Abstract
The actions of corticotropin-releasing factor (CRF) and related peptides are mediated by two receptors (CRF1 and CRF2). The respective role of each subtype in the control of food intake remains poorly known. In the present study, we examined the quantity and microstructure of ingestive behavior of knockout (KO) mice lacking CRF2 receptors and their wild-type (WT) littermates. Under basal conditions, CRF2 KO mice showed increased nocturnal food intake, evident as an increased zenith in circadian cosinor analysis of food intake. Microstructure analysis revealed that this greater food intake reflected increased meal size, rather than meal frequency, suggesting a decreased satiating value of food. Following acute restraint stress, CRF2 KO mice showed an intact immediate anorectic response with increased latency to eat and decreased meal size. However, CRF2 deletion abolished the prolonged phase of restraint-induced anorexia. CRF2 KO mice did not differ from WT controls in feeding responses to food deprivation or injection of ghrelin receptor agonists. Independent of genotype, food deprivation increased food intake, with dramatic changes in meal size, meal frequency, water : food ratio and eating rate. Acyl-ghrelin or BIM-28131, a potent ghrelin analog, dose-dependently stimulated food intake by increasing meal size (ghrelin, BIM-28131) and meal number (BIM-28131), while slowing the average eating rate (BIM-28131) similarly in WT and KO mice. These results suggest that the CRF2 receptor is involved in the control of meal size during the active phase of eating and following acute exposure to stress.
Keywords: eating behavior, food deprivation, ghrelin, knockout mice, stress-induced anorexia
Introduction
Considerable evidence suggests a role for the corticotropin-releasing factor (CRF) system in the control of food intake (Richard et al., 2002; Zorrilla et al., 2003). Central and peripheral administration of CRF and related peptides, urocortin (Ucn) 1, Ucn2 and Ucn3, reduce food intake (Spina et al., 1996; Hsu & Hsueh, 2001; Inoue et al., 2003; Zorrilla et al., 2004). Actions of CRF peptides are mediated by two G-protein-coupled receptors (CRF1 and CRF2), which have distinct anatomical distributions and affinities for CRF peptides, and serve separate functions (Bale & Vale, 2004). As the CRF system may be involved in stress-associated disorders (Koob & Heinrichs, 1999; Connan et al., 2006), and CRF receptors are current drug targets (Doyon et al., 2004; Zorrilla & Koob, 2004), defining the respective roles of CRF1 vs. CRF2 in the control of food intake remains a key question. Putative CRF2 antagonists (Cullen et al., 2001), but not CRF1 antagonists (Pelleymounter et al., 2000; Sekino et al., 2004), block the anorectic effects of CRF administration. Neither genetic deletion nor functional antagonism of the CRF1 receptor pathway eliminates the anorectic effects of CRF and Ucn1 (Bradbury et al., 2000; Contarino et al., 2000; Pelleymounter et al., 2000). Such studies suggest that the anorectic actions of CRF and Ucn1 are preferentially mediated by the CRF2. However, only few studies using highly selective CRF2 ligands have been published (Rivier et al., 2002; Zorrilla et al., 2003; Sekino et al., 2004; Jochman et al., 2005) and the relevance of pharmacological administration of CRF peptides to assess the physiological regulation of feeding behavior in response to stress is questionable.
Recent findings suggest interplay between the CRF and ghrelin systems. Ghrelin expression is present in afferents to CRF-expressing neurons (Cowley et al., 2003) and ghrelin injection increases hypothalamic CRF mRNA in rodents (Asakawa et al., 2001; Johnstone et al., 2005). Functional interactions between these systems in the control of gastrointestinal motility and anxiety-like responses to stressors have also been demonstrated (Asakawa et al., 2001, 2005; Chen et al., 2005). However, to our knowledge, no study has yet examined the interaction between the CRF and ghrelin systems in the control of food intake.
The availability of mice with genetic deletion of CRF receptors allowed the observation of distinct roles for these receptors (Contarino & Gold, 2002; Bale & Vale, 2004). However, little information on the feeding behavior of CRF2 knockout (KO) mice is available (Bale et al., 2000, 2003; Coste et al., 2000). Elsewhere, the assessment of meal patterning is fundamental to understanding the mechanisms that regulate ingestive behavior (Geary, 2005). In order to provide a detailed analysis of the specific role of the CRF2 in the control of feeding behavior, we studied CRF2 KO mice using an original microstructural assessment of prandial food and water intake. We describe herein the impact of CRF2 receptor deficiency upon the quantity and organization of spontaneous food intake, as well as the feeding-related effects of several challenges, including restraint stress, food deprivation and administration of ghrelin receptor agonists.
Materials and methods
Animals
The CRF2 KO mice were generated as previously described (Bale et al., 2000). The genetic background of the mice was C57BL / 6J×129Sv-Ter. The breeding of mice was continued and maintained via heterozygote × heterozygote non-sibling matings. All studies were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and the European Communities Council Directive of 24 November 1986 (86 / 609 / EEC), and were approved by the Animal Care and Use Committee (of the University of Bordeaux 1 and 2). Twenty-four mature (5–6 months of age at the start of the study) male mice were used throughout all of the experiments. Wild-type (WT) littermate mice were used as controls.
Experimental procedures
Apparatus
For the microstructural analysis of feeding, mice were individually tested in a custom-developed operant nosepoke experimental apparatus (Imetronic, Pessac, France), adapted from the system that we developed in rats (Zorrilla et al., 2005). Briefly, Plexiglas experimental cages (22 × 15.5 × 19.5 cm) were located in ventilated, sound-attenuating enclosures equipped with temperature control (23 °C) and a 12 / 12-h light / dark cycle (lights on from 06:00 to 18:00 h), and connected to a computer for continuous recording of all events. Two nosepoke holes (1 cm in diameter, 8.5 cm apart, 2.5 cm from the grid floor) were situated on the same wall of the cage, each equipped with infrared photobeams monitored by a computer. One nosepoke hole controlled the delivery of food and the other that of water. A precision pellet (20 mg, Formula AI, 65.8% carbohydrates, 10.3% fat, 23.8% protein, 3.2 kcal / g; Research Diets P.J. Noyes Company Inc., Lancaster, NH, USA) delivery occurred when the photobeam of the ‘food’ nosepoke hole was interrupted for at least 500 ms. Pellets were delivered by an automated dispenser situated outside the experimental cage into a food trough situated 3 cm from the nosepoke hole and 2 cm from the grid floor. The food trough was equipped with additional photobeams, which allowed the monitoring of pellet removal. An additional pellet was not delivered until the removal of the previously delivered pellet, thereby allowing resolution of food-directed behavior at the unit of an individual pellet, similar to a classical ‘eatometer’ (Kissileff, 1970) or ‘panel-push’ system (Balagura & Coscina, 1969). The wire grid floor of the cage allowed passage of uneaten food pellets to a sliding drawer, making storage impossible and allowing evaluation of food spillage. Time-course analysis of recorded events showed that nosepoke responses for food were immediately followed by removal of a pellet by both genotypes (mean ± SEM interval between nosepoke and successive interruption of trough photobeams: 1.1 ± 0.2 and 1.6 ± 0.6 s for CRF2 WT and KO, respectively). Similarly, the interruption of photobeams in the ‘water’ nosepoke hole resulted in a water delivery of 44 µL by an external syringe into a drinking reservoir situated 2.5 and 2 cm from the grid floor. A 30-V potential difference existed between the metallic drinking reservoir and the (null) grid floor. Therefore, tongue contacts with the drinking trough were detected as a fall in the potential difference, with each individual lick recorded. Ten consecutive licks were regarded as sufficient to empty the drinking reservoir of a unit of water delivery. Time-course analysis showed that nosepoke responses for water were immediately followed by the consumption of water in both genotypes [mean interval between nosepoke and (onset or offset of) consecutive water consumption: 1.1 ± 0.6 and 0.4 ± 0.1 s for CRF2 WT and KO, respectively]. For simplicity, nosepokes that initiated the delivery of food or water were considered for microstructural analysis.
Testing procedure
Mice were individually housed (23 h / day) in experimental cages. For 1 h/ day beginning at 16:00 h, mice were placed in individual external holding cages with only water available to allow cleaning of experimental cages, evaluation of food / water intake, spillage and weighing of mice. Mice were first allowed to obtain nosepoke-contingent pellets on a continuous, fixed-ratio 1 schedule of reinforcement. In order to better specify nosepokes as being ingestion-directed, the response requirement was then increased to 2 (fixed-ratio 2) with a maximum internosepoke interval of 5 s. Training was considered to be complete when each mouse demonstrated for three consecutive days: (i) a stable body weight [coefficient of variation (CV) < 3%]; (ii) stable food and water intake (CV < 20%); (iii) a poke : distribution ratio, an index of target-directed behavior, > 80% for both food and water; and (iv) a spillage of food pellets < 3%.
