Abstract
Background & Aims
Cholesterol cholelithiasis is one of the most prevalent and most costly digestive diseases in developed countries and its incidence has markedly increased in Asian countries due to the adoption of Western-type dietary habits. Because animal experiments showed that high efficiency of intestinal cholesterol absorption contributes to gallstone formation, we explored whether the potent cholesterol absorption inhibitor ezetimibe could prevent gallstones and promote gallstone dissolution in mice and reduce biliary cholesterol content in humans.
Methods
Male gallstone-susceptible C57L mice were fed a lithogenic diet and concomitantly administered with ezetimibe at 0, 0.8, 4 or 8 mg/kg/day for 8 or 12 weeks. Gallbladder biles and gallstones were examined by microscopy. Gallbladder emptying in response to CCK-8 was measured gravimetrically. Biliary lipid outputs were analyzed by physical-chemical methods. Cholesterol absorption efficiency was determined by fecal dual-isotope ratio and mass balance methods. Lipid changes in gallbladder biles of gallstone patients vs. overweight subjects without gallstones were examined before (day 0) and at 30 days after ezetimibe treatment (20 mg/day).
Results
Ezetimibe prevented gallstones by effectively reducing intestinal cholesterol absorption and biliary cholesterol secretion, and protected gallbladder motility function by desaturating bile in mice. Treatment with ezetimibe promoted the dissolution of gallstones by forming an abundance of unsaturated micelles. Furthermore, ezetimibe significantly reduced biliary cholesterol saturation and retarded cholesterol crystallization in biles of patients with gallstones.
Conclusions
Ezetimibe is a novel approach to reduce biliary cholesterol content and a promising strategy for preventing or treating cholesterol gallstones by inhibiting intestinal cholesterol absorption.
Keywords: bile, bile flow, bile salt, biliary secretion, cholesterol absorption, chylomicron, Lith gene, nutrition
INTRODUCTION
Cholesterol gallstone formation represents a failure of biliary cholesterol homeostasis in which the physical-chemical balance of cholesterol solubility in bile is disturbed.1,2 Lithogenic bile is mainly caused by persistent hepatic hypersecretion of biliary cholesterol, which has both hepatic and small intestinal components. Obviously, in a person receiving no dietary cholesterol, all biliary cholesterol could be derived mainly from de novo synthesis. However, the contribution of de novo cholesterol synthesis to biliary cholesterol secretion is small, possibly less than 15%.3,4 The small intestine is a unique organ providing dietary and re-absorbed biliary cholesterol to the body.5
Epidemiological investigations have demonstrated that cholesterol cholelithiasis is prevalent in cultures consuming a “Western” diet consisting of high total calories, cholesterol, saturated fatty acids, refined carbohydrates, proteins, and salt, as well as low fibers, and its incidence in North and South American as well as European populations is significantly higher than that in Asian and African populations.6,7 Several clinical studies have found an association between the increased incidence of cholesterol gallstones in China and a “westernization” of the traditional Chinese diet.8–10 In Japan, cholesterol cholelithiasis once was rare, but over the past 40 years with the adoption of Western-type dietary habits, the incidence has increased markedly.11,12 Because biliary cholesterol hypersecretion is an important prerequisite for cholesterol gallstone formation,1,2 it is reasonable to assume that by inhibiting cholesterol absorption and/or hepatic uptake of chylomicron remnants, biliary cholesterol secretion and saturation could be significantly reduced. More recently, we observed that there is a significant and positive correlation between the efficiency of intestinal cholesterol absorption and the prevalence of cholesterol gallstone formation in 15 strains of inbred mice,5 suggesting that high efficiency of intestinal cholesterol absorption and high dietary cholesterol are two independent risk factors for cholesterol gallstone formation.
In this study, we investigated whether the potent cholesterol absorption inhibitor ezetimibe could prevent the formation of cholesterol gallstones, and facilitate the dissolution of gallstones in gallstone-susceptible C57L mice carrying Lith1 and Lith2 genes. Also, we studied whether ezetimibe may act as a potent biliary cholesterol-desaturating agent in patients with gallstones.
MATERIALS AND METHODS
Animals and diets
Male C57L/J mice, 6–8 weeks old, were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were provided free access to water and normal rodent chow containing trace (<0.02%) cholesterol (Harlan Teklad F6 Rodent Diet 8664, Madison, WI). For gallstone prevention studies, mice were divided into five groups (n=20 per group) fed 8 or 12 weeks with a lithogenic diet (1% or 2% cholesterol plus 0.5% cholic acid and 15% butter fat) supplemented with ezetimibe (Merck/Schering-Plough Pharmaceuticals, North Wales, PA) in doses of 0, 0.8, 4, or 8 mg/kg/day. We previously observed that at 12 weeks on 2% cholesterol plus 0.5% cholic acid and 15% butter fat, 100% of C57L mice formed cholesterol gallstones.13 For gallstone dissolution experiments, additional groups (n=20 per group) of mice that have formed cholesterol gallstones due to the 12-week feeding of 2% cholesterol plus 0.5% cholic acid and 15% butter fat, were fed the chow diet supplemented with ezetimibe in doses of 0, 0.8, 4, or 8 mg/kg/day for 8 weeks. All procedures were in accordance with current National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of Harvard University (Boston, MA).
Human study design
To observe whether ezetimibe reduces bile cholesterol content and saturation in humans, we studied patients with gallstones (n=7; 40.3±14.5 years old; 1 male and 6 females) and overweighted subjects without gallstones (n=5; 38.2±13.6 years old; 2 males and 3 females). All participants were examined by ultrasonography, which confirmed the existence of gallstones in the gallbladder. All subjects took 20 mg/day of ezetimibe daily for 30 days. During the study period, daily dietary cholesterol intake was approximately 500 mg. Gallbladder bile samples were collected endoscopically before (day 0) and at day 30 after medication. In brief, after an overnight fast and during a routine upper endoscopy, the tip of the endoscope was advanced to the second portion of the duodenum and the ampulla of Vater was visualized. Bile samples were harvested by stimulating gallbladder emptying using an intraduodenal infusion of 30 ml of an 80 g/L amino acid solution.14 A 20- to 30-ml sample of duodenal bile was obtained from each subject. Bile samples were frozen and stored at −20°C for further lipid analyses. Cholesterol saturation indexes (CSI) were determined after biliary cholesterol, phospholipid and bile salt concentrations, as well as bile salt species were measured.5,15 Detection times of plate-like cholesterol monohydrate crystals were investigated in these bile samples according to published methods.16 These studies were approved by the Human Subjects Committees at The Medica Sur Clinic and Foundation (Mexico City, Mexico), as conforming to the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from all participants before entry.
