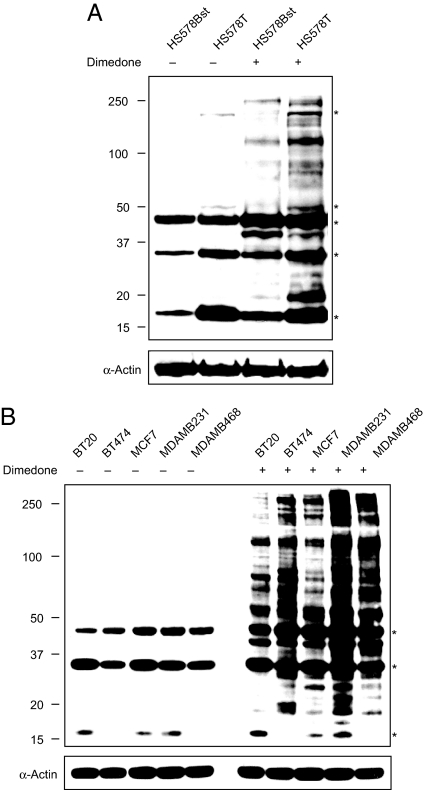
Fig. 5.
Profiling sulfenic acid modifications in breast cancer cells. (A) Comparative sulfenic acid analysis of matched breast cell lines HS578Bst (normal) and HS578T (carcinoma). HS578Bst and HS578T cells were cultured in media containing 5 mM dimedone or DMSO (2.5% vol/vol) for 2 h at 37 °C. After washing with PBS, cells were harvested in lysis buffer and sulfenic acid-modified proteins were analyzed by Western blot with the α-hapten antibody. Equal protein loading was verified by α-actin (bottom). Asterisks denote a background immunoreactive band. (B) Profiling sulfenic acid-modified proteins in breast cancer cell lines. BT20, BT474, MCF7, MDA-MB231, and MDA-MB468 cell lines were cultured in media containing 5 mM dimedone or DMSO (2.5% vol/vol) for 3 h at 37 °C. After washing with PBS, cells were harvested in lysis buffer and sulfenic acid-modified proteins were analyzed by Western blot using the α-hapten antibody. Equal protein loading was verified by α-actin (bottom). Asterisks denote a background immunoreactive band.
Protein Microarray-Based Approach to Profile Thiol Oxidation.
Protein microarrays are used as a high-throughput method to identify protein-protein interactions, kinase substrates, targets of biologically active small molecules, and profile post-translational modifications such as phosphorylation (27, 28). To the best of our knowledge, however, this approach has not been applied to investigate protein thiol oxidation. To explore the utility of the protein microarray platform to profile sulfenic acid levels, we first tested whether the α-hapten antibody could detect thiol oxidation on surface-immobilized GAPDH. Protein was applied directly to PVDF as a dot. In subsequent steps, the membrane was treated with H2O2 and dimedone to form and trap the sulfenic acid modification on GAPDH. The α-hapten antibody recognized only oxidized GAPDH when modified by dimedone (Fig. S3), analogous to data obtained for GAPDH in solution (Fig. 1C).
