Abstract
The identification of lung tumor-initiating cells and associated markers may be useful for optimization of therapeutic approaches and for predictive and prognostic information in lung cancer patients. CD133, a surface glycoprotein linked to organ-specific stem cells, was described as a marker of cancer-initiating cells in different tumor types. Here, we report that a CD133+, epithelial-specific antigen-positive (CD133+ESA+) population is increased in primary nonsmall cell lung cancer (NSCLC) compared with normal lung tissue and has higher tumorigenic potential in SCID mice and expression of genes involved in stemness, adhesion, motility, and drug efflux than the CD133− counterpart. Cisplatin treatment of lung cancer cells in vitro resulted in enrichment of CD133+ fraction both after acute cytotoxic exposure and in cells with stable cisplatin-resistant phenotype. Subpopulations of CD133+ABCG2+ and CD133+CXCR4+ cells were spared by in vivo cisplatin treatment of lung tumor xenografts established from primary tumors. A tendency toward shorter progression-free survival was observed in CD133+ NSCLC patients treated with platinum-containing regimens. Our results indicate that chemoresistant populations with highly tumorigenic and stem-like features are present in lung tumors. The molecular features of these cells may provide the rationale for more specific therapeutic targeting and the definition of predictive factors in clinical management of this lethal disease.
Keywords: ABC transporters, cancer stem cells, chemoresistance, CXCR4, xenografts
Lung cancer is the leading cause of cancer deaths worldwide because of its high incidence and mortality, with 5-year survival estimates ≈10% for nonsmall cell lung cancer (NSCLC) (1). Refined investigation on the mechanisms of tumorigenesis and chemoresistance of lung cancer is needed to improve survival rate.
Recently, the cancer stem cell (CSC) theory has been proposed to explain the tumor heterogeneity and the carcinogenesis process (2, 3). According to this model, tumor can be viewed as a result of abnormal organogenesis driven by CSCs, defined as self-renewing tumor cells able to initiate and maintain the tumor and to produce the heterogeneous lineages of cancer cells that compose the tumor (4, 5). The existence of CSCs was first proved in acute myeloid leukemia (6), and more recently in glioblastoma (7–9), melanoma (10, 11), and epithelial cancers (12–18).
CSCs were identified by using flow cytometry-based cell sorting of tissue-specific surface markers or sphere-forming assay in selective serum-free medium. CD133 (prominin-1), a five-transmembrane glycoprotein, was initially described as a marker specific for CD34+ human hematopoietic progenitor cells (19, 20), normal stem cells of the neural (21, 22), epithelial (23, 24), and endothelial lineages (25), and their tumoral counterparts (14–16, 26, 27). However, it is still a matter of debate whether CD133+ cells truly represent the ultimate tumorigenic population, particularly in colon (28) and brain (29, 30) cancer.
Long-term cultures of sphere-growing cells derived from human lung tumors were shown to be highly enriched for CD133 expression, able to self-renew, and able to be the only tumorigenic population in vivo (18). A recent report further demonstrated that Oct-4 expression plays a critical role in maintenance of stem-like properties in lung cancer CD133+ cells (31).
CSCs may be inherently resistant to the cytotoxic effect of chemotherapy because of their low proliferation rate and resistance mechanisms, such as the expression of multidrug transporters of the ATP-binding cassette (ABC) superfamily. ABCB5 was found to be expressed on a distinct subset of chemoresistant CD133+ melanoma cells (32), and its selective targeting caused tumor growth inhibition in xenograft models (11). Expression of ABCG2 (BCRP1), a transporter involved in resistance to multiple drugs (33), was reported in normal lung undifferentiated cells (34) and lung cancer cell lines (35).
By using both in vitro systems and implemented in vivo models of direct xenografts of human primary lung cancers in mice, we provide evidence that lung tumor CD133+ cells are highly tumorigenic, are endowed with stem-like features, and, importantly, are spared by cisplatin treatment.
Results
Identification of CD133+ Cells in Lung Cancer.