Microscopic studies of gallbladder biles and gallstones
At 8 or 12 weeks on the lithogenic diet supplemented with various doses of ezetimibe, a cholecystectomy was performed in overnight fasted mice (n=20 per group). Fresh gallbladder bile were examined for mucin gel, liquid crystals, plate-like cholesterol monohydrate crystals, sandy stones, and real gallstones, all of which were defined according to previously established criteria.17
Determination of biliary lipid outputs and bile salt pool sizes
The first hour collection of hepatic biles in additional groups of mice (n=5 per group) were used for biliary lipid secretion studies as described previously.18
Lipid analyses
Biliary cholesterol, phospholipid, total and individual bile salts, bile cholesterol, cholesterol content in chow and gallstones, and fecal neutral steroids were determined as described previously.16–20 The CSI values in gallbladder and hepatic biles were calculated from the critical tables.15
Measurement of intestinal cholesterol absorption by fecal dual-isotope ratio method
Nonfasted and nonanesthetized mice (n=10 per group) were given by gavage an intragastric bolus of 150 µl of medium-chain triglyceride containing 1 µCi of [14C]cholesterol and 2 µCi of [3H]sitostanol. The ratios of the two radiolabels in the fecal extracts and in the dosing mixture were used for calculating the percent cholesterol absorption.19
Cholesterol balance analysis
Because high dietary cholesterol exerts the effect of the radioisotope dilution on specific activity of cholesterol in the upper small intestine, we used cholesterol balance analysis19 to determine cholesterol absorption efficiency in mice challenged to the lithogenic diet. Mice (n=5 per group) were fed the lithogenic diet supplemented with various doses of ezetimibe for 7 days. After cholecystectomy was performed and the common bile duct was cannulated, hepatic bile was collected for the first hour of biliary secretion. Percent cholesterol absorption was calculated as described previously.19
Quantitative real-time PCR assay
Total RNA was extracted from fresh jejunum tissues of mice (n=4 per group) using RNeasy Midi (Qiagen, Valencia, CA). Primer Real-time PCR assays of Npc1l1, Abcg5 and Abcg8 for all samples were performed in triplicate.20 To obtain a normalized target value, the target amount was divided by the endogenous reference amount of rodent Gapdh as the invariant control.
Gallbladder contraction study
To explore whether ezetimibe has a protective effect on gallbladder motility function, we measured gallbladder contraction in response to exogenously administered sulfated CCK octapeptide (CCK-8) in mice (n=5 per group) fed the lithogenic diet supplemented with varying doses of ezetimibe for 8 weeks according to published methods.21 Gallbladder contractile function was determined by comparing the areas under the curves of bile flow rates and bile salt outputs, respectively, in the collected samples with mixed gallbladder and hepatic biles.
Statistical method
All data are expressed as means±SD. Statistically significant differences among groups of mice were assessed by Student’s t-test, Mann-Whitney U-tests, or Chi-square tests. If the F-value was significant, comparisons among groups of mice were further analyzed by a multiple comparison test. Analyses were performed with a SuperANOVA software (Abacus Concepts, Berkeley, CA). Statistical significance was defined as a two-tailed probability of less than 0.05.
RESULTS
Effect of ezetimibe on biliary cholesterol secretion in mice and humans
At 8 weeks on the lithogenic diet, administration of ezetimibe to mice resulted in a clear dose-dependent decrease in biliary cholesterol outputs during the first hour of biliary secretion of interrupted enterohepatic circulation (Figure 1A). Similarly, biliary phospholipid outputs were significantly reduced in a dose-dependent fashion (Figure 1B). However, hepatic outputs of biliary bile salts varied slightly and were not significantly different among four groups of mice (Figure 1C). We also observed a dose-dependent decrease in cholesterol/phospholipid ratios and cholesterol/bile salts ratios, indicating that bile cholesterol saturation was significantly reduced by ezetimibe.
Figure 1. Effect of ezetimibe on the prevention of cholesterol gallstones.
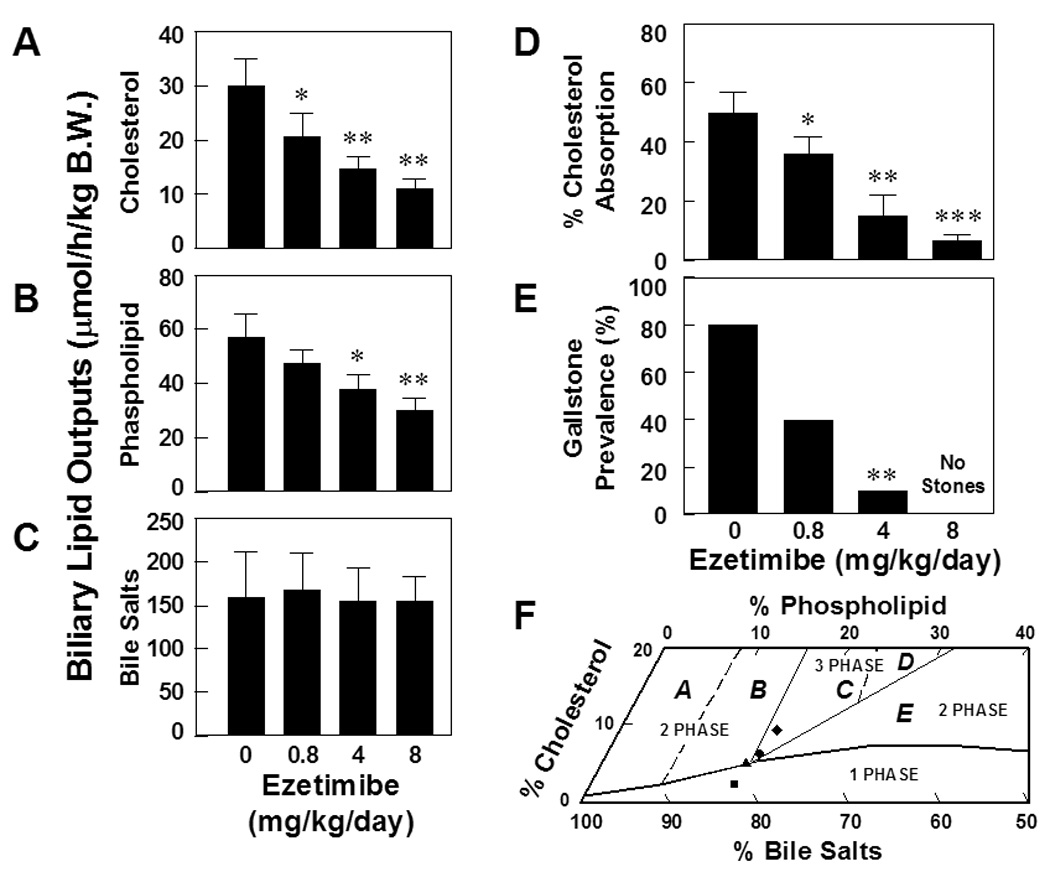
Ezetimibe significantly reduces, in a dose-dependent fashion, hepatic outputs of (A) biliary cholesterol and (B) phospholipid, but not (C) bile salts. *P<0.05, **P<0.01 and ***P<0.001, compared with mice fed the lithogenic diet and receiving no ezetimibe. (D) There is a clear dose-dependent reduction in intestinal cholesterol absorption efficiency from 50±6% to 4±2% in chow-fed mice, as measured by the fecal dual-isotope ratio method. (E) When doses of ezetimibe are increased from 0 to 4 mg/kg/day, gallstone prevalence rates are reduced from 80% to 10% in mice fed the lithogenic diet for 8 weeks. No gallstones are found in mice treated with ezetimibe at 8 mg/kg/day. (F) The relative lipid compositions of pooled gallbladder biles from mice fed the lithogenic diet and receiving no ezetimibe are located in the central three-phase zone, where biles are composed of solid cholesterol monohydrate crystals, liquid crystals, and saturated micelles at equilibrium.16 In contrast, administration of the highest dose (8 mg/kg/day) of ezetimibe results in the relative biliary lipid compositions of pooled gallbladder biles plotted in the one-phase micellar zone, even upon the lithogenic diet feeding for 8 weeks. By phase analysis, these biles are composed of unsaturated micelles at equilibrium.16 A symbol ♦ represents relative lipid compositions of pooled gallbladder biles at 8 weeks on the lithogenic diet supplemented with ezetimibe at 0; ● 0.8; ▲ 4; and ■ 8 mg/kg/day.