Microstructural analysis of food and water intake
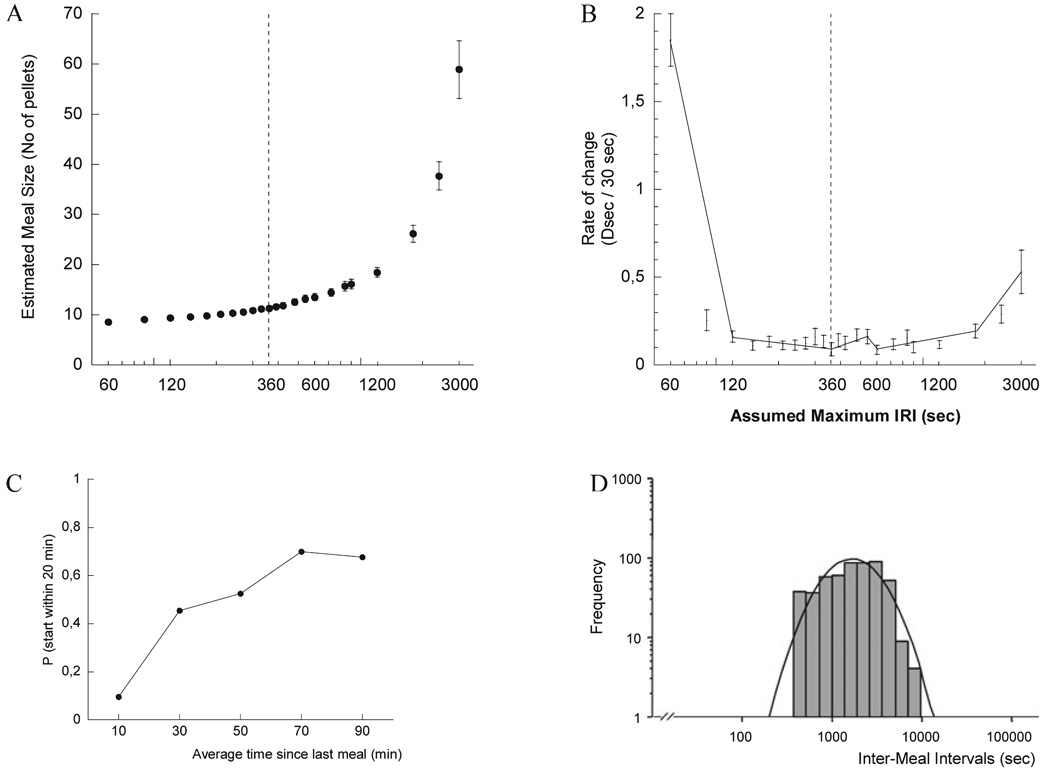
A meal was defined as any burst of responses for food or water that contained at least three food-directed responses. The meal threshold criterion was estimated by determining the inter-response interval between feeding and drinking events that obtained the most stable estimates of meal structure as detailed elsewhere (Zorrilla et al., 2005). Briefly, estimated meal characteristics were calculated for each of a series of maximum inter-response intervals ranging from 30 s to 30 min (Fig. 1A); we calculated local rates of change in the slope as the difference in the value of the zero-order function for consecutive intervals per standardized unit of time (30 s). Two approaches were used to define the minimum inflection point(s) that marked the threshold meal interval in the ‘first-order’ function. First, the minimum local value was identified visually. Second, multivariate adaptive regression splines was applied to the aggregate, first-order individual data (Fig. 1B). A maximum inter-response interval (i.e. threshold meal interval) of 360 s for the dark phase and 720 s for the light phase was determined by each method. The estimated threshold meal interval was used to calculate descriptive statistics of average meal structure. We have validated this method in rats, showing it to provide a better estimate of meal structure than log-survivorship analysis (Zorrilla et al., 2005). The reliability and validity of the meal definition in our mouse model were supported by the following four findings. First, consistent with predictions of satiety, very few meals were initiated shortly after a meal was judged to have terminated, with the average probability of imminent meal initiation subsequently increasing to a maximum likelihood of 50–70% at 50–90 min after the previous meal (Fig. 1C). Second, the aggregate distribution of post-meal intervals resolved to a single log-normal distribution of intermeal intervals (Fig. 1D). Third, a significant pre-prandial correlation (r = 0.353, P < 0.0001, d.f.-weight fixed effect meta-analysis) was observed. Fourth, the majority of food eaten and water drunk occurred within meals (mean ± SEM; food, 97.6 ± 1.4 and 99.0 ± 0.3%; water, 84.1 ± 3.0 and 85.0 ± 3.6% for CRF2 WT and KO mice, respectively).
Fig. 1.
Zero (A) and first-order (B) functions of estimated meal size for mice as a function of the maximum inter-response interval (IRI) between nosepoke responses for food or water considered to continue a meal. The first-order function, which depicts local rates of change in the slope of the zero-order function, has a minimum value with minimum SEM at an IRI of 360 s (IRI-360, dashed line). Multivariate adaptive regression spline analysis (B) provided adequate fit of individual values (F3,608 = 127, P < 1 × 10–14, r2 = 0.386, linear generalized cross-validation = 1.188). The resulting segmented function had five reliable breakpoints in the slope, the most predictive of which was the minimum inflection point determined visually and by linear regression analysis. Error bars reflect SE values for observed results. Data were obtained from three 12-h nocturnal nosepoke sessions of 12 mature wild-type (WT) mice with day as the unit of analysis. (C) Average probability (P) of initiating a meal within the next 20 min as a function of the average time since completion of the previous meal. The ‘instantaneous’ probability of meal initiation was calculated as 100 × (the incremental number of mice that initiated their second meal within the time bin of interest / the number of mice that had not yet initiated a second meal at the onset of the time bin of interest). Data were obtained from three nocturnal nosepoke sessions of 12 WT mature mice. (D) Distribution of inter-meal intervals calculated with IRI-360. Data represent all (n = 524) nocturnal inter-meal intervals from three test sessions of 12 WT mice.
Meal parameters
The parameters included the number of meals; average size, duration and response rate of meals; average intermeal interval; food : water ratio; and satiety ratio. The meal duration was calculated as the total time from the first to last response of a meal, and the duration of eating and drinking within the meal was calculated as the duration of consecutive responses for food or water, respectively. Thus, transitions between eating and drinking were included in the total meal duration but not in the specific durations of eating or drinking. Meal sizes for eating and drinking were calculated separately as the average number of food- or water-directed responses during meals. The rates of eating and drinking were calculated by dividing each meal size by its respective duration. The intermeal interval was defined as the interval from the last feeding response of a meal to the first feeding response of the next meal. For the ghrelin and BIM-28131 experiments, mice that did not eat during the 6 h following injection were assigned a latency to first meal value of 360 min. The food : water ratio, an index of the balance between food and fluid intake, was defined as the ratio between the quantities of food and water consumed per meal. Finally, the satiety ratio, an index of the ‘satiety’ (i.e. non-eating) time produced by each gram of food consumed, was calculated as the average intermeal interval divided by the average meal size for food. The interpretation of microstructural changes in meal organization was performed according to the following definitions: ‘satiation’ refers to processes that promote meal termination thereby limiting meal size, whereas ‘satiety’ refers to post-prandial events that affect the interval to the next meal, thereby regulating meal frequency without affecting meal size (Strubbe & Woods, 2004).
Cosinor analysis
The period of rhythm, midline estimating statistic of the rhythm (rhythm-adjusted mean), amplitude (one-half of the difference between the highest and lowest point of the mathematical model), acrophase (highest point of the fitted model in relation to a phase reference chosen by the investigator), zenith and nadir were calculated with Time Series Analysis Serial Cosinor 6.0 software (Laboratory View, Esvres, France) (Gouthiere et al., 2005). Food (number of pellets) and water (units of water) intakes were calculated for 30-min intervals during four consecutive days for the analysis.
Drugs
Human Ser3-acyl-ghrelin and BIM-28131 were obtained from Biomeasure, Inc. / IPSEN Group (Milford, MA, USA). BIM-28131 is a potent selective, small molecule ghrelin receptor agonist with a 10-fold longer circulating half-life as compared with ghrelin peptide. Radioligand binding studies have shown that BIM-28131 binds potently (Ki, 0.42 ± 0.06 nm) and very selectively to the GHS-1a receptor, with three-fold greater affinity than human ghrelin. Furthermore, BIM-28131 was six-fold more potent than human ghrelin in activating the GHS-1a receptor, as determined by an intracellular calcium mobilization assay (Biomeasure, Inc., personal communication).
Study design
All experiments were conducted in the same mice in the following order.
Experiment 1: Analysis of basal ingestive behavior
After achieving stable food and water intake, the meal microstructure of WT and KO mice was analysed during three consecutive 24-h periods of free feeding and drinking.
Experiment 2: Acute restraint stress
Mice were subjected to two stress conditions (no stress vs. restraint) in counterbalanced order using a within-subject design. Experimental days were separated by four non-treatment days, as per our previous observations (Tabarin et al., unpublished observations). For stress, mice were restrained for 30 min in a clear, vented semi-cylindrical Plexiglas tube fitted with tail slot to prevent unnatural body positions but designed to restrict nearly all movement. Restraint was performed in a procedure room separate from the feeding test room at 16:30 h. Stressed mice returned to the nosepoke apparatus at the end of the stress, 1 h before the dark phase. Data for 21 mice were available for statistical analysis (11 WT, 10 KO).
Experiment 3: Food deprivation
Mice were food deprived for 26 h in home cages with water available beginning from 16:00 h and spanning to 18:00 h of the following day. Mice were returned to the microstructure apparatus at the onset of the dark phase. The day before the deprivation session was used as the control day. Data for 21 mice were available for statistical analysis (11 WT, 10 KO).
Experiment 4: Effects of human Ser3-acyl-ghrelin and a ghrelin receptor agonist (BIM-28131) on ingestive behavior
Within genotype, mice were assigned to receive ghrelin (n = 6) or BIM-28131 (n = 6), according to a full Latin-square design, wherein each mouse was randomly treated with all of the peptide doses used here. Two days elapsed between exposures to the different peptide doses. The peptides were administered at 10:00 h, a time period when food / water intake is low, and ingestive events were monitored throughout the following 6-h period. The solutions of human acetylated ghrelin and BIM-28131 were prepared according to the manufacturer’s instructions, aliquoted, stored at −20 °C and then dissolved in 0.25% bovine serum albumin physiological saline just prior to being administered. Ghrelin (0, 50, 200, 400 nmol / kg) or BIM-28131 (0, 50, 200, 400 nmol / kg) was administered intraperitoneally in a volume of 10 mL / kg of body weight. Thus, ghrelin and BIM-28131 data from a total of 24 mice were available for statistical analysis.
Statistical analysis
For the analysis of cosinor parameters, non-parametric Mann–Whitney U-tests were used. To determine the time-course of the effects of stress or food deprivation on intake, three-way split-plot anovas were performed on the incremental number of nose-poke responses for food and / or water during 1-h time bins, with Experimental Condition and Time as within-subject factors and Genotype as a between-subject factor. Cumulative intake functions were plotted and subsequent analysis was limited to the experimental treatment’s duration of incremental anorexia or hyperphagia. Further analyses included two-way (Genotype and Experimental Condition) mixed design anovas of the number and duration of prandial nosepoke responses as well as measures of meal microstructure. Due to the skewed and inhomogeneous variance for the satiety ratio, water : food ratio, and eating and drinking rates, these measures were subjected to log transformation prior to parametric analysis. The total amount of food (number of pellets) or water (ml) ingested during the 6-h period following vehicle, ghrelin or BIM-28131 injection was examined by a two-way anova, with genotype (WT, CRF2 KO) as a between-subject factor and peptide dose (0, 50, 200, 400 nmol / kg) as a within-subject factor. The same statistical analysis was applied to ingestive behaviors displayed during each 1-h interval following vehicle or peptide injection as well as to the different meal microstructure parameters. Post-hoc individual group comparisons were performed using the Neuwman-Keuls test. The Student’s t-test was used to compare two groups. The results were expressed as mean ± SEM. Statistical significance was set at P < 0.05.