In an attempt to identify lung cancer-initiating cells, we analyzed 60 primary tissue samples derived from a consecutive series of lung cancer patients (Table S1). By using the surface marker CD133 alone or performing double staining with the epithelial-specific antigen (ESA) marker to exclude potential contamination by hematopoietic and endothelial progenitors, we noted a very similar frequency of CD133+ and CD133+ESA+ populations in all cases except one (LT23; Table S1), indicating that most CD133+ cells were of epithelial origin. We demonstrated the presence of a rare (mean, <1%) CD133+ESA+ population in normal lung tissues of the patients, whereas tumor samples consistently showed a noticeable (mean, 5%; P = 0.0004) CD133+ESA+ population (Fig. S1). FACS analysis showed the presence of a variable fraction of CD133+ESA+ cell populations in 47 of 56 (83.9%) tumor samples, varying from 0.02% to a maximum of 35%. However, 60% of the cases showed small amounts (≤2%) of CD133+ cells. Further investigation of CD133 expression by immunohistochemistry (IHC) on paraffin-embedded tumor sections confirmed the existence of CD133+ cells and identified 32 of 58 (55.2%) positive cases (Fig. 1 and Table S1). Positivity of tumor cells was defined as membranous staining or staining of membrane and cytoplasm, and the intensity was always specific and strong. Because the immunoreactivity was heterogeneous, no cutoff was applied, and only cases with no CD133 immunoreactivity were scored as negative. IHC generally confirmed the results obtained with FACS analysis; however, FACS was more sensitive in detecting positive cases with a very small percentage of CD133+ cells. Indeed, by categorizing FACS results into four classes (negative or positive, with 2% and 5% positivity cutoffs), the frequency of positive samples at IHC gradually increased from 11% to 92% (test for trend P = 0.0005). When data were analyzed in terms of discrimination between negative and positive IHC results on the basis of FACS classes, the areas under the receiver-operating characteristic curve (nonparametric estimate) were 0.764 (P < 0.0001; or 0.761, P < 0.0001, by using FACS score for computation as a continuous variable), value between 1 (perfect discrimination) and 0.5 (lack of discrimination). Cross-tabulation of CD133 expression data using IHC staining and clinicopathological features indicated that low-grade (G1–G2) tumors (P = 0.0356) and adenocarcinoma histology (P = 0.0441) were more frequently represented among CD133-positive cases (Table S2). Follow-up information was too limited (median follow-up duration, 14 months; eight recorded tumor progressions and two tumor deaths) for investigation of CD133 expression prognostic value.
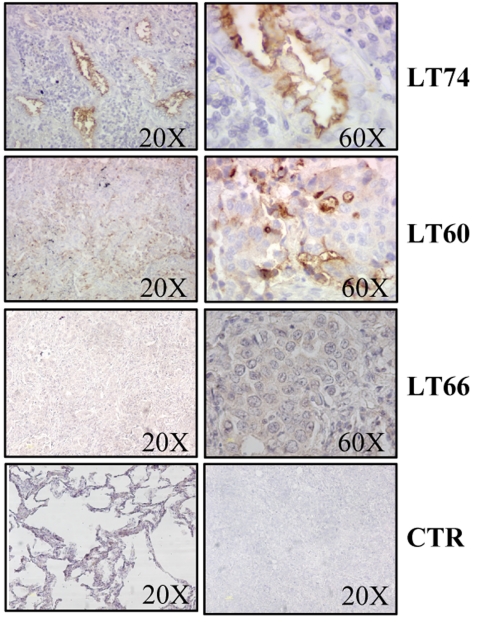
Fig. 1.
IHC analysis of CD133 expression in two representative positive (LT74 and LT60) and one negative (LT66) adenocarcinoma lung tumor samples at low and high magnifications. As controls (CTR) for staining specificity, a normal lung sample negative for CD133 (Bottom Left) and a control antibody-stained tumor sample (Bottom Right) are also shown.
Establishment of Xenograft Models of Lung Primary Tumors.
To develop novel preclinical models, we successfully grew 10 xenografts in nude mice starting from 29 human lung primary tumors. These xenografts were serially maintained in vivo as tumor lines that represented a continuous source of tumor tissue for subsequent functional studies. IHC for CD133 and for a panel of lung cancer antigens showed high similarity between parental tumors and mouse xenografts (Fig. 2A and Fig. S2) and a similar fraction of CD133+ESA+ cells by FACS (Fig. 2B) that was maintained over time in serial transplantations. No association was observed between the levels of CD133 expression in the original primary tumor and the ability to grow and propagate xenografts in mice.