As shown in Table 1, at day 30 after ezetimibe treatment, plasma concentrations of total cholesterol in overweight subjects without gallstones and LDL cholesterol in gallstone patients were significantly reduced. Other parameters of plasma lipids were decreased slightly and did not reach significant statistical differences. Furthermore, mole percent cholesterol and cholesterol saturation index were significantly reduced in patients with gallstones, and to some extent, in overweighted subjects without gallstones. As a result, crystal detection times were significantly retarded in patients with gallstones and overweight subjects without gallstones. Mole percent phospholipid and bile salts in gallbladder biles were not influenced in humans treated with ezetimibe at 20 mg/day.
Table 1.
Plasma and biliary lipids before (day 0) and at day 30 after ezetimibe treatment in humans (20 mg/day)a
| Parameter | Overweight subjects without gallstones |
Gallstone patients |
||
|---|---|---|---|---|
| Before | After | Before | After | |
| BMI (kg/m2) | 31.5±3.8 | 31.4±3.4 | 27.0±2.8 | 27.1±2.3 |
| Plasma lipid concentrations | ||||
| Total Ch (mg/dL) | 220±41 | 168±29b | 223±32 | 193±26 |
| LDL Ch (mg/dL) | 144±53 | 99±36 | 145±26 | 115±23b |
| HDL Ch (mg/dL) | 44±13 | 37±13 | 45±11 | 45±11 |
| TG (mg/dL) | 164±88 | 160±104 | 166±64 | 165±76 |
| Biliary lipid compositions of gallbladder biles | ||||
| Ch (mole%) | 7.4±0.7 | 6.8±1.9 | 9.3±1.9 | 7.2±1.2b |
| PL (mole%) | 20.2±2.4 | 21.8±2.5 | 19.3±2.8 | 20.0±3.5 |
| BS (mole%) | 72.4±2.9 | 71.4±3.9 | 71.4±4.3 | 72.8±4.2 |
| Ch/PL | 0.37±0.03 | 0.31±0.08 | 0.48±0.05 | 0.37±0.06c |
| Ch/BS | 0.10±0.01 | 0.10±0.03 | 0.13±0.03 | 0.10±0.02 |
| [TL] (g/dL) | 5.3±0.4 | 5.0±0.9 | 5.5±0.7 | 5.3±0.8 |
| CSI | 1.2±0.1 | 1.0±0.2 | 1.6±0.2 | 1.3±0.2b |
| CDT (days) | 6.4±1.1 | 10.4±1.1c | 4.0±1.2 | 7.0±1.3c |
Values were determined from overweight subjects without gallstones (n=5) and gallstone patients (n=7).
P<0.05
P<0.01, compared with before ezetimibe treatment (paired t test).
Abbreviations: BMI, body mass index; TG, triglycerides; Ch, cholesterol; PL, phospholipids; BS, bile salts; [TL], total lipid concentrations; CSI, cholesterol saturation index; and CDT, crystal detection time.
Prevention of cholesterol gallstones by ezetimibe
Administration of ezetimibe induced a significant dose-dependent inhibition of intestinal cholesterol absorption in chow-fed mice as measured by the fecal dual-isotope ratio method (Figure 1D). Compared with the chow diet, cholesterol absorption efficiency was significantly increased in mice fed the lithogenic diet, because of the dietary cholic acid as discussed elsewhere.22,23 However, there was a significant dose-dependent inhibitory effect on intestinal cholesterol absorption by ezetimibe in mice challenged to the lithogenic diet, as measured by the mass balance method (Table 2). As expected, the lithogenic diet significantly increased mole percent cholesterol in gallbladder biles.17 In contrast, mole percent cholesterol in biles was gradually decreased with increasing doses of ezetimibe. Thus, the CSI values of pooled gallbladder biles were obviously reduced from 1.56 to 0.37 by ezetimibe (Table 3). As a result, there was a dose-dependent decrease in gallstone prevalence rates from 80% to 10% in mice treated with ezetimibe from 0 to 4 mg/kg/day (Figure 1E). Of special note is that no gallstones were found in mice treated with the highest dose of ezetimibe at 8 mg/kg/day. This protective effect of ezetimibe was also observed in mice fed the lithogenic diet with even higher (2%) amounts of cholesterol for a longer (12 weeks) period.
Table 2.
Cholesterol balance data in ezetimibe-treated mice in the lithogenic state
| Ezetimibe (mg/kg/day) | Cholesterol intake (mg/day) | Biliary cholesterol (mg/day) | Steroid excretion (mg/day) | Absorbed cholesterola(mg/day) | Cholesterol absorptionb(%) |
|---|---|---|---|---|---|
| 0 | 38.98±2.27 | 7.25±1.37 | 21.81±2.59 | 24.42±2.97 | 62.5±4.6 |
| 0.8 | 39.63±1.50 | 4.77±0.95c | 28.47±1.74d | 15.94±1.63d | 40.2±3.0e |
| 4 | 38.68±1.90 | 3.52±0.77d | 35.20±1.95e | 6.99±1.02f | 18.1±2.5g |
| 8 | 39.17±1.89 | 2.52±0.46e | 39.71±1.31f | 1.98±0.65f | 5.0±1.4g |
Absorbed cholesterol was determined by subtracting the daily fecal neutral steroid output from the daily cholesterol intake and the daily biliary cholesterol output as measured by the HPLC methods.19,20
The percent cholesterol absorption was determined by the cholesterol balance analysis according to published methods.19,20
P<0.05
P<0.01
P<0.001
P<0.0001
P<0.00001, compared with mice fed the lithogenic diet and receiving no ezetimibe.
Table 3.
Biliary lipid compositions of gallbladder and hepatic biles after gallstone formationa
| Ezetimibe (mg/kg/day) | Cholesterol (Mole%) | Phospholipid (Mole%) | Bile Salts (Mole%) | Cholesterol Phospholipid Ratio | Cholesterol Bile Salts Ratio | Total Lipid Concentration (g/dL) | CSI |
|---|---|---|---|---|---|---|---|
| Pooled gallbladder biles | |||||||
| 0 | 9.36 | 16.72 | 73.92 | 0.56 | 0.13 | 9.42 | 1.56 |
| 0.8 | 6.68 | 16.21 | 77.11 | 0.41 | 0.09 | 9.23 | 1.16 |
| 4 | 5.30 | 16.22 | 78.48 | 0.33 | 0.07 | 9.88 | 0.92 |
| 8 | 2.12 | 16.14 | 81.73 | 0.13 | 0.03 | 10.44 | 0.37 |
| Individual hepatic biles | |||||||
| 0 | 12.50±2.82 | 24.04±4.06 | 63.46±6.02 | 0.52±0.10 | 0.20±0.06 | 2.02±0.27 | 2.18±0.40 |
| 0.8 | 8.96±2.61 | 20.56±8.02 | 70.49±9.89 | 0.47±0.19 | 0.13±0.06 | 1.88±0.24 | 1.99±0.66 |
| 4 | 7.25±1.98b | 18.95±4.16b | 73.80±5.80 | 0.38±0.07b | 0.10±0.03b | 1.70±0.21 | 1.62±0.33 |
| 8 | 5.63±1.54d | 15.20±2.57c | 79.17±3.39 | 0.37±0.10b | 0.07±0.02c | 1.58±0.27 | 1.58±0.41b |
Values were determined from pooled gallbladder biles (n=20 per group) and five hepatic biles (the first hour of biliary secretion) per group.