Results
Experiment 1: Analysis of basal ingestive behavior
Genotypes had similar body weights at the onset (30.2 ± 0.6 and 30.6 ± 0.6 g for CRF2 WT and KO mice, respectively) and completion of the training period that lasted for 52 days. At the end of training, both genotypes demonstrated across three consecutive days: (i) stable body weight (CV, 0.7 ± 0.1 and 1.0 ± 0.2% for CRF2 WT and KO mice, respectively); (ii) stable 24-h food intake (CV, 4.6 ± 0.5 and 6.5 ± 1.0%, respectively) and water intake (CV, 7.2 ± 1.1 and 8.3 ± 1.5%, respectively); (iii) a very high nosepoke to consumption percentage, indicating that almost all nosepoke behavior was directed towards food-seeking (97.8 ± 0.1 and 95.9 ± 0.1%, respectively); and (iv) a low spillage of food pellets (0.07 ± 0.04 and 0.11 ± 0.04%, respectively).
The analysis of total 24-h food and water intake during the three baseline days revealed no difference between genotypes (food, 4.1 ± 0.10 and 4.4 ± 0.13 g; water, 4.1 ± 0.1 and 4.6 ± 0.3 mL for CRF2 WT and KO, respectively). However, separate repeated-measure anova analysis for dark and light phases showed increased food intake in KO mice exclusively during the dark phase (main interaction, F1,22 = 4.37, P < 0.05).
Microstructural analysis of eating behavior was performed for the nocturnal and diurnal phases of the second day of basal recording, which showed a total intake representative of the 3-day period. As shown in Table 1, no reliable microstructural differences between genotypes were found when the whole dark and light phases were analysed. In both genotypes, quantitative variations in food and water intake between dark and light phases were mainly due to changes in meal frequency and less to changes in meal size. Thus, increased food and water intake during the dark cycle reflected a mean 44.4 and 57.7% decrease in the intermeal interval of CRF2 WT and KO mice, respectively (F1,20, P < 0.0001) as compared with only an 11.8 and 17.1% increase in meal size in CRF2 WT and KO mice, respectively (F1,20 = 61.72, P < 0.0001). These changes were reflected by a dramatic decrease in the satiety ratio during the dark phase (F1,20 = 36.74, P < 0.0001). The proportion of food and water that was prandial (i.e. consumed within ‘meals’) was also similar between genotypes (food, 97.6 ± 1.4 and 99.0 ± 0.3%; water, 84.1 ± 3.0 and 85.0 ± 3.6%, for CRF2 WT and KO mice, respectively).
TABLE 1.
Prandial food and water intake and meal microstructure during a representative 24-h period of basal ingestive behavior of CRF2 wild-type (WT) and knockout (KO) mice
| Dark phase | Light phase | |||
|---|---|---|---|---|
| WT | KO | WT | KO | |
| Food and water | ||||
| No. of meals*** | 15.9 ± 1.1 | 17.3 ± 1.2 | 10.1 ± 0.6 | 8.4 ± 0.6 |
| Meal size (pellets and units of water / meal)* |
11.4 ± 0.9 | 12.3 ± 0.7 | 10.2 ± 0.7 | 10.5 ± 0.8 |
| Meal duration (min)* | 7.3 ± 0.8 | 7.6 ± 0.7 | 9.6 ± 1.4 | 9.3 ± 0.8 |
| Intermeal interval (min)*** | 37.6 ± 2.5 | 36.5 ± 3.3 | 67.6 ± 5.6 | 86.3 ± 6.8 |
| Water : food ratio* | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Meal rate of intake (pellets and units of water / min)*** |
1.8 ± 0.2 | 1.9 ± 0.1 | 1.5 ± 0.2 | 1.6 ± 0.1 |
| Food | ||||
| Food intake (g) | 2.5 ± 0.1 | 3.0 ± 0.1† | 1.5 ± 0.1 | 1.3 ± 0.1 |
| Meal size (pellets / meal) | 8.1 ± 0.6 | 8.8 ± 0.4 | 7.3 ± 0.5 | 8.1 ± 0.4 |
| Meal duration (min)* | 4.2 ± 0.3 | 4.1 ± 0.2 | 5.1 ± 0.6 | 5.0 ± 0.5 |
| Eating rate (pellets / min)** | 2.1 ± 0.2 | 2.3 ± 0.1 | 1.9 ± 0.2 | 2.0 ± 0.1 |
| Satiety ratio (min / g food eaten)*** |
280 ± 23 | 226 ± 17 | 616 ± 79 | 693 ± 88 |
| Water | ||||
| Water intake (ml) | 1.9 ± 0.2 | 2.3 ± 0.3 | 1.1 ± 0.2 | 0.9 ± 0.2 |
| Meal size (units of water / meal) |
3.3 ± 0.3 | 3.5 ± 0.3 | 2.8 ± 0.3 | 2.6 ± 0.5 |
| Meal duration (min) | 1.7 ± 0.3 | 1.8 ± 0.4 | 1.8 ± 0.5 | 1.8 ± 0.5 |
Values are means ± SEM for selected meal-related parameters. Genotype was a between-subject factor and time (dark vs. light phase) was a within-subject factor.
Significant time effect P < 0.0001
P < 0.01
P < 0.05
Significant main genotype × time interaction (P < 0.05).
CRF, corticotropin-releasing factor.
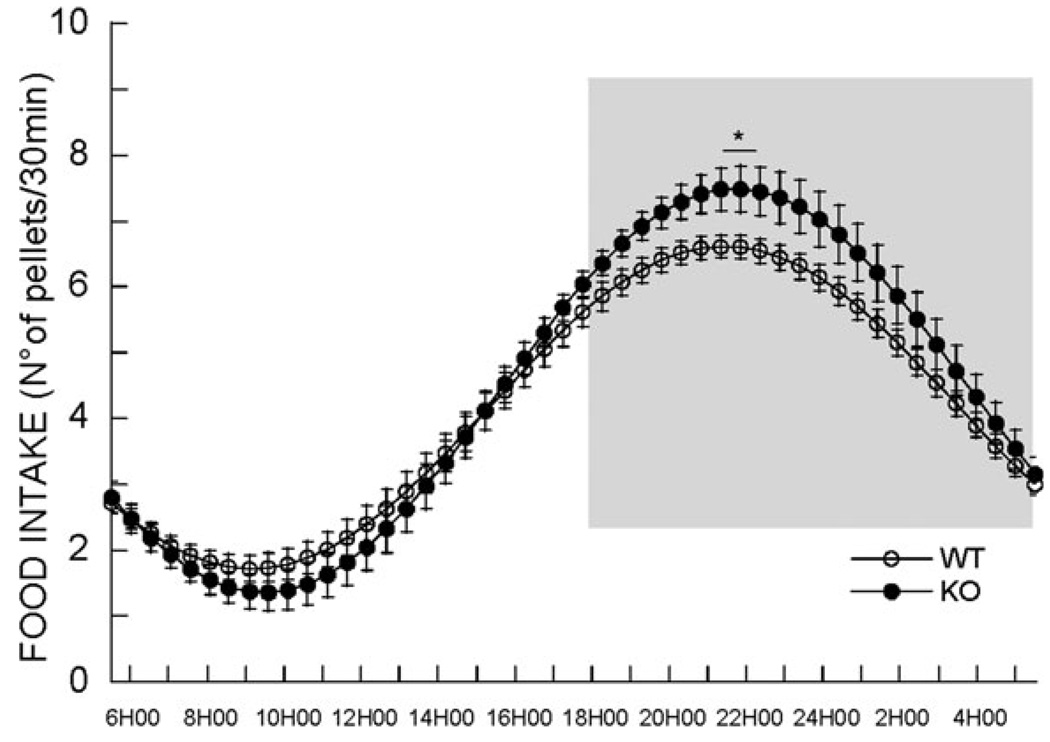
Cosinor analysis was therefore performed in order to assess more precisely the time-related differences in food intake between genotypes (Fig. 2). Cosinor analysis revealed a circadian rhythm of eating that was similar in amplitude, acrophase, midline estimating statistic of the rhythm and nadir between genotypes (Fig. 2). However, consistent with the increase in food intake in KO mice detected by anova during the dark phase, cosinor analysis showed a ~15% increased zenith (peak) in KO mice (6.7 ± 0.2 and 7.7 ± 0.4 pellets / 30 min for CRF2 WT and KO mice, respectively, P < 0.05). Subsequent microstructural analysis was therefore performed during the period of maximal differences in food intake between genotypes highlighted by cosinor analysis (i.e. 1–7 h following the onset of the dark phase) (Fig. 2). During this period, KO mice exhibited an increased meal size for food (7.2 ± 0.5 vs. 9.4 ± 0.5 pellets for CRF2 WT and KO mice, respectively; P < 0.01), with similar meal number (12.1 ± 0.9 vs. 11.9 ± 1.0, respectively) and intermeal intervals (33.4 ± 2.4 vs. 34.2 ± 3.0 min, respectively).
Fig. 2.
Cosinor analysis of the rhythm of intake of 20-mg food pellets in CRF2 wild-type (WT) (n = 11) and knockout (KO) mice (n = 10) (*P < 0.05 in zenith. Gray zone, dark phase of the light cycle). CRF, corticotropin-releasing factor.