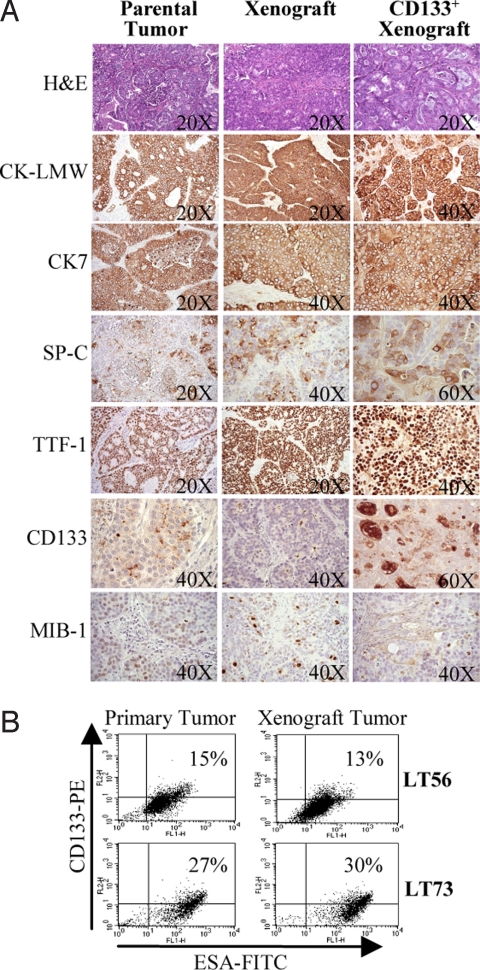
Fig. 2.
Tumor xenografts resemble the original primary tumor. (A) IHC of low-molecular weight cytokeratins (CK-LMW), cytokeratin 7 (CK7), surfactant protein C (SP-C), transcription thyroid factor 1 (TTF-1), CD133, and MIB-1 performed in LT45 parental tumor, corresponding xenograft, and xenograft tumor derived from injection of CD133+ cells. (B) FACS analysis of CD133+ESA+cells in xenografts compared with parental tumors.
Tumorigenic in Vivo Potential of Isolated CD133+ Cells.
Because the definition of cancer-initiating cells relies mainly on functional properties, like the ability to sustain tumor growth recapitulating the original cellular heterogeneity, we investigated the tumorigenic ability of lung tumor-derived CD133+ and CD133− cell populations.
After depletion of mouse cells expressing the H2K-MHCI antigen by immunomagnetic separation (when necessary), CD133+ cells were isolated by FACS or magnetic cell sorting (Miltenyi Biotech) from four xenografts and one primary tumor. Considerable enrichment of CD133+ cells was observed in the positive fraction (>80% purity), as determined by FACS (Fig. S3A). The same numbers of CD133+, CD133−, and unsorted cells mixed with Matrigel were injected in the flank of SCID mice by using limiting doses derived from preliminary experiments (104 down to 102). Palpable tumors were visible after a variable time interval in the majority of CD133+-injected mice, whereas no tumor growth was observed in CD133−-injected mice (Fig. S3B and Table S3) except for LT73 (see below). Tumorigenicity of the unsorted population was variable for the different cases, and for the xenografts it appeared to be related to the percentage of CD133+ cells in the bulk population; however, tumor formation of enriched CD133+ cells was faster and resulted in increased tumor take compared with that observed after injection of CD133− or unsorted cells. Morphological and IHC analyses revealed that tumors derived from CD133+ cells faithfully reproduced the original tumor (Fig. 2A). Similar results were observed after s.c. injection of FACS-isolated CD133+ cells from the A549 lung adenocarcinoma cell line (Table S3). These data suggest that the CD133+ cell population is enriched in cells capable of initiating lung cancer in SCID mice.
To investigate whether lung cancers originating from CD133+ cells possess higher long-term tumorigenic potential compared with the rarely observed tumors originating from the CD133− fraction, we performed serial transplantation assays in SCID mice of cells isolated from LT73 tumor xenografts originally derived from CD133+ or CD133− cell injection (Fig. S3C). Cells derived from CD133+ tumors were able to generate tumors maintaining the original morphology and proportion of CD133+ cells in primary, secondary, and tertiary transplantation, whereas cells from CD133− tumors lost tumorigenic potential during serial transplantations (Fig. S3C). In principle, the generation of tumors from CD133− cells could be caused by contamination of the negative fraction by CD133+ cells during cell sorting. However, it is likely that for this specific highly aggressive tumor the CD133− fraction also had some tumor-initiating potential at low dose. Here, we demonstrated that these CD133− cells could not maintain tumor growth in serial transplantations, indicating a lower and fading tumorigenic ability compared with CD133+ cells.
Biological and Molecular Features of Human Lung Cancer-Initiating Cells.
Down-regulation of CD133 expression was observed in short-term adherent cultures (n = 7) grown in serum-supplemented medium compared with the original cell suspension (Fig. 3A).