P<0.05
P<0.01
P<0.001, compared with mice fed the lithogenic diet and receiving no ezetimibe.
Physical-chemical analysis of gallbladder and hepatic biles
Analyses of individual bile salt species by HPLC revealed that all bile salts in gallbladder and hepatic biles of mice were taurine conjugated with a similar distribution of bile salt compositions. In mice challenged to the lithogenic diet, taurocholate (58.7–70.1%) was the major bile salt of biliary pool, followed by taurodeoxycholate (6.9–17.6%) and taurochenodeoxycholate (7.8–14.0%). There was a low concentration in tauro-β-muricholate (2.9–6.4%), tauro-ω-muricholate (1.7–4.1%) and tauroursodeoxycholate (2.4–4.0%). Hydrophobicity indexes of bile salts in biles were essentially similar (−0.01 to +0.10) among four groups of mice. These results indicate that ezetimibe does not influence bile salt species in bile. The relative lipid compositions of pooled gallbladder biles from mice fed the lithogenic diet supplemented with ezetimibe at 0 or 0.8 mg/kg/day were located in the central three-phase area denoted Region C (Figure 1F). These biles were composed of solid cholesterol monohydrate crystals, liquid crystals and saturated micelles.16 Furthermore, with an increase in doses of ezetimibe, the relative lipid compositions of pooled gallbladder biles progressively shifted downward and to the left of the phase diagrams. These alterations were caused by a significant reduction in cholesterol content (Table 3). Consequently, administration of ezetimibe at 8 mg/kg/day resulted in the biliary lipid compositions plotted within the one-phase micellar zone. By phase analysis, these biles were composed of one phase, namely micellar bile, exactly as was observed experimentally in model biles.16 It indicates that the highest dose of ezetimibe can successfully prevent the formation of cholesterol gallstones in mice challenged to high dietary cholesterol.
Dissolution of cholesterol gallstones by ezetimibe
At 12 weeks on the lithogenic diet containing 2% cholesterol, 100% of male C57L mice formed cholesterol gallstones, with stone size being 0.72±0.33 mm. After the diet was changed to the normal rodent chow diet, gallstones were still present in 85% of mice (Figure 2A), with stone diameters in 0.63±0.28 mm. This indicates that spontaneous dissolution of gallstones did not occur after the lithogenic diet was replaced with the chow diet for 8 weeks (Figure 2B). When the chow diet was added with ezetimibe at 0.8 mg/kg/day, some mucin gel, solid and liquid crystals, sandy stones, and tiny real gallstones (0.27±0.14 mm in diameter) were observed in 5% of mice. Although preexisting cholesterol gallstones were completely dissolved by ezetimibe at 4 mg/kg/day, an abundance of liquid crystals and cholesterol monohydrate crystals were still detected in biles (Figure 2B). In contrast, in mice treated with ezetimibe at 8 mg/kg/day, gallbladder biles were transparent and their examination by polarizing light microscopy revealed no solid cholesterol crystals or gallstones (Figure 2B). It indicated that treatment with the highest dose of ezetimibe for 8 weeks resulted in complete gallstone dissolution rates of 100%. Compared with the chow diet, the CSI values, cholesterol/phospholipid ratios, and mole percent cholesterol of pooled gallbladder biles were decreased markedly by ezetimibe in a dose-dependent manner (Table 4). In addition, no differences in bile salt species and the hydrophobicity indexes were found in biles of these mice. Figure 2C plots the relative lipid compositions of pooled gallbladder biles before (day 0) and at week 8 after ezetimibe treatment on a taurocholate-rich bile phase diagram. The lipid compositions of pooled gallbladder biles with preexisting cholesterol gallstones in chow-fed mice still fell in the central three-phase zone. In contrast, the relative lipid compositions of biles from mice fed the highest dose of ezetimibe were located on the one-phase micellar zone in which bile is composed of unsaturated micelles.16
Figure 2. Effect of ezetimibe on the dissolution of cholesterol gallstones.
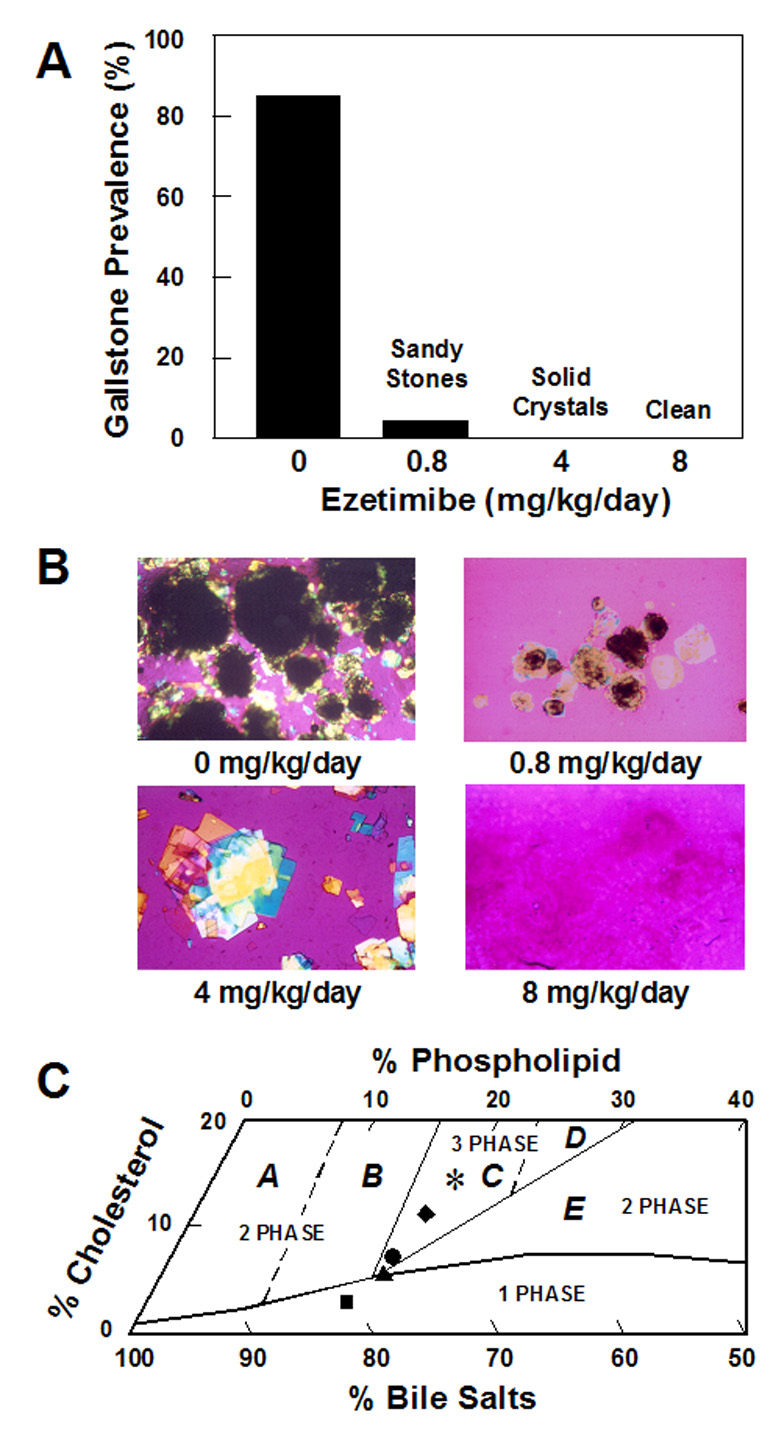
(A) For gallstone dissolution experiments, mice with the preexisting gallstones are fed the chow diet alone for 8 weeks, which does not result in a spontaneous dissolution of gallstones. In contrast, treatment with ezetimibe at 0.8 to 8 mg/kg/day induces rapid dissolution of gallstones. Gallstones are completely dissolved by the highest (8 mg/kg/day) dose of ezetimibe. (B) Representative photomicrographs of mucin gel, liquid crystals, cholesterol monohydrate crystals, and gallstones as observed in gallbladder biles at week 8 after ezetimibe treatment. All magnifications are ×800, except for ezetimibe treatment at 0 and 0.8 mg/kg/day, which are ×400, by polarizing light microscopy. (C) The relative lipid compositions of pooled gallbladder biles from mice fed 8 weeks with the chow diet supplemented with varying doses of ezetimibe are plotted on a condensed phase diagram. Because of a 12-week feeding of the lithogenic diet, the relative lipid compositions of pooled gallbladder biles from mice that have formed cholesterol gallstones are located in the central three-phase zone. Although the lithogenic diet is replaced with the chow diet for 8 weeks, the relative biliary lipid compositions of biles are still in Region C, where at equilibrium the biles are composed of solid cholesterol crystals, liquid crystals, and saturated micelles.16 By feeding varying doses of ezetimibe, the relative lipid compositions of pooled gallbladder biles gradually shift down, and finally, enter the one-phase micellar zone. These alterations explain that gallstones are dissolved through an unsaturated micelle mechanism. A symbol * represents relative lipid compositions of pooled gallbladder biles from mice that have preexisting gallstones and before ezetimibe treatment; ♦ relative lipid compositions of pooled gallbladder biles at end of gallstone dissolution study at week 8 of feeding the chow diet only (control); ● 0.8; ▲ 4; and ■ 8 mg/kg/day of ezetimibe.