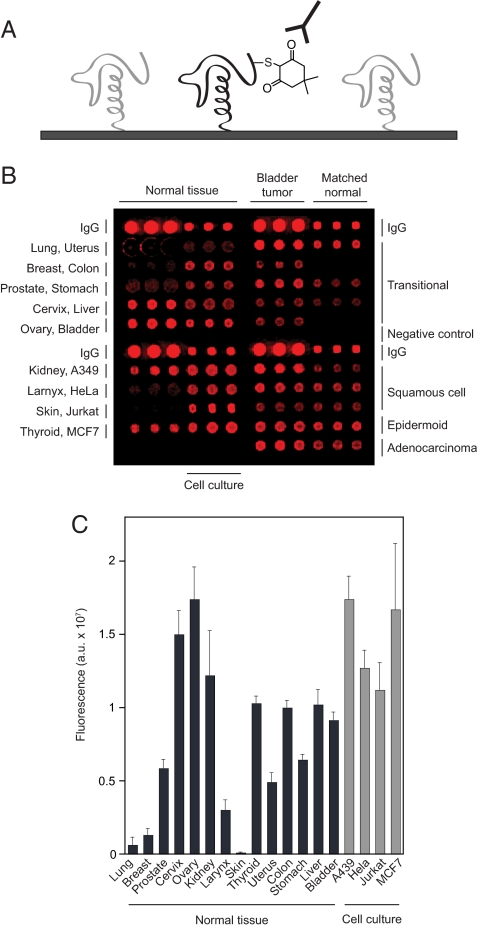
Having established that our immunochemical approach could be applied to a surface-immobilized substrate we then examined sulfenic acid levels in protein microarrays using the strategy outlined in Fig. 3A. In initial tests, we analyzed H2O2-mediated thiol oxidation using an array chip consisting of individual tissue lysate samples from 14 human organs, four mammalian cell lines as well as bladder tumors and matching normal tissue from nine individuals. After dimedone treatment, the total sulfenic acid level in each sample was assessed using the α-hapten antibody and chemifluorescence detection (Fig. 3B). Among the organs screened, the highest level of sulfenic acid was identified in the ovaries (Fig. 3C), where ROS are essential for several biological processes related to reproduction, including folliculogenesis (29). Sulfenic acid levels were also higher than the mean average in cervix, kidney, thyroid, colon, and liver tissues (Fig. 3C). BSA and buffer samples arrayed on the chip showed no background signal, as expected (Fig. 3B). The cell culture process is known to increase cellular ROS formation (1), and consistent this general observation, elevated levels of sulfenic acid were observed in all four mammalian cell lines (Fig. 3C).
Fig. 3.
Detecting thiol oxidation using the protein microarray platform. (A) Strategy for detecting sulfenic acids on protein microarrays with the α-hapten antibody. (B) Profiling protein sulfenic acid levels in tissue lysates. The protein microarray was incubated in buffer containing 2 mM dimedone for 1 h and probed for sulfenic acids (red) with the α-hapten antibody. Negative (BSA and lysis buffer) and IgG (rabbit IgG spotted at 1.0 and 0.1 mg/mL) controls are also included on the array. (C) Analysis of sulfenic acid content in human tissues and mammalian cell lines. Chemifluorescence intensity of microarray spots is indicated with bars. Error bars, the standard deviation of three replicate spots.
Sulfenic acid modifications have not been investigated in malignant tissue samples and thus, we conducted a pilot study of thiol oxidation in individuals with bladder cancer. Among the nine patient tumor tissue samples that we examined, sulfenic acid abundance varied by up to seven-fold (Fig. S4). In addition, six of seven individuals with transitional or squamous cell carcinoma exhibited a significant increase in the extent of sulfenic acid modifications, relative to matched normal tissue (P < 0.005 by paired t test; Fig. S4); adeno- and epidermoid bladder carcinomas samples were also associated with elevated levels of sulfenic acid (P < 0.001 by paired t test; Fig. S4). Although the number of paired samples on the array chip is too small to draw broad conclusions, these initial observations suggest that elevated levels of sulfenic acid might be hallmark of bladder tumor tissues. Consistent with this hypothesis, lower total thiol groups have been reported in the blood plasma of patients with bladder cancer, as compared to healthy controls (26). The current data support and extend this previous study through analysis of sulfenic acids in bladder tumor and matched normal tissue samples.
Profiling Sulfenic Acid Levels in Tumor and Normal Breast Tissue Lysates Using Protein Microarrays.