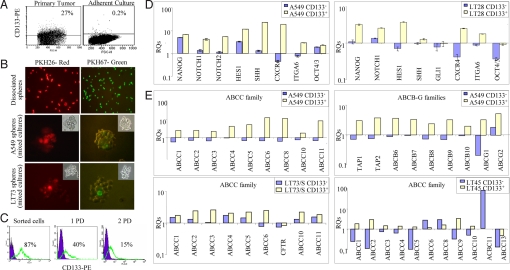
Fig. 3.
In vitro characterization of CD133+ cells. (A) FACS analysis for CD133 expression in freshly dissociated primary LT73 and corresponding adherent cell culture after four passages in vitro. (B) PKH26 staining of LT73 and A549 spheres. (Top) Single cells from dissociated spheres were separately labeled with PKH26 and PKH67 red and green fluorescent dyes, and cultures were harvested by mixing red and green labeled cells. (Middle and Bottom) A549 (Middle) and LT73 (Bottom) spheres, derived from mixed cultures, showed a separate green or red fluorescent staining, with a decreasing gradient of fluorescence intensity within the spheres. Microscopic images of spheres derived from labeled cells were acquired at 20× magnification in bright field, with rhodamine filter for PKH26 fluorescence (Left) and with fluorescein filter for PKH67 fluorescence (Right). (C) Time-course analysis of CD133+ cells sorted from A549 spheres. FACS analysis of CD133 after sorting and at the first and second population doubling (PD). (D) Real-time PCR analysis of stemness gene expression in CD133+ and CD133− fractions, sorted from LT28 xenograft (Left) and A549 spheres (Right). Unsorted cells were used as calibrator for the relative quantification of gene expression. (E) Real-time PCR analysis of transporters of ABCC-B-G families in CD133+ and CD133− cells sorted from A549/s, and ABCC family in CD133+ and CD133− cells sorted from LT73/s and LT45 xenograft. Unsorted spheres or LT45 unsorted cells were used as calibrators for the relative quantification of gene expression.
Normal and neoplastic stem-like cells from neural and epithelial organs can be expanded as sphere-like aggregates in serum-free EGF–bFGF-supplemented medium (9, 15, 36) that favors the proliferation of undifferentiated cells. However, establishment of long-term sphere cultures from primary lung tumor samples was unsuccessful. Nevertheless from adherent LT73 and A549 cell lines we were able to consistently expand cells growing as floating spheres (A549/s and LT73/s) that allowed a variety of cellular and molecular analyses.
To verify the clonal origin of A549 and LT73 spheres, we performed a label-retaining assay using vital red and green fluorescent dyes (PKH26 and PKH67, as described in SI Text). Spheres derived from mixed cultures of PKH26-labeled and PKH67-labeled cells showed a distinct red or green fluorescent staining, indicating that A549/s and LT73/s did not represent stochastic cellular aggregates. Moreover, the decreasing gradient of fluorescence intensity of labeled cells within the spheres allowed us to evaluate a difference in the rate of cell divisions because proliferating cells easily dilute the dyes, whereas slowly dividing quiescent cells retain most of the fluorescence (37). A549/s and LT73/s spheres showed a decreasing gradient of red or green fluorescence, indicating that cells within spheres have a clonal origin and are endowed with a differential proliferation rate (Fig. 3B). FACS analysis indicated that A549/s and LT73/s displayed an enrichment of CD133+ cell fraction to ≈1%, whereas it was only 0.2% in the standard adherent culture. A time-course FACS analysis of the sorted CD133+ fraction showed that CD133+ cells remained quiescent for ≈2 weeks, whereas the CD133− fraction and the unsorted A549/s population showed a constant and sustained proliferative rate. After this time CD133+ cells started to proliferate, and CD133 expression decreased along with cell divisions and returned to the original fraction in ≈50 days (Fig. 3C). In contrast, the CD133− population remained CD133 negative during the observation period.
The expression of genes involved in stem cell pathways [i.e., Sonic hedgehog (SHH, Gli-1), Notch (Notch1, Notch2, Hes-1), CD133, Oct4/3, and Nanog] was investigated by real-time PCR in CD133+ and CD133− populations sorted from A549/s and LT73/s and lung tumor xenografts (LT56, LT28, LT45). An increased expression level of genes involved in the maintenance of stemness, markedly of Oct4/3 and Nanog, and adhesion and motility genes, like α-6 integrin and CXCR4, was consistently observed in CD133+ cells compared with the CD133− counterpart (Fig. 3D; detailed procedures described in SI Text).