Table 4.
Biliary lipid compositions of pooled gallbladder biles after gallstone dissolutiona
| (Ezetimibe mg/kg/day) | Cholesterol (Mole%) | Phospholipid (Mole%) | Bile Salts (Mole%) | Cholesterol Phospholipid Ratio | Cholesterol Bile Salts Ratio | Total Lipid Concentration (g/dL) | CSI |
|---|---|---|---|---|---|---|---|
| 0 | 11.46 | 17.51 | 71.02 | 0.65 | 0.16 | 9.41 | 1.82 |
| 0.8 | 7.41 | 15.65 | 76.95 | 0.47 | 0.10 | 9.03 | 1.32 |
| 4 | 5.79 | 16.92 | 77.30 | 0.34 | 0.07 | 9.35 | 0.98 |
| 8 | 3.62 | 15.68 | 80.70 | 0.23 | 0.04 | 8.43 | 0.67 |
Values were determined from pooled gallbladder biles (n=20 per group).
Amelioration of gallbladder contraction by ezetimibe
Under lithogenic diet feeding conditions, gallbladder sizes were significantly increased compared with the chow diet (Figure 3A). In the lithogenic state, gallbladder sizes were still enlarged in mice treated even with a low (0.8 mg/kg/day) dose of ezetimibe (Figures 3A and 3B). However, these lithogenic effects on gallbladder dynamics were fully blocked by ezetimibe in doses of higher than or equal to 4 mg/kg/day. Because CCK-8 stimulated gallbladder emptying and concentrated gallbladder bile was released, biliary bile salt output and bile flow rate were increased sharply and significantly only in mice treated with the highest (8 mg/kg/day) dose of ezetimibe. In contrast, these parameters were decreased when the doses of ezetimibe were reduced from 4 to 0 mg/kg/day. These alterations suggested that during lithogenesis, gallbladder motility function was impaired completely in mice fed the lithogenic diet and partially in mice treated with lower doses (≤4 mg/kg/day) of ezetimibe. In contrast, the administration of CCK-8 induces complete gallbladder emptying in mice treated with ezetimibe at 8 mg/kg/day (Figures 3C and 3D).
Figure 3.
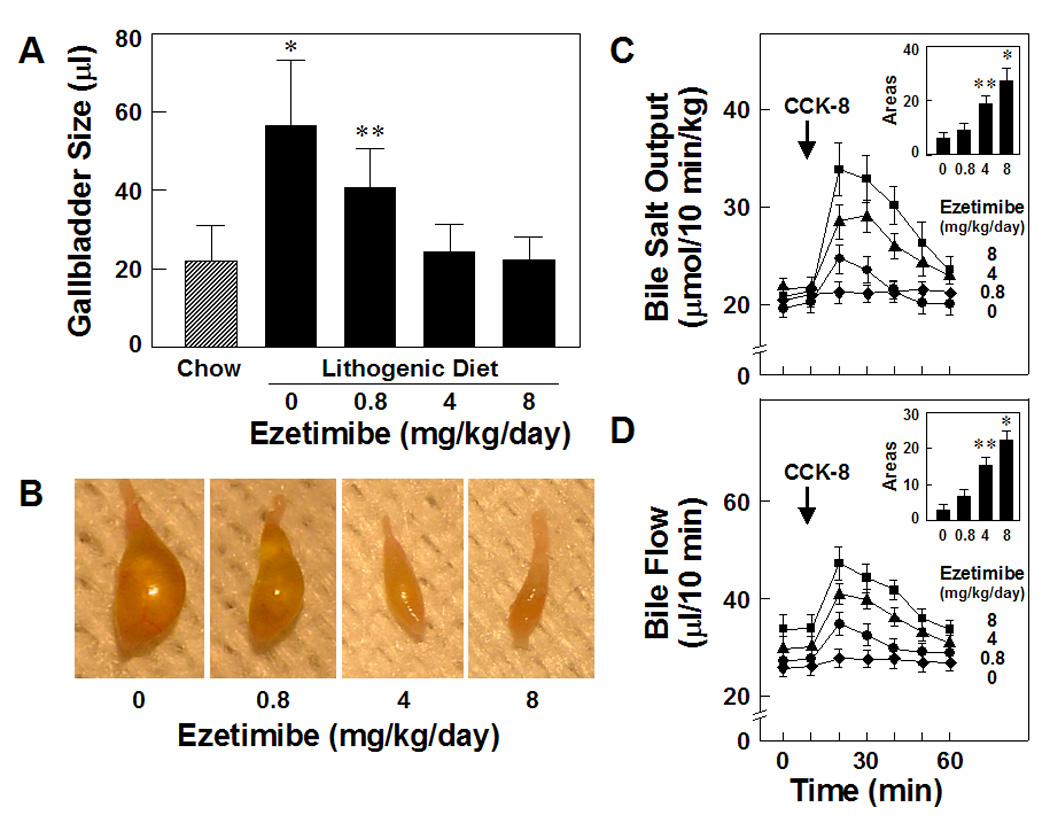
Fasting gallbladder volumes (A and B) are significantly increased by the lithogenic diet compared with the chow diet. However, these lithogenic effects on gallbladder dynamics are totally blocked by ezetimibe in doses of higher than or equal to 4 mg/kg/day. *P<0.01 and **P<0.05, compared with the chow group. (C and D) Because of gallbladder emptying and the release of a concentrated gallbladder bile, biliary bile salt output and bile flow are increased sharply and significantly in response to exogenously administered CCK-8 (as shown by arrows) in mice with bile fistulae. Gallbladder contractile function is completely impaired in mice fed the lithogenic diet for 8 weeks and partially in mice fed the lithogenic diet and concomitantly treated with ezetimibe at 0.8 or 4 mg/kg/day. In contrast, there is normal gallbladder contractile function in mice treated with the highest dose of ezetimibe at 8 mg/kg/day, even in challenge to the lithogenic diet. As shown in insets of Figures 3C and 3D, the areas under the curves of bile salt outputs and bile flow rates are significantly increased in a dose-dependent fashion in mice treated with ezetimibe. *P<0.01 and **P<0.05, compared with mice fed the lithogenic diet and receiving no ezetimibe.