Experiment 2: Effect of acute restraint stress on ingestive behavior
Time-course of effects on ingestion
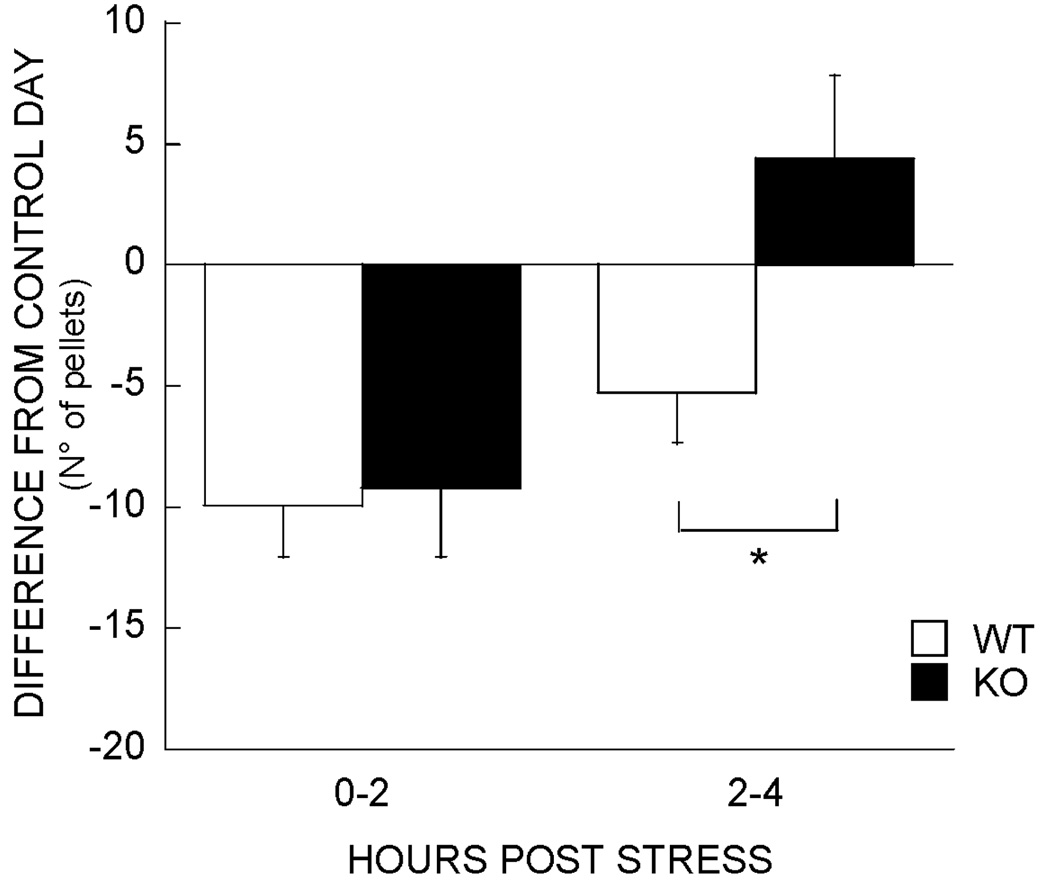
The analysis of cumulative nocturnal food intake following restraint stress revealed a main effect of stress (F1,19 = 72.9, P < 0.0001), time (F12,228 = 691.2, P < 0.0001), no genotype effect (F1,19 = 1.9) but a genotype × stress interaction (F1,19 = 4.9, P < 0.04). A stress × time interaction (F12,228 = 2.4, P < 0.01) revealed that stress-induced reduction in food intake did not increase past the fourth post-stress hour. The analysis of cumulative food intake during the first 4 h following stress revealed that CRF2 receptor KO mice showed less restraint stress-induced anorexia than WT mice (4.8 ± 2.8 vs. 15.2 ± 2.2 vs. pellet reduction in intake vs. non-stressed condition, P < 0.01). As time-course analyses have shown that acute stimulation of brain CRF2 receptors elicits anorexia that is delayed by 2–3 h in onset (Inoue et al., 2003; Fekete et al., 2006), the time-course of differential anorexia between genotypes was examined. The results showed that CRF2 KO mice showed an essentially intact immediate anorexic response to restraint stress (0–2 h, 9.9 ± 2.1 vs. 9.2 ± 2.8 pellet reductions in intake for CRF2 KO vs. WT mice, respectively) but, unlike WT mice, did not continue to show incremental anorexia thereafter [2–4 h, −4.4 ± 3.2 (i.e. refeeding hyperphagia) vs. 5.3 ± 2.1 reduction, respectively, P < 0.02]. Thus, CRF2 KO mice showed an abbreviated anorectic response to restraint stress (Fig. 3).
Fig. 3.
Cumulative food intake during the first 4 h following acute restraint stress; pellet intake following stress vs. non-stressed condition in wild-type (WT) (n = 11) and knockout (KO) mice (n = 10). KO mice show abbreviated anorexia (*P < 0.05, difference between genotypes).
Meal microstructure
As shown in Table 2, the differential anorectic response between genotypes reflected that restraint stress significantly (P < 0.05) reduced meal sizes for food of WT mice by 22%, but not those of CRF2 KO mice (3% reduction), during the 4-h observation period (see Table 2; genotype × stress interaction trend, P = 0.07). Supporting the delayed nature of differential stress-induced effects on feeding, restraint stress produced a genotype-independent increase in the latency to the first meal (F1,22 = 5.80, P < 0.05), reduced the size of the first meal in KO mice (13.7 ± 1.7 to 7.7 ± 1.2 pellets) and did so at least as much as it did in WT mice (10.5 ± 1.9 to 8.2 ± 1.0 pellets; main effect of stress, F1,19 = 12.90, P < 0.05). Apart from the differential actions of stress on average meal size between genotypes, stress produced a genotype-independent acceleration in the rate of eating within meals while shortening their length. Restraint stress did not modify the proportion of food or water consumed as meals (data not shown), the water : food ratio or measures of post-meal satiety, including the intermeal interval or meal frequency (Table 2).
TABLE 2.
Meal microstructure of CRF2 wild-type (WT) and knockout (KO) mice during the first 4 h following 30 min of restraint stress
| Control | Stress | |||
|---|---|---|---|---|
| WT | KO | WT | KO | |
| Food and water | ||||
| No. of meals | 7.9 ± 0.6 | 6.6 ± 0.3 | 7.7 ± 0.5 | 6.8 ± 0.5 |
| Meal size (pellets and units of water / meal)* |
12.5 ± 0.7 | 12.2 ± 1.5 | 10.0 ± 0.6 | 11.3 ± 1.0 |
| Meal duration (min)** | 7.5 ± 0.6 | 7.6 ± 0.9 | 6.5 ± 0.8 | 5.5 ± 0.6 |
| Intermeal interval (min) | 25.5 ± 2.7 | 31.1 ± 4.7 | 25.4 ± 2.7 | 32.2 ± 3.0 |
| Water : food ratio | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 |
| Meal rate of intake (pellets and units of water / min) |
1.9 ± 0.2 | 2.0 ± 0.1 | 2.0 ± 0.2 | 2.3 ± 0.1 |
| Food | ||||
| Food intake (g)***† | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 |
| Meal size (pellets / meal)* | 8.5 ± 0.5 | 8.6 ± 1.0 | 6.6 ± 0.4 | 8.3 ± 0.7 |
| Meal duration (min)** | 4.8 ± 0.6 | 5.0 ± 0.5 | 4.0 ± 0.5 | 3.6 ± 0.3 |
| Eating rate (pellets / min)* | 2.1 ± 0.2 | 2.1 ± 0.1 | 2.1 ± 0.2 | 2.4 ± 0.1 |
| Satiety ratio (min / g food eaten)** |
195 ± 32 | 178 ± 11 | 233 ± 24 | 251 ± 28 |
| Water | ||||
| Water intake (ml)*‡ | 1.4 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 |
| Meal size (units of water / meal)* |
4.1 ± 0.4 | 3.6 ± 0.5 | 3.4 ± 0.3 | 3.0 ± 0.5 |
| Meal duration (min) | 1.1 ± 0.2 | 1.1 ± 0.3 | 1.0 ± 0.3 | 0.8 ± 0.2 |
| Drinking rate (units of water / min) |
4.7 ± 0.6 | 5.1 ± 0.6 | 5.2 ± 0.6 | 5.8 ± 0.7 |
Genotype was a between-subject factor and stress was a within-subject factor.
P < 0.05
P < 0.01
P < 0.001 indicate genotype-independent stress effects.
Genotype difference (P < 0.05).
Genotype × stress interaction (P < 0.05). Statistical analysis also revealed a genotype × stress interaction trend (P = 0.07) on the meal size parameter for food intake only.
CRF, corticotropin-releasing factor.
Experiment 3: effect of a 26-h food deprivation on ingestive behavior
Food deprivation decreased body weight similarly in both genotypes (−11.9 ± 0.5 and −12.6 ± 0.5% for CRF2 WT and KO mice, respectively, P < 0.0001).
Time-course of effects on ingestion
The analysis of cumulative nocturnal food intake during the refeeding period revealed a main effect of food deprivation (F1,19 = 141.3, P < 0.0001) with no genotype (F1,19 = 1.42) or genotype × deprivation interaction effects (F1,19 = 0.33), indicating that CRF2 KO mice showed normal food deprivation-induced orexigenic responses. Time-course analysis revealed that food-deprived mice showed compensatory hyperphagia throughout the 12-h refeeding period. Therefore, microstructural analysis was performed on the whole dark-phase period.
Meal microstructure (Table 3)
TABLE 3.