To better define the molecular features of cancer-initiating cells with respect to cellular defense mechanisms, we analyzed the mRNA levels of transporters of the ABC superfamily in CD133+ and CD133− cell populations sorted from A549/s, LT73/s, and LT45 xenografts by using TaqMan Micro Fluidic cards (Applied Biosystems). By using this approach, we found that ≈70% of the 50 human ABC transporters were expressed at detectable levels (Fig. 3E). Compared with the CD133− fraction, CD133+ cells exhibited an increased expression of different components of the ABCC family that are known to be involved in the multidrug-resistant phenotype (i.e., ABCC1). The level of several ABCB family members and ABCG2 mRNA was also enhanced. Overall, these results indicate that CD133+ cells are slowly dividing cells, able to give rise to a CD133− cell population, thereby recapitulating the original cellular heterogeneity, and CD133+ cells express high levels of embryonic stem cell genes, motility genes, and ABC transporters, suggesting that CD133+ cells possess the features of stem-like cancer cells.
In Vitro Resistance of CD133+ Cells to Cisplatin.
Because of their low proliferation rate and the activation of defense mechanisms, the small population of CD133 lung cancer-initiating cells may thus be inherently resistant to the cytotoxic effect of chemotherapy. We therefore assayed whether standard chemotherapy treatments result in enrichment of cancer-initiating cells in the surviving cell fraction. In vitro studies were performed in adherent A549 cells that show a low fraction (0.2%) of CD133+ cells (detailed procedures described in SI Text). Exposure of cells to a cytotoxic concentration of cisplatin, corresponding to the IC80, resulted in an 8-fold enrichment of CD133+ cells (from 0.2% ± 0.05% to 1.6% ± 0.5%; Fig. 4A).
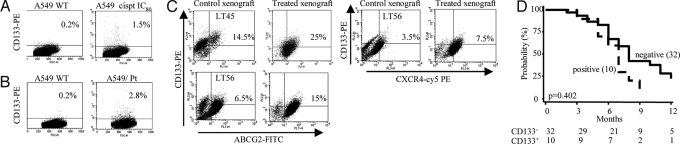
Fig. 4.
CD133+ cells survive cisplatin treatment. (A) FACS analysis of CD133 expression in A549 parental cell line and A549 treated for 1 h with cisplatin (IC80). CD133 expression increased in the A549 cell line 72 h after treatment. (B) FACS analysis of CD133 expression in stable cisplatin-resistant A549/Pt cells compared with the A549 parental cell line. (C) Enrichment of CD133+ABCG2+ (Upper) and CD133+CXCR4+ (Lower) populations in xenografts LT45 and LT56 after in vivo cisplatin treatment. (D) CD133 expression in advanced NSCLC patients treated with platinum-containing regimens. Progression-free survival curve was calculated with the Kaplan–Meier method and compared by using the log-rank test.
We then extended such an analysis to cells with acquired resistance to cisplatin. By using the A549/Pt cells, generated by chronic exposure of A549 to increasing concentrations of cisplatin and endowed with a stable drug-resistant phenotype when grown in the absence of selecting agent (degree of resistance ≈10), we found that in vitro development of drug resistance was associated with increased expression of CD133. Indeed, a 13-fold enrichment in CD133+ population (from 0.2% ± 0.05% to 2.7% ± 0.87%) was consistently observed in A549/Pt cells grown as adherent culture (Fig. 4B).
Increased ABCG2 and CXCR4 Expression of Human Lung Cancer-Initiating Cells After in Vivo Cisplatin Treatment.
To demonstrate the in vivo relevance of these findings, mice carrying six different lung cancer xenografts were treated i.v. with cisplatin, according to a weekly schedule.
Cisplatin treatment resulted in a variable tumor volume inhibition (TVI) among the xenografts, ranging from 35% to 83%, overall confirming the poor responsiveness to chemotherapy observed in the clinical setting of lung cancer (Table S4). To verify whether cisplatin treatment was able to enrich the fraction of cancer-initiating cells in the residual tumors, at 7 days after the last treatment and at the time of tumor regrowth, mice were killed. FACS analysis of cells isolated from the resected tumors showed an enrichment of 7 and 35 times in the CD133+ fraction in A549 and LT66 tumors, respectively, shortly after chemotherapy (day 7 after last treatment), which reverted to the original values at the time of tumor regrowth (Table S4). In tumors with an original large content of CD133+ cells, such as LT45 and LT56 (50% and 15%, respectively), the fraction of CD133+ cells taken as a whole did not change after cisplatin treatment, but a subpopulation of CD133+ABCG2+ cells showed a remarkable enrichment (Fig. 4C). A similar change in the CD133+ABCG2+ fraction was also noticed in A549 and LT66 treated tumors (Table S4). Overall, our findings indicate that chemotherapy, even when reducing tumor burden, may spare CD133+ tumor cells endowed with drug-resistance properties.