Effect of ezetimibe on gene expression of intestinal sterol transporters
As shown in Figure 4, the data are expressed relative to the mRNA levels of intestinal lipid transporters in mice fed the lithogenic diet and receiving no ezetimibe, and their relative expression levels are set at 1. Treatment of ezetimibe at 8 mg/kg/day resulted in significant decreases in expression levels of the intestinal Abcg5, Abcg8 and Acat2 genes in mice on the lithogenic diet. It is highly likely that these changes are a secondary phenomenon due to reduced amounts of the absorbed cholesterol entering the enterocyte in ezetimibe-treated mice. Moreover, ezetimibe treatment significantly reduced expression levels of Npc1l1 in the small intestine.
Figure 4.
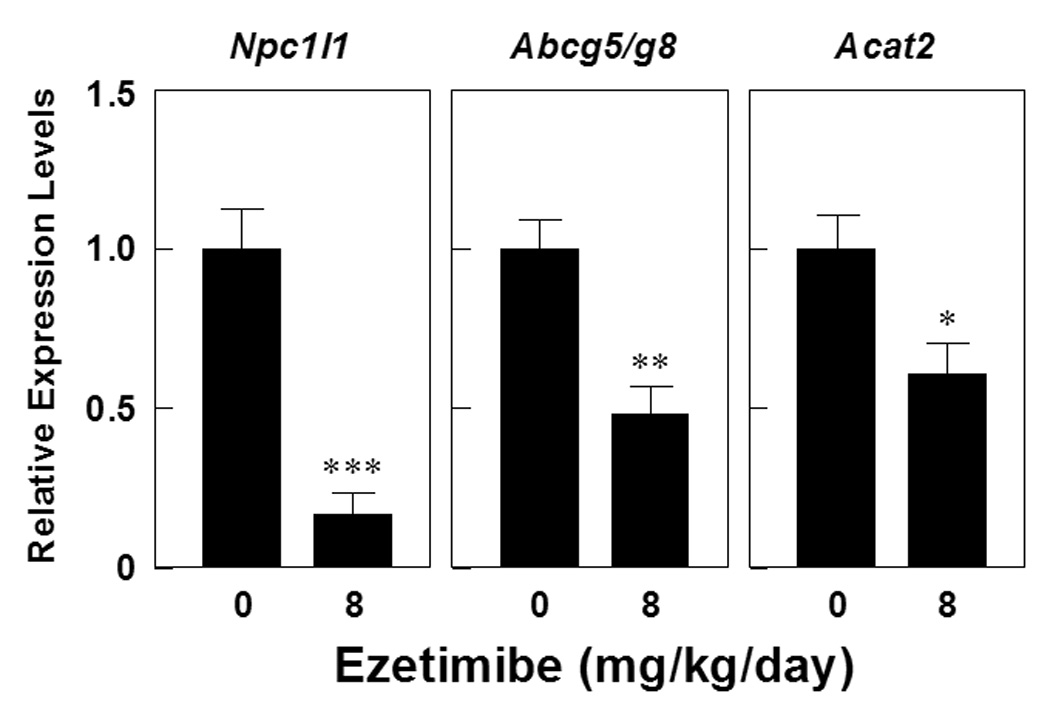
Upon the lithogenic diet feeding, compared with mice receiving no ezetimibe, mice treated with ezetimibe at 8 mg/kg/day display significantly reduced expression levels of Abcg5/g8 and Acat2, mostly attributable to secondary reaction in response to decreased amounts of the absorbed cholesterol. Also, ezetimibe treatment significantly reduces expression levels of Npc1l1 in the small intestine. *P<0.01, **P<0.001, and ***P<0.00001, compared with mice fed the lithogenic diet and receiving no ezetimibe.
DISCUSSION
Cholesterol cholelithiasis is a multifactorial disease influenced by a complex interaction of genetic and environmental factors.1,2 In mouse studies, targeted deletion of the acyl-CoA:cholesterol acyltransferase gene 2 (Acat2) results in the lack of cholesterol ester synthesis in the small intestine. This causes a marked reduction in intestinal cholesterol absorption and complete resistance to diet-induced cholesterol gallstones.24 Furthermore, absence of expression of intestinal APO-B48, but not APO-B100, reduces biliary cholesterol secretion and cholelithogenesis, possibly by decreasing intestinal absorption and hepatic bioavailability.25 Reduced gallstone prevalence in lithogenic diet-fed apolipoprotein E knockout mice may be explained by decreased availability of chylomicron-derived cholesterol in the liver for biliary secretion.26 These studies24–26 support the notion that high dietary cholesterol through the chylomicron pathway could provide an important source of excess cholesterol molecules for secretion into bile, thereby inducing cholesterol-supersaturated bile and enhancing cholelithogenesis. Since ezetimibe significantly suppresses cholesterol absorption from the small intestine via the NPC1L1 pathway,27 possibly a transporter-facilitated mechanism,28 this should diminish the cholesterol content of the liver, which in turn decreases bioavailability of cholesterol for biliary secretion. Indeed, we observed that ezetimibe induces a significant dose-dependent reduction in intestinal cholesterol absorption efficiency, coupled with a significant dose-dependent decrease in biliary cholesterol outputs and gallstone prevalence rates. In particular, even under high dietary cholesterol loads, cholesterol gallstones can be prevented in C57L mice treated with the highest dose of ezetimibe at 8 mg/kg/day.
We observed that although ezetimibe primarily reduces cholesterol content and to some extent phospholipid content but not in bile salt content in gallbladder bile, all crystallization pathways and phase boundaries on the bile phase diagram are essentially similar, regardless of whether mice are treated with or without ezetimibe. Of special note is that the relative lipid compositions of gallbladder biles from mice treated with varying doses of ezetimibe become appreciably less saturated with cholesterol and also contain relatively lower mole percent phospholipid. As a result, in company with increased doses of ezetimibe, the relative lipid compositions of pooled gallbladder biles are progressively shifted down and to the left of the phase diagram, as well as entering the one-phase micellar zone when the dose of ezetimibe is the highest at 8 mg/kg/day (see Figure 2C). The micellar zone contains an abundance of unsaturated micelles but never solid cholesterol crystals or liquid crystals.16 Under these circumstances, the micellar cholesterol solubility is dramatically increased in gallbladder bile. Thus, the cholesterol molecules could be transferred from the cholesterol monohydrate surface into unsaturated micelles so that gallstones become smaller and eventually dissolved. This provides an excellent physical-chemical explanation for the absence of solid cholesterol crystal formation in mice treated with the highest dose of ezetimibe (8 mg/kg/day) at week 8 of gallstone dissolution treatment.