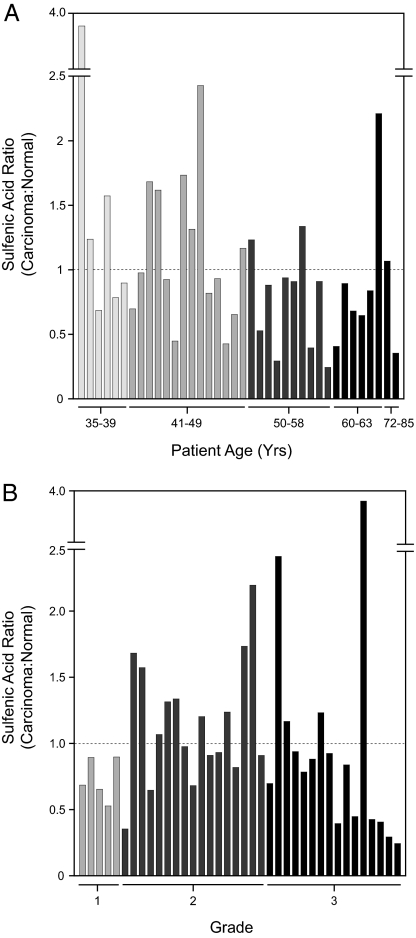
The preceding experiments led us to expand our study of sulfenic acid modifications in cancer to additional tissues, and we were specifically interested in breast carcinoma because oxidative stress is well known to modulate estrogen receptor (ER) pathways (27–30). The array chip contained 40 pairs of surgical samples (tumor and adjacent normal tissue) obtained from patients with ductal breast cancer, assigned to histological grades I–III and ranging in age from 35 to 85. Of these, 38 samples yielded a standard deviation of 15% or less and were included in subsequent comparisons. Fig. 4 shows a representative data set from these experiments and expresses the level of thiol oxidation in each sample as the ratio of sulfenic acid in breast tumor to the patient-matched normal according to age (Fig. 4A) or grade (Fig. 4B). A ratio greater than 1 indicates that thiol oxidation was elevated in the malignant tissue, relative to the paired sample.
Fig. 4.
Comparison of sulfenic acid levels by protein microarrays from tumor and normal breast tissue lysates. The level of oxidation in each sample expressed as the ratio of sulfenic acid in breast tumor to the patient-matched normal and analyzed according to age (A) or histological grade (B). A ratio greater than one indicates that thiol oxidation is elevated in malignant tissue, relative to the paired sample. Chemifluorescence intensity of microarray spots is indicated with bars. The standard deviation for three replicate spots was less than ± 15%.
Well-established markers for breast cancer such as carcinoma-associated antigen (CA15–3, also known as MUC1) and carcinoembryonic antigen (CEA) show 1.5- to 2-fold changes in abundance among breast cancer patients (30–32). By comparison, the sulfenic acid ratio among patients analyzed in this study ranged from 0.25- to 4-fold (Fig. 4). When comparing two age groups (35–49 years, n = 20 and 50–85 years, n = 18), the younger population had three times as many individuals with a sulfenic acid ratio greater than 1.2 (Fig. 4A). The lack of positive correlation between sulfenic acid ratio and age in these experiments suggests that not all cancers arising with age will exhibit excess levels of oxidative stress, relative to younger individuals. This hypothesis is supported by a recent report which indicates that expression of oxidant-responsive genes is more evident in breast cancers diagnosed at an early age, relative to those diagnosed at an older age (33). Among different tumor grades, most striking is the observation that 53% of individuals with grade II breast carcinoma (n = 17) had sulfenic acid ratios greater than 1.0, relative to 25% in grade III (n = 16) and none in grade I (n = 5) (Fig. 4B). Our observation that mid-stage breast cancer is associated with higher sulfenic acid ratios is notable and may indicate that thiol oxidation promotes tumorigenesis among a subset of patients, possibly by activating proteins that function in growth factor signaling pathways. To further validate this hypothesis, additional studies will be required.
Comparative Sulfenic Acid Profiling of Human Breast Cancer Cell Lines.