Double staining with CD133/CXCR4 antibodies also revealed a subpopulation of positive cells that increased after chemotherapy in 2 out of 3 tumors analyzed, and a persistent increase in cells expressing CXCR4 was noticed in most treated tumors and in the A549-treated cell line (Fig. 4C and Table S4). Such results suggest the survival of CD133+ chemoresistant clones also endowed with high mobility and metastatic features.
Cisplatin-treated tumors also consistently expressed higher mRNA levels of Nanog, Oct4/3, Notch, α-6 integrin, and CXCR4 genes than their corresponding untreated controls (Fig. S4A). The same was observed for ABC transporters (Fig. S4B). These findings indicate that the CD133+-chemoresistant fraction preferentially expresses genes important for the proliferation, self-renewal, homing, and drug resistance of stem cells.
CD133 Expression as a Marker of Chemotherapeutic Response in Patients.
Finally, to investigate the possible relationship between CD133 status and response to platinum-containing regimens in the clinical setting, we retrospectively analyzed CD133 protein expression by IHC in formalin-fixed biopsies obtained before treatment from 42 stage IIIB/IV NSCLC patients receiving carboplatin and gemcitabine (Table S5). CD133 expression on these archival biopsies was found in 10 (23.8%) of 42 patients.
Partial response to platinum-based chemotherapy was observed in 12 of 32 (37.5%) CD133− patients and 4 of 10 (40%) CD133+ patients. Patients with CD133+ tumors had a tendency toward a shorter median progression-free survival than patients with CD133− tumors (Fig. 4D); however, the difference did not reach statistical significance. Interestingly, 9 of 10 CD133+ patients relapsed within 9 months (the last patient at 20 months) compared with 22 of 32 (68.7%) relapses in the group of CD133− patients, suggesting that NSCLC tumors carrying higher levels of CD133+ cells might be more likely to develop early tumor recurrence after chemotherapy.
Discussion
The identification of distinct phenotypic and functional markers associated with stem-like properties of lung cancer tumor-initiating cells would be instrumental for developing therapeutic targeting strategies in lung cancer patients.
Here, we report that a CD133+/ESA+ population is increased in primary NSCLC samples compared with normal lung tissue in a prospectively collected series of lung cancer patients. Flow cytometry and IHC analyses showed a quite good concordance for the detection of the CD133+ population in surgical tumor samples, supporting the use of the latter technique for screening paraffin-embedded samples and also small archival biopsies.
CD133+ cells isolated from primary lung tumors showed higher tumorigenic potential than their CD133− counterparts and were able to reproduce the original tumor heterogeneity in SCID mice. This phenotype was associated with an increased expression level of genes involved in stemness, adhesion, and motility. An interesting finding was the enrichment for several ABC transporters in CD133+ cells compared with the CD133− fraction. Thus, CD133+ cells possess features of cancer-initiating stem-like cells, including ABCG2 expression and, moreover, they expressed additional ABC transporters that may contribute to determine a drug-resistant phenotype.
Previous studies on the putative chemoresistant features of lung cancer-initiating cells have approached this issue by using only a limited number of in vitro models of sphere-growing cells isolated from tumors or established cell lines (18, 31). Our in vitro experiments with cisplatin treatment also resulted in enrichment of CD133+ cancer-initiating cells in the surviving fraction both after acute cytotoxic exposure and in an established lung cancer cell line model of acquired stable cisplatin resistance. However, in vitro models may not perfectly mirror what is observed in primary cancer cells, whereas in vivo models are more conducive to the evaluation of the functional properties of tumor-initiating cells, particularly in the assessment of treatment responses, because they best reflect the tumor heterogeneity observed in patients and the interaction with the microenvironment. In novel preclinical models, i.e., xenografts from primary lung tumors of patients, serially maintained in vivo as tumor lines, we show that cisplatin treatment resulted in remarkable enrichment of the CD133+ cell fraction in the residual tumors, which then reverted to the original values at time of tumor regrowth. Interestingly, in tumors with a high content of CD133+ cells, only subpopulations of CD133+ABCG2+ and CD133+CXCR4+ cells displayed a clear enrichment after treatment, therefore suggesting that lung cancer CD133+ cells comprise populations of cells with a similar phenotype but different potential. It is noteworthy that if the subpopulation of tumor-initiating cells has chemoresistant features, conventional measures of treatment efficacy (such as TVI) might only reflect how the bulk tumor responds to chemotherapeutic treatment, failing to provide relevant information on long-lasting tumor-eradicating potential. In this view, additional and different endpoints should be considered to evaluate innovative therapeutic strategies.