In humans, the gallbladder is another key player in gallstone pathogenesis because enlarged fasting gallbladder volume, together with impaired postprandial and interdigestive gallbladder emptying, are frequent and distinctive features in gallstone patients.29,30 This type of “gallbladder stasis” provides time for nucleation of cholesterol crystals and their aggregation into macroscopic stones.1,2,30 It has become increasingly evident that the gallbladder epithelium could actively modify the lipid compositions of bile by secretion and absorption of lipids and water.31,32 Cholesterol-supersaturated bile facilitates gallbladder absorption of cholesterol and enhances the accumulation of excess cholesterol in the gallbladder wall.1,2 Because gallbladder absorptive cells apparently cannot assemble lipoproteins for lipid transport into plasma, the absorbed cholesterol is converted to cholesteryl ester and stored in the mucosa and lamina propria. Hence, excess cholesterol in smooth muscle cells could stiffen sarcolemmal membranes and decouple the G-protein-mediated signal transduction that usually ensues when CCK binds to its receptor, thereby further paralyzing gallbladder contractile function and consequently impairing gallbladder emptying function. In contrast, these lithogenic effects on gallbladder motility function can be totally blocked by ezetimibe in the highest dose at 8 mg/kg/day, mostly attributable to decreased biliary cholesterol content in bile.33
The hydrophilic bile acid, ursodeoxycholic acid (UDCA) has been recommended as first-line pharmacological therapy in a subgroup of symptomatic patients with small, radiolucent cholesterol gallstones,2,34 and its long-term administration has been shown to promote the dissolution of cholesterol gallstones and to prevent the recurrence of gallstones after extracorporeal shock wave lithotripsy.35 Despite major improvements in the treatment of cholesterol gallstones, optimal use of UDCA is not always achieved in clinical practice, because of failure to titrate the dose adequately. On the basis of animal studies, the natural trihydroxy hydrophilic bile acid of rodents, β-muricholic acid has recently been proposed for the prevention and the treatment of cholesterol gallstones.13 It should be emphasized that the cholelitholytic mechanism of ezetimibe is different from that of hydrophilic bile acids such as β-muricholic acid and UDCA. These hydrophilic bile acids favor the formation of vesicles in bile so that the growth of liquid crystals on the cholesterol monohydrate surface and their subsequent dispersion might occur during gallstone dissolution. As a result, liquid crystalline dissolution allows the transport of a great amount of cholesterol from stones. Our results indicate, therefore, that ezetimibe and hydrophilic bile acids promote the dissolution of cholesterol gallstones by two distinct mechanisms via the formation of an unsaturated micelle and a liquid crystalline mesophase, respectively.
Of note is that the NPC1L1 gene is expressed in livers of humans, but not in mice. More recently, Temel et al.36 found that mice transgenic for a human NPC1L1 gene display an increase in biliary cholesterol concentrations. This suggests that inhibition of NPC1L1 in the liver by ezetimibe may rescue biliary cholesterol secretion and increase CSI values in bile. However, in this study we observed that at day 30 after the medication, ezetimibe at 20 mg/day can significantly reduce cholesterol concentrations and CSI values of gallbladder biles in patients with gallstones, mostly attributable to its inhibitory effect on intestinal cholesterol absorption.27,28 As a result, cholesterol crystallization is retarded so that detection time of cholesterol monohydrate crystals is significantly delayed. Recent research on molecular transporters responsible for biliary lipid secretion suggests that the secretion efficiency of biliary cholesterol is most likely determined by the net effect between efflux and influx of cholesterol molecules across the canalicular membrane of the hepatocyte, which could be regulated by the ABCG5/G8-dependent and independent pathways as well as the NPC1L1 pathway.37 One possible reason for our results is that because biliary cholesterol secretion is a unique path for excretion of cholesterol from the body in humans and animals, hepatic ABCG5/G8 may play a stronger role in the regulation of biliary cholesterol secretion than NPC1L1. Another possible explanation is that in the gut-liver axis, the intestinal NPC1L1 plays a significant role in providing dietary and reabsorbed biliary cholesterol to the body and the inhibition of its functions by ezetimibe significantly reduces cholesterol absorption. So, the bio-availability of cholesterol from intestinal sources for biliary secretion is decreased markedly. In contrast, the blockage of the hepatic NPC1L1 by ezetimibe has a weak effect on biliary cholesterol secretion and CSI values, as we found in this study. Nevertheless, we are performing clinical studies to observe whether gallstones may completely dissolve after treating patients with ezetimibe for a longer period. To evaluate treatment time, response rate and overall cost-benefit analysis, a more detailed, long-term human study is requested as well. Furthermore, on the basis of our mouse study, it is highly likely that cholesterol gallstones could be dissolved faster by combination therapy of ezetimibe and UDCA in patients. Again, further studies are required to confirm this hypothesis.
Overall, our results show that ezetimibe prevents cholesterol gallstones through inhibiting intestinal cholesterol absorption so that biliary cholesterol secretion is reduced, and gallbladder motility function is preserved by desaturating bile in gallstone-susceptible C57L mice challenged to the lithogenic diet. Also, ezetimibe promotes the dissolution of cholesterol gallstones through a greater capacity to form an abundance of unsaturated micelles. Therefore, we conclude that ezetimibe is a novel and potential cholelitholytic agent for preventing or treating cholesterol gallstone disease. Our findings suggest a novel strategy for the prevention of cholesterol gallstones by inhibiting intestinal cholesterol absorption in humans.
Acknowledgments
This work was supported in part by research grants DK54012 and DK73917 (D.Q.-H.W.) from the National Institutes of Health (US Public Health Service), FIRB 2003 RBAU01RANB002 from the Italian Ministry of University and Research, ORBA060RME, and ORBA07Y0GT from the University of Bari (P.P.), and a grant from Medica Sur Clinic & Foundation (N.M-S). P.P. was a recipient of the short-term mobility grant 2005 from the Italian National Research Council (CNR).
Abbreviations
- ABC
ATP-binding cassette (transporter)
- CSI
cholesterol saturation index
- NPC1L1
Niemann-Pick C1 like 1 (protein)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper was presented in part at the Annual Meeting of the American Gastroenterological Association, Los Angeles, CA, in 2006 and published as an abstract in Gastroenterology 2006; 130: A85.
There is no conflict of interest to disclose for all authors.
All authors do not have a financial or other affiliation with Merck/Schering-Plough.