Meal structure of CRF2 wild-type (WT) and knockout (KO) mice during 12 h following 28 h of food deprivation
| Control | Refeeding | |||
|---|---|---|---|---|
| WT | KO | WT | KO | |
| Food and water | ||||
| No. of meals | 16.7 ± 0.6 | 17.4 ± 1.3 | 17.2 ± 1.8 | 17.3 ± 1.3 |
| Meal size (pellets and units of water / meal)*** |
11.6 ± 0.9 | 11.1 ± 0.5 | 18.4 ± 1.5 | 18.6 ± 1.6 |
| Meal duration (min)*** | 7.3 ± 0.6 | 6.7 ± 0.5 | 14.7 ± 1.7 | 14.6 ± 1.5 |
| Intermeal interval (min)* | 35.9 ± 1.4 | 36.0 ± 3.4 | 32.9 ± 4.6 | 28.4 ± 2.0 |
| Water : food ratio** | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 |
| Meal rate of intake (pellets and units of water / min)*** |
1.8 ± 0.1 | 2.0 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 |
| Food | ||||
| Food intake (g)*** | 2.7 ± 0.1 | 2.7 ± 0.2 | 3.7 ± 0.1 | 3.9 ± 0.1 |
| Meal size (pellets / meal)*** | 8.1 ± 0.5 | 8.0 ± 0.4 | 11.4 ± 1.1 | 12.0 ± 1.0 |
| Meal duration (min)*** | 4.1 ± 0.3 | 3.6 ± 0.2 | 8.5 ± 1.2 | 8.0 ± 1.1 |
| Eating rate (pellets / min)*** | 2.2 ± 0.2 | 2.5 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.2 |
| Satiety ratio (min / g food eaten)* |
247 ± 10 | 252 ± 22 | 202 ± 14 | 188 ± 12 |
| Water | ||||
| Water intake (ml)*** | 2.5 ± 0.3 | 2.3 ± 0.3 | 4.5 ± 0.3 | 4.7 ± 0.3 |
| Meal size (units of water / meal)*** |
3.5 ± 0.4 | 3.0 ± 0.2 | 6.4 ± 0.5 | 6.5 ± 0.7 |
| Meal duration (min)*** | 1.8 ± 0.4 | 1.7 ± 0.3 | 2.9 ± 0.4 | 2.9 ± 0.3 |
| Drinking rate (units of water / min) |
3.1 ± 0.4 | 2.6 ± 0.3 | 3.0 ± 0.3 | 2.8 ± 0.3 |
Genotype was a between-subject factor and experiment was a within-subject factor. No difference between genotypes was found.
Significant effect of previous food deprivation P < 0.001
P < 0.01
P < 0.05
CRF, corticotropin-releasing factor.
Food deprivation decreased the log-transformed latency to initiate the first meal similarly in both genotypes (13.5 ± 4.6 to 6.7 ± 0.8 min for WT; 20.2 ± 5.0 to 7.7 ± 0.8 min for KO) (experience effect, F1,19 = 9.6, P < 0.01). CRF2 WT and KO mice also showed dramatic, but similar, increases in the size of the first meal compared with the control day (from 8.7 ± 1.4 to 42.8 ± 4.9 pellets for WT mice, from 9.3 ± 1.2 to 40.2 ± 5.8 pellets for KO mice) (experience effect, F1,19 = 80.4, P < 0.001; genotype effect, F1,19 = 0.06, ns; genotype × experience interaction, F1,19 = 0.2, ns). As shown in Table 3, no difference in meal microstructure was found between genotypes. Exposure to food deprivation did not modify the proportion of food or water consumed as meals (data not shown). Both CRF2 WT and KO mice ate more slowly on average within meals such that their meals were not only larger but also longer. Despite the increased meal size, the duration of intermeal intervals was reduced, suggesting that food deprivation altered both satiation (within-meal) and satiety (between-meal) processes. Not only the quantity but also the proportion of drinking that was prandial was significantly increased after food deprivation (from 82 ± 6 to 93 ± 1% and from 78 ± 4 to 88 ± 3% for CRF2 WT and KO mice, respectively, P < 0.005), with the water : - food ratio increasing significantly in both genotypes (from 0.44 ± 0.04 to 0.61 ± 0.03% and from 0.42 ± 0.05 to 0.51 ± 0.03% for CRF2 WT and KO mice, respectively, F1,21 = 17.4, P < 0.01).
Experiment 4: effects of ghrelin and BIM-28131 on food and water intake
Ghrelin
Food and water intake
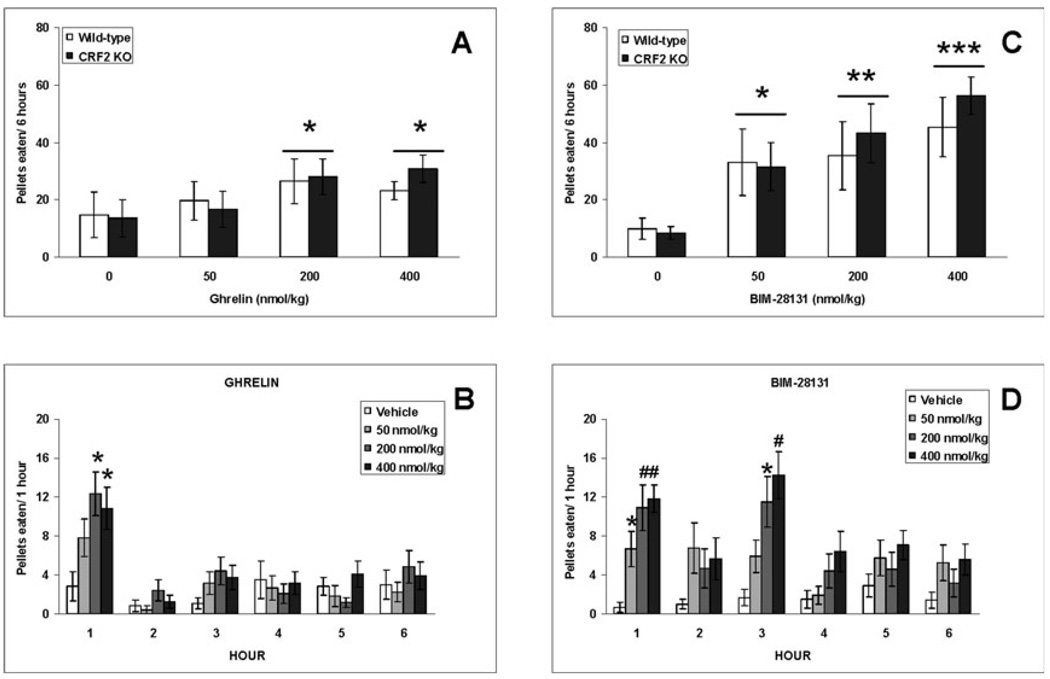
The analysis of pellets eaten during the 6-h time period following vehicle or ghrelin injection revealed a peptide dose effect (F3,30 = 5.03, P < 0.01) but neither a genotype (F1,10 = 0.05) nor a genotype × dose interaction effect (F3,30 = 0.48). Post-hoc analyses revealed that both the 200 and 400 nmol / kg doses of ghrelin increased food intake (P < 0.05 vs. vehicle; Fig. 4A). Similarly, the analysis of water intake revealed a peptide dose effect (F3,30 = 2.96, P < 0.05) but neither a genotype (F1,10 = 0.08) nor a genotype × peptide dose interaction effect (F3,30 = 1.66). Only the 200 nmol / kg ghrelin dose increased water intake (P < 0.05 vs. vehicle; data not shown). The hour-by-hour analysis of food intake revealed that the orexigenic effects of ghrelin lasted for about 1 h (peptide dose effect: F3,30 =4.03, P < 0.05; P < 0.05, 200 and 400 nmol / kg vs. vehicle; Fig. 4B), with mice taking at most one meal during this time period. Thus, further meal microstructure analyses were performed on the first meal following ghrelin injection.
Fig. 4.
Total 6-h intake of 20-mg food pellets in CRF2 wild-type (WT) and knockout (KO) mice treated intraperitoneally with different doses of human acyl-ghrelin (A) or BIM-28131 (C). Hour-by-hour effect of ghrelin (B) and BIM-28131 (D) on food intake. As there was no genotype difference, columns in B and D illustrate food intake of both CRF2 WT and KO mice. Values are means ± SEM. *P < 0.05, **P < 0.01, #P < 0.005, ***P < 0.001 vs. vehicle treatment. CRF, corticotropin-releasing factor.
Meal microstructure
As shown in Table 4, ghrelin reduced the log-transform latency to eat in both genotypes (F3,30 = 3.21, P < 0.05). Both the 200 and 400 nmol / kg doses of ghrelin reduced the latency to eat (P < 0.05 vs. vehicle). Ghrelin also increased the size of the first meal similarly in both genotypes (F3,30 = 4.50, P < 0.05). Both the 200 and 400 nmol / kg ghrelin doses increased the first meal size (P < 0.05 vs. vehicle). Ghrelin also similarly increased the first meal duration in both genotypes (F3,30 = 4.04, P < 0.05). Both the 200 and 400 nmol / kg ghrelin doses increased the first meal duration (P < 0.05 vs. vehicle). However, ghrelin did not affect meal rate (F3,30 = 2.76).
TABLE 4.
Effect of ghrelin and BIM-28131 on meal microstructure parameters displayed by CRF2 wild-type (WT) and knockout (KO) mice
| Vehicle | 50 nmol / kg | 200 nmol / kg | 400 nmol / kg | |||||
|---|---|---|---|---|---|---|---|---|
| WT | KO | WT | KO | WT | KO | WT | KO | |
| Ghrelin, First meal | ||||||||
| Latency (min) (log10)† | 2.1 ± 0.2 | 2.0 ± 0.3 | 1.6 ± 0.2 | 1.6 ± 0.3 | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.3 | 1.3 ± 0.1 |
| Meal size (pellets / meal)† | 4.3 ± 1.8 | 3.3 ± 1.4 | 8.7 ± 2.3 | 7.8 ± 3.1 | 10.7 ± 3.1 | 14.0 ± 3.5 | 11.2 ± 2.0 | 13.2 ± 3.1 |
| Meal duration (food) (min)† | 2.9 ± 1.3 | 2.4 ± 1.5 | 10.2 ± 3.6 | 8.1 ± 4.0 | 14.6 ± 4.5 | 9.7 ± 2.9 | 16.0 ± 4.4 | 12.2 ± 3.1 |
| Meal rate of intake (pellets / min) | 1.6 ± 0.2 | 2.6 ± 0.7 | 1.3 ± 0.3 | 1.6 ± 0.4 | 0.8 ± 0.1 | 1.7 ± 0.2 | 1.2 ± 0.4 | 1.3 ± 0.3 |
| BIM-28131, First 3 h | ||||||||
| Latency (min) (log10)‡ | 2.1 ± 0.1 | 2.2 ± 0.2 | 1.7 ± 0.3 | 1.8 ± 0.2 | 1.5 ± 0.4 | 1.6 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.1 |
| No. of meals* | 0.7 ± 0.3 | 0.7 ± 0.3 | 1.5 ± 0.5 | 2.0 ± 0.7 | 1.8 ± 0.7 | 2.2 ± 0.7 | 2.2 ± 0.5 | 3.0 ± 0.6 |
| Meal size (pellets / meal)* | 2.7 ± 0.9 | 1.8 ± 0.8 | 9.4 ± 3.0 | 7.8 ± 1.9 | 10.2 ± 3.6 | 12.3 ± 2.9 | 10.6 ± 2.3 | 13.1 ± 1.2 |
| Meal duration (food) (min)* | 2.6 ± 1.7 | 2.4 ± 1.1 | 23.4 ± 7.6 | 16.6 ± 4.4 | 32.8 ± 10.6 | 22.0 ± 6.3 | 34.2 ± 10.1 | 32.5 ± 4.9 |
| Meal rate of intake (pellets / min)** | 1.4 ± 0.2 | 1.4 ± 0.4 | 0.4 ± 0.02 | 0.5 ± 0.1 | 0.3 ± 0.03 | 0.6 ± 0.1 | 0.4 ± 0.05 | 0.4 ± 0.05 |
Genotypes did not differ in any of the meal microstructure parameters examined.