Although many important insights into thiol redox modifications have been gained through in vitro studies (34–38), proteins are subject to oxidation upon cell lysis (39, 40). Therefore, we extended our protein microarray analysis by profiling sulfenic acid modifications directly in human breast cell lines (Fig. 5). For these experiments, protein oxidation was characterized by Western blot analysis of whole-cell lysates derived from cells treated with dimedone, analogous to earlier experiments in HeLa cells (Fig. 3). Initially, we compared thiol oxidation in cell lines derived from primary invasive ductal carcinoma (Hs578T) and normal adjacent tissue (Hs578Bst). Fig. 5A shows that protein sulfenic acid modifications are more prevalent in the Hs578T cell line, relative to its normal counterpart. Next, we profiled protein sulfenic acid modifications across cell lines that represent distinct subtypes of breast cancer, including BT20, BT474, MCF7, MDAMB231, and MDAMB468 (Fig. 5B) [see Table S1 for source, clinical, and pathological features of tumors used to derive breast cancer cell lines used in this study (41)]. Each breast cancer cell line showed a unique pattern and intensity of protein labeling. Of particular note, the extremely invasive MDAMB231 cell line exhibited the highest level of sulfenic acid modifications. The HER2/neu-overexpressing cell line, BT474 also exhibited intense labeling and was further distinguished by prominent protein bands migrating between 50–100 kDa. Experiments are currently underway to determine the identity of sulfenic acid-modified proteins in breast cancer cell lines and will be reported in due course.
Conclusion
Understanding the complexities of protein thiol oxidation in normal function and disease states remains a major challenge. Here we have described a powerful method for detection of sulfenic acids that expands the arsenal of tools for analyzing thiol oxidation in cells. The use of antibodies that target a specific cysteine oxidation state capitalizes on the sensitivity and selectivity afforded by immunochemical methodology, combined with relatively simple preparation and potentially broad applications. A key feature of this approach is chemical functionalization of the sulfenic acid, which converts the modification into an epitope that can be recognized by the α-hapten antibody and facilitates discrimination over similar modifications, particularly sulfinic and sulfonic acid. Applying this approach to several systems, including human cancer cell lines, shows it can be used to monitor differences in thiol redox status and reveals a distinct pattern of sulfenic acid modifications across breast tumor subtypes. Together, these studies demonstrate a general strategy for producing antibodies against a specific oxidation state of cysteine and highlight the utility of this approach for profiling thiol oxidation associated with pathological conditions such as breast cancer.
Materials and Methods
Antibody Generation.
KLH (Keyhole Limpet Hemocyanin) protein was activated by adding sulfo-SMCC (sulfosuccinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate), a hetero-bifunctional crosslinker containing an amine-reactive N-hydroxysuccinimide and a sulfhydryl-reactive maleimide group. The hapten (synthesized as described in SI Appendix and Fig. S5) was conjugated to succinylated KLH overnight at pH 8 and the product was then purified over a PD10 gel filtration column (GE Healthcare). Two New Zealand White (specific pathogen-free grade) rabbits were immunized subcutaneously with conjugate in a 50:50 emulisification with adjuvant [either Freunds complete (FCA) or Freunds incomplete (FIA)] according to the following schedule: day 0 boost (FCA), day 14 boost (FIA), and day 28 boost (FIA). The rabbits were bled on days 35 and 40 after the primary boost, the red blood cells spun out by centrifugation, and the remaining antisera used for testing, without further purification.
Dimedone Labeling of GAPDH and Papain.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from rabbit muscle was obtained as a powder (Sigma). GAPDH (4 mg/mL in PBS pH 7.4) was treated with 1 mM DTT for 20 min at 0 °C. After reduction, DTT was removed by ultrafiltration using an Amicon Ultra-4 10 KDa MWCO centrifugal filter unit (Millipore). Papain was obtained as a powder (Sigma) and further purified as previously described (22). Stocks of dimedone used in labeling studies were made up in DMSO-0.1 M Bis-Tris·HCl, pH 7.4 (1:1). To obtain dimedone-tagged GAPDH or papain, protein (10 μM in PBS) was treated with 5 mM dimedone or DMSO (2.5% vol/vol) followed by 0, 10, 20, or 60 μM H2O2 for 30 min at room temperature. The reaction was terminated by adding 10% β-mercaptoethanol in XT sample buffer (Bio-Rad) and analyzed by Western blot using the α-hapten antibody.
Cell Culture.