REFERENCES
- 1.Wang DQ-H, Afdhal NH. Genetic analysis of cholesterol gallstone formation: searching for Lith (gallstone) genes. Curr Gastroenterol Rep. 2004;6:140–150. doi: 10.1007/s11894-004-0042-1. [DOI] [PubMed] [Google Scholar]
- 2.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230–239. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 3.Wang HH, Afdhal NH, Wang DQ-H. Overexpression of estrogen receptor α increases hepatic cholesterogenesis, leading to biliary hypersecretion in mice. J Lipid Res. 2006;47:778–786. doi: 10.1194/jlr.M500454-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Turley SD, Dietschy JM. The contribution of newly synthesized cholesterol to biliary cholesterol in the rat. J Biol Chem. 1981;256:2438–2446. [PubMed] [Google Scholar]
- 5.Wang DQ-H, Zhang L, Wang HH. High cholesterol absorption efficiency and rapid biliary secretion of chylomicron remnant cholesterol enhance cholelithogenesis in gallstone-susceptible mice. Biochim Biophys Acta. 2005;1733:90–99. doi: 10.1016/j.bbalip.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Diehl AK. Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am. 1991;20:1–19. [PubMed] [Google Scholar]
- 7.Mendez-Sanchez N, Zamora-Valdes D, Chavez-Tapia NC, Uribe M. Role of diet in cholesterol gallstone formation. Clin Chim Acta. 2007;376:1–8. doi: 10.1016/j.cca.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Zhang S, Huang Z. The trend of the gallstone disease in China over the past decade. Zhonghua Wai Ke Za Zhi. 1995;33:652–658. [PubMed] [Google Scholar]
- 9.Huang YC, Zhang XW, Yang RX. Changes in cholelithiasis in Tianjin in the past 30 years. Chin Med J. 1984;97:133–135. [PubMed] [Google Scholar]
- 10.Huang ZQ. Characteristic features of cholelithiasis in China. A nationwide survey of 11342 surgical cases 1983–1985. Zhonghua Wai Ke Za Zhi. 1987;25:321–329. [PubMed] [Google Scholar]
- 11.Nagase M, Tanimura H, Setoyama M, Hikasa Y. Present features of gallstones in Japan. A collective review of 2,144 cases. Am J Surg. 1978;135:788–790. doi: 10.1016/0002-9610(78)90165-4. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama F, Miyake H. Changing state of gallstone disease in Japan. Composition of the stones and treatment of the condition. Am J Surg. 1970;120:794–799. doi: 10.1016/s0002-9610(70)80052-6. [DOI] [PubMed] [Google Scholar]
- 13.Wang DQ-H, Tazuma S. Effect of β-muricholic acid on the prevention and dissolution of cholesterol gallstones in C57L/J mice. J Lipid Res. 2002;43:1960–1968. doi: 10.1194/jlr.m200297-jlr200. [DOI] [PubMed] [Google Scholar]
- 14.Zoli G, Ballinger A, Healy J, O'Donnell LJ, Clark M, Farthing MJ. Promotion of gallbladder emptying by intravenous amino acids. Lancet. 1993;341:1240–1241. doi: 10.1016/0140-6736(93)91146-d. [DOI] [PubMed] [Google Scholar]
- 15.Carey MC. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978;19:945–955. [PubMed] [Google Scholar]
- 16.Wang DQ-H, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res. 1996;37:606–630. [PubMed] [Google Scholar]
- 17.Wang DQ-H, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: physical-chemistry of gallbladder bile. J Lipid Res. 1997;38:1395–1411. [PubMed] [Google Scholar]
- 18.Wang DQ-H, Lammert F, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: pathophysiology of biliary lipid secretion. J Lipid Res. 1999;40:2066–2079. [PubMed] [Google Scholar]
- 19.Wang DQ-H, Carey MC. Measurement of intestinal cholesterol absorption by plasma and fecal dual-isotope ratio, mass balance, and lymph fistula methods in the mouse: an analysis of direct versus indirect methodologies. J Lipid Res. 2003;44:1042–1059. doi: 10.1194/jlr.D200041-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Duan LP, Wang HH, Wang DQ-H. Cholesterol absorption is mainly regulated by the jejunal and ileal ATP-binding cassette sterol efflux transporters Abcg5 and Abcg8 in mice. J Lipid Res. 2004;45:1312–1323. doi: 10.1194/jlr.M400030-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Wang HH, Afdhal NH, Gendler SJ, Wang DQ-H. Evidence that gallbladder epithelial mucin enhances cholesterol cholelithogenesis in MUC1 transgenic mice. Gastroenterology. 2006;131:210–222. doi: 10.1053/j.gastro.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Wang DQ-H, Lammert F, Cohen DE, Paigen B, Carey MC. Cholic acid aids absorption, biliary secretion, and phase transitions of cholesterol in murine cholelithogenesis. Am J Physiol. 1999;276:G751–G760. doi: 10.1152/ajpgi.1999.276.3.G751. [DOI] [PubMed] [Google Scholar]
- 23.Wang DQ-H, Tazuma S, Cohen DE, Carey MC. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am J Physiol. 2003;285:G494–G502. doi: 10.1152/ajpgi.00156.2003. [DOI] [PubMed] [Google Scholar]
- 24.Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, Farese RV., Jr Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 25.Wang HH, Wang DQ-H. Reduced susceptibility to cholesterol gallstone formation in mice that do not produce apolipoprotein B48 in the intestine. Hepatology. 2005;42:894–904. doi: 10.1002/hep.20867. [DOI] [PubMed] [Google Scholar]
- 26.Amigo L, Quinones V, Mardones P, Zanlungo S, Miquel JF, Nervi F, Rigotti A. Impaired biliary cholesterol secretion and decreased gallstone formation in apolipoprotein E-deficient mice fed a high-cholesterol diet. Gastroenterology. 2000;118:772–779. doi: 10.1016/s0016-5085(00)70147-8. [DOI] [PubMed] [Google Scholar]
- 27.Davis HR, Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2001;21:2032–2038. doi: 10.1161/hq1201.100260. [DOI] [PubMed] [Google Scholar]
- 28.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 29.Portincasa P, Moschetta A, Colecchia A, Festi D, Palasciano G. Measurements of gallbladder motor function by ultrasonography: towards standardization. Dig Liver Dis. 2003;35 Suppl 3:S56–S61. doi: 10.1016/s1590-8658(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 30.Portincasa P, Di Ciaula A, Wang HH, Palasciano G, van Erpecum KJ, Moschetta A, Wang DQ-H. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology. 2008;47:2112–2126. doi: 10.1002/hep.22204. [DOI] [PubMed] [Google Scholar]
- 31.Portincasa P, Di Ciaula A, vanBerge-Henegouwen GP. Smooth muscle function and dysfunction in gallbladder disease. Curr Gastroenterol Rep. 2004;6:151–162. doi: 10.1007/s11894-004-0043-0. [DOI] [PubMed] [Google Scholar]
- 32.van Erpecum KJ, Wang DQ-H, Moschetta A, Ferri D, Svelto M, Portincasa P, Hendrickx JJ, Schipper M, Calamita G. Gallbladder histopathology during murine gallstone formation: relation to motility and concentrating function. J Lipid Res. 2006;47:32–41. doi: 10.1194/jlr.M500180-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Mathur A, Walker JJ, Al-Azzawi HH, Lu D, Swartz-Basile DA, Nakeeb A, Pitt HA. Ezetimibe ameliorates cholecystosteatosis. Surgery. 2007;142:228–233. doi: 10.1016/j.surg.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Tokyo Cooperative Gallstone Study Group. Efficacy and indications of ursodeoxycholic acid treatment for dissolving gallstones. A multicenter double-blind trial. Gastroenterology. 1980;78:542–548. [PubMed] [Google Scholar]
- 35.Sackmann M, Niller H, Klueppelberg U, von Ritter C, Pauletzki J, Holl J, Berr F, Neubrand M, Sauerbruch T, Paumgartner G. Gallstone recurrence after shock-wave therapy. Gastroenterology. 1994;106:225–230. doi: 10.1016/s0016-5085(94)95581-6. [DOI] [PubMed] [Google Scholar]
- 36.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang HH, Portincasa P, Wang DQ-H. Molecular pathophysiology and physical chemistry of cholesterol gallstones. Front Biosci. 2008;13:401–423. doi: 10.2741/2688. [DOI] [PubMed] [Google Scholar]