P < 0.05, 200 and 400 nmol / kg ghrelin vs. vehicle
P < 0.05, 400 nmol / kg of BIM-28131 vs. vehicle
P < 0.05, 50, 200 and 400 nmol / kg of BIM-28131 vs. vehicle
P < 0.0005, 50, 200 and 400 nmol / kg of BIM-28131 vs. vehicle. n = 6 / genotype and treatment.
CRF, corticotropin-releasing factor.
BIM-28131
Food and water intake
The analysis of pellets eaten during the 6-h time period following vehicle or BIM-28131 injection revealed a peptide dose effect (F3,30 = 7.38, P < 0.001) but neither a genotype (F1,10 = 0.53) nor a genotype × peptide dose interaction effect (F3,30 = 0.28). Post-hoc analyses revealed that all of the BIM-28131 doses used here increased food intake (P < 0.05 vs. vehicle; Fig. 4C). The analysis of water ingested during the same time period revealed a peptide dose effect (F3,30 = 6.51, P < 0.005) but neither a genotype (F1,10 = 1.44) nor a genotype × peptide dose interaction effect (F3,30 = 0.36). Only the 200 and 400 nmol / kg doses of BIM-28131 increased water intake (P < 0.05 vs. vehicle; data not shown). The hour-by-hour analysis revealed that BIM-28131 increased food intake during the first (peptide dose effect: F3,30 = 7.66, P < 0.001; P < 0.05, all BIM-28131 doses vs. vehicle treatment) and third (peptide dose effect: F3,30 = 6.31, P < 0.005; P < 0.05, 200 and 400 nmol / kg vs. vehicle) hours following peptide dosing (Fig. 4D). Thus, further meal microstructure analyses were performed on the first 3-h time period following vehicle or BIM-28131 treatment.
Meal microstructure
As shown in Table 4, BIM-28131 similarly reduced the latency to eat in both genotypes (F3,30 = 4.02, P < 0.05). Only the 400 nmol / kg dose of BIM-28131 reduced the latency to eat (P < 0.05 vs. vehicle). BIM-28131 also similarly increased the number (F3,30 = 4.80, P < 0.01), size (F3,30 = 6.27, P < 0.005) and duration (F3,30 = 6.52, P < 0.005) of meals consumed during the first 3-h time period following peptide dosing. Also, BIM-28131 similarly slowed meal rate in both genotypes (F3,30 = 13.84, P < 0.0001). All of the BIM-28131 doses used here modified the number (P < 0.05), size (P < 0.05), duration (P < 0.05) and rate (P < 0.0005) of the meals consumed during the first 3-h time period following peptide dosing, as compared with vehicle treatment.
Discussion
The present experiments indicate that the CRF2 receptor limits the spontaneous peak of nocturnal feeding and mediates stress-induced anorexia in mice through the control of meal size. Previous studies had suggested an involvement of CRF / Ucn systems in the regulation of energy balance (for review see Heinrichs & Richard, 1999; Richard et al., 2002; Zorrilla et al., 2003), with dysfunctions of CRF / Ucn systems hypothesized to participate in the pathophysiology of obesity and eating disorders (Doyon et al., 2004; Connan et al., 2006). In this context, the uncertain specific roles of CRF1 vs. CRF2 pathways in the control of food intake are an important issue, especially given that CRF receptors are potential therapeutic drug targets (Doyon et al., 2004; Zorrilla & Koob, 2004). For this reason, we analysed the microstructure of food intake in mutant mice lacking the CRF2 receptor under various conditions. Meal pattern analysis is critical for understanding the mechanisms that control eating behavior (Geary, 2005). However, few previous studies have studied the impact of CRF receptor ligands on rat meal structure (Kochavi et al., 2001; Inoue et al., 2003; Fekete et al., 2006) and, to our knowledge, no meal pattern study of mice genetically engineered for CRF / Ucn system molecules has been reported. To analyse the microstructure of meal patterns in mice, we used an original approach, recently validated in rats, that integrates prandial drinking into the definition of a meal (Zorrilla et al., 2005). The meal definition, extended to our mouse paradigm, satisfied the following criteria for valid meal microstructure analysis: (i) consistent with predictions of satiety, very few meals were initiated shortly after a meal was judged to have terminated; (ii) the aggregate distribution of post-meal intervals resolved to one lognormal distribution; (iii) a significant pre-prandial correlation was observed, indicating that the time for which a mouse had not eaten predicted how much it would subsequently eat; and (iv) the overwhelming majority of food eaten and most water drunk occurred within meals.
Under basal conditions, total daily food and water intakes in CRF2 KO mice were quantitatively similar to those of WT littermates, consistent with previous studies using classical methods to measure regular food intake in CRF2 KO mice (Bale et al., 2000, 2003; Coste et al., 2000). However, our experimental approach of continuous recording of feeding events in undisturbed animals enabled us to observe that genetic deletion of CRF2 receptors increased food intake during the dark phase of the light / dark housing cycle. Specifically, cosinor analysis revealed an increased zenith of food intake in CRF2 KO mice. Microstructural analyses indicated that this orexigenic effect was due to increased meal size, a finding that mirrors our previous observation that intracerebroventricular infusion of Ucn2, a selective CRF2 agonist, made rats eat smaller meals (Inoue et al., 2003). The present results indicate that the CRF2 pathway endogenously reinforces the satiating value of food at the circadian time of greatest spontaneous intake. This suggests that the CRF2 pathway might be involved in the processing of gut-derived satiation signals or might potentiate their action (Woods, 2004). The specific site of action of the CRF2 on the control of food intake under baseline conditions remains speculative. The CRF2 is largely expressed in several hypothalamic and brainstem nuclei involved in the control of food intake, such as the ventromedial nucleus of the hypothalamus, nucleus of the tractus solitarius and area postrema (Van Pett et al., 2000). Among these, the nucleus of the tractus solitarius is the principal central site receiving inputs from short-acting satiation signals that are transmitted neurally from the gut and influence meal size (Woods, 2004). Altogether these data suggest the involvement of nucleus of the tractus solitarius / CRF circuitry in CRF2-modulated feeding. Interestingly, male Ucn2-deficient mice do not exhibit increased nocturnal food intake (Chen et al., 2006), suggesting that a different ligand may endogenously activate the satiating actions of CRF2. Elsewhere, male CRF2 KO mice had qualitatively and quantitatively similar drinking behavior to WT mice, as has been found in male Ucn2-deficient mice (Chen et al., 2006).
One may also note the apparent discrepancy between transiently increased food intake in CRF2 KO mice and similar body weight to WT mice. These findings may be interpreted as a behavioral adaptation in CRF2 KO mice to compensate for energy expenditure due to their constitutional increase in basal interscapular brown adipose tissue thermogenesis (Bale et al., 2003; Carlin et al., 2006).
The typical effect of acute stress in diverse species is to reduce food intake. The CRF / Ucn system is a candidate mediator of stress-induced anorexia (Koob & Heinrichs, 1999; Zorrilla et al., 2003) as intracerebroventricular administration of α-helical CRF9–41, a non-selective competitive CRF receptor antagonist, significantly attenuates anorexia resulting from stressors, such as restraint (Krahn et al., 1986; Shibasaki et al., 1988; Smagin et al., 1999) or indirect exposure to stressed conspecifics (Krahn et al., 1986). However, which endogenous ligand / receptor combination(s) within the CRF / Ucn system mediates stress-induced anorexia remains under debate. Pelleymounter et al. (2000) showed that CRF-induced anorexia in mice was blocked by the putatively selective CRF2 antagonist antisauvagine-30 but not by the CRF1 antagonist NBI27914. These data, together with our observation that intracerebroventricular CRF administration decreased food intake similarly in WT and CRF1 KO mice (Contarino et al., 2000), supported the hypothesis that the CRF2 pathway might be predominantly implicated in acute stress-induced anorexia (Spina et al., 1996). Only a few studies using putatively selective CRF2 antagonists in animal models of stress-induced anorexia have been published. intracerebroventricular antisauvagine-30 partly reversed restraint-, footshock- or emotional stress-induced anorexia in one study (Sekino et al., 2004), whereas the CRF2 antagonist astressin2-B injected in the basolateral amygdala of rats did not reverse emotional stress-induced anorexia in another study (Jochman et al., 2005). It should be pointed out that the in-vivo selectivity of CRF2 antagonists at tested doses remains uncertain and evidence suggests that relatively high doses of the CRF2 antagonist antisauvagine-30 produce some CRF1 blockade (Sekino et al., 2004; Jochman et al., 2005). This is of importance as administration of selective CRF1 antagonists reversed short-term anorexia induced by acute restraint (Sekino et al., 2004) or emotional stress (Hotta et al., 1999; Jochman et al., 2005). It should also be noted that most studies indicating a role for CRF2 receptors in mediating stress-induced anorexia were conducted in food-deprived rodents and are likely to reflect the impact of these peptides on the combined effects of food deprivation and stress (Pelleymounter et al., 2000; Contarino & Gold, 2002; Sekino et al., 2004). The use of non-deprived mutant mice lacking the CRF2 in the present study might be of help in circumventing these limitations of pharmacological studies.