These chemoresistant cell fractions preferentially expressed genes relevant for proliferation, self-renewal, differentiation, drug resistance, and homing of stem cells. Such results indicate that chemotherapy is effective in eliminating drug-sensitive CD133− differentiated tumor cells, whereas cancer-initiating cells with the above described phenotypes are spared by these treatments and could be responsible for tumor restoration after chemotherapy cessation. Accordingly, by using archived biopsies we observed that CD133 protein expression tended to correlate with early recurrence in a series of advanced-stage NSCLC patients treated with platinum-containing regimens, although more data are needed to draw definitive conclusions.
Our in vivo findings showing coexpression of CD133/ABCG2 markers in the cisplatin-spared cell population are in agreement with the increase in the side population fraction observed in drug-surviving cells of a H460 lung cancer cell line that can be specifically depleted by using the ABCG2 inhibitor fumitremorgin C (35). Our results suggest the interest of CD133/ABCG2 expression in relation to treatment effect in patients with lung cancer and a rationale for a new generation of ABCG2 inhibitors to be used in combination therapy. Indeed, the use of a specific antibody against ABCB5 transporter significantly inhibited tumor growth in treated melanoma xenografts (11).
An interesting finding was the elevated expression of CXCR4 in CD133+ cells compared with the CD133− fraction and a consistent enrichment of CD133+CXCR4+ population after cisplatin treatment of tumor xenografts. Our data are in line with those reported in pancreatic adenocarcinoma (38), suggesting the existence of a chemoresistant CD133+CXCR4+ subpopulation with highly tumorigenic and metastatic properties also in lung cancer. Considering that SDF-1, the specific ligand of CXCR4, is strongly expressed in the lung, targeting with specific inhibitors of the SDF1–CXCR4 axis could represent a therapeutic option to eradicate the chemoresistant CSC population.
The demonstration of the presence in lung tumors of highly tumorigenic cells with stem-like properties and exhibiting features of chemoresistant cells may be useful, together with the use of carefully selected in vivo models, to evaluate novel pathways to be targeted to increase the therapeutic response in the clinical setting of this lethal disease.
Materials and Methods
Tumor and Normal Lung Tissue Dissociation and Cell Cultures.
Clinical specimens were obtained from a consecutive series of consenting patients according to the Internal Review and the Ethics Boards of the Istituto Nazionale Tumori of Milan.
Single-cell suspensions were obtained from lung tumor and normal tissues, collected from clinical surgical specimens, and mouse tumor xenografts after mechanical and enzymatical disaggregation, as described in SI Text. For adherent cell cultures, cells were plated in conventional medium, RPMI medium 1640 supplemented with 10% heat-inactivated FCS (all from Lonza). To obtain sphere cultures, cells were plated at a density of 104 cells per milliliter in serum-free medium DMEM/F12 (Lonza), supplemented with commercial hormone mix B27 (Gibco Invitrogen), EGF (20 ng/mL; PeproTech), bFGF (10 ng/mL; PeproTech), and heparin (2 μg/mL). Floating sphere cultures were expanded by mechanical dissociation, followed by replating of single cells in complete fresh medium every 3 days.
For experiments using tumor xenografts, mouse cells were depleted by using the Dynal cell-collection Biotin Binder kit (Invitrogen) as detailed in SI Text.
Flow Cytometry Analysis.
Single-cell suspensions were washed and incubated in staining solution containing 1% BSA and 2 mM EDTA with the specific antibodies at appropriate dilutions. For CD133 staining, 106 cells were incubated with 10 μL of CD133/1-phycoerythrin antibody (50 μg/mL; AC133 clone; Miltenyi Biotech) diluted in 80 μL of staining solution and 20 μL of FcR blocking reagent (Miltenyi Biotech) for 10 min at 4 °C. Antibodies used were phycoerythrin (PE)-conjugated anti-CD133/1, PE-conjugated anti-CD133/2, and FITC-conjugated anti CD326 (EpCAM), all from Miltenyi Biotech; PE-Cy5-conjugated anti-CXCR4 (Becton Dickinson); and FITC-conjugated anti-BCRP1 (Chemicon).
Samples were acquired and analyzed by using a FACSCalibur and CELLQuest Pro software (Becton Dickinson). For time-course experiments, CD133+ and CD133− fractions were plated in serum-free medium after sorting, and the percentage of CD133+ cells was determined at each cell division by flow cytometry.
For the protocol of magnetic and cytofluorimetric CD133+ cell separation, refer to SI Text.