HeLa, Hs578Bst, Hs578T, MCF-7, BT-20, BT-474, MDA-MB-231, and MDA-MB-468 cell lines were obtained from American Type Culture Collection. HeLa, Hs578Bst, Hs578T, MCF-7, and BT-20 cell lines were grown in DMEM media supplemented 10% FBS, 1% penicillin-streptomycin-L-glutamate (PSG) and 1× non-essential amino acids (NEAA) at 37 °C in a humidified atmosphere of 5% CO2. BT-474 cells were grown in RPMI supplemented with 10% FBS and 1% PSG at 37 °C in a humidified atmosphere of 5% CO2. MDA-MB-231 and MDA-MB-468 cell lines were grown in Leibovitz's L-15 media supplemented with 10% FBS and 1% PSG at 37 °C.
Fluorescence Microscopy.
HeLa cells (∼2.0 × 105) were seeded on collagen-coated coverslips in DMEM media supplemented with 10% FBS, 1% PSG, and 1× NEAA 16 h before analysis. Cells were incubated with 5 mM dimedone or DMSO (2.5% vol/vol) for 1 h at 37 °C in DMEM media (2% FBS, 1% PSG, and 1× NEAA). Next, cells were washed with PBS (2 × 5 min) and fixed with acetone-methanol (1:1) for 10 min at −20 °C. After fixation, cells were washed with PBS (3 × 5 min), blocked with 10% FBS in PBS for 30 min at room tempertature, washed (2 × 10 min) with DMEM media (2% FBS, 1% PSG, and 1× NEAA) and then with PBS (2 × 10 min). Cells were incubated with the α-hapten antibody in PBST (1:3,000) for 30 min, washed with PBS (3 × 10 min) and incubated with α-rabbit Alexa Fluor 594 (Invitrogen, 1:1,000) in PBST for 1 h at room temperature. After washing with 5% FBS (2 × 10 min) and PBS (2 × 10 min) slides were stained with 0.1 mg/mL DAPI for 1 min, rinsed with PBS, mounted with Fluoromount G (Southern Biotech) and analyzed using an Olympus BX-51 microscope. To monitor dynamic changes in sulfenic acid formation, HeLa cells were untreated or treated with 1 mM H2O2 for 5, 15, or 30 min at 37 °C. At the end of each time point, cells were washed and cultured in fresh media containing 5 mM dimedone for 15 min at 37 °C. After dimedone treatment, cell samples were processed for fluorescence microscopy, as described above.
Detecting Protein Sulfenic Acids in Cultured Cells.
HeLa cells were trypsinized, pelleted (200 × g at room temperature) and resuspended in DMEM containing 2% FBS, 1% PSG, and 1× NEAA. Cells were then treated with 2.5, 5, or 10 mM dimedone or DMSO (2.5% vol/vol) for 2 h at 37 °C. Breast cell lines were processed as above, but resuspended in DMEM containing 0.5% FBS, 1% PSG and then treated with 5 mM dimedone or DMSO (2.5% vol/vol) for 3 h at 37 °C. After incubation, cells were harvested, washed in PBS and then resuspended in 100 μL ice-cold lysis buffer (1.0% Nonidet P-40, 50 mM Tris-HCl, pH 8, 150 mM NaCl, and protease inhibitors) and incubated for 30 min on ice with frequent mixing. The supernatant was collected by centrifugation (20,000 × g at 4 °C) and the protein concentration of the resulting lysate was determined by Bradford assay (Bio-Rad). Sulfenic acid-modified proteins were analyzed by Western blot using the α-hapten antibody.
Western Blot.
Proteins were resolved by SDS/PAGE using Criterion XT 4–12% Bis-Tris gels (Bio-Rad) in XT Mes running buffer, transferred to PVDF membrane and then blocked with 5% nonfat dried milk in TBST overnight at 4 °C or for 1 h at room temperature. The membrane was then washed in TBST (2 × 10 min) and incubated with α-hapten antibody (1:2,000–1:4,000) for 1 h at room temperature. Next, the membrane was washed with TBST (3 × 10 min) and incubated with goat α-rabbit-HRP in TBST (1:5,000–10,000) for 30 min at room temperature. After washing with TBST (3 × 10 min), the blot was developed using ECL Plus detection reagent (GE Healthcare) and the chemifluorescent signal was visualized using a Typhoon scanner (GE Healthcare). To demonstrate equal protein loading streptavidin-HRP blots were inactivated with 15% H2O2 in TBST and re-probed with α-GAPDH or α-actin (Santa Cruz Biotechnology), followed by rabbit α-mouse-HRP.