In our study, restraint suppressed the incremental food intake of non-deprived WT mice for 4 h and increased the latency to eat. In addition, stressed mice ate more rapidly within meals but ate and drank smaller meals with normal post-meal intervals. Thus, in WT mice, restraint stress had satiation-like effects on meal structure but did not seem to influence post-meal satiety processes. Such findings have not, to our knowledge, been previously described in mice but have been observed in other species (Whishaw et al., 1992; Krebs et al., 1996; Varma et al., 1999). Our current findings in mutant mice indicate that the CRF2 receptor is involved in restraint stress-induced anorexia. Importantly, the CRF2 pathway does not appear to mediate the early anorectic response to restraint stress but is involved in the prolonged phase with CRF2 KO mice showing an abbreviated reduction in meal size. In accordance with our findings, only the late-phase anorectic effects of Ucn1 are dampened by genetic inactivation of the CRF2 (Coste et al., 2000). This ‘delayed’ time-course impact of CRF2 deletion also accords with the results of our studies obtained in rats after intracranial injections of Ucn2 and Ucn3 (Inoue et al., 2003; Fekete et al., 2006). As the expression of Ucns is up-regulated during stress (Tache & Bonaz, 2007), these results suggest that the CRF2 pathway is involved in the late-phase suppression of food intake induced by endogenous release of CRF or Ucns in response to stress.
Convergent experimental reports have demonstrated that central injection of CRF peptides reproduces stress-related alterations of gut motor function in rodents, whereas CRF antagonists prevent the gastrointestinal consequences of various stressors (review in Tache & Bonaz, 2007). In the light of our findings, it should be emphasized that central injection of Ucn2 delays gastric emptying through the modulation of sympathetic nervous system activity (Czimmer et al., 2006) and that the inhibition of gastric emptying induced by restraint stress in rats is prevented by central injection of the selective CRF2 antagonist astressin-2B (Nakade et al., 2005). It is therefore tempting to speculate that the prolonged phase of restraint stress-induced reduction in meal size is mediated, at least in part, by the activation of central CRF2 pathways that modulate gastric emptying. Elsewhere, peripheral CRF2 activation by selective ligands has also been shown to delay gastric emptying and peripheral administration of CRF receptor antagonists counteracts the impact of stress on gut motility (Tache & Bonaz, 2007). However, whether the reduction in stress-induced satiation that we observed in the present study is predominantly related to brain or gut CRF2 deletion remains to be determined. To date, the only published example of a genetic deficit that disrupts stress-induced anorexia is the 5-HT4 KO mice (Compan et al., 2004). Given the reciprocal influences between the CRF2 and serotoninergic neurons (Hammack et al., 2003; Pernar et al., 2004; Staub et al., 2006), it might be hypothesized that these two systems participate in a neurochemical cascade that subserves the effect of stress on feeding behavior. Further studies using acute or chronic stress paradigms in single (CRF1, CRF2) or dual (CRF1 / CRF2) receptor KOs and mice lacking selective CRF2 ligands (Ucn2, Ucn3) are also needed to assess the respective involvement of the CRF1 and CRF2 systems in the mediation of stress-induced anorexia.
After food deprivation, a mixed metabolic and psychological stress, microstructural analysis in WT mice revealed a dramatic decrease in the latency to eat, a very large increase in the size and duration of meals (especially the first meal taken), and a smaller decrease in post-meal intervals. In accordance with our observations in rats (Zorrilla et al., 2005), food-deprived mice ate meals at a slower rate. This phenomenon has been interpreted as a reduced efficacy of satiation (Guss & Kissileff, 2000) or an adaptive response to minimize satiation signals (Spiegel, 2000). Increased food intake was associated with greater prandial drinking, as has been noted in rats (Zorrilla et al., 2005), culminating in an increased water : food ratio. Food deprivation similarly decreased body weight and altered feeding microstructure in WT and CRF2 KO, indicating that CRF2 receptor deficiency did not alter food deprivation-induced energy conservation or orexigenic responses. The results are consistent with those of Coste et al. (2000), which showed that vehicle-treated CRF2 KO and WT mice displayed similar 10-h refeeding following a 16-h deprivation period. Similarly, intracerebroventricular administration of the CRF2 antagonist antisauvagine-30 did not alter refeeding responses to 24-h food deprivation in rats (Sekino et al., 2004). In contrast, Bale et al. (2000) found that CRF2 KO mice consumed only 75% of WT food levels in the 24 h following a 24-h food deprivation. Methodological differences between studies might account for these discrepancies. In particular, the current experimental paradigm allows continuous recording of feeding and drinking in undisturbed animals. As CRF2 KO mice show increased anxiogenic-like behavior and hypersensitivity to stress (Bale et al., 2000; Coste et al., 2000; Kishimoto et al., 2000), it can be hypothesized that repeated weighing of food in previous studies may have differentially stressed mutant mice thereby attenuating their refeeding response. The mice used in our study were older than those studied in the work of Bale et al. (2000) and were individually housed, two conditions that may cause substantial differences in the impact of CRF2 deletion on the homeostatic response to acute food deprivation
From a metabolic perspective, food deprivation decreases the activity of cerebral catabolic pathways, a physiological neuroadaptation to negative energy balance (Schwartz et al., 1995). Perhaps accordingly, ventro medial hypothalamus CRF2 mRNA expression is down-regulated in starved rats (Makino et al., 1998), as well as CRF mRNA in the PVN and central amygdala (Schwartz et al., 1995; Hwang & Guntz, 1997; Timofeeva et al., 2002). Thus, the lack of difference in eating behavior between WT and CRF2 KO mice following food deprivation may reflect the decreased functional significance of CRF2 pathways in fasted WT mice. Further studies concerning the expression of CRF2 ligands and CRF2 signaling in feeding relevant areas of the central nervous system during fasts may help to elucidate this possibility.
Understanding the relationships between CRF2 signaling and known regulators of food intake may provide a clue to the physiological role of CRF2 pathways. For example, administration of non-selective CRF antagonists in the PVN of rats dramatically attenuated the anorectic effects of leptin (Gardner et al., 1998) and the melanocortin receptor type 4 receptor agonist MTII (Lu et al., 2003), suggesting that CRF pathways may be downstream mediators of several catabolic systems. Conversely, hypothalamic CRF pathways may functionally antagonize the anabolic effects of neuropeptide Y. Indeed, administration of a non-selective CRF antagonist in the PVN potentiated intra-PVN neuropeptide Y-induced feeding (Heinrichs et al., 1993; Menzaghi et al., 1993). More recent findings also suggest interplay between CRF and ghrelin systems in the control of food intake. For example, ghrelin expression is present in afferents to CRF-expressing neurons (Cowley et al., 2003). In addition, peripheral and intracerebroventricular ghrelin injection increases CRF mRNA expression in the hypothalamus of rats (Johnstone et al., 2005) and mice (Asakawa et al., 2001). Functional interactions between these systems in the control of gastrointestinal motility and anxiety-like responses to stressors have also been demonstrated (Asakawa et al., 2001, 2005; Chen et al., 2005). Therefore, we hypothesized that the CRF2 system could serve a counter-regulatory ‘brake’ function to oppose orexigenic ghrelin, as has been shown with regard to the CRF and neuropeptide Y systems (Heinrichs et al., 1993; Menzaghi et al., 1993). To address this issue, we studied diurnal food intake of non-food-deprived mice following peripheral injections of ghrelin or BIM-28131, a potent ghrelin receptor agonist. In WT mice, acute ghrelin injection transiently (007E1 h) stimulated food intake. Consistent with its greater in-vitro bioactivity and longer circulating half-life than ghrelin, BIM-28131 significantly increased food intake in WT mice at the 50 nmol / kg dose, at which ghrelin was ineffective, and induced a longer orexigenic effect. The orexigenic actions of ghrelin and BIM-28131 did not differ between genotypes, thereby not supporting the hypothesis that the CRF2 pathway limits the appetite-stimulating action of ghrelin.
In conclusion, constitutive genetic deletion of CRF2 receptors did not alter feeding behavior induced by food deprivation or acute stimulation of ghrelin receptors. In contrast, our data indicate an endogenous ‘pro-satiation’ role for CRF2 receptors in the control of meal size both during the active feeding period and after acute stress exposure.
Acknowledgements
This work was supported by grants from the Fondation pour la Recherche Médicale, Action Thématique Concertée ‘Nutrition’ from INSERM and from the Conseil regional d’Aquitaine. E.P.Z., W.W.V. and G.F.K. were supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases (DK64871, DK70118 and DK26741).