IHC.
IHC was performed on formalin-fixed, paraffin embedded samples. CD133 immunostaining was assessed on whole-tissue sections, and CD133 immunoreactivity was evaluated inside the neoplastic epithelial component. All specimens were evaluated independently by two observers (E.R. and G.S.), and interobserver agreement was reached in all cases. Technical details of immunostaining are reported in SI Text.
In Vivo Studies of Tumorigenicity.
All experiments were carried out with female CD-1 nude mice or SCID mice, 7–10 weeks old (Charles River Laboratories). Mice were maintained in laminar flow rooms, with temperature and humidity constant. Mice had free access to food and water. Experiments were approved by the Ethics Committee for Animal Experimentation of the Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Istituto Nazionale Tumori, according to institutional guidelines.
For establishment of xenograft models and tumor lines, regular small fragments were obtained by patient surgical specimens as described (39). Fragments were implanted s.c. by trocar gauge in one or both flanks of nude mice. Tumor lines were achieved by serial s.c. passages of fragments from growing tumors into healthy mice (39).
To assess the tumorigenic potential of different cell populations, after sorting, viable 103 and 104 CD133+, CD133−, and unsorted cells were suspended in Matrigel (BD Biosciences) at a ratio of 1:1, and 200 μL of cells was s.c. injected into the right flank of SCID mice. For serial transplantation assays, 103 CD133+ and CD133− cells were injected s.c. in SCID mice, and derived tumors were dissociated to single cells and serially reinjected in mice at 102 cell numbers, generating secondary and tertiary tumors.
The in vivo chemotherapy studies to evaluate response to cisplatin were performed as described in SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. Giacomo Cossa for technical assistance and Prof. Malcolm Alison for helpful discussions. F.A. is a fellow of Fondazione Ermenegildo Zegna. This work was supported by the Associazione Italiana Ricerca Cancro (to G.S. and U.P.), Italian Ministry of Health (Ricerca Finalizzata) (to G.S.), Compagnia di San Paolo di Torino, and European Community Integrated Project 037665 “CHEMORES.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905653106/DCSupplemental.
References
- 1.Jemal A, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 4.Clarke MF, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 5.Clarke MF. Self-renewal and solid-tumor stem cells. Biol Blood Marrow Transplant. 2005;11:14–16. doi: 10.1016/j.bbmt.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 7.Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 8.Hemmati HD, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galli R, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 10.Fang D, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 11.Schatton T, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 14.Collins AT, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 15.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 17.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eramo A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 19.Yin AH, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 20.Miraglia S, et al. A novel five-transmembrane hematopoietic stem cell antigen: Isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 21.Uchida N, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee A, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbeil D, et al. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275:5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 24.Richardson GD, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 25.Salven P, et al. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 26.Yin S, et al. CD133+ hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 27.Singh SK, et al. Identification of human brain tumor initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 28.Shmelkov SV, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, et al. CD133− glioma cells form tumors in nude rats and give rise to CD133+ cells. Int J Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 30.Joo KM, et al. Clinical and biological implications of CD133+ and CD133− cells in glioblastomas. Lab Invest. 2008;88:808–815. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- 31.Chen YC, et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133+ cells. PLoS ONE. 2008;3:e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frank NY, et al. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–4333. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- 33.Sarkadi B, Ozvegy-Laczka C, Nemet K, Varadi A. ABCG2: A transporter for all seasons. FEBS Lett. 2004;567:116–120. doi: 10.1016/j.febslet.2004.03.123. [DOI] [PubMed] [Google Scholar]
- 34.Summer R, et al. Side population cells and Bcrp1 expression in lung. Am J Physiol. 2003;285:L97–L104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 35.Levina V, et al. Drug-selected human lung cancer stem cells: Cytokine network, tumorigenic and metastatic properties. PLoS ONE. 2008;3:e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villa A, Snyder EY, Vescovi A, Martinez-Serrano A. Establishment and properties of a growth factor-dependent, perpetual neural stem cell line from the human CNS. Exp Neurol. 2000;161:67–84. doi: 10.1006/exnr.1999.7237. [DOI] [PubMed] [Google Scholar]
- 37.Lanzkron SM, Collector MI, Sharkis SJ. Hematopoietic stem cell tracking in vivo: A comparison of short-term and long-term repopulating cells. Blood. 1999;93:1916–1921. [PubMed] [Google Scholar]
- 38.Hermann PC, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Pratesi G, et al. Effects of 5-FU and cis-DDP combination on human colorectal tumor xenografts. Tumori. 1989;75:60–65. doi: 10.1177/030089168907500116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.