Tissue Lysate Protein Microarray.
SomaPlex reverse phase protein microarrays with cell culture and tissue lysate samples were obtained from Protein Biotechnologies (see also SI Materials and Methods). Microarray slides were washed with water, TBST (2 × 5 min) and incubated with 2 mM dimedone in TBST for 15 min at room temperature. After washing with TBST (3 × 5 min), the slide was incubated in ZeptoBlock (Protein Biotechnologies) overnight at 4 °C and washed with TBST (5 × 5 min). The slide was then incubated with α-hapten antibody in TBST (1:5,000) for 1 h at room temperature, washed with TBST (6 × 5 min) and incubated with goat α-rabbit-HRP (1:10,000) for 1 h at room temperature. After washing in TBST (6 × 5 min), the slide was developed with ECL plus detection reagent and the chemifluorescent signal was visualized using a Typhoon scanner. The resulting signal intensities were quantified using ImageQuant software.
Supplementary Material
Acknowledgments.
This work was supported by the Life Sciences Institute, Leukemia and Lymphoma Society Special Fellows Award 3100-07, and the American Heart Association Scientist Development Grant 0835419N (to K.S.C.).
Footnotes
Conflict of interest statement: The University of Michigan is presently negotiating with Assay Designs to commercialize this antibody along with the necessary reagents for labeling protein sulfenic acids.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903015106/DCSupplemental.
References
- 1.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th Ed. New York: Oxford University Press; 2007. p. 851. [Google Scholar]
- 2.Halliwell B. Oxidative stress and cancer: Have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 3.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 4.Ragu S, et al. Oxygen metabolism and reactive oxygen species cause chromosomal rearrangements and cell death. Proc Natl Acad Sci USA. 2007;104:9747–9752. doi: 10.1073/pnas.0703192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 6.Miller EW, Chang CJ. Fluorescent probes for nitric oxide and hydrogen peroxide in cell signaling. Curr Opin Chem Biol. 2007;11:620–625. doi: 10.1016/j.cbpa.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 8.Barford D. The role of cysteine residues as redox-sensitive regulatory switches. Curr Opin Struct Biol. 2004;14:679–686. doi: 10.1016/j.sbi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Cross JV, Templeton DJ. Regulation of signal transduction through protein cysteine oxidation. Antioxid Redox Signal. 2006;8:1819–1827. doi: 10.1089/ars.2006.8.1819. [DOI] [PubMed] [Google Scholar]
- 10.Jacob C, Knight I, Winyard PG. Aspects of the biological redox chemistry of cysteine: From simple redox responses to sophisticated signalling pathways. Biol Chem. 2006;387:1385–1397. doi: 10.1515/BC.2006.174. [DOI] [PubMed] [Google Scholar]
- 11.Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Curr Pharm Des. 2006;12:4427–4443. doi: 10.2174/138161206779010549. [DOI] [PubMed] [Google Scholar]
- 13.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: A breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 14.Schumacker PT. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Hirose M, Hayano T, Shirai H, Nakamura H, Kikuchi M. Isolation of anti-glutathione antibodies from a phage display library. Protein Eng. 1998;11:243–248. doi: 10.1093/protein/11.3.243. [DOI] [PubMed] [Google Scholar]
- 16.Lorch SA, et al. Immunohistochemical localization of protein 3-nitrotyrosine and S-nitrosocysteine in a murine model of inhaled nitric oxide therapy. Pediatr Res. 2000;47:798–805. doi: 10.1203/00006450-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Woo HA, et al. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J Biol Chem. 2003;278:47361–47364. doi: 10.1074/jbc.C300428200. [DOI] [PubMed] [Google Scholar]
- 18.Allison WS. Formation and reactions of sulfenic acids in proteins. Acc Chem Res. 1976;9:293–299. [Google Scholar]