Abbreviations
- CRF
corticotropin-releasing factor
- CV
coefficient of variation
- KO
knockout
- Ucn
urocortin
- WT
wild-type
References
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, Katsuura G, Makino S, Fujino MA, Kasuga M. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74:143–147. doi: 10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54:18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagura S, Coscina DV. Influence of gastrointestinal loads on meal-eating patterns. J. Comp. Physiol. Psychol. 1969;69:101–106. doi: 10.1037/h0027939. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology. 2003;144:2580–2587. doi: 10.1210/en.2002-0091. [DOI] [PubMed] [Google Scholar]
- Bradbury MJ, McBurnie MI, Denton DA, Lee KF, Vale WW. Modulation of urocortin-induced hypophagia and weight loss by corticotropin-releasing factor receptor 1 deficiency in mice. Endocrinology. 2000;141:2715–2724. doi: 10.1210/endo.141.8.7606. [DOI] [PubMed] [Google Scholar]
- Carlin KM, Vale WW, Bale TL. Vital functions of corticotropin-releasing factor (CRF) pathways in maintenance and regulation of energy homeostasis. Proc. Natl Acad. Sci. U.S.A. 2006;103:3462–3467. doi: 10.1073/pnas.0511320103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Inui A, Asakawa A, Fujino K, Kato I, Chen CC, Ueno N, Fujimiya M. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129:8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Chen A, Zorrilla E, Smith S, Rousso D, Levy C, Vaughan J, Donaldson C, Roberts A, Lee KF, Vale W. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior. J. Neurosci. 2006;26:5500–5510. doi: 10.1523/JNEUROSCI.3955-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, Dumuis A, Brunner D, Bockaert J, Hen R. Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J. Neurosci. 2004;24:412–419. doi: 10.1523/JNEUROSCI.2806-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connan F, Lightman SL, Landau S, Wheeler M, Treasure J, Campbell IC. An investigation of hypothalamic-pituitary-adrenal axis hyperactivity in anorexia nervosa: The role of CRH and AVP. J. Psychiatr. Res. 2006;41:131–143. doi: 10.1016/j.jpsychires.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Contarino A, Gold LH. Targeted mutations of the corticotropin-releasing factor system: effects on physiology and behavior. Neuropeptides. 2002;36:103–116. doi: 10.1054/npep.2002.0899. [DOI] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale WW, Gold LH. Dissociation of locomotor activation and suppression of food intake induced by CRF in CRFR1-deficient mice. Endocrinology. 2000;141:2698–2702. doi: 10.1210/endo.141.7.7653. [DOI] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cullen MJ, Ling N, Foster AC, Pelleymounter MA. Urocortin, corticotropin releasing factor-2 receptors and energy balance. Endocrinology. 2001;142:992–999. doi: 10.1210/endo.142.3.7989. [DOI] [PubMed] [Google Scholar]
- Czimmer J, Million M, Tache Y. Urocortin 2 acts centrally to delay gastric emptying through sympathetic pathways while CRF and urocortin 1 inhibitory actions are vagal dependent in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G511–G518. doi: 10.1152/ajpgi.00289.2005. [DOI] [PubMed] [Google Scholar]
- Doyon C, Moraru A, Richard D. The corticotropin-releasing factor system as a potential target for antiobesity drugs. Drug News Perspect. 2004;17:505–517. doi: 10.1358/dnp.2004.17.8.863694. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, Koob GF, Zorrilla EP. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2006;32:1052–1068. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JD, Rothwell NJ, Luheshi GN. Leptin affects food intake via CRF-receptor-mediated pathways. Nat. Neurosci. 1998;1:103. doi: 10.1038/353. [DOI] [PubMed] [Google Scholar]
- Geary N. A new way of looking at eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1444–R1446. doi: 10.1152/ajpregu.00066.2005. [DOI] [PubMed] [Google Scholar]
- Gouthiere L, Claustrat B, Brun J, Mauvieux B. Complementary methodological steps in the analysis of rhythms: search of periods, modelling. Examples of plasma melatonin and temperature curves. Pathol. Biol. (Paris) 2005;53:285–289. doi: 10.1016/j.patbio.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Guss JL, Kissileff HR. Microstructural analyses of human ingestive patterns: from description to mechanistic hypotheses. Neurosci. Biobehav. Rev. 2000;24:261–268. doi: 10.1016/s0149-7634(99)00079-2. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J. Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Richard D. The role of corticotropin-releasing factor and urocortin in the modulation of ingestive behavior. Neuropeptides. 1999;33:350–359. doi: 10.1054/npep.1999.0047. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Hauger RL, Koob GF. Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Res. 1993;611:18–24. doi: 10.1016/0006-8993(93)91771-j. [DOI] [PubMed] [Google Scholar]
- Hotta M, Shibasaki T, Arai K, Demura H. Corticotropin-releasing factor receptor type 1 mediates emotional stress-induced inhibition of food intake and behavioral changes in rats. Brain Res. 1999;823:221–225. doi: 10.1016/s0006-8993(99)01177-4. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat. Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Guntz JM. Downregulation of corticotropin-releasing factor mRNA, but not vasopressin mRNA, in the paraventricular hypothalamic nucleus of rats following nutritional stress. Brain Res. Bull. 1997;43:509–514. doi: 10.1016/s0361-9230(97)80004-4. [DOI] [PubMed] [Google Scholar]
- Inoue K, Valdez GR, Reyes TM, Reinhardt LE, Tabarin A, Rivier J, Vale WW, Sawchenko PE, Koob GF, Zorrilla EP. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J. Pharmacol. Exp. Ther. 2003;305:385–393. doi: 10.1124/jpet.102.047712. [DOI] [PubMed] [Google Scholar]
- Jochman KA, Newman SM, Kalin NH, Bakshi VP. Corticotropin-releasing factor-1 receptors in the basolateral amygdala mediate stress-induced anorexia. Behav. Neurosci. 2005;119:1448–1458. doi: 10.1037/0735-7044.119.6.1448. [DOI] [PubMed] [Google Scholar]
- Johnstone LE, Srisawat R, Kumarnsit E, Leng G. Hypothalamic expression of NPY mRNA, vasopressin mRNA and CRF mRNA in response to food restriction and central administration of the orexigenic peptide GHRP-6. Stress. 2005;8:59–67. doi: 10.1080/10253890500095283. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Kissileff HR. Free feeding in normal and ‘recovered lateral’ rats monitored by a pellet-detecting eatometer. Physiol. Behav. 1970;5:163–173. doi: 10.1016/0031-9384(70)90060-0. [DOI] [PubMed] [Google Scholar]
- Kochavi D, Davis JD, Smith GP. Corticotropin-releasing factor decreases meal size by decreasing cluster number in Koletsky (LA / N) rats with and without a null mutation of the leptin receptor. Physiol. Behav. 2001;74:645–651. doi: 10.1016/s0031-9384(01)00610-2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Grace M, Levine AS. CRF antagonist partially reverses CRF- and stress-induced effects on feeding. Brain Res. Bull. 1986;17:285–289. doi: 10.1016/0361-9230(86)90233-9. [DOI] [PubMed] [Google Scholar]
- Krebs H, Macht M, Weyers P, Weijers HG, Janke W. Effects of stressful noise on eating and non-eating behavior in rats. Appetite. 1996;26:193–202. doi: 10.1006/appe.1996.0015. [DOI] [PubMed] [Google Scholar]
- Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J. Neurosci. 2003;23:7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Nishiyama M, Asaba K, Gold PW, Hashimoto K. Altered expression of type 2 CRH receptor mRNA in the VMH by glucocorticoids and starvation. Am. J. Physiol. 1998;275:R1138–R1145. doi: 10.1152/ajpregu.1998.275.4.R1138. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Heinrichs SC, Pich EM, Tilders FJ, Koob GF. Functional impairment of hypothalamic corticotropin-releasing factor neurons with immunotargeted toxins enhances food intake induced by neuropeptide Y. Brain Res. 1993;618:76–82. doi: 10.1016/0006-8993(93)90431-l. [DOI] [PubMed] [Google Scholar]
- Nakade Y, Tsuchida D, Fukuda H, Iwa M, Pappas TN, Takahashi T. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R427–R432. doi: 10.1152/ajpregu.00499.2004. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, Murphy B, Grigoriadis DE, Ling N, Foster AC. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J. Pharmacol. Exp. Ther. 2000;293:799–806. [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neuro-chemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J. Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard D, Lin Q, Timofeeva E. The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur. J. Pharmacol. 2002;440:189–197. doi: 10.1016/s0014-2999(02)01428-0. [DOI] [PubMed] [Google Scholar]
- Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Tache Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J. Med. Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Dallman MF, Woods SC. Hypothalamic response to starvation: implications for the study of wasting disorders. Am. J. Physiol. 1995;269:R949–R957. doi: 10.1152/ajpregu.1995.269.5.R949. [DOI] [PubMed] [Google Scholar]
- Sekino A, Ohata H, Mano-Otagiri A, Arai K, Shibasaki T. Both corticotropin-releasing factor receptor type 1 and type 2 are involved in stress-induced inhibition of food intake in rats. Psychopharmacology (Berl.) 2004;176:30–38. doi: 10.1007/s00213-004-1863-1. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Yamauchi N, Kato Y, Masuda A, Imaki T, Hotta M, Demura H, Oono H, Ling N, Shizume K. Involvement of corticotropin-releasing factor in restraint stress-induced anorexia and reversion of the anorexia by somatostatin in the rat. Life Sci. 1988;43:1103–1110. doi: 10.1016/0024-3205(88)90468-7. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Howell LA, Redmann S, Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am. J. Physiol. 1999;276:R1461–R1468. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- Spiegel TA. Rate of intake, bites, and chews – the interpretation of lean-obese differences. Neurosci. Biobehav. Rev. 2000;24:229–237. doi: 10.1016/s0149-7634(99)00076-7. [DOI] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Staub DR, Evans AK, Lowry CA. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF2) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Woods SC. The timing of meals. Psychol. Rev. 2004;111:128–141. doi: 10.1037/0033-295X.111.1.128. [DOI] [PubMed] [Google Scholar]
- Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J. Clin. Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva E, Picard F, Duclos M, Deshaies Y, Richard D. Neuronal activation and corticotropin-releasing hormone expression in the brain of obese (fa / fa) and lean (fa / ?) Zucker rats in response to refeeding. Eur. J. Neurosci. 2002;15:1013–1029. doi: 10.1046/j.1460-9568.2002.01942.x. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Varma M, Chai JK, Meguid MM, Gleason JR, Yang ZJ. Effect of operative stress on food intake and feeding pattern in female rats. Nutrition. 1999;15:365–372. doi: 10.1016/s0899-9007(99)00033-7. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Dringenberg HC, Comery TA. Rats (Rattus norvegicus) modulate eating speed and vigilance to optimize food consumption: effects of cover, circadian rhythm, food deprivation, and individual differences. J. Comp. Psychol. 1992;106:411–419. doi: 10.1037/0735-7036.106.4.411. [DOI] [PubMed] [Google Scholar]
- Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am. J. Physiol. Gastro-intest. Liver Physiol. 2004;286:G7–G13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin. Invest. Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Tache Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol. Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Reinhardt LE, Valdez GR, Inoue K, Rivier JE, Vale WW, Koob GF. Human urocortin 2, a corticotropin-releasing factor (CRF) 2 agonist, and ovine CRF, a CRF1 agonist, differentially alter feeding and motor activity. J. Pharmacol. Exp. Ther. 2004;310:1027–1034. doi: 10.1124/jpet.104.068676. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1450–R1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]