- 19.Reddie KG, Carroll KS. Expanding the functional diversity of proteins through cysteine oxidation. Curr Opin Chem Biol. 2008;12:746–754. doi: 10.1016/j.cbpa.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Poole LB, et al. Fluorescent and affinity-based tools to detect cysteine sulfenic acid formation in proteins. Bioconjug Chem. 2007;18:2004–2017. doi: 10.1021/bc700257a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charles RL, et al. Protein sulfenation as a redox sensor: Proteomics studies using a novel biotinylated dimedone analogue. Mol Cell Proteomics. 2007;6:1473–1484. doi: 10.1074/mcp.M700065-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Reddie KG, Seo YH, Muse WB, III, Leonard SE, Carroll KS. A chemical approach for detecting sulfenic acid-modified proteins in living cells. Mol Biosyst. 2008;4:521–531. doi: 10.1039/b719986d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo YH, Carroll KS. Facile synthesis and biological evaluation of a cell-permeable probe to detect redox-regulated proteins. Bioorg Med Chem Lett. 2009;19:356–359. doi: 10.1016/j.bmcl.2008.11.073. [DOI] [PubMed] [Google Scholar]
- 24.Benitez LV, Allison WS. The inactivation of the acyl phosphatase activity catalyzed by the sulfenic acid form of glyceraldehyde 3-phosphate dehydrogenase by dimedone and olefins. J Biol Chem. 1974;249:6234–6243. [PubMed] [Google Scholar]
- 25.Johansson M, Lundberg M. Glutathionylation of beta-actin via a cysteinyl sulfenic acid intermediary. BMC Biochem. 2007;8:26. doi: 10.1186/1471-2091-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee SG, et al. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Hoheisel JD. Microarray technology: Beyond transcript profiling and genotype analysis. Nat Rev Genet. 2006;7:200–210. doi: 10.1038/nrg1809. [DOI] [PubMed] [Google Scholar]
- 28.Zhu H, et al. Global analysis of protein activities using proteome chips. Science. 2001;293:2101–2105. doi: 10.1126/science.1062191. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal A, Gupta S, Sekhon L, Shah R. Redox considerations in female reproductive function and assisted reproduction: From molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1375–1403. doi: 10.1089/ars.2007.1964. [DOI] [PubMed] [Google Scholar]
- 30.Kulasingam V, et al. Activated leukocyte cell adhesion molecule: A novel biomarker for breast cancer. Int J Cancer. 2009;125:9–14. doi: 10.1002/ijc.24292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffy MJ. CA 15–3 and related mucins as circulating markers in breast cancer. Ann Clin Biochem. 1999;36:579–586. doi: 10.1177/000456329903600503. [DOI] [PubMed] [Google Scholar]
- 32.Duffy MJ. Serum tumor markers in breast cancer: Are they of clinical value? Clin Chem. 2006;52:345–351. doi: 10.1373/clinchem.2005.059832. [DOI] [PubMed] [Google Scholar]
- 33.Yau C, Benz CC. Genes responsive to both oxidant stress and loss of estrogen receptor function identify a poor prognosis group of estrogen receptor positive primary breast cancers. Breast Cancer Res. 2008;10:R61. doi: 10.1186/bcr2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuangthong M, Helmann JD. The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative. Proc Natl Acad Sci USA. 2002;99:6690–6695. doi: 10.1073/pnas.102483199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saurin AT, Neubert H, Brennan JP, Eaton P. Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proc Natl Acad Sci USA. 2004;101:17982–17987. doi: 10.1073/pnas.0404762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greco TM, et al. Identification of S-nitrosylation motifs by site-specific mapping of the S-nitrosocysteine proteome in human vascular smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:7420–7425. doi: 10.1073/pnas.0600729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leichert LI, et al. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci USA. 2008;105:8197–8202. doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemble DJ, Sun G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc Natl Acad Sci USA. 2009;106:5070–5075. doi: 10.1073/pnas.0806117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Mirabal HR, Winther JR. Redox characteristics of the eukaryotic cytosol. Biochim Biophys Acta. 2008;1783:629–640. doi: 10.1016/j.bbamcr.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Neve RM, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.